-
Fine Flavor Cocoa (FFC) is characterized by a unique flavor quality with specific aromas such as fresh and browned fruit, floral, herbal, and woody (mentioned as aromatic cocoa)[1], and has a balanced chocolate (cocoa) taste[2]. So the taste and aroma are the main parameters of cocoa bean quality[3,4]. Based on aroma, there are two groups of flavor qualities in the cocoa trade, namely bulk (Forastero) and fine aroma cocoa (Criollo and Trinitario)[3,4]. The fine aroma class has a higher price and a more specific market than bulk cocoa. Therefore, increasing the added value of cocoa beans should be carried out by variety with a better fine aroma as one of the characteristics of fine flavor cocoa (FFC).
Flavor arises due to the presence of chemical compounds received by the primary receptors in the nasal and oral cavities to create taste, sensation (trigeminal), and aroma[5]. Various components of chemical compounds in cocoa beans in the form of volatile and non-volatile compounds[6] contribute in generating flavor during processing[4]. There are differences in character between non-volatile and volatile compounds. Non-volatile compounds are characterized by not being easily evaporated, and vice versa for volatile compounds. Volatile and non volatile compound and their composition have an impact on the flavor characteristics of cocoa beans. Non volatile compounds as primary metabolites are determined by secondary metabolites[7]. In addition, flavor precursors play a role in forming the final organoleptic quality of chocolate[8], therefore, it will affect the final flavor of the product.
Fermentation is a crucial phase in the processing of cocoa beans because it controls the synthesis of various flavor precursors, specifically aroma[2,3]. There are many techniques of fermentation to reach the optimum fermentation level, and it can be a strategy to induce the flavor of cocoa beans. Microbe treatment and pulp conditioning are reported to increase the flavor richness of cocoa beans[9]. Within the fermentation process, the pulp releases its contents which then enters to cotyledons and affects flavor precursors to decrease bitterness and astringency[10]. Optimal flavor development occurs at the end of the fermentation process, as indicated by the concentration and ratio of flavor-forming precursor compounds. These compounds are also closely related to the production of precursors during the roasting process[4]. A bitter and astringent base taste characterizes cocoa beans, and new specific flavors are formed during fermentation, drying, and roasting[2].
A previous study showed that there were differences in the sensory characteristics of cocoa liquor between the aromatic and non-aromatic groups which were fermented using a medium scale of fermentation[1]. The flavor of cocoa beans both on quality and intensity is influenced by genotype[2]. Genotype factor can influence the flavor precursors and it can affect to flavor formation of cocoa beans[10]. Flavor is affected by volatile (such as ester, pyrazine, pyran, pyrrole, aldehydes, etc) and non-volatile components such as polysaccharides[11], carbohydrates, polyphenols, alkaloids, proteins, and amino acids[4,12]. Flavor precursor components such as fat, protein, carbohydrates, polyphenols, amino acids, and sugars[2,13] contribute to aroma formation during roasting[13]. Polyphenols and alkaloids such as caffeine and theobromine are present in polyphenolic cells as single large[13]. Meanwhile, proteins, fats, and starch are in the lipid-protein cells, and they play a role in forming cocoa flavor and aroma character[14,15].
The composition and sensory quality of cocoa liquor comes from interaction among genotypes; fermentation and roasting[10]. During the fermentation and roasting process, there is the development of bean flavor, the removal of volatile acetic acid compounds, and decrease of water content of 1%−2%[16,17]. Differences in genotype or varieties have different chemical compounds in the cocoa beans. There were differences in the non-volatiles compounds including organic acids, levels of caffeine content, and the ratio of theobromine in fine (white beans) and bulk cocoa (purple beans)[15]. The chemical composition of each genotype influences the production of flavor precursors[8]. The differences in flavor precursors in different cocoa genotypes affect to flavor development[8]. Identification of non-volatile compounds which will be degraded to secondary metabolites is needed to identify the flavor potential between genotypes early. Various components from raw cocoa beans between aromatic and non-aromatic cocoa genotypes were hypothesized to produce different metabolite compounds that will participate in the formation of specific cocoa flavors. The study was conducted to identify the characteristics of non-volatile compounds between aromatic and non-aromatic Indonesian cocoa genotypes and it can be used as an indicator in the preliminary selection of breeding programs in Indonesia.
-
The research was conducted at the Indonesian Coffee and Cocoa Research Institute (ICCRI), Jember, East Java, Indonesia with climate type C to D according to Schmidt and Fergusson, an altitude of 45 m above sea level, average rainfall 2,400−2,500 mm/year and average temperature 24−30 °C. The study used the genetic materials that have been classified based on the sensory characteristics by the ICCRI's certified panelists following the International Standard for the Assessment of Cocoa Quality and Flavour (ISCQF). Based on the assessment the genotypes were divided into two groups based on Anita-Sari et al.[1] including aromatic cocoa genotypes consisting of 'DR 2', 'ICCRI 03', 'ICCRI 07', 'ICCRI 09', 'TSH 858', 'MCC 02', and non-aromatic genotypes including 'Sulawesi 1', 'Sulawesi 2', 'KW 516', and 'KEE 02' (Table 1)[1]. All genotypes are commercial clones and mostly have been released by the Indonesian Government. The research was arranged in a Randomized Complete Block Design (RCBD) with three blocks as replications. The experiment unit was made up of 20 trees in the productive stage (5-year-old) with a 3 m × 3 m spacing between them. Chemical compound analysis was conducted at the Food and Science Laboratory, Jember Polytechnic, East Java.
Table 1. Ten cocoa genotypes with different genetic backgrounds[1].
Genotype Description Flavor DR 2 Triniario, white beans, selection result from
the Criollo half-sib populationAromatic ICCRI 03 Trinitario, purple beans, selection result from the Trinitario population (DR 2 × Sca 6) Aromatic ICCRI 09 Trinitario, purple beans, selection result
from the Trinitario population
(TSH 858 × Sulawesi 1)Aromatic MCC 02 Trinitario, purple beans, exploration result in the Sulawesi region Aroamtic TSH 858 Trinitario, purple beans, introduction clone Aromatic ICCRI 07 Trinitario, purple beans, exploration result in North Sumatra Aromatic Sulawesi 1 Triniatrio, purple beans, exploration result in the Sulawesi region Non-aromatic Sulawesi 2 Trinitario, purple beans, exploration result in the Sulawesi region Non-aromatic KEE 02 Forastero, purple beans, introduction clone from PNG Non-aromatic KW 516 Forastero, purple beans, exploration result in North Sumatra Non-aromatic Fermentation and drying process
-
Approximately 100−150 cocoa pods were plucked to yield fresh cocoa beans for each replication. The cocoa pods were collected and broken after which the seeds and pulp were extracted from their pod layer and pooled in each experimental unit. The fresh pods did not undergo any storage treatment before the extracted bean. The process of fermentation was conducted using little wooden containers 70 cm × 70 cm × 50 cm with a capacity of 10 kg (medium scale of fermentation). The fermentation process was conducted using a natural approach. No starting culture was added during the four-day fermentation period. The fermentation mass was turned every 48 h, and the temperature ranged between 42−47 °C, depending on the specific genotypes. Following a 96-h duration, the beans were extracted and then arranged in a two-layer configuration on a tarpaulin. Then, the beans were subjected to a drying process inside a dryer until the moisture content reached a level of 7%. The fermentation results are tested by cut test methods to ensure the fermentation results. Bean sampling collected only the fully fermented beans, normal beans, and beans that were not defective (empty and destroyed by pests and disease) for further analysis.
Fermentation index analysis
-
The fermentation index (FI) of cocoa beans was determined using the methodology described by Romero-Cortes et al.[18]. A quantity of cocoa powder, about 50 mg, was combined with 5 mL of a methanolic solution containing 3% hydrochloric acid (HCl). The solution was subjected to vortexing and thereafter kept in a refrigerator for 18 h. The solution, exhibiting a reddish-brown color, was thereafter subjected to centrifugation at a speed of 2,383 g / 3,500 rpm for 10 min. The absorbance of the resulting supernatant was quantified using a Thermo Fisher SpectroNic 200 spectrophotometer at wavelengths of 460 and 530 nm. The fermentation index was determined by dividing the absorbance measured at 460 nm by the absorbance measured at 530 nm.
$\rm FI=\frac{Absorbance\;value\;at\;460\;nm}{Absorbance\;value\;at\;530\;nm} $ Values above one (1) indicate well-fermented beans, while values below 1 indicate insufficient fermentation[19]. According to SNI 2008, fermented beans may be identified by the presence of at least 3/4 of the cotyledon slice surface being brown, hollow, and possessing a distinct scent. On the other hand, unfermented beans can be recognized by at least 1/2 of the slice surface being slate gray or grayish blue and having a thick texture. Furthermore, the color of anthocyanin content is used as a reliable indication for the purpose of estimating the fermentation outcomes of cocoa beans. A cut test was conducted to determine and identify the fermentation process results and ensure that the bean samples will further analyze the fully fermented beans. The cut test was done following the SNI 2008.
Liquor sample
-
The paste samples were prepared based on the method of Misnawi & Ariza[20] from 500 g of cocoa beans sampled at each genotype for each replication. Cotyledons were roasted at a temperature of 120 °C for 12 min. The cotyledons were crushed and mashed for 15 min. The paste was packed and stored at 5 °C.
Sensory analysis
-
Sensory analysis was performed by three trained panelists according to the International Standard for the Assessment of Cocoa Quality Flavor[21]. Samples were served one by one at a temperature of 40−60 °C without sugar. The flavor attributes, including bitterness, astringency, acidity, cocoa, floral, fresh fruit, browned fruit, spicy, woody, nutty, and browned/roast, were evaluated on a scale of 0−10: 0−2: terrible; 2−4: bad; 4−6: ordinary; 6−8: good; 8−10: excellent.
Total polyphenol content
-
Total polyphenol was determined by the spectrophotometric method of Singleton & Rossi[22]. The fat-free sample and 40 mL of 80% acetone were placed in a glass beaker and sonicated for 30 min. The solution was kept cold during sonication by filling the vessel with ice water. The extract was obtained by vacuum filtration using Whatman filter paper no. 1, and a total of 1 mL extract was placed in a 100 mL glass beaker and dissolved with 70 mL distilled water. The extracted polyphenols were then reacted with 5 mL of Follin Ciocalteau 0.2 N reagent for 2 min. Furthermore, 15 mL of saturated Na2CO3 solution was added to stabilize the formed color and left for approximately 2 h before measuring at 765 nm using a spectrophotometer.
Total fat content
-
Fat content analysis was conducted at the Post-Harvest Laboratory, ICCRI using the procedure of Indonesia standard for cocoa beans[23]. Samples of 3−5 g cocoa beans were ground to a maximum size of 150 microns and then put into a 300 mL beaker. The samples were spread on filter paper and rolled to form a thimble, and the cotton was stored in a Soxhlet extractor. It was extracted for 6 h with 150 mL hexane solvent and then dried in an oven at 105 °C for 1 h. Residue was determined by weighing, and the fat content was calculated as follows:
$ \rm Fat\;content=\dfrac{Extracted\;fat\;weight}{Sample\;weight}\times 100{\text{%}} $ Caffeine and theobromine content
-
Theobromine and caffeine analysis was carried out at the Jember Polytechnic Bioscience Laboratory, using LCMS Shimadzu LCMT 2020 referring to the method of Brunetto et al.[24]. Fat-free cocoa bean powder samples were 6 g. Analytical sample preparation was conducted using 1 g of fat-free cocoa powder and 25 mL of distilled water before heating for 20 min at a temperature of 100 °C. The concentrations of caffeine and theobromine were determined using LCMS Shimadzu LCMT 2020. Furthermore, 10 microliters of sample were injected into the Water C18 column at 40 °C with a 0.8 mL/min and 1 mL/min flow rate for caffeine and theobromine, respectively.
Protein content
-
Protein analysis of cocoa beans was carried out using the Kjeldahl method according to the procedure developed by the Food Analysis Laboratory, Jember Polytechnic, East Java Province, Indonesia. First, the basis for determining nitrogen changed organic compounds by destruction using concentrated sulfuric acid in ammonium sulfate, then reacting with NaOH to form volatile NH4OH. Next, distillation was conducted using water vapor captured with boric acid, and then the results were measured by direct or back titration using acid or bases.
Carbohydrate content
-
Carbohydrate analysis of cocoa beans is the method developed by the Jember Polytechnic Food Analysis Laboratory. The analysis used 5 g of cocoa beans, with 200 mL of 3% KCl heated for 3 h before filtering the filtrate. First, measurement was carried out by making 100 ppm glucose stock solution with standard series of 0, 20, 40, 60, 70, and 100 ppm. Each sample and standard were added to 1 mL of Nelson's reagent and heated in a water bath for 20 min. After that, Arsenomolybdat reagent was added with 7 mL of Aquades, and carbohydrates were measured using a spectrophotometer at a wavelength of 540 nm.
Amino acid content
-
Amino acid analysis was carried out at the Jember Polytechnic Bioscience Laboratory through LCMS Shimadzu LCMT 2020, according to Apriyanto et al.[25]. The analysis consisted of several steps such as hydrolysis step, derivatization, and followed by chromatography analysis. The cocoa bean powder sample preparation used 500 mg with 6 N HCl. The filtrate was separated using a 0.45-micrometer membrane filter and analyzed using LCMS Shimadzu LCMT 2020. Finally, 10 microliter samples were injected into the Water C18 column at 40 °C with a 0.4 mL/min flow rate.
Statistical analysis
-
Data was analyzed by calculating the mean of non-volatile compounds in each tested genotype grouped based on Anita-Sari et al.[1], aromatic and non-aromatic genotypes group. Analysis of variance used orthogonal contrast analysis. Meanwhile, STAR 2.0.1 from IRRI for variance, Microsoft Excel 2019, R Studio for correlation analysis, PCA biplot, and heatmap were used for data analysis.
-
The cut test analysis showed that the aromatic group always reached sufficient fermentation level with 100% as categorized full fermented beans, while the non-aromatic genotypes showed different results (Fig. 1). The more the bean color shifts toward brown, the more fermentation has occurred, indicating proper flavor development. Properly fermented beans will have a uniform brown color inside, whereas under-fermented beans will retain a purplish hue. The fermentation index (FI) of the aromatic genotypes were significantly higher than non-aromatic genotypes. The fermentation index of cocoa beans (FI) is the magnitude or extent of fermentation attained, which can be quantified using several indicators including color, pH, and the degradation of bean constituents. This FI plays a vital role in establishing the ultimate taste characteristics of the cocoa beans and, as a result, the overall excellence of the chocolate. During fermentation, the color of cocoa beans changes from purple (unfermented) to brown (fully fermented). The extent of this color change indicates the degree of fermentation. The FI of the aromatic genotypes were significantly higher than non-aromatic genotypes.

Figure 1.
A cut test analysis for (a) ICCRI 09, and ICCRI 03 as aromatic groups, and (b) Sulawesi 1 and KW 516 as non-aromatic.
Non-volatile compound analysis
-
The results indicated differences in the content of non-volatile compounds between aromatic and non-aromatic genotypes (Table 2). The fat content, protein, fructose, and sucrose of the aromatic genotypes were lower than those of the non-aromatic genotypes. However, the fermented beans of aromatic genotypes showed higher in carbohydrate, caffein, and polyphenol content in non-volatile compounds and was higher in amino acid only in leucine-isoleucine content (Table 2) in amino acid compound than the non-aromatic group.
Table 2. The contrast test of non-volatile compounds between aromatic and non-aromatic clone groups.
Characters Mean ± SD F-value Aromatic Non-aromatic Non-volatile compounds Fermentation index (%) 1.27 ± 0.10 1.12 ± 0.07 * Polyphenol (%) 5.66 ± 0.24 5.17 ± 0.34 * Fat content (%) 52.25 ± 1.31 55.00 ± 0.90 * Carbohydrate (%) 22.08 ± 1.08 18.43 ± 0.93 * Caffein (%) 0.32 ± 0.02 0.30 ± 0.01 * Protein (%) 13.39 ± 0.16 14.51 ± 0.29 * Glucose (%) 1.04 ± 0.03 1.07 ± 0.03 * Fructose (%) 1.50 ± 0.04 1.53 ± 0.03 * Sucrose (%) 2.64 ± 0.04 2.74 ± 0.04 * Theobromine (%) 1.37 ± 0.05 1.38 ± 0.07 ns Amino acid compounds Aspartic acid (%) 0.2307 ± 0.0062 0.2488 ± 0.0066 ** Valine (%) 0.1341 ± 0.0041 0.1550 ± 0.0049 ** Leucine-isoleucine (%) 0.1503 ± 0.0049 0.1495 ± 0.0054 ** Alanine (%) 0.2182 ± 0.0046 0.2331 ± 0.0041 ** Lysine (%) 0.0353 ± 0.0024 0.0445 ± 0.0020 ns Methionine (%) 0.0950 ± 0.0028 0.0998 ± 0.0036 ** Proline (%) 0.1427 ± 0.0023 0.1598 ± 0.0034 * Lysine (%) 0.0353 ± 0.0024 0.0445 ± 0.0020 ns Glutamic acid (%) 0.2648 ± 0.0280 0.2729 ± 0.0077 ns Threonine (%) 0.0864 ± 0.0046 0.0954 ± 0.0068 ns ** significantly different at α = 1%, * significantly different at α = 5%, ns is not significantly different at α = 5%. Each cocoa genotype in Indonesia produced a unique flavor character and could be categorized into three groups based on flavor characters: aromatic genotypes with weak taste, aromatic genotypes with strong taste, and non-aromatic group (Table 3)[1]. Beans that are not fully fermented will retain a high level of polyphenols, resulting in astringency and bitterness. The flavor can also be acidic and raw, lacking complexity.
Table 3. Flavors attribute of aromatic and non-aromatic group of Indonesia cocoa genotypes[1].
Flavor attribute Average of flavors score Aromatic Non-aromatic Taste Cocoa 6.42 ± 0.71 5.63 ± 0.3 Acidity 2.25 ± 0.85 2.00 ± 0.20 Bitterness 3.75 ± 0.52 4.09 ± 0.37 Astringency 3.48 ± 0.46 4.06 ± 0.81 Aroma Fresh fruit 2.54 ± 0.73 1.81 ± 0.13 Browned fruit 2.19 ± 0.62 1.84 ± 0.28 Floral 1.98 ± 0.30 1.28 ± 0.48 Woody 2.10 ± 0.23 1.88 ± 0.32 Spicy 1.00 ± 0.22 0.75 ± 0.20 Nutty 2.29 ± 0.43 1.88 ± 0.43 Sweet 0.77 ± 0.67 0.63 ± 0.25 Browned/roasted 5.44 ± 0.42 5.47 ± 0.26 Dirty/dusty 0.00 ± 0.00 0.44 ± 0.24 Meaty/animal 0.00 ± 0.00 0.00 ± 0.00 Smoky (drying smoke) 0.00 ± 0.00 0.00 ± 0.00 Mouldy 0.00 ± 0.00 0.25 ± 0.29 Correlation and PCA analysis
-
The non-volatile chemicals present in cocoa beans are shown to be significantly correlated with the flavor characteristics of these beans. The fat content had a notable positive relation with bitterness, while also displaying an adverse relationship with the degree of chocolate taste. A statistically significant positive correlation was seen between the carbohydrate content and fructose acidity. Statistically significant positive correlations were seen between the bitterness level and the levels of polyphenol, fat, sucrose, and theobromine (Table 4).
Table 4. Pearson correlation of flavor (taste and aroma) profiles and non-volatile compounds of cocoa bean paste.
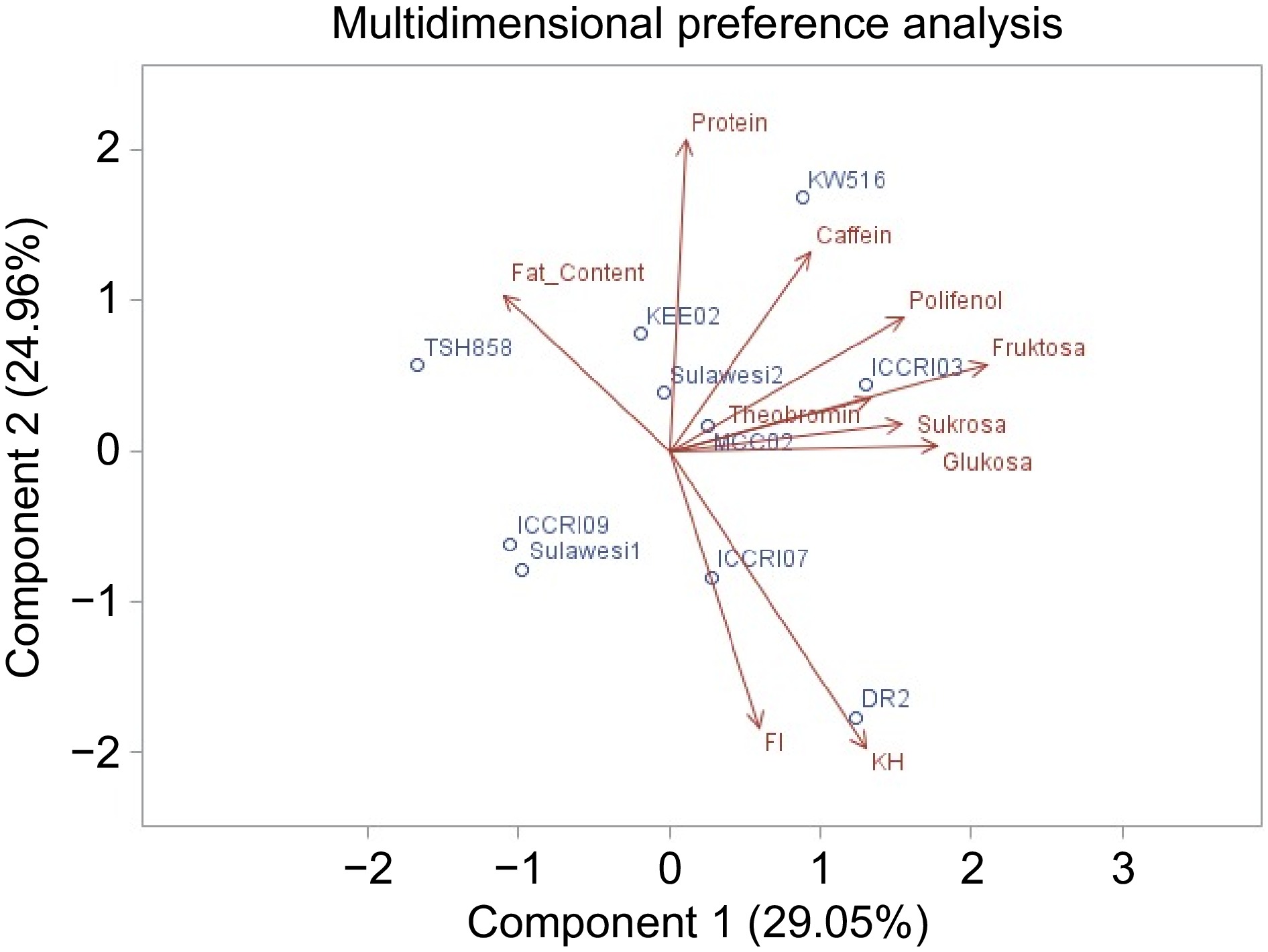
FI Pol FC Ch Thb Pro Fru Suc Taste Cocoa −0.17 0.14 −0.59* 0.19 −0.31 −0.12 0.00 −0.29 Acidity −0.52 −0.16 0.12 −0.57* −0.50 0.17 −0.58* −0.48 Bitterness 0.00 0.58* 0.56* −0.13 0.57* 0.21 0.45 0.57* Astringency −0.56* 0.37 0.47 −0.58* 0.05 0.71* 0.00 0.35 Aroma Fresh fruit −0.07 −0.02 −0.13 −0.05 −0.64* −0.23 −0.55 −0.46 Browned fruit −0.49 0.16 −0.60 0.26 −0.69* −0.15 −0.08 −0.33 Floral 0.30 0.09 −0.44 0.52 −0.13 −0.51 0.03 −0.59 Woody −0.27 0.36 −0.37 0.57* −0.12 −0.45 0.33 −0.14 Spicy 0.17 −0.18 −0.83 0.69 −0.41 −0.48 −0.01 −0.46 Nutty 0.50 −0.10 −0.24 0.46 0.10 −0.43 0.01 −0.60 Sweet 0.43 −0.16 0.14 0.09 0.07 −0.43 0.09 0.02 Browned roasted −0.59 0.15 −0.25 0.17 −0.32 0.14 0.22 0.01 Dusty −0.02 −0.36 0.34 −0.48 0.33 0.66* 0.21 0.44 Putrid −0.42 −0.08 0.05 0.18 −0.62 −0.42 −0.10 −0.10 Moldy −0.09 −0.44 0.00 −0.17 −0.09 0.17 0.00 0.17 Global quality 0.13 0.32 −0.57 0.46 −0.09 −0.35 0.05 −0.32 FI: Fermentation index, Pol: polyphenol, FC: Fat Content, Ch: Carbohydrate, Thb: theobromine, Pro: protein, Fru: fructose, Suc: sucrose, * significantly different at α = 5%, sample size (n) on all characters = 10 sample. The PCA analysis showed that each aromatic genotype had different non volatile compounds. ICCRI 09 has a similar content of non-volatile compounds close to TSH 858 as the parent (Fig. 2).
Cluster analysis was performed based on non-volatile taste precursors, including carbohydrates, fermentation index, theobromine, sucrose, fructose, and amino acids (Fig. 3). The results showed that there were four genotype groups based on the content of non-volatile taste precursors, namely group 1 (DR2), group 2 (KW 516, KEE 02, Sulawesi 2), group 3 (ICCRI 03, MCC 02), and group 4 (TSH 858, Sulawesi 1, ICCRI 07, ICCRI 09). While the cluster analysis based on sensory showed three groups of genotypes, including groups with weak and aromatic taste characters (DR 2), medium and aromatic taste characters (ICCRI 09, ICCRI 03, MCC 02, ICCRI 07, TSH 858), and strong taste characters but not aromatic (Sulawesi 1, KEE 02, KW 516, Sulawesi 2)[9]. Grouping based on non-volatile taste precursors (Fig. 3) and sensory (Fig. 4) resulted in a few different clusters, due to the Sulawesi 1 clone. This clone was in a non-aromatic group. However, based on non-volatile taste precursors, Sulawesi 1 was in a similar group with TSH 858, Sulawesi 1, ICCRI 07, and ICCRI 09. DR 2 as fine cocoa (white bean color) showed close with the non-aromatic group due to the optimum fermentation of DR 2 reached faster than Trinitario purple bean. In these results, the decreasing flavor profile of DR 2 has impacted the fermentation process. The optimum fermentation reached is essential for controlling the flavor profile. Fermentation defects can arise during the fermentation process and negatively impact cocoa bean quality including off-flavors, off-notes, or undesirable sensory attributes[26].
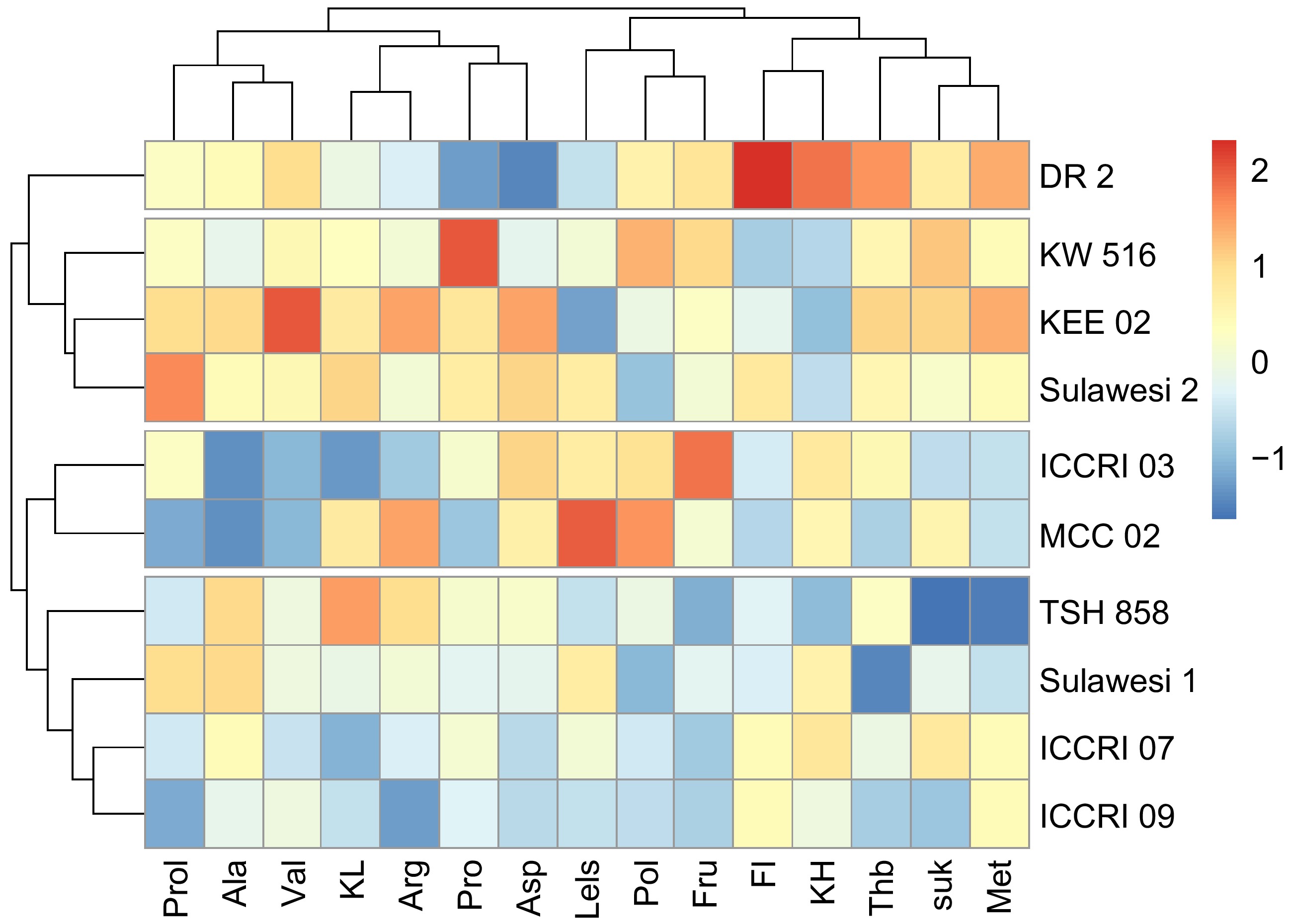
Figure 3.
Non-volatile compounds and amino acids of 10 cocoa clones based on hierarchical clustering analysis. Prol: proline (%), Ala: alanine (%), Val: valine (%), KL: fat content, Arg: arginine (%), Pro: protein, Asp: aspartic acid (%), LeIs: leucine-isoleucine (%), Pol: polyphenols, Fru: fructose, FI: Fermentation index, KH: Carbohydrate, Thb: theobromine, Suk: sucrose, Met: methionine (%).
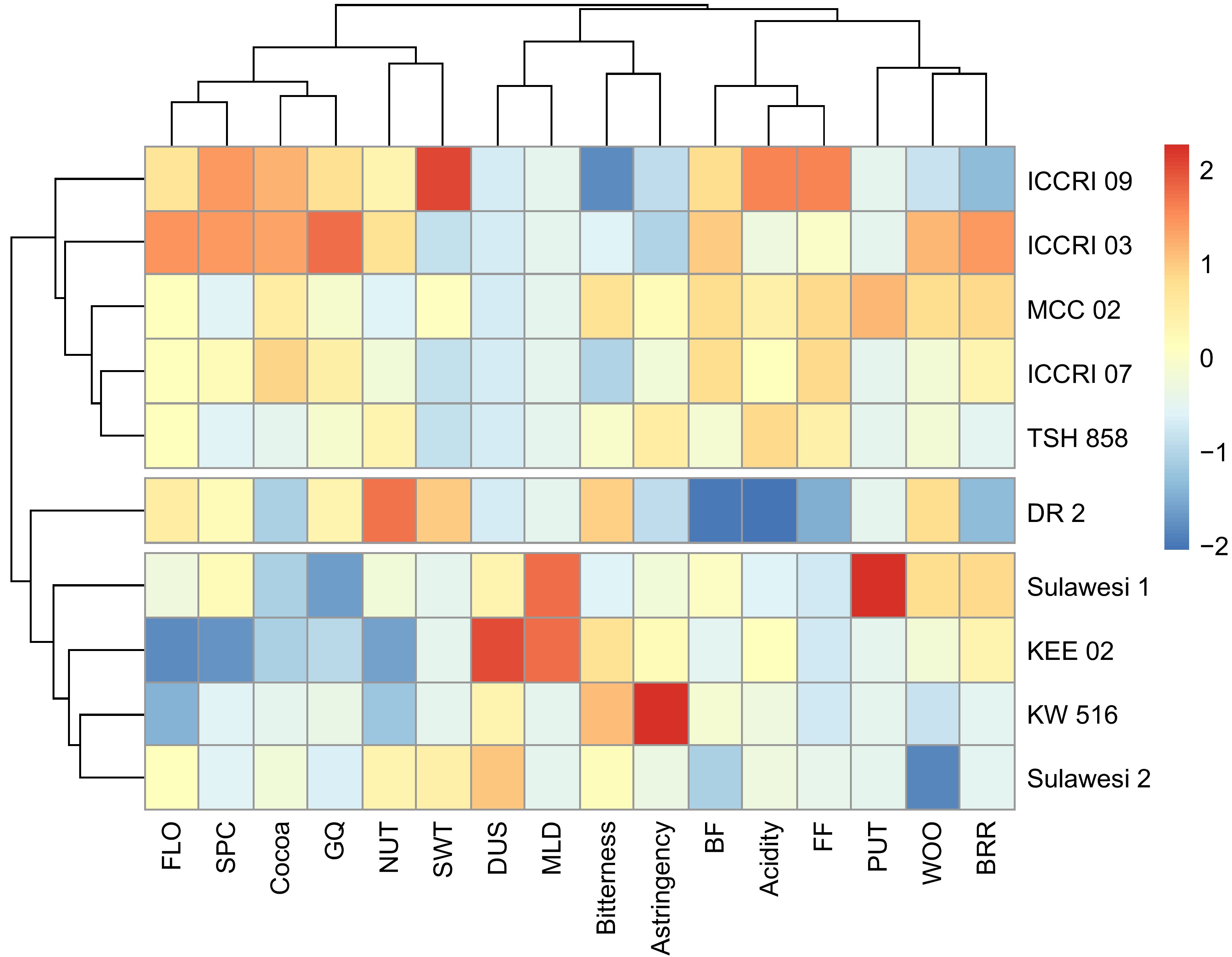
Figure 4.
Sensory compounds taste and aroma attributes of 10 cocoa clones based on hierarchical clustering analysis. FLO: Floral, SPC: Spicy, GQ: Global Quality, NUT: Nutty, SWT: Sweet, DUS: Dirty/Dusty, MLD: Moldy, BF: Browned Fruit, FF: Fresh Fruit, PUT: Putrid/Over Fermented, WOO: Woody, BRR: Browned/Roast.
-
The process of fermentation plays a vital role in the post-harvest conversion of cocoa beans, exerting a substantial impact on their biochemical makeup and ultimately shaping the flavor and taste of chocolate. The fermentation process initiates a sequence of biochemical transformations that give rise to the favorable characteristics of cocoa. During fermentation, microbial activity (mainly yeast, lactic acid bacteria, and acetic acid bacteria) breaks down the sugars and pulp surrounding the cocoa beans, leading to several critical biochemicals. Lactic acid and acetic acid produced by bacteria diffuse into the beans, altering their internal pH and leading to biochemical changes, particularly in the proteins and polyphenols (tannins) inside the beans.
Figure 1 showed the higher fermentation level in aromatic genotypes indicated that the fermentation can induce the flavor precursor development and affect taste and aroma formation. The FI differs between aromatic and non-aromatic cocoa beans due to their distinct biochemical compositions and flavor development needs. Aromatic beans require more precise control of the fermentation process to preserve their delicate flavor precursors, whereas non-aromatic beans undergo longer fermentation to develop strong, chocolatey flavors. FI is determined by pulp content per bean and shows the ratio of polyphenol and anthocyanins[27]. Non-aromatic genotypes (included in Forastero) have a greater pulp than Trinitario and Criollo[28]. The consequences, a longer period to achieve optimum fermented bean is needed. Optimum fermentation will produce better flavor, aroma, and color[28], as the impact of the flavor precursors development during the fermentation process. The previous study reported that FI has a relationship with the intensity of bitterness, sour, and astringent[29]. Optimum levels of FI decreased bitterness, sour, and astringent. Sufficient FI with a value of 1 (one) suppressed astringent intensity. The rate of cocoa bean fermentation was also reported to affect aroma precursor formation through amino acid formation, and sucrose reduction[3]. Properly fermented fine-flavor beans result in complex, layered profiles with floral, fruity, nutty, and sometimes spicy notes. Beans that have undergone optimal fermentation exhibit a balance of flavors. The bitterness and astringency are significantly reduced, and complex flavor notes such as fruity, floral, nutty, and sometimes spicy characteristics are well-developed.
The differences in non-volatile compounds between aromatic and non-aromatic genotypes indicate differences in the flavor precursors of the two genotype groups. The level and composition of compounds has an impact on the flavor characteristics of cocoa beans. Identification of the compounds that influence the flavor characteristics is necessary to support the selection program of cocoa genotypes.
Polyphenol content was indicated by a decrease in purple and an increase in brown color[28,30]. Aromatic cocoa beans have a higher concentration of polyphenols (antioxidants) compared to bulk cocoa as shown in Table 2. Polyphenols, responsible for astringency and bitternesse[19,31], undergo oxidation during fermentation. Polyphenols and theobromine affect the intensity of astringent and bitterness[17,32]. This process reduces the bean's bitterness and astringency, making the chocolate smoother and more palatable. The oxidation also contributes to the development of desirable flavor precursors. Bulk cocoa contains fewer polyphenols than aromatic beans, and the fermentation process aims to reduce bitterness and astringency. This result is in line with a previous study by Rusconi & Conti[33], the aromatic genotypes tended to have higher polyphenol content than non-aromatic groups. The National variety as fine flavor cocoa also showed higher polyphenol content than Forastero[31]. The higher amount of polyphenols in the aromatic group indicated can induce rich aromatic flavor, since polyphenols, proteins, polysaccharides, and alkaloids determine cocoa beans' color, flavor, and aroma[32,33]. At optimum conditions, a concentration of polyphenols is required to obtain the highest amount of flavor precursors, such as amino acids and sugars, which are essential attributes in the formation of cocoa flavor. The concentration of polyphenols also affects sucrose and glucose content[33] and sensory characteristics of cocoa beans, including flavor in cocoa, bitterness, astringency, and aroma, such as fruity, floral, raw/green, and smoky[34]. However, it is usually believed that the polyphenol content adds to the astringent effect and does not contribute to the flavor in tiny doses[35]. There was a relationship between polyphenol content, and the fruity aroma formed[1], however this study did not show a significant relationship between polyphenols and cocoa bean aroma. On the other hand, the theobromine had a linear relationship between the intensity of fresh and brown fruit. The fresh fruit was in line with a high level of brown fruit aroma[1].
One of the most important aspects of chocolate processing and quality is the fat content of cocoa beans, which is mostly made up of cocoa butter. The fat content of aromatic cocoa beans typically ranges from 50% to 55% of the bean's dry weight. Criollo beans are known for their slightly lower fat content compared to other varieties. In this research, the fat content of aromatic genotypes was lower than non-aromatic groups. This finding was linear with other research results that Forastero cocoa (bulk cocoa) had a higher fat content than Trinitario-Criollo[15]. The fine-flavored cocoa from Peru has also a low-fat content[36]. Cocoa butter is a carrier and solvent for particles in the paste sugar and other ingredients[37]. The fat content itself doesn't contribute directly to the flavor, the presence of high-quality cocoa butter in fine-flavor beans helps in delivering a smoother mouthfeel and enhances the overall sensory experience of the chocolate. In addition, non-aromatic beans are more suitable for large-scale chocolate production due to their consistent fat content and yield. Meanwhile, the fat content of cocoa beans affects the number of volatile chemicals released. Based on correlation analysis, the level of fat was significantly correlated to the woody aroma of cocoa beans, while protein content tended to be related to the dusty aroma.
In fine-flavor cocoa beans, the caffeine contributes to the slight bitterness, but it is not a dominant factor in the overall flavor profile. The result showed the caffeine of aromatic beans significantly more than non aromatic beans. This results in accordance with others' results where the percentage of caffeine in Java Fine Flavor Cocoa (white bean) is higher than bulk cocoa[15]. Criollo, fine flavor cocoa (white bean) has high caffeine levels and low theobromine levels, while Forastero (bulk cocoa) shows the opposite. Furthermore, Trinitario has caffeine and theobromine levels between Criollo and Forastero[15]. The content of caffeine and theobromine contributes to the bitter flavor of cocoa beans[28]. Caffeine was suspected to play more role in forming a cocoa flavor; while theobromine has a more negligible effect than caffeine[38]. In bulk cocoa, caffeine contributes to the characteristic bitterness of the beans but is the more dominant stimulant.
The sugars play an essential role in the fermentation process, as they are broken down by microorganisms to produce acids, which contribute to flavor development. Sugar components change into organic acids and volatile compounds during fermentation, which are precursors of flavor and color[28]. Fine-flavor beans may have slightly higher sugar content compared to bulk beans, which contributes to the generation of more complex flavor precursors during fermentation. The Criollo and Trinitario groups showed a higher reduction in sugar concentration than Forastero[16]. The finding in this research is in line with the aforementioned, in which sucrose and fructose content in the aromatic group was lower than the non-aromatic groups, as the impact of decreasing sugar content (fructose and glucose) during the fermentation process[2,39]. In fine-flavor beans, the naturally occurring sugars, primarily glucose and fructose, are consumed by yeast and bacteria during fermentation. This process produces ethanol, lactic acid, and acetic acid, which help develop the delicate fruity, floral, and nutty flavors characteristic of fine-flavor cocoa. Sugars, specifically fructose, decreased during roasting[17]. A decrease in sugar content is critical in developing chocolate flavor through Maillard reactions involving amino acids during the roasting process[40]. The low levels of fructose and sucrose in aromatic beans are limiting factors in flavor development[12,17]. Fructose content of cocoa beans did not affect the taste. The content of both compounds influences hydrolysis enzymatic reactions and are involved in non-enzymatic Maillard browning reactions. Cocoa beans contain free reducing sugars that could also participate in these important flavor reactions[41]. The sugar content helps drive the fermentation process, which is critical for flavor development. The acids produced during fermentation help reduce bitterness and astringency, while the remaining sugars contribute to a slight sweetness and enhance the overall flavor complexity of fine-flavor cocoa. In non-aromatic cocoa, the fermentation process focuses on developing the characteristic chocolate flavor, with less emphasis on complexity.
Fine-flavor beans like Criollo may have slightly lower protein content compared to bulk beans like Forastero, but the difference is not substantial. The result in this research showed the protein content of aromatic genotypes was lower than the non-aromatics group. Forastero tends to be higher in protein content than Criollo[39]. Furthermore, the protein content is influenced by location and plant genetics. Protein content in cocoa beans controlled bioactive compounds and flavor in cocoa products[42]. Proteolysis is a critical bio-catalyzed process because it generates flavor precursors such as peptides and amino acids[9,8], and causes the difference in flavor during fermentation[33,40]. Proteins contribute to the formation of sensory qualities and aroma and flavor precursors. Proteins in aromatic cocoa beans are broken down into amino acids during fermentation. These amino acids, especially those like arginine and aspartic acid, react with reducing sugars during roasting in the Maillard reaction, which is essential for developing the complex flavor profile of fine-flavor cocoa. This reaction produces flavor compounds that contribute to the fruity, floral, and nutty notes characteristic of fine-flavor beans. In non-aromatic cocoa beans, proteins also break down into amino acids during fermentation, but the flavor compounds produced during roasting tend to be simpler. The catabolism is mediated by the action of aspartate-endoprotease and carboxypeptidase enzymes as factors responsible for releasing hydrophilic amino acids and polypeptides associated with aromatic flavor[32]. However, it was inversely proportional to the intensity of dusty and moldy, which was affected by the intensity of the protein content in the cocoa paste.
Amino acids play a critical role in the flavor development of both aromatic (fine-flavor) and non-aromatic (bulk) cocoa beans. During fermentation and roasting, amino acids react with sugars in the Maillard reaction, producing a wide range of flavor compounds responsible for the final taste of chocolate. The amino acid profile of these beans is essential for the development of their fruity, floral, and nutty notes. The amino acids in fine-flavor beans interact with sugars during the Maillard reaction, leading to the production of volatile compounds that are responsible for the rich, fruity, floral, and nutty aromas. These beans have a more complex amino acid breakdown during fermentation, which is key to their delicate flavor profiles. They are associated with forming a specific cocoa bean aroma through the Maillard reaction and Strecker degradation during the roasting and conching process. The amino acid content is influenced by genetics and the environment. Amino acid content of Ecuador, which belongs to the refined cocoa group with Arriba genetics, was low. Amino acid content will also be different when fermented using various varieties and a batch of fermentation[43]. Lower amino acid content in aromatic genotypes was feasible due to earlier proteolysis. Furthermore, low content in aromatic cocoa paste may be due to a decrease in amino acids and a decrease in sugar during roasting. The hydrophobic amino acids, including alanine, tyrosine, valine, isoleucine, leucine, and phenylalanine, are specific precursors for cocoa aroma. Proteins and amino acids control sensory quality and are responsible for forming aroma and cocoa flavor[15].
Various chemical compounds in cocoa beans contribute to flavor formation during bean processing. Over fermentation can result in undesirable flavors such as musty or moldy aromas, or a depletion of beneficial chocolate flavor profiles. Beans may similarly acquire an excessive level of acidity, resulting in a sour or vinegar-like taste. The fermentation index of cocoa beans differs between aromatic ('fine-flavor' cocoa) and non-aromatic ('bulk' cocoa) varieties. Cocoa beans can be classified into aromatic and non-aromatic classes depending on the components of compounds[44].
In Fig. 3, the DR 2 was split alone caused by higher fermentation index, carbohydrate, theobromine, and methionine. Carbohydrates, methionine, and theobromine each contribute uniquely to the flavor and aroma profile of cocoa. The combination of carbohydrates, methionine, and theobromine in cocoa creates a complex balance of sweetness, bitterness, and roasted flavors, which are essential to the characteristic taste of cocoa and chocolate products. The balance of these compounds, along with others such as fats and additional amino acids, defines the final flavor profile of cocoa. The result is a deep and layered aromatic profile with a perfect balance between light and dark elements, making the cocoa aroma more sophisticated and appealing.
The second cluster is KW 516, KEE 02, and Sulawesi 1 with higher content of proline, valine, protein, and arginine. All these amino acids (proline, valine, and arginine) are involved in the Maillard reaction, interacting with sugars during the roasting of cocoa beans to create a wide range of aroma compounds. The equilibrium of sweetness (derived from sugars and proline), nuttiness (attributed to proline and valine), bitterness (resulting from arginine), and umami (also originating from arginine) enhances the complexity of cocoa's flavor profile. It would create a rich, complex, and well-rounded cocoa aroma, with intensified roasted, nutty, sweet, malty, and savory notes. This aroma profile would be deeper, more robust, and multidimensional, offering a luxurious sensory experience.
While TSH 858, Sulawesi 1, ICCRI 07, and ICCRI 09 contained low in methionine, sucrose, theobromine, arginine, polyphenol and proline. Cocoa with low levels of methionine, sucrose, theobromine, arginine, polyphenols, and proline would taste less bitter, less sweet, and less roasted with reduced depth and complexity. Low methionine, sucrose, theobromine, and polyphenols reduce bitterness, sweetness, and roasted notes, the high-fat content, fermentation, and amino acids (especially leucine, isoleucine, and alanine) will create a rich, smooth, and mildly sweet cocoa with enhanced umami and nutty flavors. The flavor will be complex but less intense in bitterness and caramelization, appealing to those who prefer a milder, more rounded chocolate experience. The combination of high amino acids, fermentation, and fat content results in a smooth, nutty, and complex aroma that's rich and multi-layered, with hints of sweetness and fruitiness.
The specific flavor and aroma characters are highly dependent on interaction among genotypes, growing environmental conditions, and processing processes[4,2,11]. These beans contain higher concentrations of flavor precursors (such as amino acids and sugars) that contribute to the development of fine, floral, fruity, and nutty notes. Differences in cocoa genotypes influence the formation of flavor which is determined by differences in the type and number of chemical compounds such as protein, carbohydrates, polyphenols, and enzyme activity in cocoa beans[8]. Chemical compounds generate flavor precursors such as amino acids, peptides, and sugar[8] ICCRI 07, with sensory characters of fresh and browned fruit, woody, and nutty[1], was dominated by the fermentation index. Furthermore, TSH 858, which has sensory characteristics of fresh fruit, woody, and nutty[1], was dominated by fat content. Non-volatile compounds as flavor precursors such as proteins, lipids, polyphenols, sugars, and carbohydrates undergo breakdown during fermentation, leading to flavor formation. The unique taste of cocoa is attributable to its constituent chemical compounds, both aroma-forming compounds and taste-determining compounds[45]. Genotypes included in the non-aromatic category with strong sensory characteristics to taste of bitter, astringency, acidity, and cocoa[1] was dominated by protein compounds (KEE 02 and Sulawesi 2) and caffeine (KW 516). The protein content of bulk cocoa, specifically the Forastero, was higher than the aromatic group (Criollo)[39]. Additionally, the compound content of Sulawesi 1 has similarities with ICCRI 09 since they are from similar families. ICCRI 09 is genetically related to Sulawesi 1 and TSH 858, and ICCRI 09 has a taste precursor characteristic similar to its parent. This study showed that the non-volatile compounds of progeny (ICCRI 09) indicated as the effect of the parental both female parent (TSH 858) and male parent (Sulawesi 1). Related to the previous study on rice has demonstrated that the metabolite profiles of the progeny and crossing parent were similar[46]. The metabolites profiles can be influenced by both genetics and environment[47].
-
The aromatic and non-aromatic cocoa genotypes produce differences in non-volatile compounds that affect their flavor profiles. The aromatic group shows a higher fermentation index, carbohydrate, polyphenol, and lower fat, theobromine, protein, fructose, and sucrose content than non-aromatic. The amino acid has an important impact on cocoa flavor. The composition and intensity of non-volatile compounds of each genotype affect flavor precursors and determine the flavor characteristics.
The authors would like to thank Fitratin, Ariza Budi Tunjungsari, Fuad Rijalul Fikrie, Avan Nur Diyansyah, Ninik Kusmiarsih, Arik Ermawan from ICCRI for research support.
-
The authors confirm contribution to the paper as follows: experiments conception: Anita Sari I, Murti RH, Misnawi, Putra ETS, Susilo AW; experiments conducting: Anita Sari I, Murti RH, Misnawi, Putra ETS; result analysis: Anita Sari I, Setyawan B, Akbar MR; figures and tables preparation: Anita Sari I, Nuringtyas TR, Setyawan B. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Anita Sari I, Murti RH, Misnawi, Putra ETS, Setyawan B, et al. 2025. The differences non-volatile compound and flavor attributes in several Indonesian cocoa genotypes using medium scale fermentation. Beverage Plant Research 5: e009 doi: 10.48130/bpr-0024-0041
The differences non-volatile compound and flavor attributes in several Indonesian cocoa genotypes using medium scale fermentation
- Received: 13 July 2024
- Revised: 25 November 2024
- Accepted: 17 December 2024
- Published online: 02 April 2025
Abstract: Flavor is the main parameter of cocoa bean quality. The characteristic of flavor is influenced by the composition and intensity of metabolites both volatile and non volatile. It was hypothesized that non-volatile compounds impacted the flavor preference of whole cocoa beans. This study aimed to identify the differences in the non-volatile compounds between aromatic and non-aromatic genotypes. The study used the genetic materials that have been classified as aromatic and non-aromatic genotypes based on sensory characteristics. The six aromatic cocoa genotypes, including DR 2, ICCRI 03, ICCRI 07, ICCRI 09, TSH 858, and MCC 02, and four non-aromatic cocoa genotypes consisting of Sulawesi 1, Sulawesi 2, KW 516, and KEE 02 were used. The results showed that the non-volatile compounds influenced the cocoa flavor both taste and aroma. There were differences in the content of non-volatile compounds in aromatic and non-aromatic genotypes. The fermentation index, carbohydrate, and polyphenol content of the aromatic genotypes were higher than the non-aromatic group. The fermentation index showed an important indicator of the flavor precursor development of cocoa beans. Polyphenol compounds have a contribution to bitter and astringent taste. The carbohydrate and theobromine content influenced the strength of the fine aroma, such as fresh fruit, browned fruit, woody, and spicy.
-
Key words:
- Non-volatile /
- Compounds /
- Flavors /
- Attributes /
- Indonesian /
- Cocoa