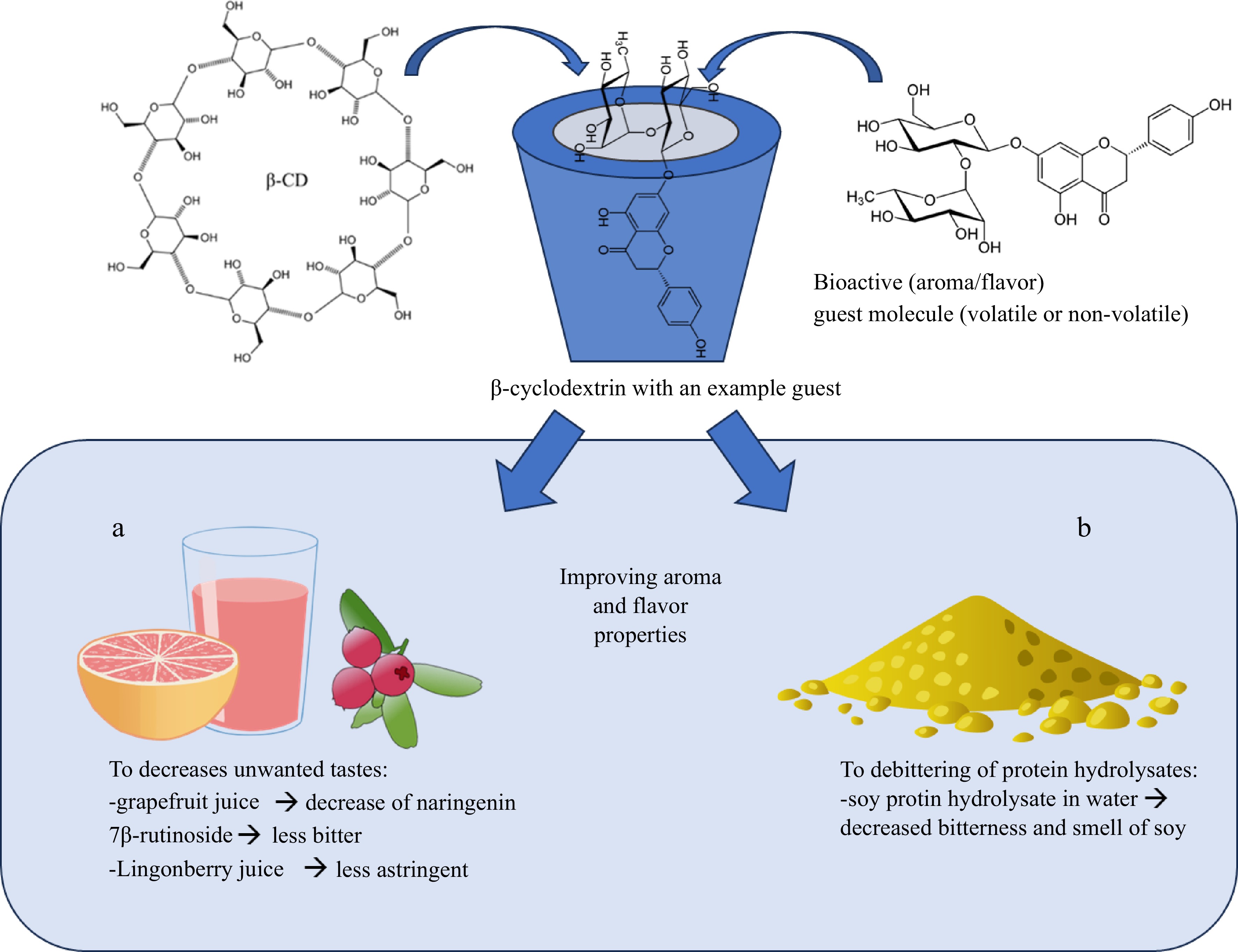
-
Cyclodextrins (CDs) are tapered cyclic oligosaccharides composed of six (α-CD), seven (β-CD), or eight (γ-CD) α-1,4-linked α-d-glucopyranoside units. α-, β-, and γ-CDs (Fig. 1) are the so-called native cyclodextrins, because they can be produced from starch by a relatively simple enzymatic conversion by cyclodextrin glucanoltransferase (EC 2.4.1.19, CGTase). CGTase is a multifunctional enzyme, which is capable of catalyzing both hydrolyzation of starch and cyclisation of cyclodextrin. CGTase forms all native, α-CD, β-CD, and γ-CD, CDs in different ranges[1]. The cavity sizes of the three types of CDs increases by the number of glucopyranoside units, with cavity size of 0.56, 0.70, and 0.88 nm for, α-CD, β-CD, and γ-CD, respectively[2].
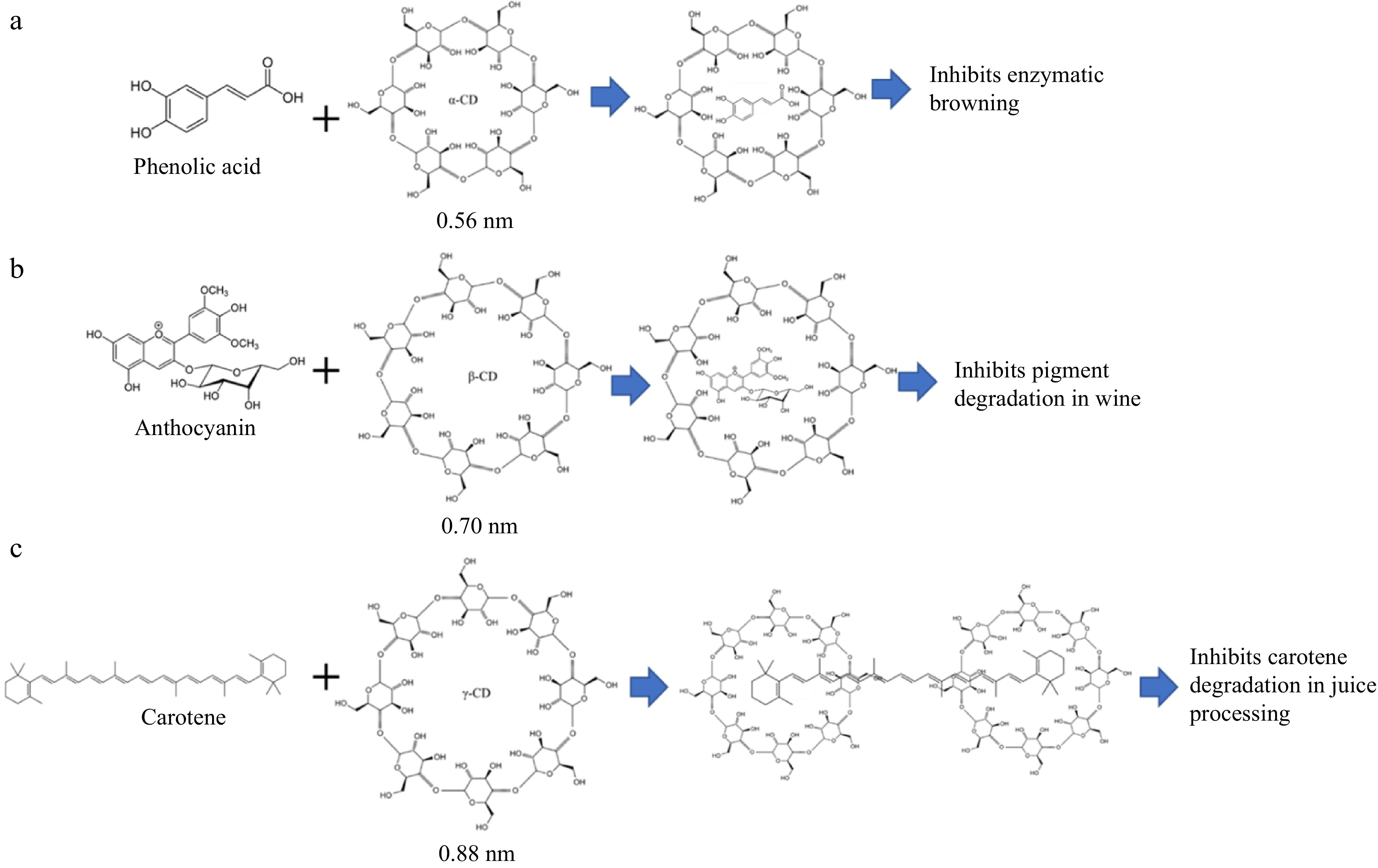
Figure 1.
Chemical structure of native cyclodextrins, α-CD, β-CD, and γ-CD, and examples of their possible use in improving sensory properties of food products. (a) α-CD can be used to prevent enzymatic browning in juice processing due it is capable to encapsulate substrate of polyphenol oxidases[3]. (b) β-CD can be used to prevent degradation of anthocyanin pigments in wine making[4]. (c) γ-CD can prevent carotene degradation during homogenization of juice[5].
Although native CDs and their complexes are hydrophilic, their solubility in aqueous solutions is a key characteristic limiting the application of CDs in foods, especially for β-CD which has the lowest water-solubility. To overcome this limitation, CD derivatives are produced by chemical modification. Many studies have shown that the modification of CDs increases their usage potentially by, for example, increasing stability of inclusion complex, compared to the native CDs[6−11]. The derivation of the CD changes the water solubility, extends the cavity size and thus, increases the van der Waal interactions with the guest compound, improves stability against light and oxygen, and reduces effective cavity polarity. Examples of the CD derivatives are hydroxypropyl-β-cyclodextrin (HP-β-CD), sulfobutylether-β-cyclodextrin (SBE-β-CD), randomly methylated α-, β-, γ-cyclodextrins, and thiol-β-cyclodextrin[6,12−14]. The use of some cyclodextrin derivatives in the food industry is limited by their possible toxicity and high costs; therefore only a few of them are produced at an industrial level. However, some of CD derivatives have been evaluated, such as HP-β-CD and SBE-β-CD, and considered safe[15].
The European Union regulates use of CDs in food products in EU countries. β-CD is approved as a food additive (E 459) in the EU with the acceptable daily intake (ADI) at 5 mg/bw kg/d[16]. The α- and γ-CD are considered as novel foods, of which the ADI has not been specified[17]. The Joint FAO/WHO Expert Committee on Food Additives (JECFA) have approved CDs as General Food Standard Additives (GFSA). JECFA does not specify ADIs for α- or γ-CD, meaning their use is not limited in food products. Similar to European Union regulation, JECFA has set ADI of β-CD at 0–5 mg/bw kg/d[18−20]. The US Food and Drug Administration (FDA) considers all native CDs as Generally Recognized as Safe (GRAS). Toxicity of α- and γ-CD has been studied intensively and no toxicological findings were filed by FDA[21].
Sensory properties, such as color, smell, flavor, and taste, effect on the consumers consumption choices. Appearance of the food is the first thing which consumers can use when making consumption decisions. Color of fruits and berries are mainly a result of anthocyanin and carotenoid pigments[22,23], whose content can decrease significantly during food processing. In addition, browning of fruits not only negatively effect their appearance but also their taste and nutritional quality[24]. Smell and flavor of the food are mainly caused by the volatile compounds. Food processing has an effect on the volatile compound composition[25]. Negative taste properties, such as bitterness and astringency, affect consumers preferences, but at the same time, bitter and astringent compounds are important bioactive compounds, which can lower the risk of, for example, cardiovascular disease and cancer[26].
The CDs may potentially be used to micro-encapsulate various compounds in food products with the aim of improved sensory properties: (1) protection of oxygen, light, or temperature sensitive compounds from degradation; (2) stabilization of flavors; (3) prevent enzymatic browning; (4) suppression of unpleasant tastes and odors, such as bitterness and off-flavors[12,24,27−31]. The present review aimed to present applications of cyclodextrins to improve sensory properties of food after processing. Emphasis has been paid to the effects of cyclodextrins on the different color changes, such as prevention of color loss and browning, taste properties, such as decreased bitterness, and flavor properties.
-
Since the CD cone has the hydrophilic outer surface and hydrophobic inner surface, the CDs are water-soluble and able to form an inclusion complex with the hydrophobic molecule (guests) or the hydrophobic part of the compound in the aqueous environment (Fig. 1.). The guest molecule can be in a solid, liquid, or gaseous state. The inclusion complex does not break or form any covalent bonds. In an aqueous solution, the CD cavity is occupied by water molecules, which is readily substituted by more hydrophobic guest compounds. Due to the hydrophobicity of the cavity, the hydrophobic compound is more energetically favorable for the apolar-apolar association. Other binding forces, such as van der Waals interaction, hydrogen bonding, hydrophobic interactions, changes in solvent-surface tension, and release of ring strain in the CD molecule, may have importance in the binding of the guest molecule[12,32,33].
In the inclusion complex, one molecule is enclosed within another molecule or an aggregation of molecules[32]. Encapsulation of the guest molecule is not fixed or permanent, but a more dynamic equilibrium. Inclusion complex kinetics between the CD and the guest molecule depends on the relative size, shape, and hydrophobicity rate between the guest molecule and the CD[12,33]. Moreover, smaller molecules have greater complexing activity with CD and larger compounds are more dependent on the suitable functional group or ring to enter and interact with the CD cavity (Fig. 1)[32]. Stability of the inclusion complex is also an important factor to consider in the use of CDs. The water-solubility of the guest molecule effects the stability of the formed complex with the CD: more water-soluble molecules, such as sugars and organic acids, form a less stabile complex compared to hydrophobic compounds[34].
Several studies have shown that CDs form 1:1 stoichiometry complexes with flavonoids (Fig. 1b) and volatile aroma compounds[8,14,34,35], but other ratios, such as 2:1 and 1:2 (CD:guest; Fig. 1c), are also possible. The optimal ratio for forming the complexation depends on the structural features of the molecules such as the length of the carbon chains and the types of functional groups[36]. The formation of an inclusion complex can be determined by many methods, for example, with the oxidative differential scanning calorimetry (DSC)[37] or the Fourier transform infrared spectroscopy[25].
-
CDs have been used to improve the stability of natural colorants in food during food processing and storage, or to prevent the formation of browning reactions by binding certain pigment compounds, such as anthocyanin, or by binding the substrate of enzymatic reaction, such as phenolic compounds in the enzymatic browning.
Effects on anthocyanin and carotenoid stability
-
The color is the first thing a consumer perceives about food products, making the appearance of food and beverages one of the most important characteristics. Anthocyanins are natural blue, red, and purple pigments (Fig. 1b). Fliszár-Nyúl et al. studied the effects of different β-CD bead polymer (BBD) concentrations on the color parameters of five red and three white wines from different grape varieties using the color-related Glories parameters: color intensity, tonality, Red%, Blue%, Yellow%[4]. Treatment at a concentration of 1.0 mg/mL BBP did not have any effect on the color properties of any of the wines studied, whereas BBP at concentration range of 1.0–15 mg/mL dose-dependently decreased the color intensities of the wines. Not surprisingly, greater changes were observed in the red wines than in the white wines. In addition, one red wine (portugieser) was clearly affected more by the β-CD treatments compared with the wines of other grape varieties. The results clearly indicate that the impact of treatment depends on the structures of anthocyanins.
Anthocyanins are labile compounds sensitive to impact by several environmental factors, such as pH, temperature, light, oxygen, co-pigment formation, enzymes, metal ions, and antioxidants[22,30]. Howard et al. studied the effects of pH (2.8, 3.2, and 3.6), β-CD (in the range of 0–3%, w/v), and storage temperature on the anthocyanins of chokeberries (Table 1)[30]. Their results showed that pH, β-CD concentration, and pH × β-CD interaction positively affected the anthocyanin content compared to the control juices that were not treated. They concluded that the juice containing 3% of the β-CD at natural pH of chokeberry (3.6) had 49% more anthocyanins than the juice without β-CD addition after 8-month storage. They discussed that anthocyanin structure changes in low pH has an important role in β-CD stabilization. Mourtzinos et al. studied the thermal stability of the anthocyanins of the roselle extract with and without the β-CD (Table 1)[38]. β-CD was mixed with anthocyanins at the 1:1 ratio (w/w). They observed higher thermal degradation with higher temperatures and longer treatment time, but the presence of the β-CD decreased the degradation rate and nearly doubled the half-life of the anthocyanins. In addition, Fernandes et al. reported increased thermal stability of the blackberry anthocyanins and decreased degradation of the anthocyanins under simulated gastrointestinal conditions when β-CD was used[39]. Lachowicz et al. studied the effect of the yeast strain, β-CD (1 g/L), and storage time (3 months at 4 ºC) on the physiochemical parameters, phenolic compound concentrations, sensory properties, and antioxidative activity of red apple cider[40]. They observed that fermentation with the β-CD had a positive effect on the pH value, color, antioxidative potency, and most of the polyphenols. However, fermentation with the β-CD resulted in red apple cider with the darkest color. In addition, additional β-CD resulted in the highest anthocyanin concentration and increment in the phenolic acid and flavan-3-ol concentrations after 3 months storage, whereas the fermentation solely with the yeast decreased these contents.
Table 1. Effects of the cyclodextrins on the individual compounds and sensory properties (appearance, flavor, taste) of food products.
Raw material Type of used cyclodextrin Methodology Analysed parameters Effects on chemical/food products Reference Instrumental analyses of sensory-active compounds Apple β-cyclodextrin
thiol-β-cyclodextrin700 µM (in 0.1 M sodium acetate buffer, pH 4.6) of β-CD or thiol-CD was added to apple slices, incubated at RT for 24 h. Inhibition of the enzymatic browning:
The CIE coordinatesThiol-CD exhibited considerably higher inhibition of the enzymatic browning. Thiol-CD treatment resulted lighter, less red and yellow color of apple slices, indicating less browning. [6] Apple M-β-cyclodextrin
(0, 30, 60, or 90 mM)CD in the 25 mL of distilled water. Color by the CIE coordinates Increased level of MBCD decreased more the total color difference and slowed down the changes of L*, a*, and b*. [51] Apple α- (10/30/40 mM) and β-cyclodextrins (5/10/15 mM) Apple juice with CD was treated with HPP (0/300/400/500 Mpa; 5 min, 22 °C). Browning index and phenolic compounds α-CD at 30 mM and β-CD at 15 mM level reduced the most HPP induced browning. [11] Chokeberry β-cyclodextrin
(0, 0.5%, 1%, or 3%)
in pH levels
(2.8, 3.2, 3.6)Different level of β-CD in different pH chokeberry juice. Storage at 25 and
4 °C for 2, 4, 6, 8 months.Monomeric anthocyanins Both pH and β-CD amount affected stability of anthocyanins during storage. Ambient storage temperature effected more on the degradation of anthocyanins than refrigerator temperature. [30] Peach α-cyclodextrin (0,
10, 30, and 60 mM)
M-β-cyclodextrin (MBCD, 0, 10, 20, and 30 mM)
β-cyclodextrin (0, 3, 5, and 10 mM)CD in 25 mL of distilled water. Color by the CIE coordinates Increased level of α-CD slowed down the changes of L* and ΔE*, and 60 mM of α-CD eliminated the change of L*. β-CD did not have effect on the color in any concentration. Increased level of MBCD slowed down the changes of L* and ΔE*. [81] Pomegranate β-cyclodextrin,
HP-β-cyclodextrin (0.5%, 1%, or 2%)Pomegranate juice treated with CDs and stored 3 months at 25 °C. Monomeric anthocyanins, TPC, FRSA CD type and level affected the degradation rate of anthocyanins. HP-CD stabilized more anthocyanins than β-CD. β-CD did not show protective effect on the TPC. 0.5% of HP-β-CD significantly increased TPC. FRSA did not change after 3-month storage in any sample. [9] Roselle extract β-cyclodextrin: anthocyanin (1:1) in extract Extract heated to 60, 70, 80, and 90 °C for 10−110 min. Total anthocyanin content The degradation of anthocyanins increased with increased heat and time, but presence of β-CD decreased the degradation. β-CD nearly doubled the half-time values of anthocyanins. [38] Tangerine β-cyclodextrin (1, 3, and 5 g%) Batch process, packed bed column process, and fluidized bed process with varying CD concentrations and process parameters were used. Limonin content Batch processing: direct correlation between CD content and limonin complexation. Column processing: 3 g% CD had better debittering result (94% limonin reduction) than batch processing. Fluidized bed process: juice flow rate effected on the binding rate of limonin, CD content did have only a little effect on limonin reduction. [60] Assessments by sensory panels and instrumental analyses Amanatsu
Grapefruit
Orange
β-cyclodextrin 0.3% (w/w) CD and 8% (w/w) of sucrose were added to the juice, heated to 95 °C for 10 min and cooled to RT. Bitterness (trained panel) Addition of CD decreased bitterness significantly of all studied citrus fruits. [80] Bitter gourd β-cyclodextrin
(0.25%−2%)β-CD was added to freshly pressed juice, stirred for 1 h at 25 °C, pH was adjusted to 3.5 and 2 g/L of stevia was added. Juice was pasteurized (95 °C, 2 min) Effect on sensory quality (trained panel), TPC, TAC, and antidiabetic potential All studied CD concentrations decreased bitterness. Addition of 1.5% of CD resulted the most acceptable juice. Increased level of CD increased TPC and TAC. Marginal reduction in antidiabetic activity was observed. [31] Grapefruit
Navel orangeβ-cyclodextrin 1 g/L in 50 mL of juice Cyclodextrin in the continuous flow fluid-bed or in batch process Concentration changes of limonin, nomilin, and naringin and impacts on the sensory properties (trained panel) Cyclodextrin polymer treatment decreased approximately 50% of bitter composition and sensory panel preferred debittered juices over non-treated. [82] Mandarin juice enriched with pomegranate and goji berries β-cyclodextrin and
HP-β-cyclodextrinJuice (mandarin 96%, goji berries 2%, pomegranate extract 1%, CD 1%) was pasteurized (98 °C, 30 s) and stored for 75 d at 4 °C. Stability of vitamin C, color, and retinol equivalents, antioxidant capacity and sensory properties (trained panel) Control juice without CDs had the most intense fresh mandarin aroma, β-CD had the second highest and HP-β-CD lowest. HP-β-CD had the best overall quality, the highest value of color intensity, vitamin C content, and retinol equivalents. [47] Orange juice β-cyclodextrin 13 mM of β-CD was added before juice pasteurization (98 °C for 20 s). Samples were stored at 20 °C for 145 d. TTS, acidity, pH, vitamin C, color by the CIE coordinates, carotenoids, antioxidant capacity, sensory quality (trained panel) Addition of β-CD did not have significant effect on the measured parameters. [46] Pear α-cyclodextrin (0,
15, 45, and 90 mM)Freshly pressed pear juice was mixed with distilled water containing CD. All juice samples were oxidized at stirrer for 20 min. Color by the CIE coordinates, volatile composition, and sensory evaluation (trained panel) Increased CD content delayed color changes. Oxidation increased concentrations of certain volatile compounds. Use of CD decreased contents of volatiles in concentration dependent manner. Only high CD content significantly modified volatile profile. 15 mM of CD had a significant positive effect on the sensory quality and 90 mM led to the deterioration of aroma and odor attributes. [83] Pear α-, β-, γ-cyclodextrins 15 mM of each CD was added to 25 mL of pear juice and mixed for 20 min. Enzymatic browning, volatile compounds, color, sensory properties (trained panel) CDs slower enzymatic browning by complexation of phenolic compounds. γ-CD significantly decreased the aroma and odor intensities, β-CD provided best color, and α-CD resulted increased global quality of pear juice. [26] CD(s) cyclodextrin(s); CIE coordinates: lightness (L*), red-green (a*), yellow-blue (b*), hue (H*), chroma (C*) total color difference (ΔE*), browning index (BI*); FRSA free radical scavenging activity; HP hydroxy propyl; HPP high pressure processing; MBCD maltosyl-β-cyclodextrin; TPC total phenolic content; TAC total antioxidant capacity; RT room temperature; TSS total soluble solids. Many studies have reported CDs' ability to prevent anthocyanin degradation. However, the effectiveness of CD also depends on anthocyanin structure. Kulcan et al. reported that β-CD and HP-β-CD did not effectively prevent analytical color loss of clear pomegranate juice during 3 months storage (Table 1)[9] .
Encapsulation of the anthocyanins in the CDs may increase the retention during food processing and the shelf life, but it can also reduce the intensity of the anthocyanin color[41]. Color fading is also known as anti-copigmentation phenomenon. It occurs when the colorless forms of anthocyanins, quinoidal base, hemiketal, and chalcone, are more preferred in the inclusion complexation. This leads to a shift in the pigment hydration equilibrium towards the formation of more colorless forms of anthocyanins. Environmental pH, concentration of the β-CD and anthocyanin structure has an effect on the rate of anti-copigmentation[41].
Carotenoids are natural orange, yellow, and red pigments, which occur in fruits, vegetables, algae, and photosynthetic bacteria. They have an important role in human diet due to their vitamin A, immune regulating, antioxidant, and intracellular communication activities. Carotenoids are lipid-soluble and instable, limiting their use in food products as colorants[23]. Inclusion complexation of carotenoids with cyclodextrins can improve water-solubility[42,43], general storage stability[43], and stability against light, oxide, or ozone induced degradation[44] of carotenoids, thus, improve color stability, when used in aqueous food products (Fig. 1c)[45]. However, Navarro et al. (Table 1) did not observe significant improvements in studied quality parameters with only slight increment in the contents of individual carotenoids, when they studied effects of β-CD (in both studies at the 1.5% rate) on the quality of ultra-frozen mandarin juice and pasteurized orange juice after storage for 145 d, respectively[46,47].
Effects on browning of fruits and vegetables
-
Browning of fruits and vegetables during processing effects their appearance, taste properties, and nutritional values. The browning is typically caused by the polyphenol oxidases (PPOs), which catalyze the oxidation of mono- and o-diphenols to their corresponding quinones, which are polymerized with protein or amino acids resulting in high molecular weight structures called melanin or melanoidin[48]. For tropical and subtropical fruits, the browning causes as high as 50% of the losses[49]. CDs can be used to slow down the enzymatic browning after juice processing by binding the enzyme substrates (Fig. 1a)[3,50] or they may act as secondary antioxidants by preventing premature oxidation of the primary antioxidant, such as an ascorbic acid[51]. The effectiveness of the CDs to inhibit the enzymatic browning is highly dependent on the enzyme substrates and, furthermore, the stability constant between the CD and substrates: the higher the stability constant, the better the inhibition activity[52,53]. The studies about the impact of CD treatment on the browning of banana pulp resulted in observations contradictory to the findings in other fruit juices, and the use of CDs was even found to enhance the browning in crude banana extracts[7,51]. Both of these banana studies concluded that this phenomenon was caused by CD complexation of the natural browning inhibiting substances. Furthermore, Ghidelli et al. reported that CD used at concentrations of 10–50 mM did not significantly affect the browning of persimmon extract or precipitate compared to the control sample[54].
In their study, Rho et al. used both β-CD (10 mM) and large ring cyclodextrin (LR-CD; cycloamylose; 10 mM) successfully to reduce oxidation of chlorogenic acid, caffeic acid, 3,4-dihydroxy-l-phenylalanine, cathecol, 4-methylcathecol, and pyrogallol (0.1–10 mM) and browning of apple juice[55] . Apple juice was treated with CDs in the range of 0–15 mM. They observed that β-CD and LR-CD presented similar inhibition efficiency against the oxidation of studied phenolic compounds by PPO, and on browning. In their storage study, both studied polymers decreased the degradation rate constant and increased the half-life of the studied phenolic compounds. In addition, Rho et al. studied effects of the β-CD and LR-CD on the color properties of apple juice[55]. Both polymers reduced color changes in the treated juice compared to the control juice. While the effects of both polymers showed a strong dependence on their concentration, LR-CD significantly delayed the browning rate of apple juice in smaller concentrations compared to β-CD.
In addition to their inhibition effects on intensities of browning, type of used CD also affects the rate of browning. Andreu-Sevilla et al. observed that treatment with three native CDs (15 mM) led to different rates of the color changes in pear juice (Table 1): α-CD slowed down the browning the most and γ-CD the least[29]. On the other hand, López-Nicolás et al. observed that maltosyl-β-CD (0–30 mM) and β-CD (0–10 mM) reduced browning of apple juice more than α-CD (0–60 mM; Table 1)[51].
Furthermore, CDs are reported to be effective against browning when they are used together with other polymers. Alvarez-Parrilla et al. studied the effects of the 4-hexylresorcinol (HR; 0.5 mM), β-CD (5 mM), and methyl jasmonate (MJ; 2 mM), or their combinations at the same concentration levels as in the individual treatments on the PPO-catalyzed oxidation of chlorogenic acid extracted form Red Delicious apples[52]. They observed higher inhibition of the catalytic activity of the PPO when HR and β-CD were used together, indicating a synergic effect between HR and β-CD. They suggested the synergic effect to be caused by different inhibition pathways: HR inhibits the oxidation by a competitive mechanism whereas β-CD inhibits oxidation by reducing the concentration of substrates with complexation. Any synergic effect was not observed between MJ and β-CD, which may have been due to the β-CD complexing MJ. However, de la Rosa et al. reported contrary results for the synergic inhibition effect of HR and β-CD[53]. They studied the inhibition effects of HR (0.5 mM) and β-CD (10 mM) on PPO extracted from Prisco peaches with catechol, 4-methyl catechol, and chlorogenic acid as substrates. When HR and β-CD were used together, they observed a decreased inhibition activity as regards PPO oxidation of the chlorogenic acid compared to single inhibitor treatments.
Additional β-CD may enhance the results of non-thermal technologies used to inhibit browning of fruit products. Zhang et al. studied the synergistic effects of high-intensity ultrasound (HIU; 0–400 W) and β-CD (0, 0.002, 0.004, 0.006, and 0.008 g/mL) on the browning degree of apple juice[56]. Addition of β-CD decreased the browning degree compared to HIU treatment used alone. In addition, additional β-CD in HIU increased a total phenolic compound content compared to HIU treatment alone. Finally, authors reported HIU to alter the structure of PPO and, thus, inhibiting enzyme activity, and β-CD to form inclusion complex with phenolic compounds and PPO inhibiting browning.
The browning of fruits and juices can also occur non-enzymatically. Karangwa et al. studied the effects of HP-β-CD and γ-CD treatments (1–5 g/100 mL) on non-homogenized and homogenized carrot-orange juices[5]. They observed that the studied CDs had different effects on the non-enzymatic browning rates: the HP-β-CD treatment significantly increased the non-enzymatic browning in both studied juice types and decreased the juice clarity, whereas the γ-CD treatment significantly decreased the browning in the non-homogenized juice.
-
Flavor is simultaneously sensed from the gustatory, olfactory, and somatosensory systems. Taste sensations, sweet, sour, salty, bitter, and umami, are sensed in the oral cavity. Olfactory receptors are located within the nasal cavity. Retronasal olfaction is achieved when volatile compounds from food arise to the nasal cavity and bind with olfactory receptors. Retronasal olfaction is the smelled 'taste', it defines the flavor of objects giving flavors their identity (Fig. 2).
Fliszár-Nyúl et al. studied five red wines and three white wines from different grape varieties treated with five different β-CD concentrations[4]. The impact on taste properties was studied using e-tongue. Surprisingly, the e-tongue was able to distinctively classify different β-CD concentrations in one of the red wines with 100% accuracy indicating notable sensory property changes. The clearest changes in taste properties were observed with β-CD treatment at the two highest (10 and 15 mg/mL BBP) concentrations, but not in all the wines, again highlighting structural specific interaction between CDs and the key compounds contributing to taste properties.
Effects on bitterness and astringency
-
The unpleasant taste properties, such as bitterness and astringency of fruit and berry juices may limit their consumption. It is well known that certain phenolic acids and flavonoids cause bitter and astringent sensations in the oral cavity[57−59]. Sugar has good masking properties, but excess consumption of sugar increases the risk of diseases, such as type II diabetes and obesity. The CDs can be used to reduce the bitterness of fruits, vegetables, and berries (Table 1)[27,31,60,61]. The effectiveness of the CDs is based on the formation of the inclusion complex between the CD and the bitter compound: when the bitter compound is bound to the CD cavity, the interaction between the compound and the bitter receptors is inhibited[62]. Use of the CDs as binders of the bitter compounds, naringin and limonin, in citrus fruits have been widely studied. Konno et al. used 0.01%–0.5% of β-CD to bind naringin and limonin in the aqueous solution[27]. In addition, they used 0.3% and 0.5% of β-CD in the amanatsu concentrate and 0.5% in the heated and non-heated Citrus iyo juice. In the aqueous solutions, 0.5% of β-CD reduced 50% of the bitterness with both studied flavonoid compounds. β-CD significantly reduced the bitterness in both studied juices. Similar results have been reported with navel orange and grapefruit juices[61] and with Thai tangerine juice (Citrus reticulate Blanco; Table 1)[60]. β-CD has also been used successfully to de-bitter bitter gourd juice (Momordica charantia; Table 1)[31]. In the study, they were able to reduce the bitterness with low concentrations of CD (0.25%–2%, w/v), increasing the overall quality of the bitter gourd without effecting the color or aroma properties. However, Kelanne et al. was not able to reduce the bitterness of commercial lingonberry (Vaccinium vitis-idaea) juice using β-CD (8.8 mM) and γ-CD (7.7 mM) alone and β-CD also after gelatine treatment[63]. However, they observed reduction in bitterness in lingonberry juice after sequential treatments of gelatine and β-CD, but not with CD treatments.
Protein hydrolysates, peptides, and amino acids are used in the fortification of food products. Hydrolysates are preferred over proteins in fruit and berry products due to their enhanced pH stability range, better solubility properties, and digestibility properties. Protein hydrolysates can be prepared from plant- and animal-based proteins[64,65]. However, protein hydrolysates and peptides have characteristic bitter taste and astringent mouthfeel, which limits their use in food products. Using β-CD (0.15% of total protein, w/w), Yadav et al. successfully masked bitterness of whey protein concentrate and hydrolysates (0−4%, w/w), used to fortify mango beverage, expect the mango beverage consisting 4% of whey protein hydrolysate (Fig. 2)[64]. Linde et al. demonstrated capability of β-CD to mask the taste and mouthfeel properties of individual amino acids (used in 1:1 rate)[66]. However, complexation between β-CD and phenylalanine seemed to cause steric modification in amino acids, modifying taste properties of phenylalanine from being 'slightly acidic' to 'slightly sweet with bitter aftertaste'. In addition, Linde et al. studied the effects of β-CD on soy protein hydrolysate in water. Without addition of β-CD, the protein hydrolysate solution was evaluated as strongly bitter and residual umami tastes and with a characteristic smell of soy. Addition of 3% (w/v) β-CD already decreased intensity of bitterness and smell of soy, and 5% of β-CD decreased bitterness intensity by 90% and further reduced the smell of soy. β-CD can be used together with other treatment to lower the bitter taste of protein hydrolysates. Xia et al. studied effects of different methods (sec-butanol, enzymatic hydrolysation, and β-CD) and their synergic effects (sec-butanol and β-CD) on the bitterness of pea protein hydrolysate[67]. They used both trained sensory panel and e-tongue to measure the changes in the taste properties. Both sec-butanol and β-CD treatment alone lowered the bitter taste, but when these treatments were combined the bitterness was lowered even further.
Effects on the off-flavors
-
Off-flavors have several sources, such as microorganisms growth, lipid oxidation, environmental pollutants, and endogenous enzymatic decomposition, and they typically indicate a physical health danger associated with contamination or spoilage[68]. Off-flavors can be reduced with the CDs. Yang et al. studied the effect of β-CD (0.05%–0.25%) and other polymers on the thermal treatment of watermelon juice[69]. They observed β-CD to be the most effective in reducing off-flavors after thermal treatment compared to other studied polymers. In addition, they observed differences in the timing of the addition: addition of the β-CD after thermal treatment was more successful in reducing the content of the off-flavor compounds compared to the treatment which β-CD was added before thermal treatment. Lee et al. were able to reduce beany off-flavor compounds in the yuba film using isolated soy protein and the addition of β-CD (0, 1%, 2%, and 4%, w/w; Fig. 2). In addition, the effect of the removal of the β-CD after complexation was studied[70]. An addition of 2% and 4% of β-CD significantly reduced the content of the compounds responsible for the beany flavor and significantly reduced the beany flavor observed by a trained sensory panel. However, the total flavor intensity decreased simultaneously with reduction of the beany flavor. Furthermore, they observed that increasing the level of β-CD led to a decrease in the puncture strength and deformation of the yuba film; however, when the CD complexes were removed, there was no significant difference between the treatments. Similar results were reported by Suratman et al. in their study of the effects of γ-CD or α-CD on the beany flavor of two soymilks[71]. Using γ-CD or α-CD at 0.5% (w/v) or both CDs together (0.25% for each CD). The number of beany flavor compounds significantly decreased by the treatments but did not significantly reduce the beany flavor of the two soymilks as evaluated by trained sensory panel.
Off-flavors and undesirable tastes, such as bitterness, can be further eliminated when CDs are used with other inhibitors or maskers. This kind of synergic reduction of the undesirable flavors and tastes have been reported, for example, in goat cheese treated with β-CD (0.3%, w/v) and polymerized whey protein (0.6%, w/v)[72], (+)-catechin bitterness treated with β-CD (0.3%, w/v) and rebaudioside A (sweetener; 0.26 mM)[73], soy protein isolate treated with β-CD (8 mM) and phospholipase A2[74], and alcalase treated salmon frame protein hydrolysate treated with β-CD and 2-butanol[75].
Effects on the volatile compounds in food products
-
Volatile compounds typically influence the aroma of food products, but not all volatile compounds are important for aroma. Different food processing methods, packaging materials, and storage conditions influence flavor intensity, off-flavors, and volatile composition. CDs can be used to encapsulate volatile compounds, which improves the retention of these compounds and, hence, improves the shelf-life of the food products and beverages[14,76,77]. CDs are also used to increase the solubility of the volatile compounds, making them easier to use as flavoring agents[78]. In addition, CDs, especially β-CD, can improve the retention of volatile compounds after an elevated temperature process. Reineccius et al. studied the effects of the encapsulated benzaldehyde, citral, l-menthol, and vanillin on the retention of these compounds and sensory properties in hard candies, fruit leather, and angel food cake[76] . The use of β-CD improved the heat stability of the studied compounds. The result of sensory evaluation of the mint flavored (l-menthol) hard candy, the citrus flavored (citral) fruit leather, and the cherry flavored (benzaldehyde) angel food cake showed that β-CD enhanced the flavor of citral and benzaldehyde compared to the liquid formulated flavor containing certain aroma compound (benzaldehyde, citral, l-menthol, or vanillin) without encapsulation. However, the liquid formulation of l-menthol in the hard candy resulted in a significantly more intensive minty taste than that encapsulated by the β-CD. This study demonstrated the differences in the inclusion complex stability between different guest compounds and the effects of the stability on the release of the guest compounds.
Volatile compounds, such as allicin, have reported to present health and well-being promoting bioactivities. However, utilization of volatile compounds in functional purposes can be prevented by their strong and even irritating organoleptical properties. Zhou et al. studied effects of α-CD complexation on the sensory properties of allicin, which has strong irritating odor and stimulates gustatory mucosa, with the in vivo and in vitro methodologies[79]. In addition, they studied thermal stability of α-CD:allicin complex compared to physical mixture containing α-CD and allicin. They observed that the α-CD:allicin complex was effective to mask unpleasant odor and flavor compared to physical mixture. Furthermore, allicin complexation to α-CD remarkably enhanced the thermal stability of allicin and, thus, improves its usability in food processing as a functional ingredient.
-
Treatment with cyclodextrins is an effective way to reduce undesirable tastes, such as bitterness, and off-flavor properties in some food products. In addition, cyclodextrins can be used to improve the stability of many groups of compounds, such as anthocyanins and volatile compounds during processing of food. However, many studies use notably higher cyclodextrin concentrations than is recommended by food authorities. Even though the safety of some cyclodextrins are well evaluated and described, they may contain residual solvents, such as trichloroethylene (classified as carcinogenic to humans), making their use in such concentrations, in which they have shown to impact sensory properties, difficult. Encapsulation of hydrophobic compounds improves their water solubility, which has many beneficial effects, such as improved bioavailability and antioxidative activity of the target compounds. Studies have shown that successful use of the CD requires good planning and in-depth understanding of the chemical composition of the food product. Finally, more studies with lower cyclodextrin concentrations and synergic effects with other flavor masking or binding agents are needed.
Funding for this research from the Finnish Cultural Foundation (00190487, 2019, Finland).
-
The authors confirm contribution to the paper as follows: conceptualization: Kelanne N, Laaksonen O; resources: Yang B; investigation; methodology; visualization; roles/writing - original draft: Kelanne N; supervision, project administration: Yang B, Laaksonen O. All authors reviewed the results and approved the final version of the manuscript.
-
The data that support the findings of this study are available in the Niina Kelanne repository. These data were derived from the following resources available in the public domain: https://www.webofscience.com/wos/woscc/basic-search.
-
The authors declare that they have no conflict of interest. Baoru Yang is the Editorial Board member of Food Innovation and Advances who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and the research groups.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Kelanne N, Yang B, Laaksonen O. 2024. Potential of cyclodextrins in food processing for improving sensory properties of food. Food Innovation and Advances 3(1): 1−10 doi: 10.48130/fia-0024-0001
Potential of cyclodextrins in food processing for improving sensory properties of food
- Received: 15 October 2023
- Revised: 16 January 2024
- Accepted: 22 January 2024
- Published online: 29 January 2024
Abstract: Cyclodextrins are tapered cyclic oligosaccharides, which are used to encapsulate a wide range of compounds, such as phytochemicals and drugs. They can be divided roughly into native, modified, and large-ring cyclodextrins: native- and large-ring cyclodextrins are prepared from starch by cyclodextrin glycosyltransferase and are further chemically modified, improving their chemical properties, such as water-solubility. Cyclodextrins have many possible applications in food processing due to their inclusion complexation characteristics. Cyclodextrins can be used to improve the color properties of food by protecting natural pigments from degradation during storage or by inhibiting enzymatic browning. In addition, encapsulation of bitter compounds inhibits their interactions with taste receptors in the oral cavity, decreasing undesirable taste properties. Finally, encapsulation of hydrophobic compounds improves their dispersion in the aqueous matrix, increasing the bioavailability and antioxidative activity of the target compounds. Studies have shown that successful use of the cyclodextrin requires good planning and understanding of the chemical composition of the food product.
-
Key words:
- Cyclodextrin /
- Encapsulation /
- Color /
- Flavor