-
The characteristic of color enables the differentiation of various food commodities types and qualities. The perception that color is linked to taste, freshness, shelf life, and nutritional value has driven the demand for coloring materials in food processing and storage[1]. The utilization of naturally derived colors in food is believed to have started long back in prehistoric times, then came the 19th century when chemically synthesized colorants were brought to the forefront and gained more popularity[2]. Despite their low production cost and good chemical stability, increasing dissatisfaction with synthetic colorants' detrimental and allergic effects gradually led to their limited use[3]. The public's awareness of safety and environmental conservation has rekindled enthusiasm for research on natural pigment sources. Advantageous characteristics such as the biodegradable nature and additional function of naturally derived pigments over their synthetic counterparts enhance their acceptability for utilization as additives not only in food systems but also in the cosmetics and pharmaceutical industries[4,5].
Natural pigments ubiquitously exist in plant tissue, animal structure components, and microorganisms, including colorants and bioactive compounds. Fruits and vegetables constitute a significant part of our diet and serve as the primary source of chlorophyll, carotenoids, flavonoids, and betalains, the main types of edible pigments found in plants[6]. Pigments used for food coloration or nutraceutical purposes such as carminic acid (E120) and astaxanthin are derived from insects and microorganisms[7]. Besides the health benefits, natural pigments' antioxidant and antimicrobial efficacy have gained immense importance in recent years, demonstrating the potential to be a promising health-promotional ingredient and a food quality enhancer. As more and more attention is paid to their functional application in food preservation and quality improvement, the primary concern lies in maintaining the stability and biological properties[8]. These naturally derived pigments typically carry some limitations in food application when exposed to process stress (e.g., thermal processing, high-pressure, oxygen, or extreme pH values) and in-vivo environment (presence of enzymes and low-pH in the gastrointestinal tract). In this regard, various nanocarriers or stabilizers and encapsulation techniques are investigated as stabilizing methods to offer more options for utilizing natural pigments[9].
Aquatic products are regarded as one of the important sources of dietary nutrients, providing nearly 20% of the global consumption of animal protein with high economic benefits[10]. The high nutritional value, palatability, and delicacy gained aquatic products increasing popularity. As a global food commodity, transporting and storing recently captured aquatic species for an extended time is necessary, followed by various processing methods to cater to the requirements of manufacturers, businesses, and customers. However, owing to the rich content of proteins, polyunsaturated fatty acids, and high water content, this type of product is more likely to undergo sensory and nutrition degradation than other muscle foods, which is induced by lipid and protein oxidation, enzymatic change, and bacterial spoilage[11]. Many antioxidants, cryoprotectants, and novel techniques have been used in aquatic food processing and storage[12,13]. The significant role of natural pigments in this context is emerging. For a while, pigments were applied in fish and shrimp products for color-protecting or coloration purposes. Now, they are more often used in quality enhancement, preservation, and packaging as their diverse functions have been widely perceived[14]. To our knowledge, a comprehensive overview of incorporating natural pigments into aquatic products has not been summarized. Here, recent research progress on natural pigments application with a specific emphasis on aquatic products is overviewed, in the hope of providing directions for further development of pigments in improving the quality of aquatic products.
-
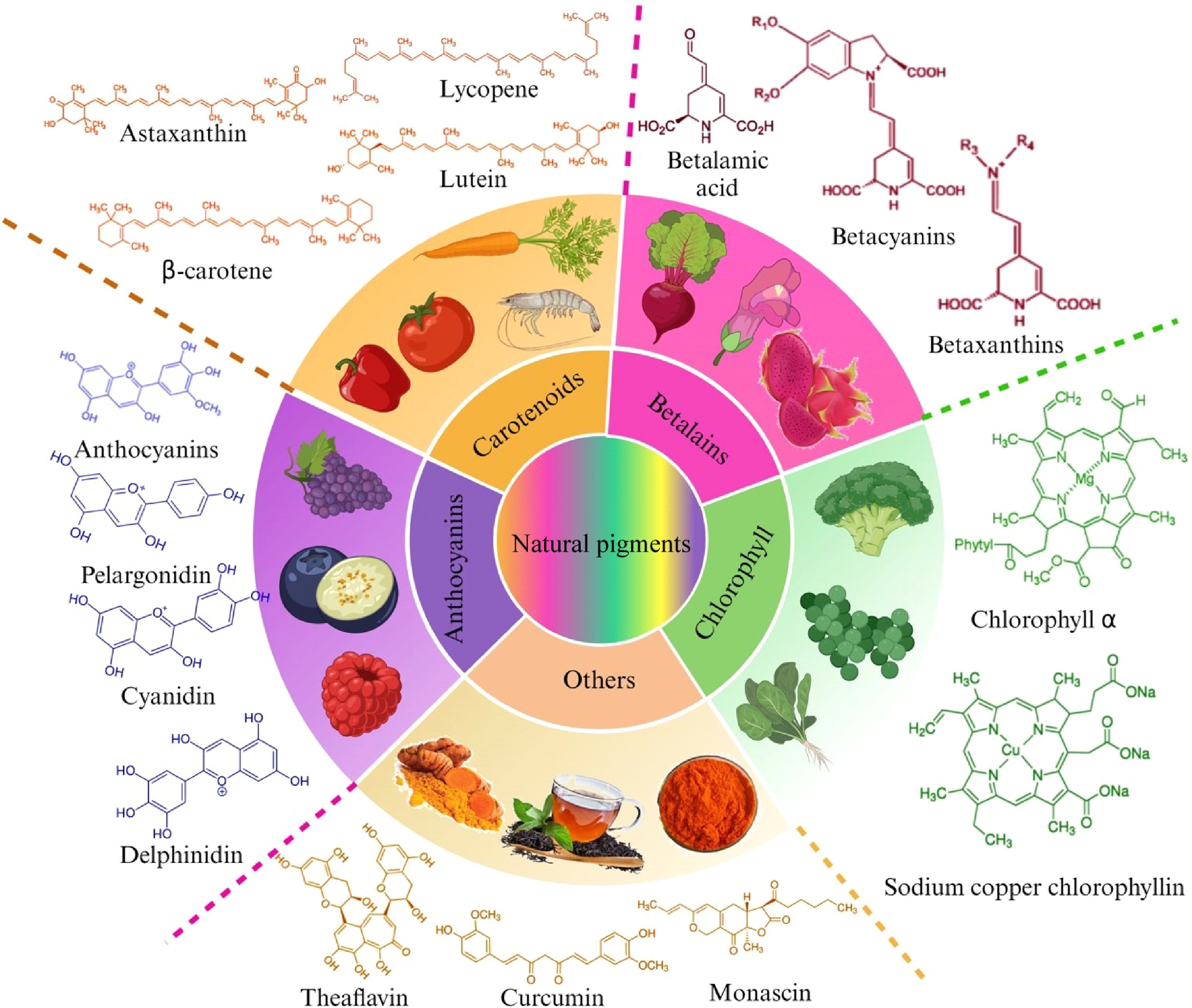
Natural pigments' characteristics and chemical properties influence their applications and regulations in certain environments. Depending on the features of the resource, color, solubility, and chemical structure, pigments can be classified into different categories. Considering the molecular polarity and basic properties, they can be divided into water-soluble, lipid-soluble, and ethanol-soluble pigments. Generally, betalains and anthocyanins are soluble in water, while chlorophylls and carotenoids are soluble in lipids. From the perspective of diverse structures, these compounds contain categories such as carotenoids, iridoids, indoles, polyphenols, pyridines, pyrroles, etc. Based on natural occurrence, the classification of carotenoids, chlorophylls, betalains, and anthocyanins is currently adopted and investigated in most studies (Fig. 1).
Carotenoids
-
Showing colors include yellow, orange, red, and purple, carotenoids (E160) provide vibrant and attractive appearances for various vegetables, fruits, and flowers due to their chromophores consisting mainly of a chain of conjugated double bonds[15]. As a class of polyene terpenoids, there are two types of carotenoids: carotenes (α, β, γ-carotene and lycopene) and xanthophylls (lutein, zeaxanthin, astaxanthin, capsaicin, peridinin, etc.) distinguished by the existence of oxygen functional groups in the polyunsaturated hydrocarbon[7]. Up to now, with a wide distribution that includes algae, photosynthetic bacteria, insects, fish, and crustaceans, approximately 700 carotenoids have been identified, of which 40 can be consumed by the human body[16]. Astaxanthin, an extensively investigated carotenoid widely distributed in aquatic organisms, can be synthesized by shrimps and crabs from β-carotene, acting as an antioxidant and enhancing protection from lipid peroxidation[17]. Carotenoids have been employed as a coloring component in various foods, including butter, popcorn, fruit juice, ice cream, and fish products[4]. Especially in aquatic species, astaxanthin, and other biosynthesized carotenoids provide accurate color and higher consumer acceptance for fish fillets and surimi[18].
Chlorophylls
-
Chlorophyll (E141) is built of a symmetrical cyclic tetrapyrrole with a centralized magnesium atom. The two types of chlorophylls that are commonly used in industry and present in higher plants are chlorophyll a (with a methyl group) and chlorophyll b (with an aldehyde group). The structure variation leads to the appearance of a yellow-green color in chlorophyll a and a green-blue color in chlorophyll b. Another member of the porphyrin group of natural pigments is chlorophyllin, which is more stable than chlorophylls. Ester bond hydrolysis leads to the formation of chlorophyllin, resulting in a shift of the pigment from lipophilic to hydrophilic[4]. However, both types show low resistance to acid and heat and are easily degraded compared to other natural pigments. While treated with weak acid, the central magnesium ion would be displaced by hydrogen ions, forming the pigment called pheophytin with an olive-brown color[19]. Among the green colorants, sodium copper chlorophyllin (SCC) or copper chlorophyllin (E141) is the most used with qualifications worldwide[20]. This kind of semi-synthetic product derived from edible resources has been applied in vegetable and fruit preserves, fruit juice, alcoholic drinks, jelly, and candy dyeing.
Anthocyanins
-
Anthocyanins (E163) are accepted as the glycoside form of anthocyanidins in chemical terms, with enhanced stability due to glycosylation and acylation. As an important subclass of flavonoids, one of the common groups of phenolic compounds of plant origin, anthocyanins possess a basic structure of two benzene rings linked with different sugar residues, differing in degree of hydroxylation and methoxylation, glycosidic substitution, and possible acylation[21]. Found in all tissues of higher plants, they impart brilliant red or blue color to flowers and fruits, which is different from the range of colors (usually yellow) derived from other groups of flavonoids[22]. Purple grapes, berries, black carrots, and red cabbage are the primary sources for extracting anthocyanins. Due to the biological potential of elimination of superoxide free radicals, evidence from clinical trials and epidemiology studies has suggested the beneficial effects of anthocyanins on human health[23,24]. Furthermore, the capacity of anthocyanins to inhibit the growth of bacteria, such as E. coli, has received increased attention from researchers interested in improving stability for a broader application in the industry. Since there are some restrictions on the application potential under certain circumstances when exposed to heat, oxygen, enzymes, light, etc., co-pigmentation and encapsulation have emerged as increasingly prominent solutions to strengthen the color and stabilize the bioactivity.
Betalains
-
Belongs to a class of N-heterocyclic compounds, betalains (E162) are ammonium derivatives of betalamic acid. Typically found in red beet, there are two major groups of betalains: red-violet betacyanins and yellow-orange betaxanthins. The categorization of the pigment is determined by the specific composition of the extra residues. Betaxanthins show high similarity in function with anthocyanins, yet these two pigment types have never been discovered in the same plant, indicating their mutually exclusive characteristic[25]. After the identifications of betacyanin from red beet[26] and betaxanthin from yellow-orange cactus pear[27], both types were addressed as betalains in the 1960s. Compared to anthocyanins, betalains possess an extended pH stability range of 3 to 7 and higher tinctorial strength. Its high hydrophilicity renders betalains more appropriate for incorporation into food. Depending on the pH, they change color from blue and violet to red in alkaline to acidic environments. However, it turns yellow when treated at temperatures over 70 °C, which is why betalains are exclusively suitable for coloration in non-thermal processing food.
Others
-
Other prominent pigments commonly found in nature and employed in food processing involve polyphenolic pigments and pigments of microbial origin. The most representative pigment of polyphenols is curcumin (E100), which is widely used as a bioactive ingredient, functional additive, and herbal medicine. Strong antioxidants, anticancer, antibacterial activity, and dyeing properties with low toxicity are the advantages of its various utilization in the food industry[28]. For its lipophilic nature, curcumin utilized as a food coloring agent includes the following types: water-dispersible curcumin oils, oil-soluble purified curcumin, and curcumin powder. In the past few years, considerable attention has been paid to the fresh-keeping effects of curcumin on food, such as shelf-life extension and maintenance of nutrition and senses, especially the application of curcumin-loaded materials in preserving fruit, meat, and seafood. Nanocarriers and encapsulation technology have been used to increase curcumin's stability and solubility, thus ensuring its adaptability for practical uses[29].
Monascus pigments are the secondary metabolites from Monascus species. They are widely used in China, Japan, and many other Asian countries, and they have a long history as edible colorants owing to their bright colors, excellent solubility, and high safety. These pigments are traditionally added as flavoring and coloring agents to Chinese food, such as fermented bean curd, fish cake, roast duck, and preserved dry meat and fish products. In Europe, they are considered a partial substitute for nitrate salt in meat and sausage preservation[30]. The pigments primarily present three colors: red, orange, and red, depending on the absorbance maxima at different wavelength ranges, and they can be converted into each other by reactions.
-
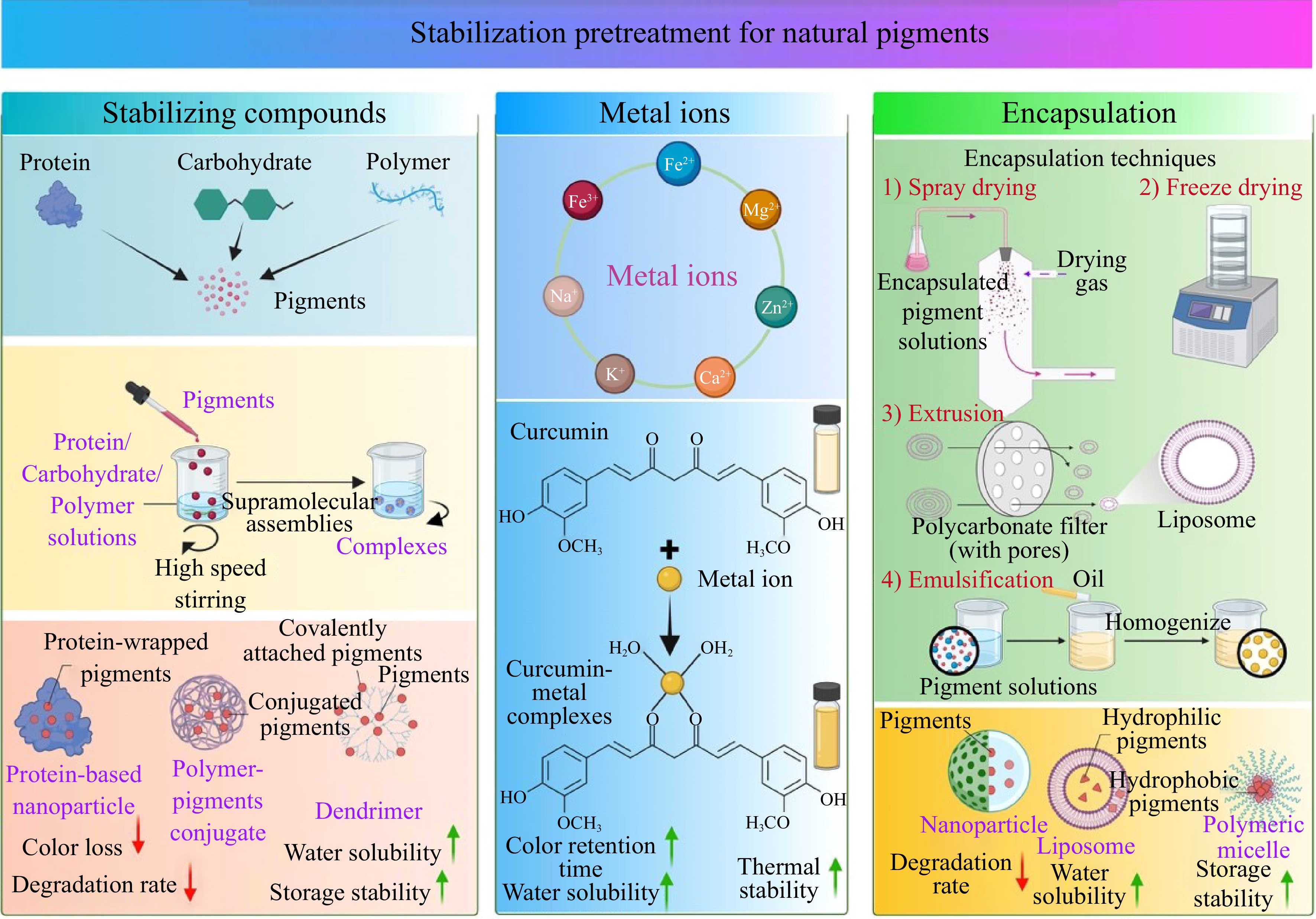
For commercial exploitation, it is imperative to avoid color fading and activity loss of natural pigments to ensure product quality and consumer satisfaction. Therefore, advanced approaches and progress are needed to stabilize natural pigments and increase their bioavailability. Numerous studies have been dedicated to devising strategies aimed at protecting pigments against degradation under various application conditions, such as the addition of stabilizing compounds, the use of metal ions, and encapsulation (Fig. 2).
Stabilizing compounds
-
Hydrocolloids are excellent candidates in the stabilization enhancement of pigments via non-covalent interactions. In contrast to the physical barrier of encapsulation, this interaction allows the formation of supramolecular assemblies of pigments and hydrocolloids. Pectin, an anionic polysaccharide, shows pH-dependent interaction and constant hydrogen bond, which is suggested to improve anthocyanins' stability and bioavailability[31]. Acacia gum and alginate are also used to form complexes with anthocyanins, betalains, and chlorophylls for stabilization, lowering color loss, and pigment degradation rat[32]. Supramolecular complexation with hydrocolloids is now attracting researchers' attention as a feasible method to increase pigment stability. Nevertheless, the addition of hydrocolloids in food products may lead to a change in textual properties and appearance, additional research is needed.
Binding to protein or carbohydrates provides another strategy for stabilizing and applying pigments to the food system. Various proteins from aquatic species have been shown to bind astaxanthin in different binding ways, such as lipoprotein, muscle protein, and glycoprotein. Zhang et al.[33] demonstrated that the interaction of shrimp ferritin and astaxanthin provided defense against thermal conditions and oxidative damage induced by Fe2+. It is worth noting that one ferritin molecule is estimated to bind 48 astaxanthin molecules, and the improved water solubility facilitates its applications in the food industry. In microalgae, AstaP (astaxanthin-binding protein) is identified as an efficient carotenoid solubilizer, which provides potential carotenoid delivery approaches[34]. Moreover, catechin and β-cyclodextrin contribute to the deceleration of pigment degradation processes. Both of them were found to show promotive effects on the storage stability of betacyanin besides maintaining the visual color attributes in the model beverage[35].
Metal ions
-
Metal ions are essential for the structural integrity of certain natural pigments. According to the study by Cao & Dong[36], adding Na2CO3 salt could extend chlorophyll color retention time to 119 d while increasing recovery efficiency by up to 150% during extraction. Furthermore, some pigment molecules are capable of metal chelation and, in the presence of polysaccharides, achieve enhanced stability. The binding of metal cations are attributed to several hydroxyl groups present on the B-ring of anthocyanins[37]. In a comparison study, at the same concentration, more charged metal ions were suggested to have better protective effects on natural pigments, probably due to their increased capacity to bind free hydroxyl groups[38]. Although the metal cations cannot solely prevent pigments from degrading under heating conditions, they stabilize pigments in the presence of polysaccharides. Interaction with polysaccharides enables the formation of a more stable complex, thus preventing the dissociation of anthocyanin-metal chelates. Luna-Vital et al.[39] evaluated the color and chemical stability of colored corn anthocyanins in the beverage model with the addition of zinc ions and alginate. Alginate could protect anthocyanins from thermal degradation under lower temperatures, while the combination of these two additives showed higher efficacy in promoting chroma stability of the incubated beverage. Meanwhile, the combination of 0.02% zinc and alginate was capable of prolonging the half-life of anthocyanins during 12 weeks of 25 °C storage.
Encapsulation
-
Encapsulation is a promising technology aimed to protect easily degradable compounds and control the release of natural bioactive components. It serves as an effective means to mitigate the impact of light, temperature, acidity, alkalinity, and other factors on natural pigments, thereby ensuring their stability. The functional barrier was achieved by a matrix or polymeric system, with carbohydrates, cellulose, gum, lipids, and proteins as the main types of materials. Depending on their size, encapsulated particles are classified as microparticles/microcapsules (1−1,000 μm) and nanoparticles/nanocapsules (< 100 nm). The encapsulation efficiency depends on the capsule material and the encapsulation method. Multiple techniques can be used for encapsulation, including freeze drying, spray drying, extrusion, ionic gelation, and emulsification. Spraying drying is the most widely studied method and is shown to efficiently encapsulate pigments in combination with polysaccharides such as maltodextrins[40]. To increase the stability of lutein, Álvarez-Henao et al.[41] obtained micro-particles with spray-drying, using maltodextrin, Arabic gum, and a modified starch as the encapsulating agent. Under the storage of 20 °C, the highest retention rate of lutein was achieved by the formulation of Arabic gum : maltodextrin : modified starch (33.3% : 33.3% : 33.3%), where Arabic gum is the key substance to improve the encapsulation efficiency. In comparison, freeze-drying is considered more suitable for sensitive pigment encapsulation under lower temperatures, which can offer an efficiency of up to 95%[42]. Ionic gelation is a novel technique that is appropriate for various component types. In a recent study by Tekin et al.[43], alginate/calcium-chloride ionic gelation was carried out to encapsulate red beet concentrate. It was found that betacyanins were more stable than betaxanthins after 6 weeks of storage at room temperature, and 79.48% of the betalain was preserved under the optimum encapsulation parameters. In addition to avoiding degradation, nanocarriers are also employed to incorporate lipophilic pigments in aqueous systems to extend their applicability in food products. Peng et al.[44] fabricated the curcumin nanoparticles by pH-shift method and evaluated the performance of different kinds of coating biopolymers (sodium caseinate, whey protein isolate, soy protein isolate, and Arabic gum). All four biopolymers could enhance aqueous dispersions of curcumin nanoparticles and showed high loading capacity, with casein showing the highest (27%). Among them, the best pH and salt stability were achieved by Arabic gum coatings, whereas the best heat stability was offered by sodium caseinate and soy protein isolate.
In summary, the stability of the extracted pigment can be improved through appropriate treatment, with the selection of a pretreatment method depending on the type of pigment. Combining different approaches, such as metal ions and stabilizing compounds, can be performed to achieve better effects. Enhanced heat/light/pH stability will allow natural pigments to be more effectively applied in food processing.
-
Natural pigments find application in various seafood sectors, including preservation, packaging, production, and processing (Fig. 3), which are detailed in upcoming sections. Freshness and safety are among the most demand-driven challenges of aquatic products[45].
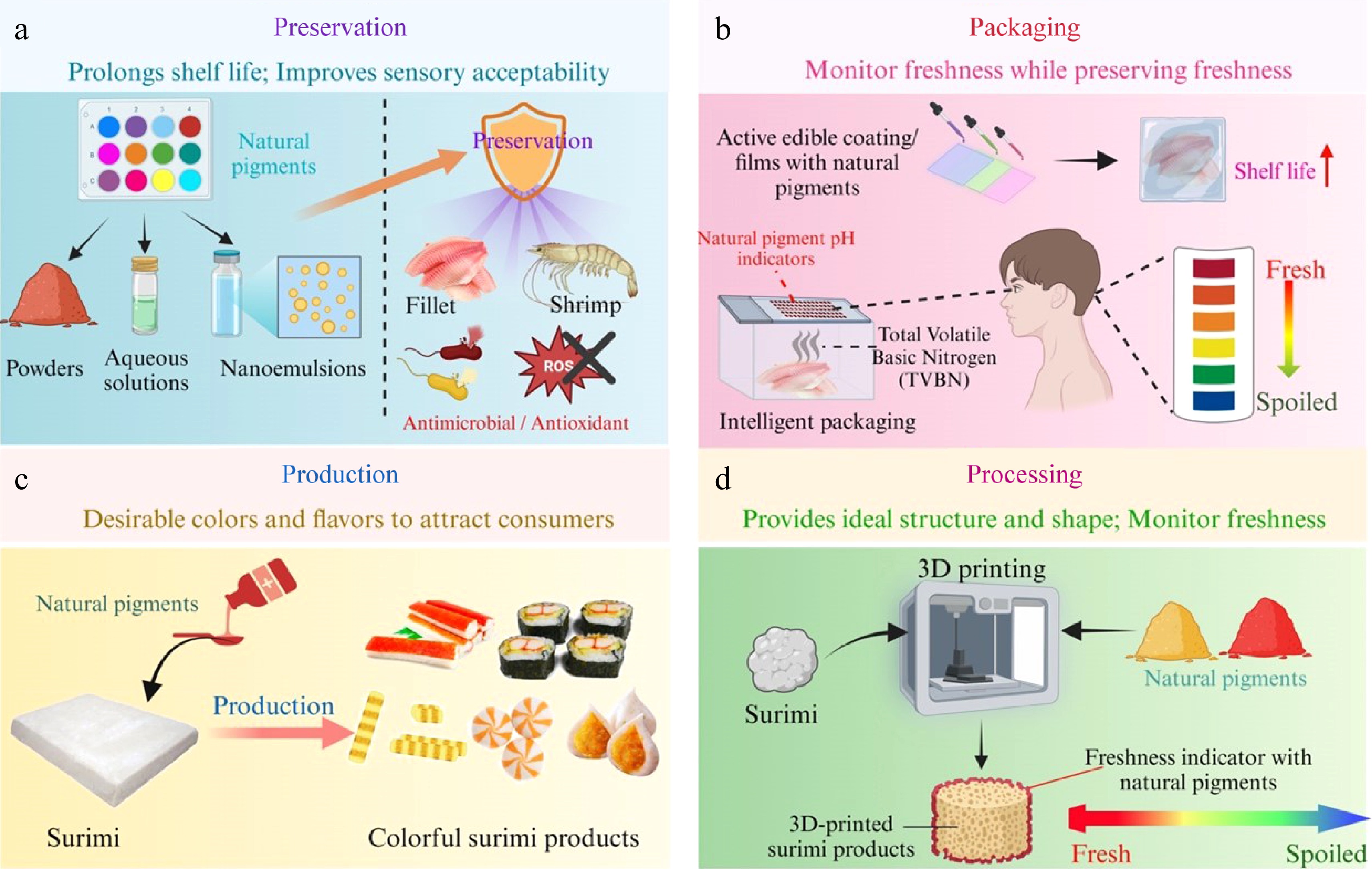
Figure 3.
Applications of natural pigments in preservation, packaging, production and processing of aquatic products.
Antimicrobial and antioxidant effect in preserving aquatic products
-
Despite the delicacy and palatability resulting from high protein content and accessibility of high-water activity, seafood products are exceptionally perishable after death and thus necessitate treatment and cold storage to enhance their quality and shelf life. Lipid oxidation, protein degradation, and formation of amine products under the effect of microorganisms and enzymes begin immediately after aquatic species die, compromising commercial products' nutritional quality and flavor profile[46].
Many bioactive compounds preserve fish fillets or shrimp by being retained as nanoemulsions, aqueous solutions, or powders. They are summarized in Table 1. Currently, lycopene, curcumin, and theaflavin are the most common pigments employed for storing aquatic products, with relatively higher acceptance in sensory evaluation. Curcumin and rosemary oil nano-emulsion produced by sonication techniques have a pronounced effect in protecting rainbow trout fillets from contamination by bacteria such as P. aeruginosa, E. coli, and S. typhimurium[47], reducing peroxide value and prolonged shelf life after the storage. Meral and co-workers[48] fabricated nisin and curcumin-loaded nanomaterials to improve the acceptability of the fish fillets during the cold storage period. They observed the limited total mesophilic aerobic count growth and extended storage time of up to 10 d with acceptable sensory attributes of the fish fillet. To suppress protein and lipid oxidation, theaflavins are made into an aqueous solution for pre-soaking treatment on yellow croaker (Pseudosciaena crocea) fillets for 5 d at room-temperature and 40 d of chilled storage[49]. In addition to inhibiting myofibrillar protein and lipid oxidation, the theaflavins solution positively impacts the texture and color stability of the fillet samples.
Table 1. Utilization of natural pigments in aquatic products.
Characterization Source Concentration Food product Method Application condition Metrics E number Ref. Curcumin / 1 g/L Rainbow trout Nanoemulsion 4 °C storage Specific pathogenic microorganism inhibition; delaying total mesophilic bacteria growth E100 [47] Curcumin / 0.1 g/L Rainbow trout Nanomats 4 ± 1 °C storage Extending shelf life of coated fillets to 12 d;
high antimicrobial activity[48] Theaflavins / 0.5 g/L Yellow croakers Solution immersion for 30 min 4 ± 1 °C storage Protein and lipid oxidation degree reduction; myofibrillar protein stability improvement NA [49] Lycopene Tomato 360 ppm Rainbow trout Solution immersion for 30 min 4 ± 1 °C storage Shelf life extension; maintaining sensory attributes; delaying lipid oxidation E160d [50] Carotenoids/
flavonoids/ anthocyaninsPotato / sweet potato / red beet 0.1% Rainbow trout Powder Ice storage Sensory and chemical quality improvement;
low costE160/ NA/ E163 [53] Flavonoids/ betalains Red beetroot 1 g/L (crude extract) Tilapia fish Dipping solution (5 min) 5 °C storage Antioxidant activity; reducing TBA; high safety NA/ E162 [54] Astaxanthin Algae / Rainbow trout Dipping solution (30 s) 4 ± 1 °C storage Delaying microbial growth and lipid oxidation; maintaining meat color NA [55] Proanthocyanidins Grape seed / Salmon Film with microcapsules 5 °C storage Antimicrobial effect; maintaining the luminosity value; extend shelf-life to 4−7 d NA [58] Carotenoid Shrimp and tomato by-product 0.1 g/100 mg protein / Film 22 °C Edible; antioxidant; high stability; low carotenoid degradation E160 [60] Curcumin / 0.4 mg/mL Grass carp Emulsion 4 °C storage Shelf-life extension by 6 d; lipid oxidation suppression; reducing microbial coruption E100 [62] Anthocyanins Sweet potato 4% Bighead carp Film 4 °C storage Color change respond to pH; real-time monitoring of freshness; stability E163 [65] Anthocyanins Echium amoenum 19 mg/L Shrimp Film 4 °C storage Visually-distinguishable color change; TVC and TVB-N change indication; high sensitivity [66] Anthocyanins Purple corncob 0.8% Shrimp Film 4 °C storage Antimicrobial and UV-blocking properties;
pH-responsive colorimetric indicator; biodegradability[67] Phycocyanin and anthocyanin Ipomoea nil and red cabbage 1 g/L Grass carp Film 4 °C storage High sensitivity to ammonia; fish freshness indication; non-destructively tracing [68] Betalains Cactus pears 3% Shrimp Film 20 °C, 48 h Antioxidant and ammonia-sensitive properties;
high water vapor barrier propertyE162 [69] Betalains Red pitaya peel 1% Shrimp Film 20 °C, 48 h UV–vis light and water vapor barrier ability; antioxidant and antimicrobial properties;
freshness indication[70] Carotenoid Shrimp waste 10 ppm Fish sausage Powder Frozen storage Color and flavor improvement; quality enhancement E160 [73] Astaxanthin Shrimp waste 1% Minced tilapia Oil soluble astaxanthin 4 ± 1 °C storage Extending shelf-life up to 20 d; antimicrobial activity; coloring ability; reducing lipid peroxidation; NA [74] Curcumin / 400 nmol/g surimi Shrimp surimi Solution 4 °C storage Bacterial growth inhibition; delaying quality deterioration E100 [78] Curcumin / 1 mg/mL Surimi Nanoparticle −3 °C storage Oxidation resistance and relative release efficiency; microbial growth inhibition; shelf-life extension [79] Lutein/ anthocyanin / 0.25%, / 3D-printing surimi Powder 4 °C storage Fresh-keeping effect; bacterial growth inhibition; freshness monitoring E161b/ E163 [80] Lutein / 0.5% 3D-printing surimi Nanoparticle 4 °C storage Prolonged lutein release; gel quality improvement; antioxidant function; E161b [82] Similarly, lycopene solution is demonstrated to be very effective in stabilizing the freshness of trout fillets during refrigeration[50]. Following immersion in various concentrations of lycopene (w/v) solutions, the rainbow trout fillets exhibited reduced PV, TBA, and FFA values compared to the control group. Fillet samples with extra lycopene, especially higher levels, remained acceptable throughout the test, suggesting lycopene's efficiency in extending the trout fillets' shelf-life. Nirmal & Benjakul[51] used a catechin solution to treat Pacific white shrimp (Litopenaeus vannamei) before 10-d ice storage. The retarded growth of microorganisms, lower increases in total volatile base (TVB) content, and delayed formation of melanosis that was observed in the results indicate a promising melanosis inhibitor as well as an antimicrobial and an antioxidant in ice-stored shrimp.
Extracts derived from the peels, roots, and seeds of fruits and vegetables, which are abundant in polyphenolic pigments, effectively preserve shrimp and fish while increasing the value of the by-products[52]. Icing with sweet potato, sugar beet, and red beet peel extract as a source of antioxidants was employed to store rainbow trout fillets[53]. These peel extracts extended the shelf life by 4 d. It provided positive features on sensory, chemical, and microbiological quality, which can be used as an alternative technology for food preservation. Similar outcomes were obtained from investigating tilapia fish fillet preservation using red beetroot peel extract[54]. During the cold storage of the rainbow trout fillets, extract from Haematococcus pluvialis (Hp) among different algae extracts showed the most evident effect in delaying microbial growth and lipid oxidation processes. More importantly, the Hp extract contributed to the appearance of trout fillets by preventing a* values (redness) from decreasing throughout refrigeration[55].
Application in active novel packaging of aquatic product
-
Packaging is an essential safeguard against environmental threats during the transportation and storage of processed aquatic products. Addressing sustainable development and the health hazards of plastic products to living organisms, the current research topic in food packaging is the development of biodegradable packaging[12]. By integrating active ingredients/intelligent compounds into packaging or directly onto the surface of aquatic products, protection that contributes to the assured quality or timely information regarding the quality is provided (Fig. 4a). For monitoring the freshness of food products, additional functional substances such as natural pigments are embedded in films[56] to both preserve the food and indicate quality change (Fig. 4b).
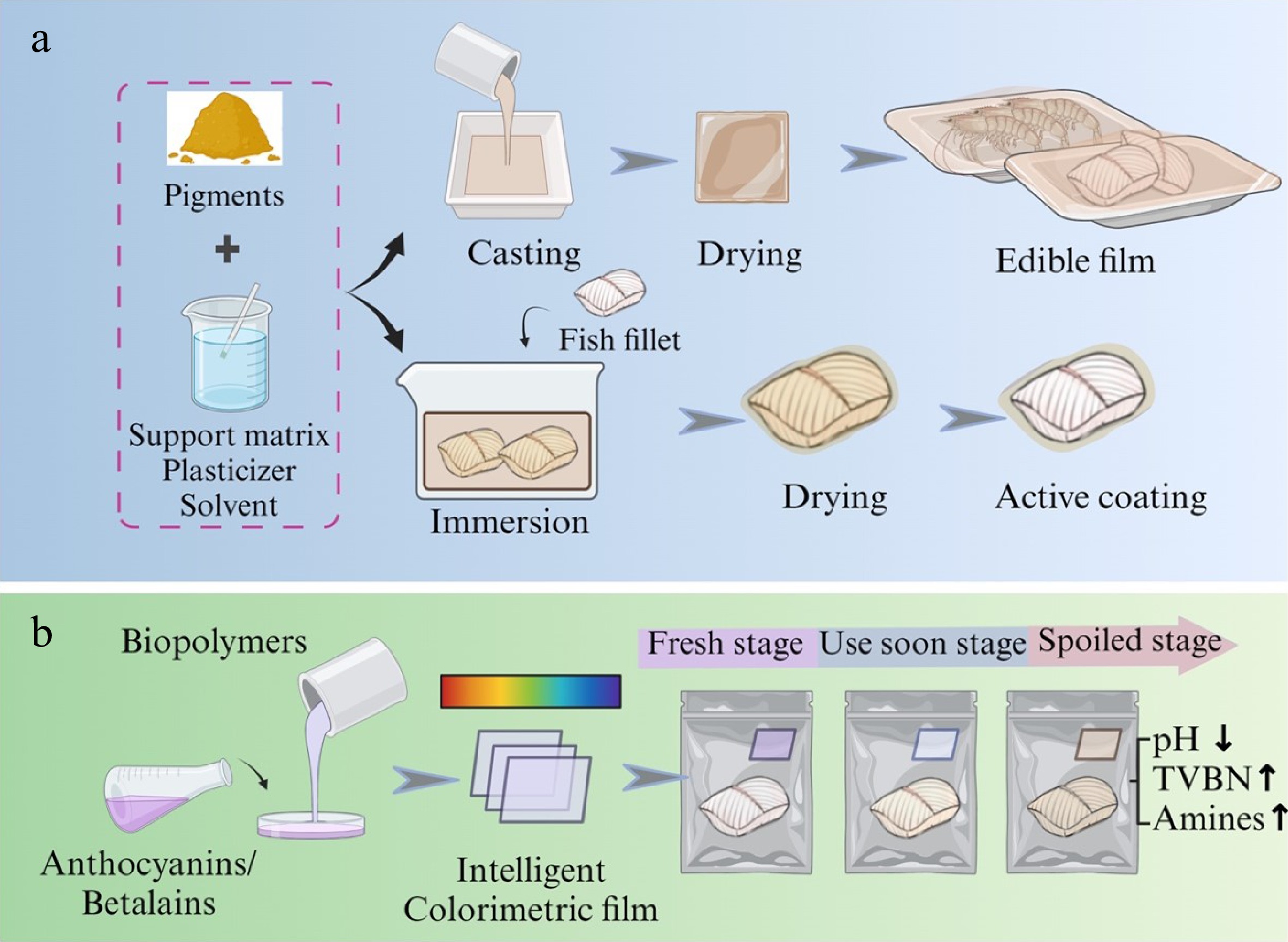
Figure 4.
Natural pigments as active coating and intelligent colorimetric film for freshness indicator of aquatic products.
Active edible coating
-
Aiming for food preservation, edible coatings are made as a thin layer that wraps the food directly by submersion or spraying[57]. The coatings are initially formulated using a biopolymer matrix, with the option of including functional ingredients, and the resulting food-grade suspensions are then applied in a liquid form onto the surface of the food product, followed by drying. In terms of fishery product preservation, the coated film's strong antimicrobial and antioxidant capabilities are key factors in extending the shelf life on the premise of high quality. Chitosan is mostly employed in edible films among various biopolymers due to its superior performance, which is prepared with grape seed extract to preserve salmon fish[58]. Grape seed extract contains abundant phycocyanin and anthocyanins, commonly employed as natural antioxidants to strengthen coatings. Zhao et al.[59] introduced vacuum impregnation to fish gelatin-based coating containing grape seed extract for chilled seabass fillets storage. This coating effectively postponed the microbial spoilage and discoloration of the seabass fillets and its ability to impede the water migration sustained the fillet's water-holding capacity.
Furthermore, pigments derived from aquatic waste are employed for preservation. Lipid-extracted astaxanthin from shrimp has been utilized to produce edible membranes with antioxidant properties that remain stable during refrigeration. Compared to lycopene and beta-carotene, astaxanthin exhibited the lowest degradation rate of 17% following a one-month storage period[60]. Arancibia et al.[61] developed a coating solution containing chitosan and enriched shrimp waste extract concentration and applied it to shrimp preservation during cold storage. The antioxidant activity of the extract delayed microbial growth and the onset of melanosis, resulting in a desirable color and taste on the shrimp. Sun et al.[62] reported an edible coating prepared by fish gelatin enriched with curcumin/β-cyclodextrin, which exhibited considerable potential in preserving grass carp fillets at 4 °C. With the addition of curcumin, the oxidation degree, microbial spoilage, and color change were suppressed in the fish fillet during storage. The curcumin/β-cyclodextrin emulsion coating treatment extended the shelf life of grass carp fillets for 6 d.
Intelligent packaging
-
Unlike active films that contain natural compounds with bioactivities and physicochemical properties, it is not necessary for intelligent packaging to release specific elements. Intelligent packaging consists of three types: indicators (integrity, freshness, time, and temperature), data carriers, and sensors (gas sensors, chemical sensors, etc.)[63]. One of the main parts of the intelligent packaging system, freshness indicators based on pH sensing have found fast growth in the aquatic food industry due to their affordability, adaptable production, and easy visual observation detection of color changes[64]. The pH-sensitive films function by the released volatile amines from food products, which leads to an elevation in the pH of the package headspace.
Recently, anthocyanins, betalains, curcumin, and phycocyanin from plant extracts have been primarily used solely or compositely as natural pH indicators. These pigments are typically immobilized into a solid-based platform, the natural or synthetic polymers including cellulose nanofibers, starch-chitosan, starch-polyvinyl alcohol gelatin, and alginate. Combined anthocyanins with curcumin, Chen et al.[65] produced starch and glycerol-based composite film that exhibited durability for no less than 180 d, which non-destructively indicated the different degrees of bighead carp fillet at 4 °C. It was found that films containing a 2:8 ratio of curcumin to anthocyanins exhibited a higher degree of precision in responding to pH variations. The bacteria cellulose film containing Echium amoenum extracted anthocyanins was fabricated and used to monitor packaged shrimp's freshness[66]. Three distinguishable colors were observed as the shrimp aged: violet (fresh stage), gray (use soon stage), and yellow (spoiled stage), indicating the suitability of anthocyanins from E. amoenum for quality indication of protein-rich food. Normally regarded as an agricultural waste product, purple corncob is a suitable raw material for preparing pH-sensitive packaging. Pigments and lignin-containing cellulose nanocrystals endowed the packaging film with a reversible color response and strong mechanical properties, which were proven to act well as a freshness indicator of shrimp and meat products[67]. Tavakoli et al.[68] immobilized anthocyanins and phycocyanin into composite gelatin/soybean polysaccharide matrices and obtained a highly sensitive colorimetric film. An obvious correlation was observed between the label color and the fish's pH, TVB-N, and bacterial growth, which can be used to trace the spoilage of grass carp refrigerated at 4 °C. Smart film formed by combining betalains from Cactus pears (Opuntia ficus-indica) with quaternary ammonium chitosan/polyvinyl alcohol blends exhibit favorable water vapor barrier properties and tensile strength, which also changes color in response to volatile nitrogen compounds, indicating shrimp freshness[69]. By adding betalains-riched red pitaya (Hylocereus polyrhizus) peel extract into starch/polyvinyl alcohol film matrix, the water vapor barrier, UV barrier, mechanical properties, antioxidant, and antimicrobial properties of the film can be effectively enhanced. The film is also sensitive to ammonia and can be used as a freshness test for shrimp[70]. In summary, natural pigments derived from plants are predominantly employed in smart and active packaging applications to assess the freshness of aquatic products. This utilization stems from the ability of natural pigments to undergo color changes due to pH variations caused by the release of volatile ammonia compounds during the storage of aquatic products.
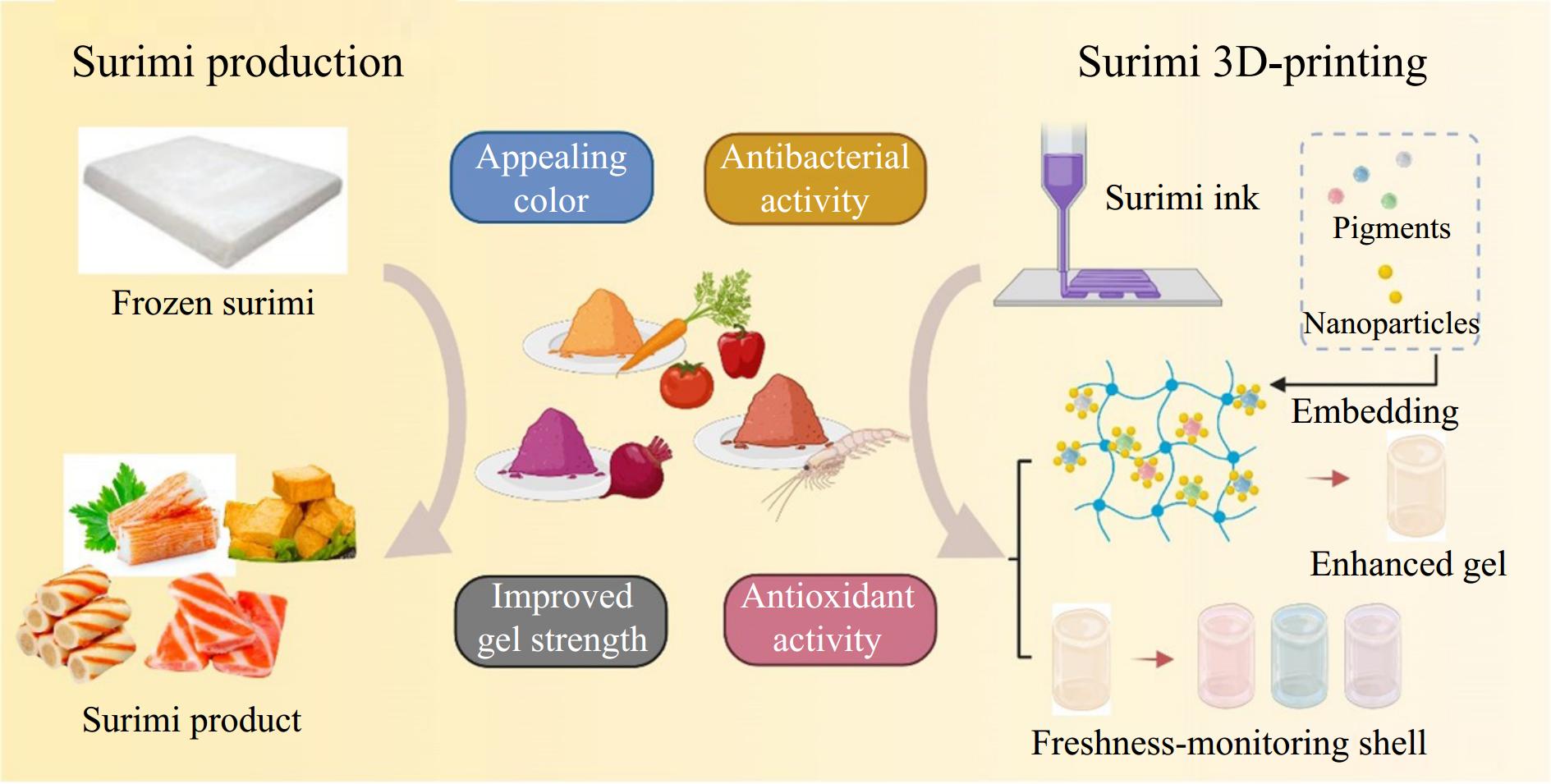
Incorporation in surimi product and surimi-based 3D-printing
-
Surimi products like fish balls, fish sausages, fish tofu, and shrimp surimi are becoming daily food with increasing popularity worldwide due to their high nutrition and elastic texture. Nevertheless, their quality is constrained by protein oxidation, fat oxidation, and contamination with foodborne bacteria during refrigeration because of their high protein content and perishable nature[71]. Regarding frozen surimi, measures have been taken to improve the quality and gel strength and reduce oxidation during extended freezing by employing several additives like cryoprotectants[72]. However, as for the prepared surimi-based products (ready to cook) mostly sold in hot-pot restaurants, attractive colors and designed shapes are the key sensory attributes that increase consumer preference and acceptance. Surimi products, including simulated crab sticks, shrimp cakes, and fish sausages, are often orange or red-colored. Typically, the desired color of the surface is achieved by adding colorants like carmine, monascus, paprika, caramel, and lycopene (Fig. 5).
Carotenoids recovered from shrimp waste had been applied to the fish sausage, which had a positive impact on the color and flavor of fish sausage[73]. A previous study demonstrated the use of edible oil extraction to recover astaxanthin from shrimp processing waste[74]. In application, the recovered astaxanthin improved the storage quality and stabilized the color of minced tilapia fish. Suryaningrum et al.[75] reported the good performance of beetroot pigment extracts in improving catfish surimi's gel quality and appearance. Moreover, curcumin among the pigments extracted from colored plants is proven to be most effective in increasing microbial resistance and augmenting the sensory properties of tilapia fish surimi[76]. More vulnerable to deterioration under frozen conditions, shrimp surimi requires refrigerated storage between 0 and 4 °C[77]. Curcumin-mediated sono/photodynamic demonstrated superior bactericidal activity in preserving shrimp surimi quality, which can be used as a reliable non-thermal sterilization method[78]. Regarding the limited solubility in water and the poor chemical stability of curcumin, encapsulation with chitosan nanoparticles was incorporated into its application, providing improved oxidation resistance and maintaining the nutrient content of surimi[79]. Natural pigments also provide additional opportunities for the creation of colorful and diverse shapes, as well as the improvement of the quality of ready-to-eat snacks that are based on surimi.
The antibacterial and antioxidant properties of these pigments exhibited the potential to extend the shelf life and monitor the freshness of printed food[80]. Because of its homogenous and suitable fluid properties, surimi is suitable for 3D printing technology as the 'ink' matrix, generating customized functional foods for the elderly, patients, and children[81]. Lutein is a potent addition for inhibiting oxidation and an effective quality enhancer, providing surimi with better structure and shapes. The addition of 0.5% lutein combined with nano starch imparted a visually appealing red hue to Pennahia argentata surimi, delaying the decrease of L* and increase of a* and b* caused by the addition of lutein separately[82]. The charged group of lutein contains electrons that could interact with radicals to exert antioxidant properties. Thus, controlled release becomes imperative when lutein is engaged in processing or digesting. With nanoparticles, pigments with antioxidant activity are released better for longer-lasting functions[83]. Comparable to the functional film, anthocyanins exert the role of pH indicator in the shell of 3D-printing surimi for non-destructive quality monitoring, turning green and yellow gradually during refrigeration to indicate that the freshness is getting worse. For ease of understanding, the utilization of different natural pigments are listed in Table 1.
In summary, numerous studies have demonstrated the preservation value of natural colors in seafood products such as fish fillets and fresh shrimp, which provides possibilities for the development of ready-to-eat seafood. Commodities such as ready-to-eat cooked fish fillets, dried shrimp, and scallops are very popular and possess good economic benefits in China. Natural colors have good potential for their application in color enhancement and quality retention. At the same time, fat-soluble colors can be combined with fish oil and other nutrients rich in polyunsaturated fatty acids to develop nutritionally enhanced high-end aquatic products. This combination attracts consumers seeking minimally processed and natural products and ensures the benefits of the aquatic industry as well. These pigments' multifunction and nontoxic nature confirms their ubiquitous application range in the aquatic industry as natural additives and preservatives.
-
Natural pigments are always more favored by consumers than synthetic ones when applied in the food industry, which can be exemplified by the inclination of people to take pigments (anthocyanin, etc.)-rich foods to attain health advantages. Their ability to counter oxidants also attracted intensified interest from food researchers to find more approaches to unlock their application potential. Among numerous food product categories, aquatic products have relatively higher economic and nutritional value but are particularly sensitive to spoilage. Nevertheless, the stability, cost, color range, and safety are still the main concerns in the scale-up from lab to commercial application:
(1) Regarding stability, susceptibility to some common conditions limits their incorporation efficiency, and the resulting color change may also affect the meat texture. More studies are needed to understand the degradation, color retention degree of pigment, and impact on the food matrix during storage and processing. Including encapsulation, advanced techniques need to be used to control the release of pigments. To solve the problem of low thermal resistance of pigments used for coloring purposes, potential integration approaches involve optimizing the food processing methods and incorporating the pigments directly into the final product.
(2) As food additives, natural pigments generally require higher costs for extraction and larger amounts compared to synthetic alternatives due to the complexity of their extraction process. Further improvements in extraction techniques, such as supercritical fluid extraction and enzymatic extraction, and more sustainable pigment sources are also required to enhance their competitiveness in the marketplace. It is practicable to employ pigments derived from microorganisms, algae, and microalgae to preserve aquatic products, meet sustainable development needs and mitigating the seasonal influence on plant resources. The future may see the development of cost-effective biotechnological processes, such as fermentation and genetic engineering, and large-scale cultivation techniques that could drastically reduce production costs and ensure a steady supply of high-quality pigments.
(3) The limited availability of natural pigments has led to a constrained color options for food products. While current natural colors continue to be extensively employed and favored by numerous consumers, the incorporation of a broader range of colors presents a promising opportunity for expanding the utilization of natural pigments in aquatic products. The ideal approach involves the physical blending of established natural pigments to achieve the desired color of ready-to-eat seafood and the combination of fat-soluble colors with fish oil to develop nutritionally enhanced high-end aquatic products. These advancements could transform the food industry by providing more vibrant and stable natural coloring options, thus enhancing the visual appeal and perceived quality of food products while aligning with consumer preferences for natural ingredients. However, this necessitates optimal stability and a sufficient level of purity, both of which are closely associated with the extraction method employed for obtaining the natural pigment.
(4) The utilization of natural colors in both edible and smart packaging necessitates adherence to food safety and regulatory standards. There will be a growing emphasis on conducting thorough safety assessments to identify and mitigate potential allergens or hazardous substances in natural pigments. As consumer demand for natural and safe food additives increases, regulatory frameworks will likely evolve to support and ensure the safe use of these pigments, including establishing clear guidelines and standards for their production, application, and labeling. Commercial application of smart packaging technology that integrates natural pigments could enhance consumer confidence and satisfaction.
Overall, the integration of natural pigments into food products holds promising potential for transforming the food industry. Addressing these challenges, pigment applications emerge as a key facet in shaping the future of high-quality, visually appealing, and nutritionally rich aquatic offerings.
-
Aquatic products are highly susceptible to quality deterioration, which may lead to economic loss and health risks. The prospect of incorporating natural pigments in aquatic processing and storage is increasingly realized, which includes enhancing visual appeal and customer perception, maintaining the overall quality of aquatic products, prolonging their shelf life, and indicating freshness. These pigments' multifunction and nontoxic nature confirms their ubiquitous application range in the aquatic industry as natural additives and preservatives. With increased focus on transparency in food labeling, natural pigments will play a crucial role in providing recognizable ingredient lists and preservatives without harming the human body.
-
The authors confirm contribution to the paper as follows: conceptualization: Ding N, Tan Y; visualization: Ding N, Zhou Y; formal analysis, investigation: Zhou Y; writing – original draft: Ding N; writing – review & editing: Chang S, Feng R, Hong H, Luo Y; Tan Y; funding acquisition: Luo Y, Tan Y. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
This review was supported by the National Key R&D Program of China (2023YFE0122800) and the Earmarked Fund for China Agriculture Research System (CARS-45).
-
The authors declare that they have no conflict of interest.
-
Authors contributed equally: Ning Ding, Yongjie Zhou
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Ding N, Zhou Y, Dou P, Chang SKC, Feng R, et al. 2024. Colorful and nutritious abundance: potential of natural pigment application in aquatic products. Food Innovation and Advances 3(3): 232−243 doi: 10.48130/fia-0024-0023
Colorful and nutritious abundance: potential of natural pigment application in aquatic products
- Received: 22 May 2024
- Revised: 04 July 2024
- Accepted: 07 July 2024
- Published online: 23 July 2024
Abstract: The promising future of natural colors in the food industry aligns with the shift in consumer preference toward healthier food options. These naturally derived ingredients gradually replace their artificial counterparts and find applications in a wide range of food categories, and aquatic products have emerged as one of them. In this work, we introduced the characteristics and extraction of several main types of natural pigments and also explored the positive outcomes of integrating the pigments, such as carotenoids, curcumin, anthocyanins, and betalains, in aquatic product processing and preservation. Their outstanding antioxidant and dyeing properties contribute to the production and storage of various aquatic products. This review aims to provide a comprehensive understanding of the current state of natural pigment applications in aquatic products and to provide inspiration for future research and industry practices.
-
Key words:
- Natural pigments /
- Anthocyanins /
- Betalains /
- Carotenoids /
- Aquatic products