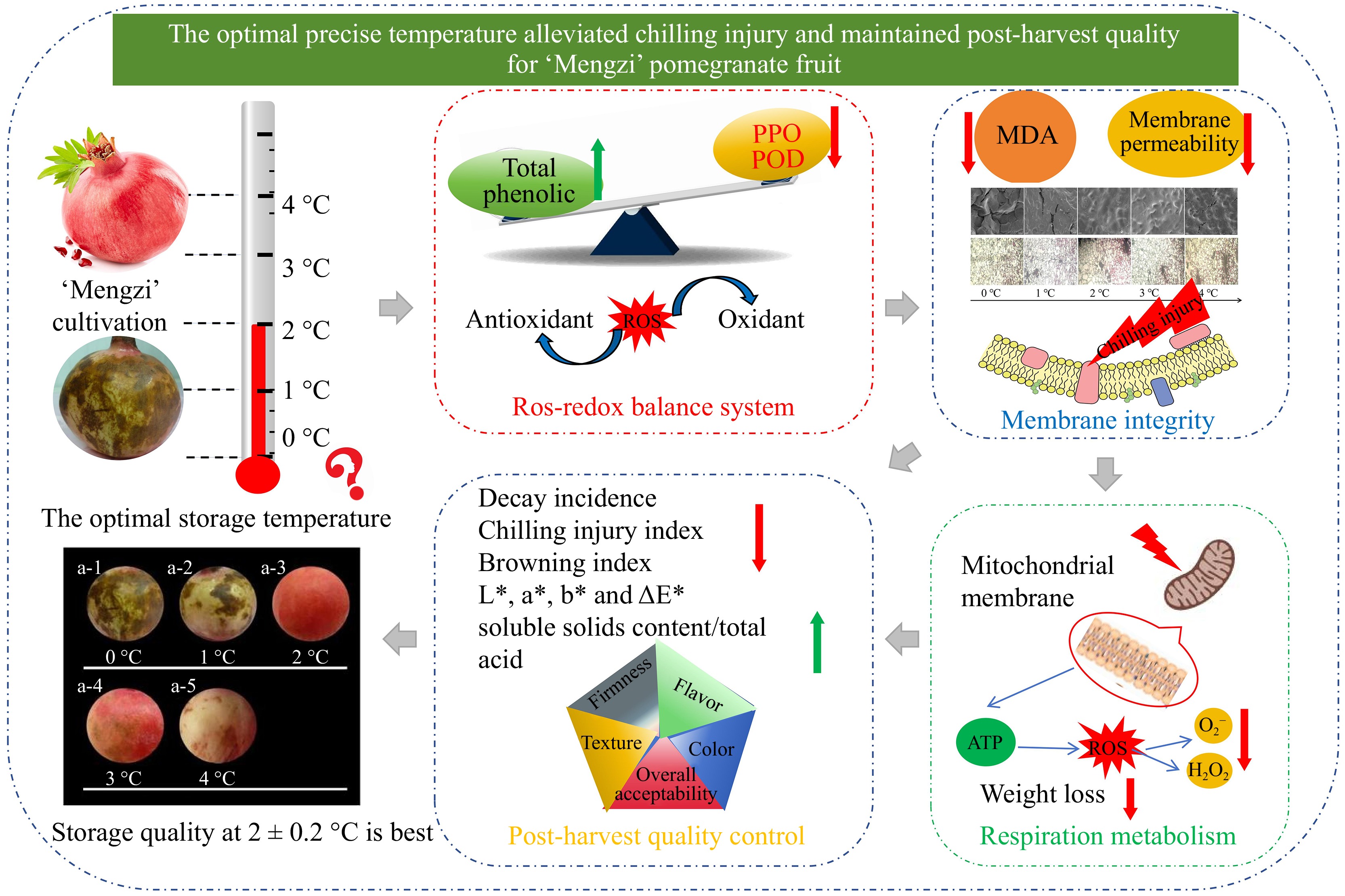
-
The pomegranate (Punica granatum L.), a berry of the pomegranate family (Punicaceae), is a medicinal and edible fruit[1]. In addition to numerous vitamins and minerals[2], pomegranates are also rich in valuable bioactive substances, including anthocyanins, carotenoids, tannins, polyphenols[3], and flavonoids[4]. It is proven that these substances have anti-diabetic, anti-inflammatory, antioxidant[5], anti-tumour, anti-hyperglycaemia, and anti-hypertensive properties in vivo and in vitro[6].
Pomegranates are highly sensitive to temperature and are typically stored in cold storage at a range of 0−5 °C[7]. Higher temperatures lead to obvious water loss, browning, and decay. While, under unfavorable low temperatures condition, the fruits are vulnerable to chilling injury (CI), thus leading to metabolic disorder and decay during post-harvest storage[8]. Besides, low temperature fluctuation (LTF) ranging from ± 0.1 to ± 0.2 °C was discovered to significantly alleviate the CI in peach during 60 d of storage, as opposed to a temperature fluctuation ranging from ± 0.5 to ± 1 °C[9]. Similarly, LTF storage was demonstrated to be an effective approach to maintaining flesh quality and retarding water core dissipation in 'Fuji' apples[10]. There are many publications revealing that the occurrence of CI is accompanied by an increase in oxidative enzyme levels, including polyphenol oxidase (PPO) and peroxidase (POD) activity, as well as total phenolics. Specifically, the chilling temperatures exerted a stimulating effect on the activities of PPO and POD and resulted in a decrease in the content of phenolic substances in olives[11] and peaches[12].
It is well known that the membranes guarantee the biochemical and physiological reactions are carried out orderly and efficiently[13]. There is a hypothesis that CI begins with internal cell membrane injury. Kong et al.[14] proposed that the increased incidence of CI in bell peppers is accompanied by enhanced membrane permeability, and it was further demonstrated that the incidence of CI is closely related to the integrity of the cell membrane. Likewise, Wang et al.[15] found more malondialdehyde (MDA) accumulated along with CI occurrence in peaches. Also, the impaired function of cell membranes led to an insufficient supply of metabolic energy, which was closely associated with respiratory abnormalities. This led to the excessive buildup of MDA and reactive oxygen species (ROS)[16]. The accumulation of these harmful substances disrupted a series of physiological and metabolic disturbances, ultimately resulting in further exacerbation of the development of CI symptoms[17].
To date, a diversity of methods have been explored to mitigate the CI among pomegranates and other fruits. Physical treatment for controlling CI is primarily achieved through temperature conditioning[18], near-freezing temperature storage[19], and heat treatment[20]. A study in which sweet potato tuberous roots were pre-cooled at 10 °C for 5 d before cold storage demonstrated that low-temperature conditioning could inhibit the CI progression[21]. Fan et al.[22] proved that the near-freezing point temperature of 0 °C extended the storage time of apricot from 45 to 90 d without CI symptoms. Furthermore, it was demonstrated that pomegranates were soaked with hot water at 45 °C for 4 min, and subsequently stored at 2 °C for 3 months, leading to a significantly reduced incidence of CI symptoms[23]. Additionally, chemical treatments, such as sodium nitroprusside[24], hydrogen sulfide[15], brassinolides[25], and 1-MCP[26] are also the research hotspots for mitigating CI among fruits and vegetables.
Although many techniques have been proposed to reduce CI and increase storage-ability, the temperature always remains the key environmental factor that governs the extent of CI. An appropriate and precise preservation temperature prominently maintains pomegranate physiological quality[27,28]. Further, it has been observed that the chilling injury of pomegranates was remarkably different in different cultivars and different harvest periods[20]. While there are few researches on the influence of precise storage temperature for 'Mengzi' pomegranate. This research investigated the optimal temperature for 'Mengzi' pomegranate during 130 d of storage. Not only their storage parameters were determined, but also the indicators related to micro-structure and ROS-redox balance in all storage temperatures were evaluated. The aim is to provide a reference for the long-term commercial storage of 'Mengzi' pomegranates.
-
The 'Mengzi' pomegranates (Punica granatum L.) were harvested at 80% maturation (the color of pomegranate surface was green-red, and the single fruit weight was 330−350 g) in October from the Heyuan Ten Thousand Acres Pomegranate Manor of Honghezhou Heyuan Agricultural Development Co., Ltd located at Liwu Village, Jianshui County, Honghezhou City, Yunnan Province, China (23°37′32″ N, 102°49′16″ E). Then they were wrapped with single-fruit net bags, placed in foam boxes with ice packs (the ice packs were covered with a foaming net), and airlifted to the Agricultural Products Processing and Preservation Laboratory of Tianjin University of Science and Technology (Tianjin, China). To ensure the transportation environment was as uniform as possible, the same ice packs, and the same quantity of pomegranates were placed in each box during cold chain transportation. With a bright red color, uniform size, smooth surface, no mechanical lesions, no cracks and no disease spots, pomegranates were selected for the experiment. After pre-cooling at 3 °C for 12 h, the samples were packaged into 0.02 mm PE bags, containing 20 samples per bag. Each treatment comprised 10 bags and were subsequently stored at 0 ± 0.2 °C, 1 ± 0.2 °C, 2 ± 0.2 °C, 3 ± 0.2 °C, and 4 ± 0.2 °C, respectively.
Chilling injury (CI) index
-
The CI indicator was evaluated in light of the proportion and severity of visible symptoms area[29]. Fifteen individual fruits were taken and calculated using five grades and the following formula: 0 = no CI, 1 = slight (0 < CI ≤ 5%), 2 = regular (5% < CI ≤ 15%), 3 = moderate (15% < CI ≤ 25%); 4 = severe (CI > 25%).
$ CI\; index\; (CI)=\displaystyle\sum_{ }^{ }\dfrac{Number\; of\; fruits\; with\; CI\; symptom\, \times\, CI\; score}{Total\; number\; of\; eachtreatment\, \times\, 4} $ Browning (BI) index
-
The BI indicator was evaluated, in light of the browning area. The extent of BI was categorized into these five levels given the approach of Luo et al.[30]: 0 = no BI, 1 = slight (0 < BI ≤ 10%), 2 = regular (10% < BI ≤ 25%), 3 = moderate (25% < BI ≤ 50%), 4 = severe (> 50%). The BI indicator was computed as below:
$ BI\; index\; (BI)=\displaystyle\sum_{ }^{ }\dfrac{Number\; of\; fruits\; with\; BI\; symptom\, \times\, BI\; score}{Total\; number\; of\; eachtreatment\, \times\, 4} $ Colour parameters and decay incidence
-
L*, a*, b* and ΔE*, as peel colour parameters, were assessed through a color meter (HP-200, China). Decay incidence was the ratio of decay fruits to the total fruits denoted as per
Gao et al.[12].Scanning electron microscope (SEM) and optical microscope observation
-
Microscopic morphology of pomegranate peel was observed based on the approach of Andrade et al.[31]. Several pieces of pomegranate husk (5 mm × 5 mm) were randomly cut and fixed in 4% glutaraldehyde solution (pH 6.8) prepared with 0.1 mol·L−1 phosphate buffer. After 3 h, they were rinsed with 0.1 mol·L−1 phosphate buffer three times and subsequently dehydrated using isoamyl acetate for 20 min. Finally, the specimens were freeze-dried using one vacuum freezing dryer (FD-1A-50, China) for 1 d. The slices were sprayed with gold sputtering coater for 60 s and then were observed with SEM (LEO, Germany).
Similarly, the microstructure of pomegranate samples was observed in accordance with the approach of Kato et al.[32] with some adjustments. Pomegranate peel tissue samples were cut into cubes with the size of 1 cm3 and fixed in FAA solution (5% acetic acid, 40% formaldehyde, and 70% ethanol) at low temperatures (4 °C). After 48 h, the samples were successively put into 70%, 80%, 90%, 95%, and 100% ethanol solutions (2 h per solution) for dehydration. Subsequently, the samples were successively put into 100% alcohol: 100% xylene (1:1) solution, 100% xylene, and 100% xylene solution (1.5 h per solution) for transparency. After that, the samples were placed in xylene: paraffin (1:1) solution, and subsequently embedded in pure paraffin. Once solidified, the paraffin wax was cut into pieces with 10 μm in thickness. Finally, the slices were stained with eosin solution and micro-photographed using an inverted fluorescence microscope (TE2-PS, Japan).
Cell membrane permeability
-
The permeability of the cell membrane was assessed according to the method of Guo et al.[33] with some adjustments. Ten cylindrical tissue samples (10 mm in diameter) were extracted from pomegranate peel using one punch and rinsed three times using distilled water. Then, tissues were placed in a triangular bottle containing distilled water (20 mL) at 25 °C for 1 h. The initial conductivity P1 was determined through one digital conductometer (DDS-11A, China). Subsequently, the specimen was boiled for 10 min and then cooled at 25 °C. The conductivity of P2 was determined. The conductivity of distilled water was P0. The cell membrane permeability (%) = (P1 - P0) × 100 / (P2 - P0). Throughout the entire process, all utensils were rinsed with distilled water, and the slice samples were not touched directly by hand. Before measurements, the electrode was calibrated using a blank solution. Additionally, before each measurement, the electrode was rinsed with distilled water and then washed with the liquid to be measured three times.
Respiratory rate and weight loss
-
Respiratory rate was measured through one fruit and vegetable respiration detector (FS-GH100, China). The results were represented in CO2 mg·kg−1·h−1. Specifically, after weighted, one pomegranate fruit was placed into an air-tight jar (1 L) and detected at room temperature (25 °C) for 3 min. Then the respiration rate was calculated automatically by inputting the weight of the pomegranate. Weight loss was measured as the distinction between the initial weight and the weight of each storage time, then divided by initial weight.
Malondialdehyde (MDA) content
-
MDA concentration of pomegranates was measured according to the method of Si et al.[34]. As well as pomegranate peel (5 g), 10% trichloroacetic acid (TCA) solution (10 mL) was taken into pre-cooled mortar and ground into homogenizing pulp under the condition of ice bath. After grinding, the homogenizing pulp was centrifuged at 5,000× g for 10 min under 4 °C. Two mL supernatant was blended with 0.6% of 2 mL thiobarbituric acid (TBA) solution and bathed in water for 5 min under 100 °C. After that, it was cooled to room temperature and then centrifuged at 5,000× g at 4 °C for 10 min. Absorbance of samplers was determined at 600, 532, and 450 nm, respectively. The MDA content (μmol·g−1·FW) = [(OD532 − OD600) − 0.56 × OD450] × V/(Vs × m × 100), where, V represented the volume of the extract (mL); Vs indicated the volume of the extract utilized for the measurement (mL); and m represented the weight of the sample (g).
Total phenolic content
-
The total phenolic content of pomegranates was measured as described by Chen et al.[35]. Pomegranate tissues (0.2 g) and 70% methanol (4 mL) were ground into a homogenate in a mortar and then bathed at 70 °C for 15 min. Then, the homogenate were centrifuged at 5,000× g for 20 min after cooled to room temperature. The supernatant was added up to 25 mL using distilled water. After that, the above supernatant (1 mL) was taken and blended with Folin-Ciocalteus (1 mL) and 7.5% sodium carbonate solution (1 mL). After the blend was incubated at 25 °C for 1 h, the phenolic content was determined at an absorbance of 760 nm and denoted as mg·g−1.
Assay of enzyme activity
-
The peroxidase (POD) activity and polyphenol oxidases (PPO) activity were measured according to the method of Si et al.[34]. The pomegranate peel (3 g) and 10 mL PBS solution (1 mM PEG 6000, 4% PVPP, 1% Triton X-100) were taken into pre-cooled mortar and ground into homogenate under the condition of ice bath. Afterward, the mixture was centrifuged at 5,000× g at 4 °C for 30 min. The supernatant was collected as enzyme extracts and stored at 4 °C for later tests. As for POD activity, 0.5 mL enzyme extracts were mixed with 1.0 mL of 5 mM guaiacol solution and 0.5 mL 2% H2O2. At 15 s of the reaction, the absorbance was measured at 470 nm six times (once per 10 s). POD activity (U·g−1·FW) = ΔA470 × Vt/0.01 × m × Vs × t, here in, ΔA470 represented value change in absorbance, t denoted the reaction time, m signified the mass of the sample, Vt indicated the total volume of supernatant, and Vs stood for the volume of supernatant utilized for the measurement. As for PPO activity, 0.1 mL enzyme extracts were blended with 2.9 mL phosphate buffer (pH 5.5) and 1mL of 1 mM catechol solution, and then the mixture was incubated at 37 °C for 10 min. At 15 s of the reaction, the absorbance was read at 420 nm for 6 min (once per 10 s). PPO activity (U·g−1·FW) = ΔA420 × d/0.01 × t, where, ΔA420 represented the value change in absorbance, t denoted the reaction time, and d signified the dilution multiple. In the control group, 0.5 mL distilled water was added to replace the enzyme extracts.
Soluble solids content (SSC) and titratable acidity (TA)
-
As claimed by Wei et al.[36], SSC was determined through one pocket refractometer (PAL-3, Japan). TA was evaluated in accordance with the approach of Moon et al.[37].
Sensory evaluation
-
Sensory evaluation was conducted in line with the approach of Li et al.[38] with minor adjustments. Pomegranates were eliminated from low-temperature storage after 130 d and stored at ambient conditions (25 °C). The 20 panelists were invited to evaluate the pomegranate samples that were stored at 25 °C on day 1, day 4, day 7, and day 10. The evaluation panelists (10 males and 10 females) were members of the Agricultural Products Logistics Preservation Laboratory. They were between the ages of 20 and 30, and had extensive experience in food sensory evaluation. The members have all received systematic training in the specialized course of food sensory evaluation. Meanwhile, the processing methods of the samples were confidential, and the samples were randomly numbered for sensory evaluation. The sensory evaluated attributes of pomegranates included color, flavor, firmness, texture, and overall acceptability. The preference scale is as follows: very low intensity (scores ≤ 3); low intensity (3 < scores ≤ 5); moderate intensity (5 < scores ≤ 7); elevated intensity (7 < scores ≤ 9); excessively elevated intensity (9 < scores ≤ 10).
Statistical analysis
-
Both technical replications (repeated measurements of the same sample) and biological replications (different samples from the same treatment group) experiments were conducted in triplicate for analysis of each treatment. The SPSS 27 (SPSS Inc., USA) was used to analyze the significance of differences. Moreover, mean values with standard errors were represented. Further, digraph analysis was implemented through Origin 2021 (Microcal Software, MA, USA). Duncan's multiple range tests were applied for analysis of variance (ANOVA). In addition, p < 0.05 is significant.
-
The appearance of pomegranates was diverse after 130 days cold storage. Apparent CI symptoms of pomegranates at 0 °C were observed as follows: depression, browning of the husk, pitting, and scald (Fig. 1a). As depicted in Fig. 1b, the CI gradually rose as storage temperature decreased and storage time extended. The results demonstrated that the CI index exhibited a distinctly elevated trend among the pomegranate samples stored at 0 and 1 °C, as compared to the other treatment groups. On day 130, the values reached 0.523 and 0.278, respectively. Similar results have been published by Zhang et al.[23]. The pomegranates stored at 2 °C resulted in a 30 d postponement of the CI occurrence, accompanied by a markedly reduced CI indicator on day 130 in comparison to the group stored at 0 °C. Similar publications have been released in persimmon, where the alleviation of CI was achieved at 4 °C but aggravated at lower storage temperatures[26].
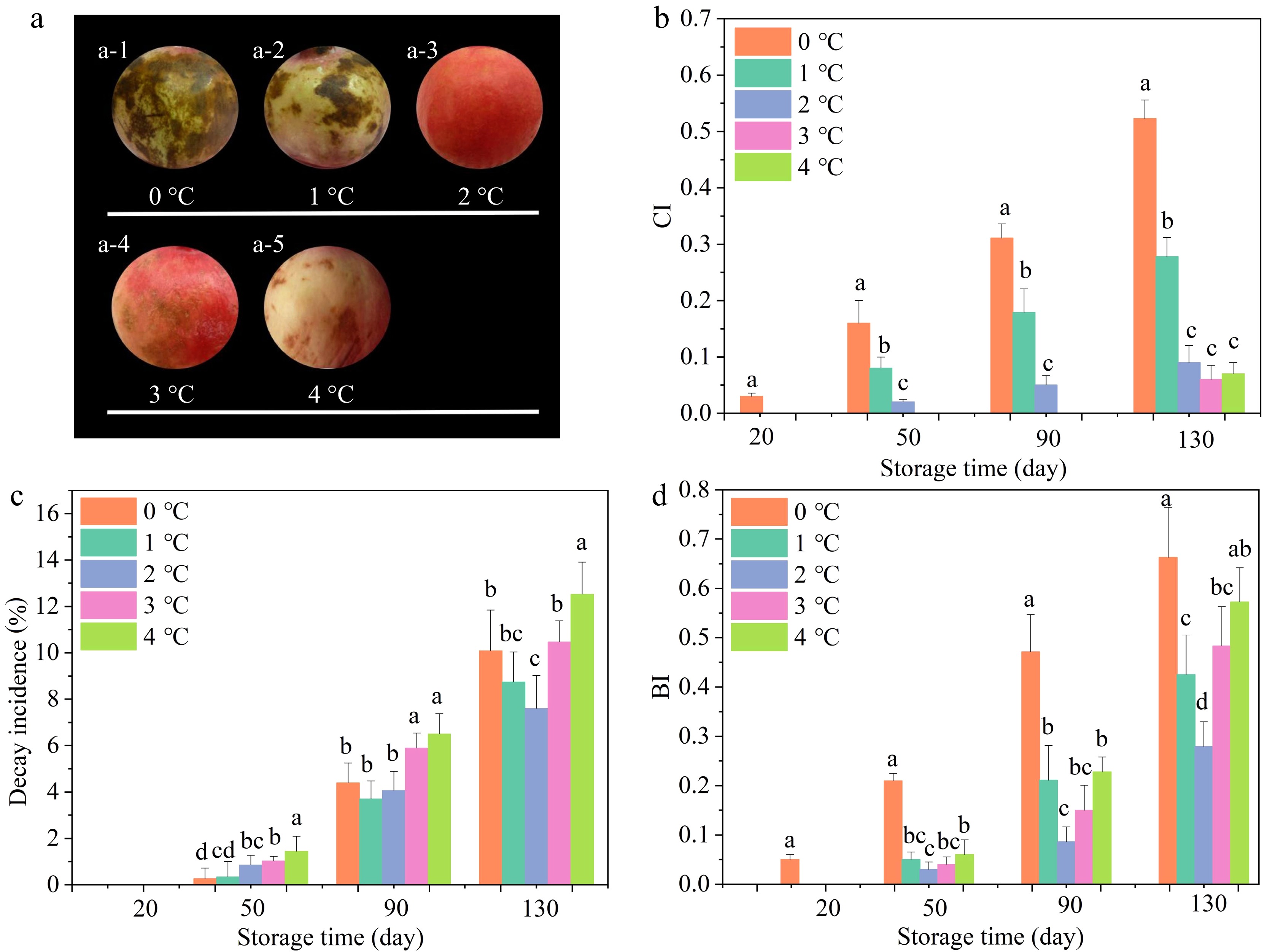
Figure 1.
Effects of storage temperatures on (a) appearance, (b) CI, (c) decay incidence, along with (d) BI. Values of the figures were exhibited as means ± standard error (n = 3). Vertical bars denoted the standard errors of the means. Disparate small letters denoted significant differences in the treatments for each sampling period at p ≤ 0.05.
The surface decay on the pomegranate was another pervasive CI symptom[39]. The variation of decay incidence is shown in Fig. 1c. Similar to the trend of the BI index, significantly lower decay incidence was noted from 90 to 130 d for pomegranates stored at 2 °C as compared with other groups. On day 130, the pomegranates stored at 0 °C exhibited a decay incidence of 10.09%, while those stored at 2 °C had the lowest decay incidence at 7.65%. In CI fruit, the amino acids, sugars, and some minerals were released from chilling-injured cells, which could offer the substrate to microorganisms, particularly fungi. The pathogens then infected the fruit and led to decay[27].
Colouration parameters L*, a*, b*, and ∆E* for pomegranates during storage as displayed in Fig. 2. The L* values of the fruit peel exhibited a decline as the storage time increased. The L* value of pomegranates stored at 0 °C showed a dramatic decline of 37.50% on day 130, while those stored at 4 °C showed a reduction of 26.49% (Fig. 2a). The results suggested that the storage of pomegranates at 0 and 4 °C resulted in the CI and browning, respectively, which in turn led to a reduction in fruit surface luster. The occurrence of CI and browning both led to the decrease of gloss of the pomegranate surface[40], which may be the reason for the higher L* value of samples stored at 2 °C than those stored at other temperatures. Furthermore, there was a similar study in which the decreasing L* values of water core 'Fuji' apples indicated increasing browning[38].
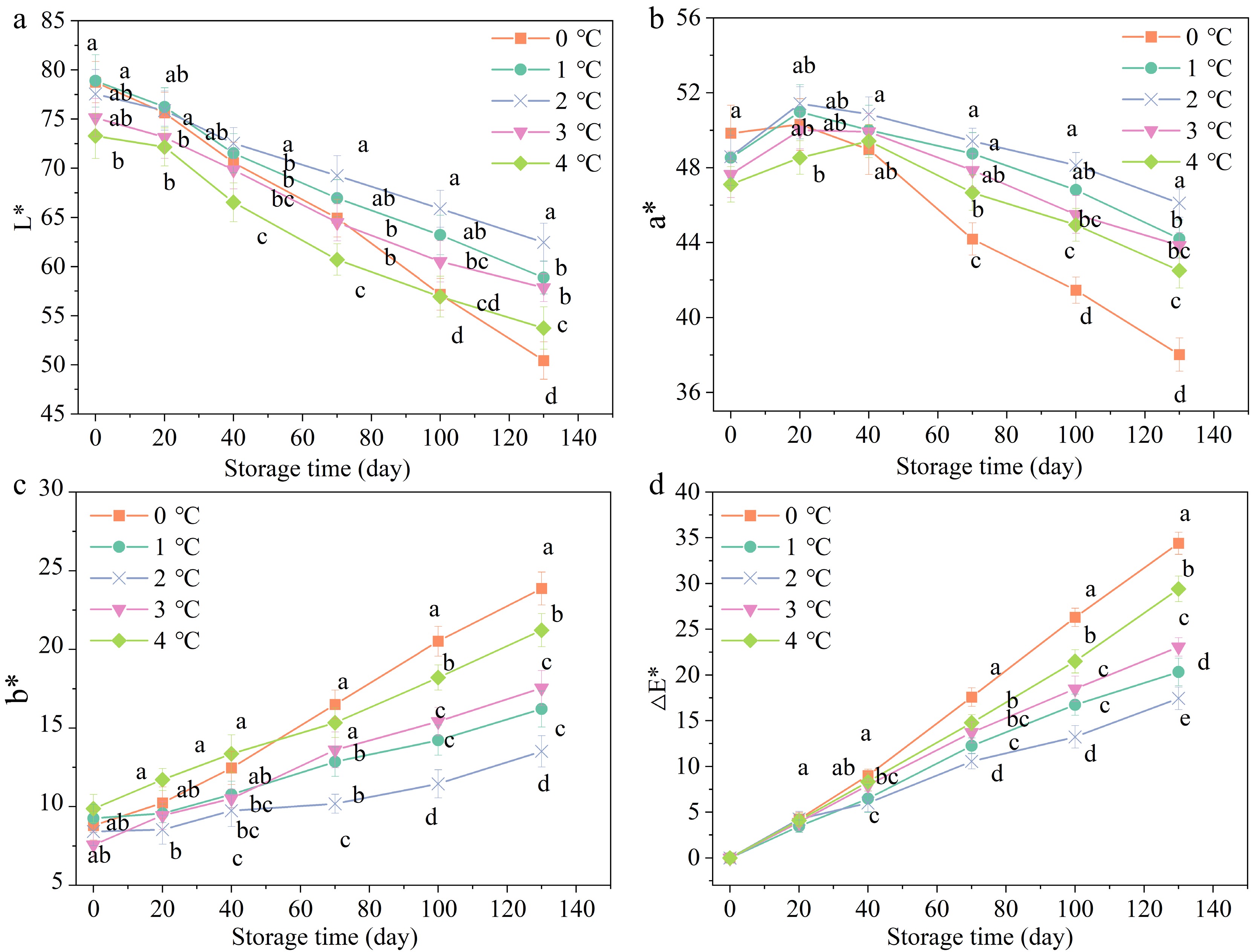
Figure 2.
Effects of storage temperatures on the (a) L* value, (b) a* value, (c) b* value, and (d) ∆E* value of pomegranates appearance. Values of the figures were displayed as means ± standard error (n = 3). Vertical bars denoted the standard errors of the means. Disparate letters denoted prominent differences in the treatments for each sampling period at p ≤ 0.05.
The a* values raised constantly in all treatments during the initial 20 d, followed by a gradual decline (Fig. 2b). The a* values of pomegranates stored at 2 °C exhibited a higher level during 130 d of storage than those of other treatments. The a* values of pomegranates stored at 2 °C exhibited the greatest increase, while those stored at 0 °C had the smallest increase during the initial 20 d. It might be that chilling temperature could significantly inhibit the ripening of fresh fruits[20]. Failure to maturity was another ordinary CI symptom among fruits like bananas, tomatoes, and melons[41]. Besides, the a* values of pomegranate samples stored at 3 and 4 °C were found to be lower than those at 2 °C, suggesting that higher storage temperature (3 and 4 °C) may contributed to a higher degree of browning. This was consistent with the results of BI.
Moreover, the b* values (Fig. 2c) and ∆E* (Fig. 2d) values showed an increasing trend with time. The b* and ∆E* values of pomegranate stored at 0 °C increased the most, while those stored at 2 °C was the least. These changes might correlate to maturity and browning[42]. The results demonstrated that the optimal storage temperature (2 °C) was beneficial to maintain the appearance of pomegranate and delay the browning and senescence.
Membrane integrity
-
SEM observations showed varying degrees of cracking on the surface of the peel at different temperatures (Fig. 3a). By comparison, pomegranates stored at 0 °C exhibited considerable cracks, a rough surface, and severely damaged wax layer. Crack extension might result in further water loss and microbial aggression, which might accelerate fruit decay[43]. Pomegranates stored at 2 °C exhibited no epidermal cracks and retained a complete waxy layer compared to other treatments. Generally, the significant decrease in waxy cuticle thickness might result from the wax falling off the peel surface during fruit storage[43]. The waxy cuticle is a momentous barrier for fruits, which contributes to anti-mechanical damage, resistance to moisture loss, and pathogen penetration[44].
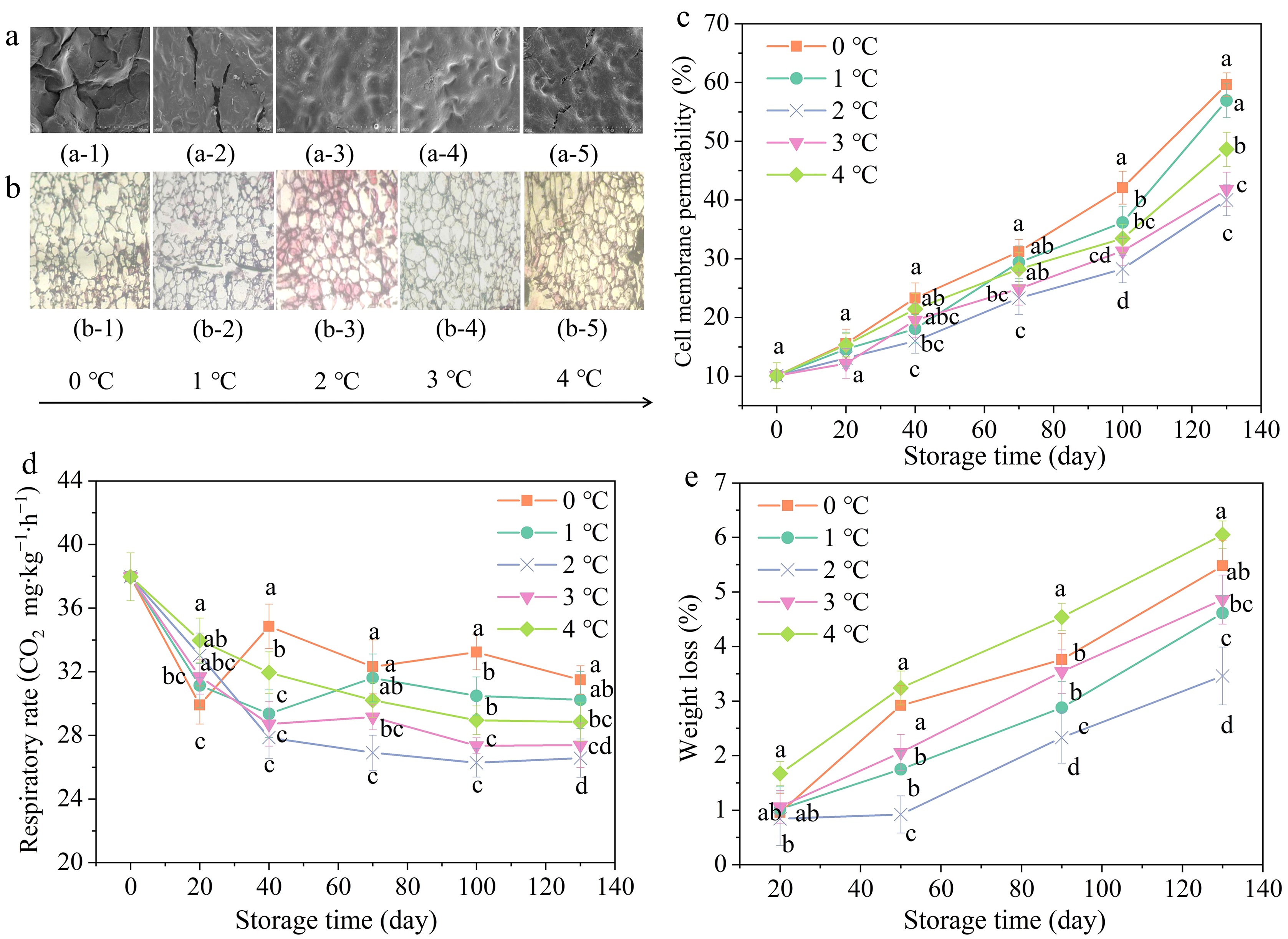
Figure 3.
Effects of storage temperatures on micro-structure ((a) SEM images, (b) paraffin section images), (c) cell membrane permeability, (d) respiratory rate, and (e) weight loss. Values in the figures were exhibited as means ± standard error (n = 3). Vertical bars stood for the standard errors of the means. Disparate letters denoted prominent differences in treatments for per sampling period at p ≤ 0.05.
It was noteworthy that cells with CI typically exhibited an irregular and collapsed shape, as illustrated in Fig. 3b. The vascular bundle cells of pomegranates stored at 0 °C were damaged, and the surrounding stone cells were irregularly arranged. Besides, no apparent boundaries emerged between epidermal cells (Fig. 3b.b-1), suggesting that the cell membranes were degraded[17]. Similar findings were observed in CI samples of zucchini, which could be ascribed to dissolution and degradation of cell walls[45]. During 130 d of storage at 2 °C, the majority of cells retained their integrity and original shape (Fig. 3b.b-3).
The changing patterns of cell membrane permeability are represented in Fig. 3c. Similar to the trends of CI, the cell membrane permeability of pomegranates stored at 0 °C exhibited a notable increase in comparison to the other treatments, particularly within the final 30 d. Notably, cell membrane permeability of pomegranates stored at 0 °C exhibited the greatest increase, reaching 57.46%, while those stored at 2 °C displayed the smallest increase, reaching only 33.68% on day 130. Reportedly, cell membrane lipids experienced a physical variation from a liquid-crystalline to a solid gel state at chilling temperature, resulting in an elevation of membrane permeability and ions leakage[46].
Respiration, as a basic metabolic activity, was a crucial factor in maintaining the fruit quality[15]. The respiratory rate was presented in Fig. 3c. On day 40, the respiratory rate of pomegranates stored at 0 °C increased significantly and reached a respiratory peak of 34.85 mg∙kg−1∙h−1, which was roughly 1.17 times as high as that of the samples on day 20. Subsequently, respiratory rate slowly declined over times, yet remained higher than other groups. The respiratory rate of fruit stored at 2 °C was decreased steadily and was always lower than other groups throughout the storage time. Thereby, it was indicated that the CI occurrence might trigger a prominent burst in respiratory rate by combining the CI index. CI induced the abrasion of cell membrane and other cell organelles, especially the mitochondrial membrane, resulting in abnormally high respiration rate[46]. The aforementioned findings consisted with previous reports on cucumber and persimmon[46].
Weight loss is chiefly attributed to the water evaporation from stored pomegranates[46]. Weight loss of pomegranates significantly increased during 130 d of storage (Fig. 3d). On day 130, the pomegranates stored at 4 °C demonstrated the highest weight loss at 6.05%. Following that, the fruit stored at 0 °C exhibited a weight loss of 5.48%. In contrast, the pomegranates stored at 2 °C exhibited the lowest weight loss, at approximately 3.46%. A robust relationship between weight loss and pomegranates respiration rate has been established and the natural barrier of the waxy layer in reducing the fruit weight loss has also been confirmed by Ali et al.[47]. Therefore, less weight loss of pomegranates at 2 °C might be ascribed to stable respiratory rate and slight CI. Pomegranates stored at 0 °C exhibited an abnormal respiratory rate and a destructed waxy layer of peel, which might lead to excessive water loss[48].
ROS-redox balance
-
MDA and cell membrane permeability are important indicators of fruit senescence and chilling injury caused by low temperatures[17]. The MDA content raised continuously during the storage of 130 d, as depicted in Fig. 4a. On day 20, the MDA content of pomegranates stored at 0 °C rose to 6.56 μmol·g−1·FW, exhibiting a rise of 3.08 μmol·g−1·FW compared to the initial value, while the samples stored at 2 °C increased by only 1.29 μmol·g−1·FW. The increased MDA content indicated that mild chilling injury had occurred in the pomegranates under the storage condition of 0 °C. It was also reported that both loquat and banana fruits with chilling injury exhibited higher levels of MDA accumulation and electrolyte leakage[49]. The MDA content of pomegranates stored at 2 °C was relatively lower compared to other groups on day 130, reaching only 13.23 μmol·g−1·FW. Notably, the MDA content of pomegranates stored at 0 °C was almost twice as much as that stored at 2 °C on day 130. Accordingly, it was indicated that the formation of MDA in the 'Mengzi' pomegranate was aggravated by the cold stress. The present findings are in agreement with reports on bell peppers and peaches[12].
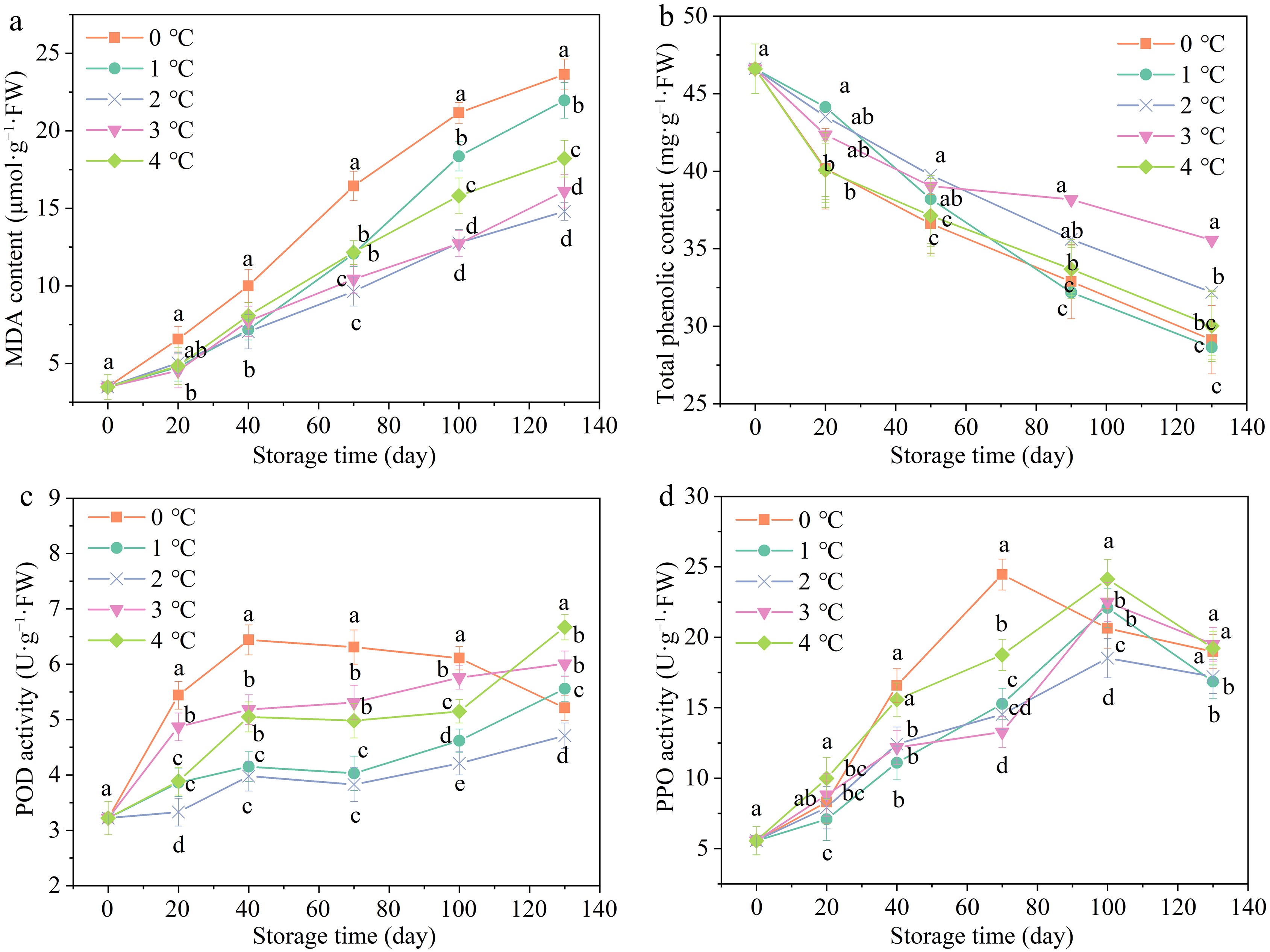
Figure 4.
Effects of storage temperatures on (a) MDA, (b) total phenolic content, (c) POD, and (d) PPO. Values of the figures were displayed as means ± standard error (n = 3). Vertical bars denoted the standard errors of the means. Disparate letters represented remarkable differences in the treatments for each sampling period at p ≤ 0.05.
All treatments exhibited a declined tendency in the total phenolic content during a storage of 130 d (Fig. 4b). The total phenolic content of pomegranates stored at 2 °C declined slower than others, accumulating 35.56 mg·g−1·FW on day 130. In particular, the total phenolic content of pomegranates stored at 0 °C declined dramatically, decreasing by 38.53% on day 130. The total phenolic content of pomegranates stored at 3 and 4 °C were also lower than those stored at 2 °C, which may be due to the increased tissue aging and browning (Fig. 1d) of pomegranates with increasing storage temperature[50]. These results suggested that storage temperature (2 °C) improved the retention of phenolic content in pomegranates. In turn, the high phenolic content increased the resistance to senescence and CI during the post-harvest storage in pomegranates. This may be because the total phenolics can prevent the membrane lipid peroxidation by inhibiting the occurrence and propagation of oxidative chain reactions[12].
The pattern of POD activity is presented in Fig. 4c. The POD activity of pomegranates stored at 0 °C increased significantly, peaking at 6.44 U·g−1·FW on day 40, and then decreased steadily until day 130. The POD activity of pomegranates stored at 2 °C, by contrast, increased steadily and changed minimally during the storage of 130 d. The changes might be attributed to the accumulated toxic substances (free radicals and MDA) binding to proteins such as defense-related enzymes, destroying structure and function[51]. Consistently, the PPO activity of pomegranates in these groups showed a similar rising tendency with POD activity during storage of 130 d (Fig. 4d). The PPO activity of pomegranates at 0 °C increased to a maximum peak (24.45 U·g−1·FW) on day 70, while other groups peaked on day 100. Notably, the changes in PPO activity of samples stored at 2 °C was always the smallest compared to other groups. Our results corresponded to the former report on phenolase metabolism of Chinese olives under CI storage temperature[11].
POD and PPO, two key defense-related enzymes, were highly associated with alleviating enzymatic browning. They were also considered as important factors causing browning in post-harvest agricultural products[52]. It was considered that PPO and POD exerted an antagonistic influence over phenolic anabolism in the case of CI. The phenols were oxidized to the quinones by the action of two enzymes (PPO and POD), resulting in typical CI in peaches[51].
Flavor and sensory evaluation
-
The differences in TA, SSC, and SSC/TA of pomegranates during the storage of 130 d are shown in Table 1. Compared to the initial value, all values of pomegranates declined to varying degrees. Specifically, the SSC content of pomegranates stored at 0 °C was 12.50%, while those stored at 2 °C was 14.34% on day 130. A few soluble sugars could be adopted as signal molecules, adjusting progression and growth, which were strongly associated with maturity and senescence of horticultural products[53]. The possible reason is that the storage temperature of 2 °C could reduce the metabolic intensity and the consumption of the organic matrix, while the reduced SSC in CI pomegranates was possibly attributed to sugars as an energetic response to cold stress[50]. Pomegranates stored at 2 °C exhibited remarkably higher TA values than those at 0 °C. The higher TA loss in pomegranates could be related to increased respiration intensity, in which the organic acid was severely depleted during the high respiration[41]. The solid-acid ratio increased on day 130, however, there were no significant differences among treatments.
Table 1. Effects of storage temperatures on soluble solids content (SSC), titratable acids (TA), and SSC/TA of pomegranates.
Parameter Time (d) Storage temperature 0 °C 1 °C 2 °C 3 °C 4 °C SSC content (%) 0 16.80 ± 0.32 20 17.31 ± 0.48a 17.43 ± 0.40a 17.97 ± 0.40a 17.65 ± 0.47a 17.01 ± 0.54a 40 17.54 ± 0.70ab 17.74 ± 0.27ab 18.32 ± 0.31b 17.89 ± 0.44ab 17.34 ± 0.31a 70 16.01 ± 0.30c 16.31 ± 0.50bc 17.21 ± 0.49a 17.11 ± 0.40ab 15.67 ± 0.56c 100 14.89 ± 0.40c 15.26 ± 0.40bc 16.46 ± 0.50a 15.66 ± 0.30b 13.57 ± 0.40d 130 12.50 ± 0.36c 12.75 ± 0.26c 14.34 ± 0.29a 13.40 ± 0.33b 11.83 ± 0.41d TA content (%) 0 2.01 ± 0.23 20 1.66 ± 0.05b 1.75 ± 0.12ab 1.97 ± 0.28a 1.85 ± 0.04ab 1.83 ± 0.04ab 40 1.54 ± 0.15b 1.64 ± 0.08b 1.93 ± 0.06a 1.71 ± 0.15b 1.67 ± 0.07b 70 1.43 ± 0.07c 1.55 ± 0.06bc 1.87 ± 0.09a 1.62 ± 0.11b 1.57 ± 0.09b 100 1.26 ± 0.07d 1.38 ± 0.06c 1.71 ± 0.04a 1.51 ± 0.08b 1.48 ± 0.05bc 130 1.16 ± 0.04c 1.24 ± 0.05c 1.57 ± 0.07a 1.45 ± 0.05ab 1.39 ± 0.04b SSC/TA ratio (%) 0 8.46 ± 1.32 20 10.44 ± 0.60a 10.02 ± 0.89a 9.28 ± 1.52a 9.55 ± 0.46a 9.30 ± 0.50a 40 11.43 ± 0.66a 10.86 ± 0.67ab 9.49 ± 0.14ab 10.55 ± 1.16ab 10.39 ± 0.25b 70 11.23 ± 0.30a 10.55 ± 0.16ab 9.19 ± 0.16c 10.60 ± 0.44ab 9.99 ± 0.90bc 100 11.85 ± 0.98a 11.06 ± 0.19ab 9.61 ± 0.10cd 10.37 ± 0.71bc 9.17 ± 0.04d 130 10.81 ± 0.05a 10.32 ± 0.53a 9.13 ± 0.54b 9.25 ± 0.11b 8.54 ± 0.51b The results denoted mean ± standard deviations, n = 3. Different letters denoted prominent differences in the treatments for each sampling period at p ≤ 0.05. The sensory evaluation is presented in Fig. 5. Results demonstrated that pomegranates stored at 2 °C exhibited the highest scores, which were higher than those stored at 0 °C during room temperature storage. Loss of flavor and softening were typically macroscopic CI symptoms, which were usually not easily detected until the fruits were subsequently transferred from cold storage to ambient temperatures[17]. A similar result was found in tomatoes, and the potential mechanisms leading to the specific CI phenomenon were also elucidated. To be specific, the water was absorbed by the middle lamellar when its movement from the symplast to the apoplast occurred, which subsequently reduced the cell turgor pressure and exacerbated the softening induced by CI[23].
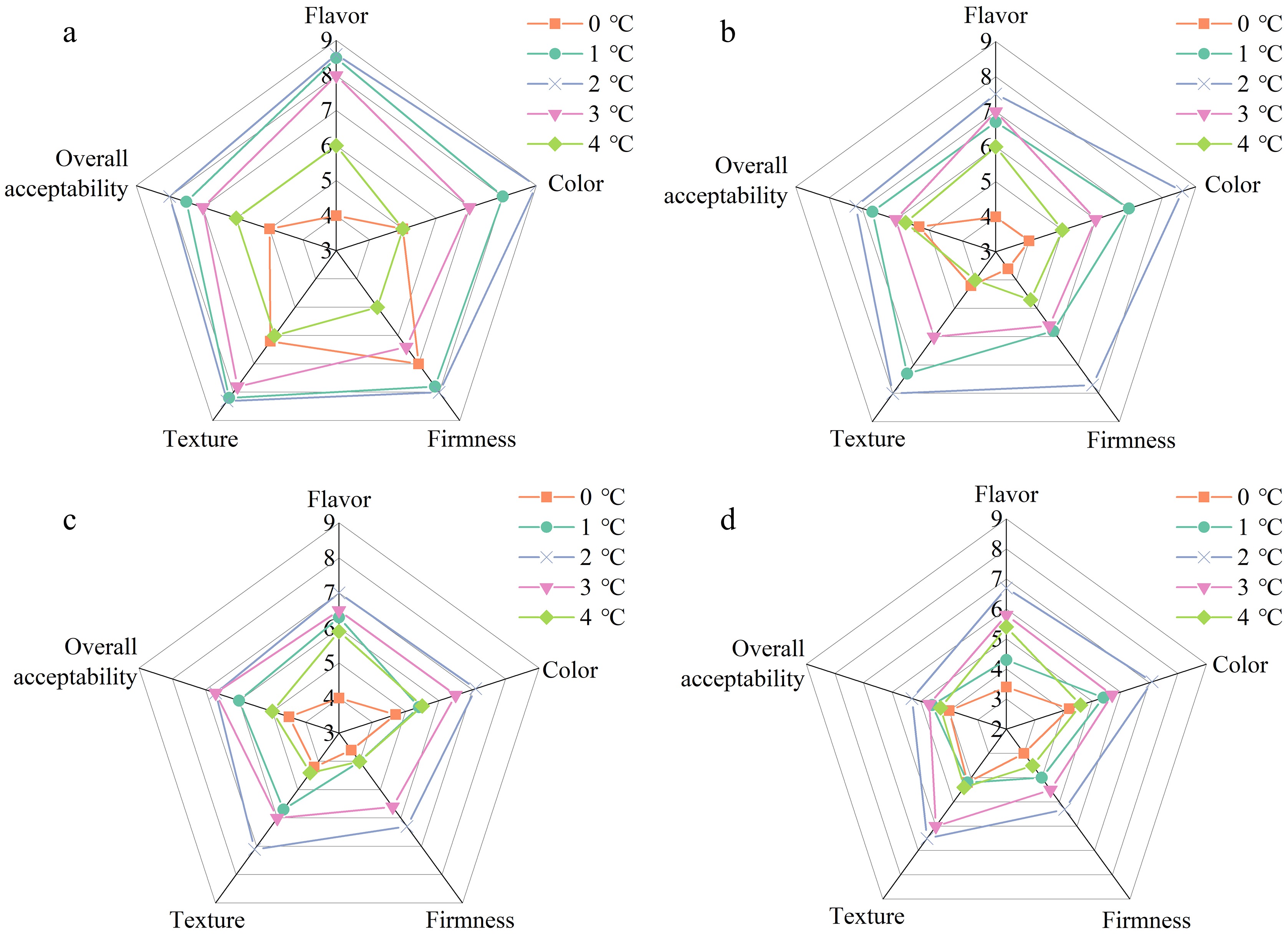
Figure 5.
Pomegranates were stored at low temperatures for 130 d and further stored at 25 °C for 10 d. The pomegranates sensory scores were performed on (a) day 1, (b) day 4, (c) day 7, and (d) day 10 when stored at 25 °C.
Correlation analysis and possible mechanism
-
The associations among different quality attributes for pomegranates (Fig. 6) were evaluated using Pearson's correlation analysis. It was found that the rising CI index was positively correlated with BI, b* value, ∆E* value, decay incidence, cell membrane permeability, MDA, POD, PPO, and weight loss, while negatively related to L* value, a* value, SSC, and TA, respiratory rate, and total phenolic content.
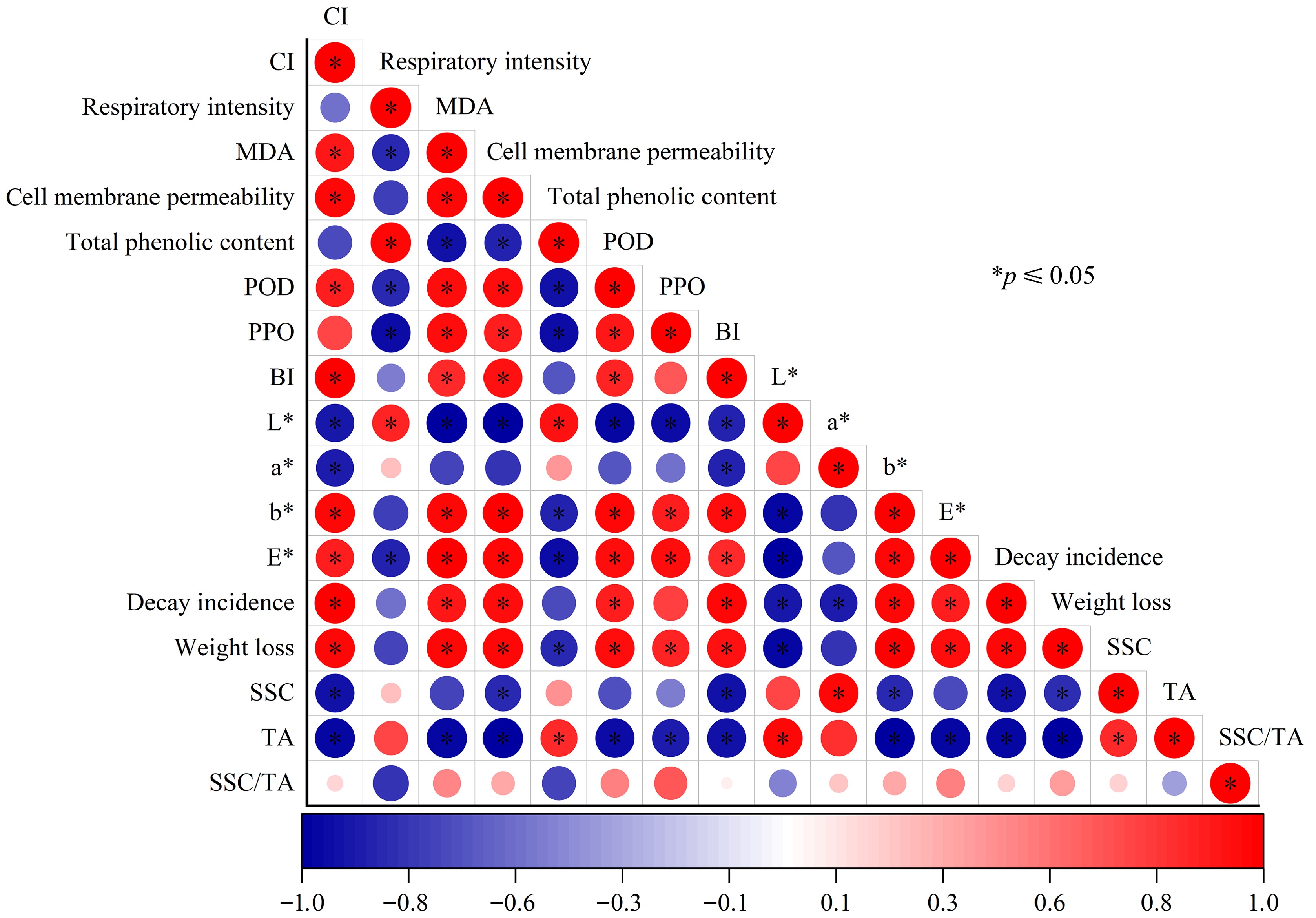
Figure 6.
Pearson's correlation coefficients on the quality attributes of pomegranates stored at 2 °C. The red dots stood for positive correlation; and the blue dots stood for negative correlation. The mark * on dots represented a significant correlation at the level of p ≤ 0.05.
Consequently, our study corroborated that CI symptoms first appeared around the vascular bundle cells and epidermic cells. The cell membranes were degraded due to the cold stress, and then the total phenolics were oxidized to the quinones by the action of PPO and POD, leading to browning and aging[41]. For another, cell membranes and mitochondrial membranes were degraded, which resulted in the leakage of metabolites and salt ions. Thus, these metabolic disorder induced by the accumulation of toxic substances like ROS and MDA, which accelerated the senescence of fruit and the infection of pathogenic bacteria (Fig. 7)[23].
-
In this study, the optimal temperature for the long-term cold storage of the 'Mengzi' pomegranates was determined to be 2 °C among the range of 0 to 4 °C. This temperature storage maintained the best appearance quality, with minimal color change and browning index. Not only was the CI index minimized, but the decay rate was also the lowest after 130 d of storage. Meanwhile, the respiratory rate of pomegranates was suppressed, along with a reduction in weight loss when stored at 2 °C. Additionally, the levels of oxidants, including PPO, POD, and MDA, were reduced, while the levels of antioxidants (the total phenolics) were increased. SEM observation and membrane permeability examination revealed the cell integrity of pomegranates stored at 2 °C were the highest. Furthermore, pomegranates stored at 2 °C produced a relatively higher content of soluble solids and titratable acid, as well as better sensory scores. Overall, the results indicated that storage at 2 °C had a significant effect on moderating the antioxidant-oxidant balance and respiration metabolism, contributing to the maintenance of cell integrity and thus alleviating chilling injury. This report may provide the guiding significance for long-term storage of 'Mengzi' pomegranates under precise temperature.
This research was funded by the National Natural Science Foundation of China (3200161737), Science and Technology Action Project of Rural Revitalization Industry Development of Xinjiang Uygur Autonomous Region (2022NC119), Postdoctoral Research Foundation of China (2022M712375), and Open Project Program of State Key Laboratory of Food Nutrition and Safety (SKLFNS-KF-202316).
-
The authors contributed the article as below: Li L: compiling the first draft & software. Luo J: validation. Li X: resources & methodology. Pang L: editing. Jia X: visualization. Liu L: software & editing. Kačániováe M: review. Song J: Supervision. Qiao L: editing & review. All authors checked the results and approved the final version.
-
All data generated or analyzed during this study are included in this published article.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Li L, Luo J, Li X, Pang L, Jia X, et al. 2024. The optimal precise temperature alleviated chilling injury and maintained post-harvest quality for 'Mengzi' pomegranate fruit. Food Innovation and Advances 3(4): 385−395 doi: 10.48130/fia-0024-0036
The optimal precise temperature alleviated chilling injury and maintained post-harvest quality for 'Mengzi' pomegranate fruit
- Received: 30 May 2024
- Revised: 27 October 2024
- Accepted: 28 October 2024
- Published online: 28 November 2024
Abstract: Chilling injury (CI) is a highly common physiological disorder in pomegranates during cold storage. Although several approaches have been investigated to mitigate the CI symptoms among some pomegranate cultivars, the fundamental and crucial environmental factor — the precise storage temperature for the 'Mengzi' cultivation remains unknown. This research evaluated the impact of storage temperatures of 0, 1, 2, 3, and 4 °C on the post-harvest quality of pomegranates. Results indicated that pomegranates stored at 2 °C exhibited the slightest color change and browning index. After storage of 130 d, pomegranates stored at 2 °C exhibited the lower CI index (82.79% reduction) and the lowest decay incidence (24.68% reduction) compared to those stored at 0 °C. The respiratory rate of pomegranates (2 °C) was also evidently suppressed (16.60%), along with a reduction in weight loss (3.46%). Furthermore, pomegranates stored at 2 °C exhibited the lowest activities of polyphenol oxidase (PPO) and peroxidase (POD), accompanied by the highest total phenolic content, which contributed to a reduction in malondialdehyde (MDA) accumulation. Relatively higher concentrations of soluble solids and titratable acid, as well as a higher sensory evaluation, were found in pomegranates stored at 2 °C. Consequently, it was inferred that the optimal temperature maintained cell membrane integrity modulated normal respiratory metabolism, and oxidative balance, and therefore alleviated CI and deterioration. This report can provide the guiding significance for the long-term storage of 'Mengzi' pomegranates under the condition of precise temperature control in phase temperature storage.
-
Key words:
- Pomegranate /
- Chilling injury /
- Optimal temperature /
- Storage quality