-
Certain medicinal plants have demonstrated potency in managing and treating various ailments, and the pharmaceutical industry has made extensive use of their secondary metabolites, which are a repertoire of physiologically potent compounds[1]. Sida linifolia L. is a species of plant in the Malvaceae family; it is also commonly known as flaxleaf sida or narrowleaf fanpetals. Sida linifolia is native to regions such as North America, South America, and the dry forest areas of West Africa, particularly Nigeria[2]. The genus Sida has been employed in ethnomedicine to address a variety of illnesses, such as liver problems, urinary tract infections, asthma, headaches, and snake bites. It has also been used to treat digestive ailments and antifertility and used as a diuretic[3]. This is because of its hepatoprotective, antioxidant, analgesic, antibacterial, anti-arthritic, antispasmodic, hypoglycemic, and anti-inflammatory pharmacological properties[4,5]. As a result, phytocompounds with potential for therapeutic use can be found in abundance in Sida linifolia. Previous studies both in vitro and in vivo have discovered that the ethyl acetate fraction, crude ethanol extract, crude aqueous extract, and ethanolic fraction of S. linifolia possess anti-inflammatory properties[6,7].
Malaria as a life-threatening disease still causes several deaths in many regions of the globe despite the efforts at controlling and eliminating the causative parasite and vectors. It caused about 619,000 deaths and 247 million cases worldwide in 2022[8]. Among the factors that contribute to this high rate of mortality due to malaria is the issue of drug resistance[9]. Due to the high rate of resistance to the existing antimalarials, efforts are being made to identify and possibly isolate new compounds from plants such as Piper nigrum, Zingiber officinale, Nigella sativa, Myristica fragrans, Terminalia arjuna, etc., to serve as lead compounds in the development of a fresh class of antimalarials with little or no resistance. From T. arjuna, compounds such as 3-carboxyphenol, gallic acid, pyrane, ethyl oleate and furfural were identified.
Inflammation is an intricate biological reaction of bodily tissues to detrimental stimuli, including microbial infection, cellular damage, tissue damage, and irritability from chemicals, it is a defense mechanism that involves blood vessels, molecular mediators, and immune cells[10]. At the location of tissue damage, chemical mediators are released, and immune cells migrate out of blood vessels to start this process[11]. The inflammatory process can be stimulated or inhibited by the inflammatory cytokines produced during inflammation. Several inflammatory-promoting cytokines and chemokines, such as monocyte-chemoattractant protein-1, tumor necrotic factor-α (TNF-α), interleukin 1beta (IL-1β), IL-6, and IL-8 have been shown to have decreased expression in various cell types by several flavonoids[12].
Flavonoids are a class of secondary metabolites found in a large variety of plants that build up in plant organs such as leaves, fruits, roots, and stems. Flavonoids are thought to be health-promoting and disease-preventing dietary supplements. They have a wide range of health-promoting properties and are used in a wide range of nutraceutical, pharmaceutical, medical, and cosmetic products. This is because of their capacity to modify the activities of cellular enzymes as well as their antioxidant, anti-inflammatory, anti-mutagenic, antibacterial, and other therapeutic qualities[11]. The synthesis and functions of several pro-inflammatory mediators, including adhesion molecules, cytokines, eicosanoids, and C-reactive protein, are inhibited by flavonoids as part of their anti-inflammatory properties[12]. The anti-inflammatory properties of flavonoids may be attributed to various molecular mechanisms, such as the inhibition of pro-inflammatory enzymes like activating protein-1 (AP-1), the activation of phase II antioxidant detoxification enzymes such protein kinase C, mitogen-activated protein kinase (MAPK), and nuclear factor-erythroid 2-related factor 2; and cyclooxygenase-2, lipoxygenase, and inducible nitric oxide (iNO) synthase. Based on scientific evidence, flavonoids may be able to suppress the activation of nuclear factor kappa B (NF-kB), AP-1, and MAPK, which in turn may inhibit the expression of enzymes and proteins related to inflammation[13,12].
Terpenoids are among the most abundant and diverse classes of secondary metabolites identified in plants[14]. The major role of terpenoids is to protect plants from pathogenic microbes and herbivores by being directly toxic to them, deterring herbivores, or attracting enemies of herbivores[15]. It has been demonstrated that terpenoids have a variety of therapeutic applications, including anti-inflammatory properties. Research has shown that terpenoids ameliorate a range of inflammatory symptoms by inhibiting different stages of the inflammatory process[14]. Most terpenoids' anti-inflammatory effects have been demonstrated to be mediated by a reduction in pro-inflammatory mediator levels, including NO, interleukins, TNF, and prostaglandin E2 (PGE2)[15].
The alkaloid 3-formyl-6-methoxycarbazole, which was isolated from the root of Clausena lansium showed impressive anti-inflammatory potency, according to an in vitro study conducted by Rodanant et al.[16]. The anti-inflammatory property of 3-formyl-6-methoxycarbazole was demonstrated by its remarkable ability to suppress the release of TNF-a, from monocytes stimulated by lipopolysaccharide (LPS).
Ethnomedicinally, S. linifolia is employed in the treatment of malaria but there is little or no scientific proof to this claim. The therapeutic properties of certain medicinal plants are due to their contents of secondary metabolites[17]. Nwankwo et al.[18] had previously reported that ethyl acetate leaf fraction of S. linifolia which was rich in phytochemicals, such as phenols, flavonoids, terpenoids, tannins, steroids, and alkaloids exerted anti-inflammatory properties but did not narrow it down to the specific phytochemicals involved. Therefore, it is of interest to investigate the antimalarial properties and in vitro anti-inflammatory properties of the flavonoid-, terpenoid-, and alkaloid-rich fractions of Sida linifolia leaves.
-
In this study, the plant materials used were leaves of Sida linifolia. Mr. Alfred Ozioko, a taxonomist at the Bioresources Development and Conservation Programme (BDCP) Research Center Nsukka, Enugu State, Nigeria, identified and confirmed the leaves. The leaves came from Nsukka, Nsukka Local Government of Enugu State. A sample of the specimen was deposited at the herbarium (voucher no: BDCP20210724).
Chemicals and reagents
-
The analytical grade chemicals employed in this investigation were all products of Sigma Aldrich, USA; May and Baker, England; and Burgoyne, India. The assays employed commercial kits and products from Teco (TC), USA and Randox, USA as reagents.
Equipment and instruments
-
The instruments and equipment utilized in this investigation were acquired from Divine Chemicals and the Analytical Laboratory in Nsukka (Nigeria), as well as the laboratory unit of the Department of Biochemistry, University of Nigeria, Nsukka (Nigeria). Others were bought from Nsukka, Enugu State, (Nigeria) commercial vendors.
Methods
Extraction procedure
-
Fresh leaves of Sida linifolia were washed to remove dirt. After being chopped into tiny bits, the leaves were shade-dried for two weeks at room temperature. A known mass (2,000 g) of the pulverized leaves was blended with an electric blender (High-Speed Grinder, China). The pulverized leaves were macerated using 3.2 L of absolute ethanol for 48 h in a tightly corked conical flask. Thereafter, it was filtered using Whatman paper. The ethanol content of the filtrate was further evaporated until constant mass using a soxhlet extraction set-up to obtain a dark green viscous mass of ethanolic leaf extract of Sida linifolia (ELES). The resultant crude extract was stored in a well-labeled sterile screw-capped vessel at 4 °C in a refrigerator until when needed for the study.
Isolation of flavonoid-rich fraction
-
After re-dissolving the ethanol extract, ethyl acetate was used to isolate flavonoids. This was done 3−5 times. All fractions were concentrated as flavonoid-rich.
One % hydrochloric acid (HCl) was added to the extracting solvent to break the glycosidic linkage of flavonoids which led to obtaining a high yield of flavonoids.
Isolation of terpenoids-rich fraction
-
A quantity (1 g) of the crude ethanol extract of S. linifolia was measured out and added to a glass container as plastic containers will leach into the organic solvent. The extraction solvent (85:15, v/v) of ethyl acetate was added. The mixture was then transferred to a flask and was shaken for about 3−4 h overnight. It was separated and purified in column chromatography. Afterward, the eluent (terpenoid-rich fraction) was dried using nitrogen steam.
Isolation of alkaloids-rich fraction
-
Using n-hexane, ethyl acetate, and ethanol as solvents, column chromatography was used to separate the dried ethanol extract (40 g). A laboratory mortar was used to homogenize the 500 g of silica gel with the dried ethanol extract before being placed within a column for column chromatography. An exact amount (1.3 L) each of ethanol, ethyl acetate, and n-hexane were used to elute the column in turn. To prevent the inactivation of the active components, the fraction was concentrated and dried with a rotary evaporator set at the proper temperature. The samples were kept chilled in preparation for further research.
Experimental design
-
Animals were consigned into six groups (n = 3/group 6), and different treatments were meted on the mice in the various groups. Induction of malaria was performed by inoculation using Plasmodium berghei parasite on mice. The extract (ELES) was administered orally in doses of 100, 200, and 400 mg/kg b.w (per oral), for 3 d. The experimental design was as follows:
Group 1: No malaria parasite inoculation and received regular saline treatment (vehicle) (Normal control);
Group 2: Inoculated with the malaria parasite without treatment (Positive control);
Group 3: Inoculated with malaria and administered with 80 mg/kg body weight of artesunate (Standard control);
Group 4: Administered with 100 mg/kg body weight of ELES after receiving a malaria parasite inoculation;
Group 5: Administered with 200 mg/kg body weight of ELES after receiving a malaria parasite inoculation;
Group 6: Administered with 400 mg/kg body weight of ELES after receiving a malaria parasite inoculation.
Sample collection and preparation
-
The animals were administered various treatments in their respective group via oral intubation for 3 d. Twenty-four hours after the last treatment, all the animals were euthanized using a diethylether inhalation jar. Afterward, they were dislocated from their cervical vertebrae, and blood samples were taken by venipuncture into lithium-heparinized specimen bottles for evaluation of biochemical status. The liver of the animals were also harvested and used for liver enzyme analysis.
Acute toxicity (LD50) study
-
The plant fraction's acute toxicity (LD50) investigation was carried out using Lorke's[19] method. Before the trial began, six groups of mice- three animals in each group- were starved of food for 12 h. Oral dosages of the fraction or aspirin were given to the animals in each group by gastric gavage at different rates (10, 100, 1,000, 1,600, 2,900, and 5,000 mg/kg bw). Animals were examined for behavioral changes (such as incoordination, dullness, and anxiety) or death for 24 h after the treatment.
Procurement of parasitemia
-
The malaria parasite (Plasmodium berghei) was procured from a malaria-infected mouse at the Department of Veterinary Parasitology and Entomology, Faculty of Veterinary Medicine, University of Nigeria (Nsukka, Nigeria). The blood sample was obtained using a capillary tube from the ocular region of the mice. Ten drops of the parasitized blood, were diluted with 1 mL of normal saline. An aliquot, of 0.2 mL of the mixture was used for each of the mice.
Determination of malaria parasitemia
-
The technique of Dacie & Lewis was used to determine the malaria parasitemia (Mp+)[20].
Hematological studies
Determination of total red blood cell count
-
The method of Dacie & Lewis was utilized to determine the total red blood cell (RBC) count[20].
Determination of total white blood cell count
-
The method of Dacie & Lewis was utilized to determine the total white blood cell (WBC) count[20].
Determination of packed cell volume (PCV)
-
The PCV of the RBC was determined using the technique of Dacie & Lewis[20].
Determination of hemoglobin (Hb) concentration
-
Hemoglobin (Hb) concentration was determined using the hemoglobin cyanide (HCN) technique outlined by Dacie & Lewis[20].
In vitro anti-inflammatory studies
Hypotonicity-induced hemolysis
-
This was determined using the method of Oyedepo & Famurewa[21] with slight modifications.
Principle:
Lysosomal enzymes, released during inflammation, cause various disorders. Anti-inflammatory drugs inhibit these lysosomal enzymes or stabilize the lysosomal membrane. This study was conducted to assess the stability of the Human RBC membrane using the extracts to estimate the anti-inflammatory activity in vitro since the Human RBC membrane is like the lysosomal membrane.
Procedure:
Three milliliters of blood were drawn from healthy volunteers, put into an ethylenediaminetetraacetic acid (EDTA) bottle, centrifuged for 10 min at 3000 rpm, and then rinsed three times with an equivalent volume of normal saline. Following measurement, the blood volume was reconstituted as a 40% (v/v) solution using normal saline.
The hypotonic solution consisted of distilled water dissolved in samples of the fraction and diclofenac sodium. One milliliter of the fraction at different concentrations (0.1, 0.2, 0.4, 0.6, and 0.8 mg/mL) was placed in each of the five test tubes. One milliliter each of 0.2, 0.4, 0.6, and 0.8 mg/mL of diclofenac sodium was put into three more tubes. The vehicle (distilled water) was added to fill each tube to a capacity of 4.9 mL. For this test, two control tubes were employed. The vehicle was placed in a control tube, and 4.9 mL of normal saline (isotonic solution) was placed in another tube. Each tube was filled with 0.1 mL of the suspension of human red blood cells. The mixtures were then gently mixed and allowed to sit at room temperature (37 °C) for 1 h. Following incubation, each tube's reaction mixture was centrifuged for 10 min at 3000 rpm to determine the supernatant's absorbance at 418 nm. There were three duplicates of each test run. For each test, reaction media with 1 mL of various fractions of diclofenac sodium concentrations created up to 5.0 mL in normal saline without human red blood cell (HRBC) suspension were utilized as the corresponding blank. The control tube blank was made of normal saline and did not include any suspension of red blood cells. The following relationship was used to calculate the % inhibition of hemolysis:
$ {\text{%}}\;{\rm Inhibition\; of\; haemolysis}=\left[1-\left\{\dfrac{O.D2-O.D1}{O.D3-O.D1}\right\}\right]\times100 $ Where, optical density 1 (OD1) is the absorbance of control I; OD2 is the absorbance test sample; OD3 is the absorbance of control II.
Protein denaturation
-
The Mizushima & Kobayashi technique[22] was followed to investigate the anti-inflammatory efficacy through the prevention of protein denaturation.
The reaction mixture was made up of 1% aqueous solution of bovine albumin fraction and test fractions at various concentrations. One N HCl solution in small amounts was used to modify the pH of the reaction mixture. The typical medication used was diclofenac sodium. After 20 min of incubation at 37 °C, the sample fractions were heated for 30 min at 57 °C. After cooling the samples, the turbidity was measured at 660 nm. Three copies of the experiment were run. To calculate the percentage of protein denaturation inhibition, the following formula was used:
$ {\rm Percentage\;inhibition\;}({\text{%}})=\left[ \dfrac{(O.D\;o f\,control-O.D\;o f\,sample)}{O.D\;o f\;control} \right]\times 100 $ Anti-proteinase activity
-
This test was conducted, with a few minor modifications, as described by Sakat et al.[23]. An exact quantity 0.06 mg trypsin, 20 nM Tris HCl buffer pH 7.4, and 1-mL test sample with varying concentrations (0.1, 0.2, 0.4, 0.6, and 0.8 mg/mL) were included in the reaction mixture (2 mL). The mixture was then incubated for 20 min. Two milliliters of 70% perchloric acid was added to halt the reaction. The absorbance of the supernatant was measured at 210 nm using buffer as a blank after centrifuging the hazy suspension. The experiment was performed three times. The following formula was used to determine the percentage inhibition of the anti-protease activity:
$ {\rm Percentage\;inhibition\;}({\text{%}})=\left[ \dfrac{(O.D\;o f\,control-O.D\;o f\,sample)}{O.D\;o f\,control} \right]\times 100 $ Inhibition of phospholipases A2 activity
-
Using a modified Vane's[24] approach, the fraction's inhibitory effect on phospholipase A2 activity was ascertained.
Principle:
The erythrocyte membrane is the site of phospholipase A2 activity measurement. Its action results in the release of free fatty acids and leakage, which permits hemoglobin to enter the medium. The concentration of hemoglobin in the medium, which absorbs at its maximum at 418 nm, is directly correlated with this enzyme activity.
Enzyme preparation:
A preparation of fungal enzymes was obtained using a culture of Aspergillus niger. Once 15 g of Sabouraud dextrose agar had been dissolved in 1,000 mL of distilled water, homogenizing the mixture for 10 min in a water bath, and then pouring the mixture into 250 mL conical flasks, and the nutritional broth was created. Cotton wool and foil paper were used to seal the conical flasks. After that, the broth was autoclaved for 15 min at 121 °C. After allowing the broth to reach room temperature, the organisms in the Petri dishes were aseptically injected into it and left to incubate for a full 72 h at room temperature. Afterward, the culture was moved into test tubes each with 3 mL of phosphate-buffered saline, the tubes were centrifuged for 10 min at 3,000 rpm. The test tube's supernatant was utilized to prepare crude enzymes, while the fungal cells sank to the bottom.
Substrate preparation:
New blood samples were centrifuged for 10 min at 3,000 rpm, and the blood plasma supernatant was disposed of. After three equal washes in normal saline, the red blood cells were quantified and reconstituted as a 40% (v/v) suspension in saline buffered with phosphate. This was going to be phospholipase A2's substrate.
Assay procedure:
Test tubes were incubated for 1 h with calcium chloride (2 mM) (0.2 mL), human red blood cells (0.2 mL), the crude enzyme preparation, and different quantities of normal saline, the fraction, and the reference medication. CaCl2, a free enzyme, and a suspension of human red blood cells were present in the control. Separately, 0.2 mL of boiling enzyme was applied to the blanks. The reaction mixtures used for incubation were centrifuged for 10 min at a speed of 3,000 rpm. The absorbance of the solutions was measured at 418 nm after 1.5 mL of the supernatant sample was diluted with 10 mL of normal saline. The comparison medication was diclofenac, a well-known phospholipase A2 inhibitor. The following relationship was used to compute the percentage of maximum enzyme activity and the percentage of inhibition:
$ {\text{%}}\;maximum\;enzyme\;activity=\left\{\dfrac{O.D\;o f\;test}{O.D\;o f\;control}\right\}\times 100 $ % inhibition = 100 − % maximum enzyme activity.
Statistical analysis
-
Version 20.0 of the Statistical Package for Social Science (SPSS) was used to analyze the data, and the findings were reported as mean ± standard error of the mean. One-way and two-way ANOVA was used to determine whether there was a significant difference in the results, and p < 0.05 was the acceptance threshold for significance for all results.
-
The acute toxicity study's results are displayed in Table 1. The administration of ELES did not show any toxicity up to 5,000 mg/kg bw (p.o.) during the observation period, according to the results.
Table 1. Acute toxicity study.
Treatments
(mg/kg bw, po, ELES)No. of animals used No. of deaths recorded 10 3 0 100 3 0 1,000 3 0 1,700 3 0 3,000 3 0 5,000 3 0 n = 3; po = per oral treatment; bw = body weight. Effect of ELES on malaria parasitemia
-
The effect of ELES on the level of parasitemia in Plasmodium berghei-inoculated mice are shown in Table 2. From the result, mice challenged with the malaria parasite and left untreated showed high parasitemia load after inoculation. However, mice administered with various doses (80−400 mg/kg bw) of ELES for 3 d after malaria induction, showed a significant (p < 0.05) decrease in the parasitemia load, and was comparable with the group administered 80 mg/kg b.w. Artesunate. In addition, the peak anti-malaria effect (16.67 ± 5.56) of ELES was observed at the highest dose (400 mg/kg bw ELES), moreover, the extract exerted a significantly (p < 0.05) higher effect at a similar dose.
Table 2. Effect of ELES on malaria-infected mice parasitemia.
Groups Before treatment 3 d post-treatment Normal control 0.00 ± 0.00a 0.00 ± 0.00a Positive control 80.00 ± 5.77b 70.00 ± 5.77e 80 mg/kg bw Arthesunate 73.33 ± 13.33b 30.00 ± 3.33c 100 mg/kg bw ELES 63.33 ± 8.82b 46.67 ± 3.33d 200 mg/kg bw ELES 63.33 ± 12.02b 26.67 ± 3.33bc 400 mg/kg bw ELES 56.67 ± 2.02b 16.67 ± 5.56b n = 3. Results are presented as Means ± SEM. Mean values that differ in superscripted alphabets down the columns are deemed significant (p < 0.05). Hematological parameters
Effect of treatment with the extract of S. linifolia on hematological parameters of malaria-infected mice
-
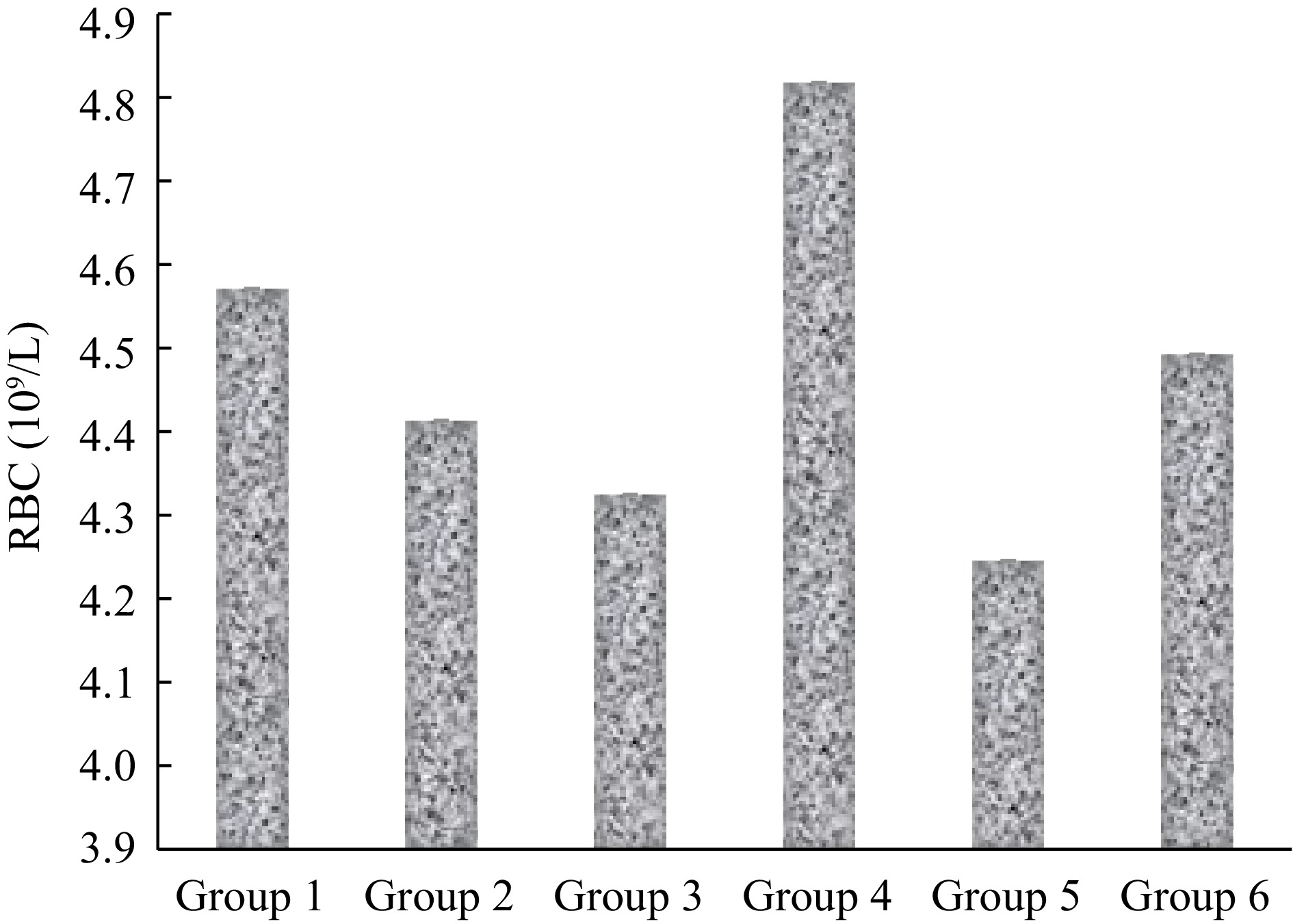
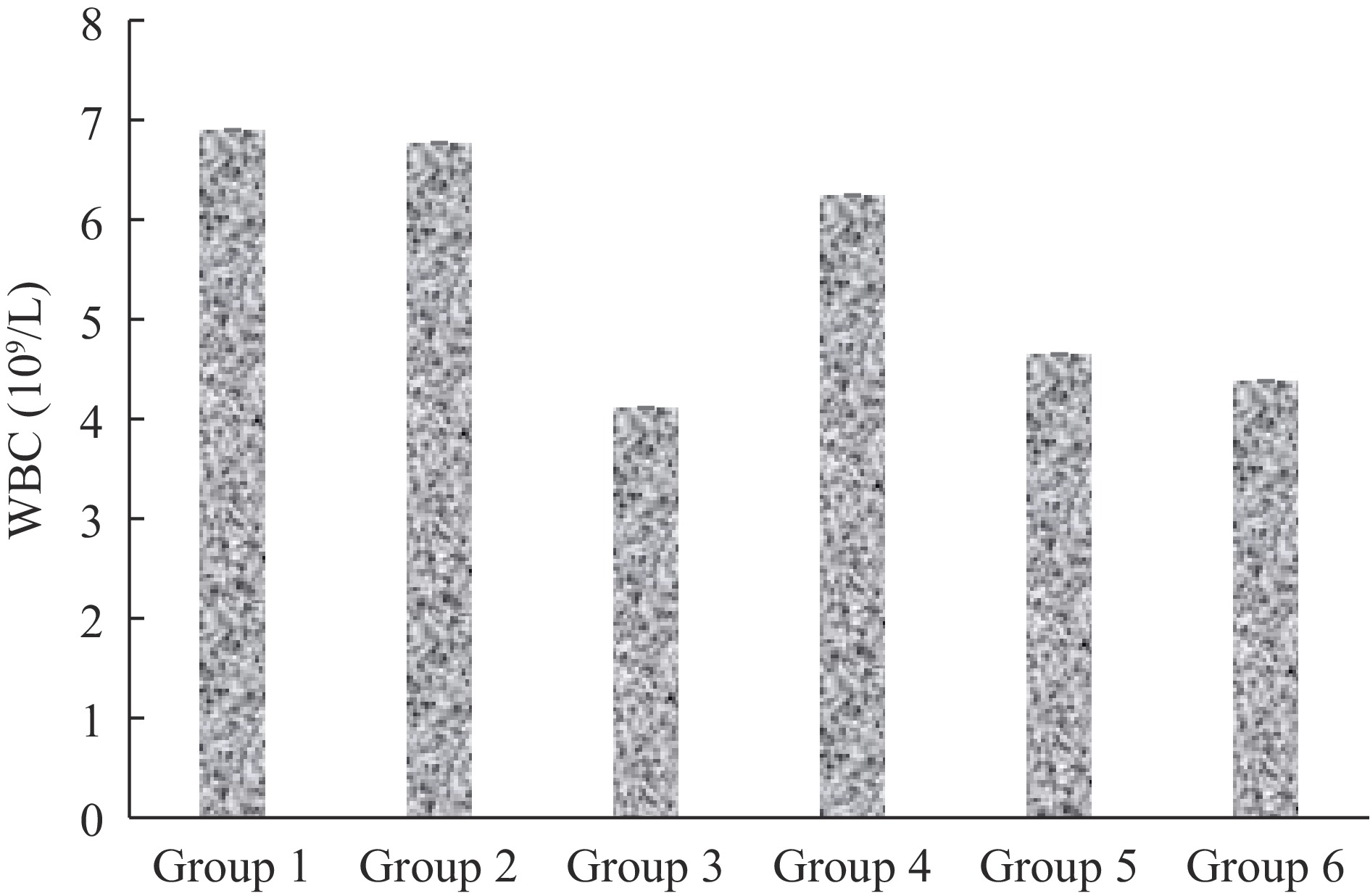
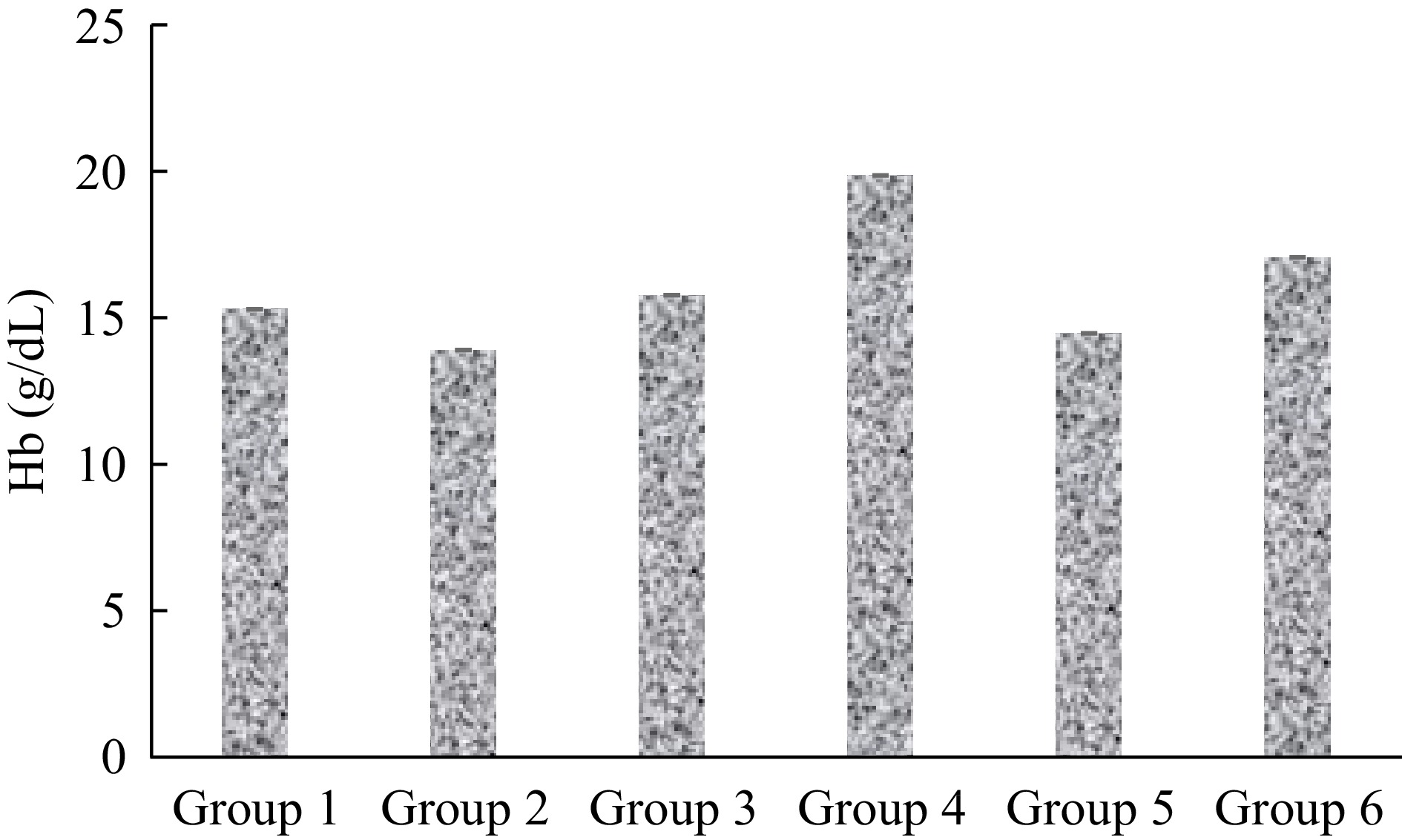
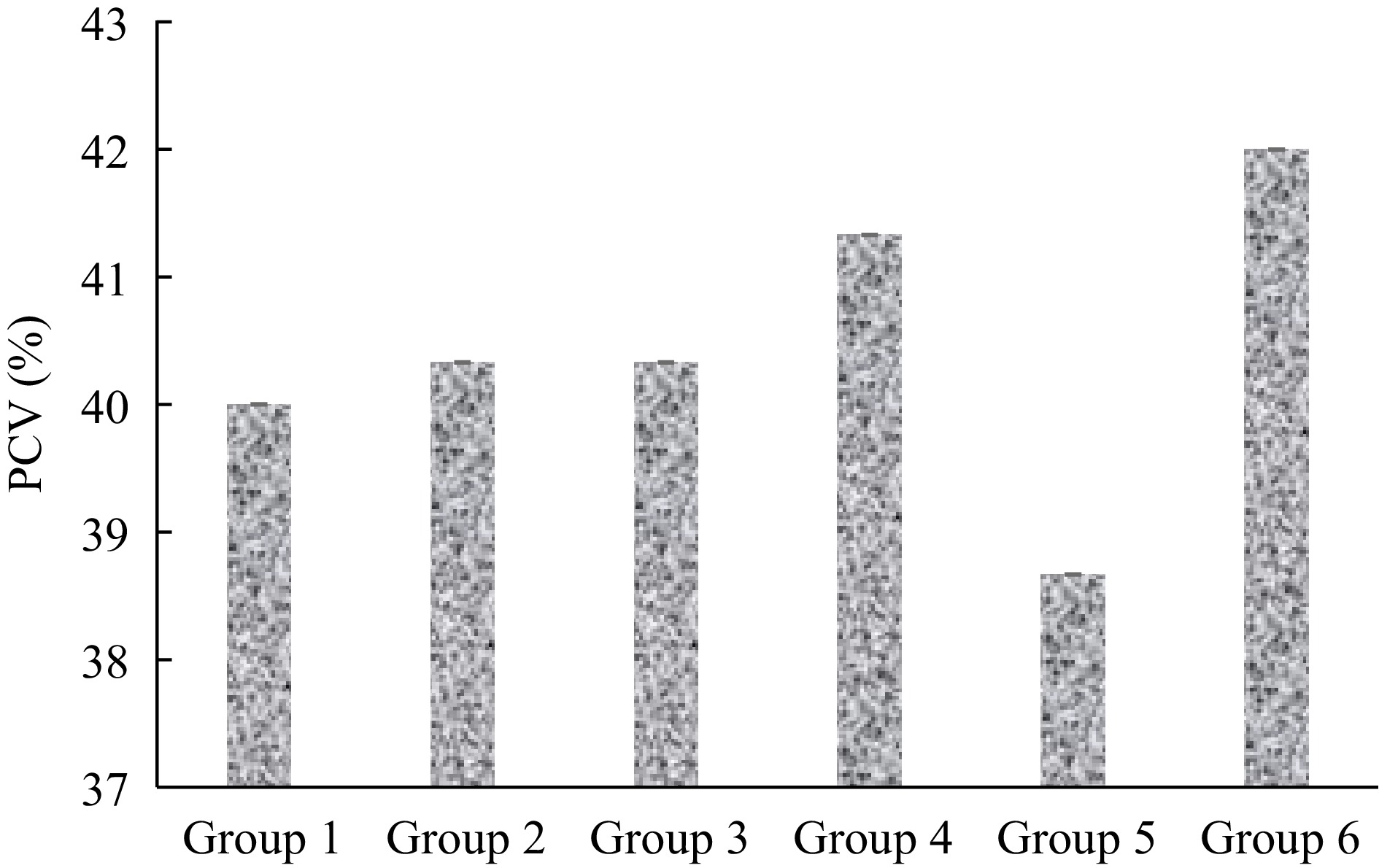
The effect of treatment with the ELES on the hematological parameters of malaria-infected mice is presented in Figs 1−4.
From Fig. 2, the highest white blood cell (WBC) count was noticed in the normal control group which is 6.93 ± 0.48 109/L, while the lowest count was in group 3 (4.13 ± 0.46 109/L). The WBC counts of all extract-treated groups on 3 d post-treatment were non-significantly (p > 0.05) higher when compared to the standard control.
The highest packed cell volume (PCV) percentage was observed in group 6 which is 42.00% ± 4.163%, while the lowest percentage was in group 5 (38.67 ± 1.856) (Fig. 3). The PCV percentage of groups 4 and 6, 3 d post-treatment was non-significantly (p > 0.05) higher when compared to the standard control.
The highest red blood cell (RBC) count was observed in group 4 which is 4.83 ± 0.22 109/L, while the lowest count was in group 5 (4.25 ± 0.38 109/L) (Fig. 1). The RBC counts of groups 5 and 6, 3 d post-treatment were non-significantly (p > 0.05) higher when compared to the standard control.
As shown in Fig. 4, the highest hemoglobin (Hb) concentration was observed in group 4 which is 19.90 ± 0.800 g/dL, while the lowest concentration was in the positive control group (13.93 ± 0.811 g/dL). The Hb concentration of groups 4 and 6, 3 d post-treatment was significantly (p < 0.05) higher when compared to the standard control.
Effect of the flavonoid-rich fraction (FRF), terpenoid-rich fraction (TRF), and alkaloid-rich fraction (ARF) on hypotonicity-induced hemolysis
-
The % inhibitions of hypotonicity-induced hemolysis exhibited by FRF, TRF, and ARF of S. linifolia leaves are presented in Table 3. The result revealed that the highest % inhibition was exhibited by 200 µg/mL while the lowest % inhibition was exhibited by 800 µg/mL of the FRF. Compared to the standard (50 µg/mL), there was a significant (p < 0.05) increase in hypotonicity-induced hemolysis inhibition of the fraction at 50 µg/mL.
Table 3. Membrane stabilization capacity of FRF, TRF, and ARF of S. linifolia on hypotonicity-induced hemolysis and protein denaturation.
Concentrations
(µg/mL)FRF TRF ARF Hypotonicity-induced hemolysis (% inhibition) 50 43.15 ± 2.45a 51.50 ± 399.40a 61.20 ± 1.20b 100 78.45 ± 8.15b 89.734 ± 194.04b 88.50 ± 201.50a 200 80.65 ± 3.45c 64.10 ± 5.40c 75.30 ± 12.30c 400 67.45 ± 6.45d 69.70 ± 124.88d 73.45 ± 26.75d 800 70.10 ± 174.10e 53.80 ± 260.80e 68.15 ± 2.15e Aspirin (50 µg/mL) 79.76 ± 8.72b 79.76 ± 8.72f 79.76 ± 8.72f Protein denaturation (% inhibition) 50 32.44 ± 41.10a 35.12 ± 52.40a 22.99 ± 3.66a 100 94.64 ± 1.86b 77.02 ± 10.57b 80.95 ± 4.04a 200 85.12 ± 1.81c 53.27 ± 8.87c 84.00 ± 18.09c 400 62.50 ± 2.73d 66.07 ± 2.73d 71.73 ± 23.90d 800 79.82 ± 7.80e 76.84±21.60b 88.02 ± 18.04e Aspirin (50 µg/mL) 79.76 ± 8.72e 79.76 ± 8.72e 79.76 ± 8.72f Values are presented as mean ± standard error of the mean (SEM). Values with different letter superscripts are significantly (p < 0.05) different. The % inhibition of hypotonicity-induced hemolysis exhibited by TRF of S. linifolia revealed that the highest % inhibition was exhibited at 100 µg/mL, while the lowest % inhibition was exhibited at 800 µg/mL. Compared to the standard (50 µg/mL) there was a significant (p ˂ 0.05) decrease in hypotonicity-induced hemolysis inhibition by the fraction at 50 µg/mL. There was also a significant (p ˂ 0.05) decrease in hemolysis inhibition by the TRF with an increase in concentrations (200, 400, 800 µg/mL).
The percentage inhibition of hypotonicity-induced hemolysis exhibited by the ARF of S. linifolia revealed that the highest percentage inhibition was exhibited at 50 µg/mL, while the lowest percentage inhibition was exhibited at a concentration of 800 µg/mL of the alkaloid-rich fraction.
Effect of the FRF, TRF, and ARF on protein denaturation
-
The % inhibition of protein denaturation exhibited by the FRF, TRF, and ARF of S. linifolia leaves are presented in Table 3. The results revealed that the highest % inhibition was exhibited by 100 µg/mL while the lowest % inhibition was exhibited by 800 µg/mL of the FRF. Compared to the standard (50 µg/mL), there was a significant (p < 0.05) decrease in protein denaturation inhibition of the fraction at 50 µg/mL. There was a significant (p < 0.05) increase in the protein denaturation inhibition of the fraction at 100 µg/mL compared to that of the standard (50 µg/mL).
The result of the % inhibition of protein denaturation exhibited by the TRF of S. linifolia revealed that the highest % inhibition was exhibited at 100 µg/mL, while the lowest % inhibition was exhibited at 800 µg/mL. Compared to the standard (50 µg/mL) there was a significant (p ˂ 0.05) decrease in protein denaturation inhibition by the fraction at the same concentration. There was a significant (p < 0.05) decrease in protein denaturation inhibition with increasing concentrations.
The percentage inhibition of protein denaturation exhibited by the ARF of S. linifolia leaves revealed that the highest percentage inhibition was exhibited at 50 µg/mL, while the lowest percentage inhibition was exhibited at a concentration of 800 µg/mL of the alkaloid-rich fraction. However, there was a significant (p < 0.05) difference down the column with increasing concentration of the alkaloid-rich fraction and there was no significant (p > 0.05) difference in the standard.
Effect of the FRF, TRF, and ARF on proteinase activity
-
The % inhibition on anti-proteinase activity exhibited by the FRF, TRF, and ARF of S. linifolia leaves are presented in Table 4. The result revealed that the highest % inhibition was exhibited by 50 µg/mL while the lowest % inhibition was exhibited by 800 µg/mL of the FRF. Compared to the standard (50 µg/mL) there was a significant (p < 0.05) increase in anti-proteinase activity inhibition of the fraction at 50 µg/mL.
Table 4. Effect of FRF, TRF, and ARF of S. linifolia on proteinase and phospholipase A2 activities.
Concentrations
(µg/mL)FRF TRF ARF Proteinase (% inhibition) 50 20.80 ± 0.42c 14.40 ± 0.29b 15.84 ± 2.30b 100 23.71 ± 0.79b 18.75 ± 1.56a 18.13 ± 2.20a 200 30.46 ± 8.34a 20.78 ± 3.14c 20.92 ± 1.81c 400 33.27 ± 6.25d 27.09 ± 8.00d 33.44 ± 3.09d 800 39.96 ± 9.37e 32.04 ± 5.63e 39.85 ± 2.93e Aspirin (50 µg/mL) 62.64 ± 3.84f 62.64 ± 3.84f 62.64 ± 3.84f Phospholipase A2 (% inhibition) 50 59.85 ± 24.63b 41.00 ± 3.12a 42.25 ± 5.69b 100 82.57 ± 5.89c 58.87 ± 2.41b 61.78 ± 0.72a 200 84.61 ± 2.51a 69.69 ± 4.767c 62.00 ± 6.86c 400 73.77 ± 8.96d 70.63 ± 4.03d 62.47 ± 10.47c 800 85.90 ± 2.67a 60.08 ± 6.44e 69.82 ± 7.14d Prednisolone (50 µg/mL) 76.01 ± 1.52e 76.01 ± 1.52f 76.01 ± 1.52e Values are presented as mean ± standard error of the mean (SEM). Values with different letter superscripts are significantly (p < 0.05) different. The result of the % inhibition of proteinase activity exhibited by the TRF of S. linifolia revealed that the leaf extract was effective in inhibiting protease activity in a dose-dependent manner. The highest % inhibition was exhibited at 50 µg/mL, while the lowest % inhibition was exhibited at 800 µg/mL. At a lower concentration of 50 µg/mL, there was a significant (p ˂ 0.05) increase in the proteinase activity inhibition compared to the standard at the same concentration.
The percentage inhibition of anti-proteinase activity exhibited by the ARF of S. linifolia leaves revealed that the highest percentage inhibition was exhibited by 800 µg/mL while the lowest percentage inhibition was exhibited by 50 µg/mL of the ARF. Although there was a significant (p < 0.05) difference in the alkaloid-rich fraction at different concentrations, lower activities were observed with increasing concentrations. However, the standard showed no activity and no significant (p > 0.05) difference with increasing concentration.
Effect of the FRF, TRF, and ARF on phospholipase A2 Activity
-
The % inhibition of phospholipase A2 (PLA2) activity exhibited by the FRF, TRF, and ARF of S. linifolia leaves are presented in Table 4. The result revealed that the highest % inhibition was exhibited by 800 µg/mL while the lowest % inhibition was exhibited by 50 µg/mL of the FRF. Compared to the standard (50 µg/mL) there was a significant (p < 0.05) decrease in PLA2 activity inhibition of the fraction at 50 µg/mL. There was a significant (p < 0.05) increase in the PLA2 activity inhibition of the fraction at 800 µg/mL compared to that of the standard (50 µg/mL).
The % inhibition of PLA2 activity exhibited by the TRF of S. linifolia revealed that the TRF of S. linifolia was effective in inhibiting PLA2 activity in a dose-dependent manner. The highest % inhibition was exhibited at 400 µg/mL, while the lowest % inhibition was exhibited at a concentration of 100 µg/mL. At a lower concentration of 50 µg/mL, there was a significant (p ˂ 0.05) decrease in PLA2 activity inhibition compared to the standard at the same concentration.
The percentage inhibition of PLA2 activity exhibited by the ARF of S. linifolia leaves revealed that the highest percentage inhibition was exhibited at 400 µg/mL while the lowest percentage inhibition was exhibited at a concentration of 800 µg/mL of the ARF.
-
There was no sign of toxicity, including mortality observed in any of the treated animal groups during the LD50 study even at the dose level of 5,000 mg/kg of ethanol extract of S. linifolia leaf, thus an indication that the extract is safe for consumption and could cause very minimal damage to the organ even at high dosages. The extract was able to significantly reduce the number of malarial parasites in the blood, thus validating the antiplasmodial capacity of the plant extract. In a study conducted by Enechi et al.[25], the ethanol leaf extract of Sida acuta, when administered to Plasmodium berghei ANKA-65, significantly decreased parasitemia and suppressed the malaria parasite. Hematological parameters such as RBC count, WBC count, PCV and hemoglobin were improved upon administration of the ethanol leaf extract of S. linifolia. Leaf extracts of some medicinal plants such as those of Mucuna pruriens have been observed to boost hematological parameters (RBC, WBC, PCV, and Hb) in Albino rats[26].
The result of the present study revealed that the FRF of Sida linifolia leaves inhibited hypotonicity-induced hemolysis at all the concentrations but was most effective at 200 µg/mL. Compared to the standard (Aspirin) at 50 µg/mL, there was a significant increase in the fraction of inhibiting hypotonicity-induced hemolysis at 200 µg/mL. The results indicate that S. linifolia TRF significantly inhibited hypotonicity-induced hemolysis at a lower dose (100 µg/mL) than the FRF. At 100 µg/mL, ARF exhibited better activity compared to the FRF. The highest percentage inhibition of hypotonicity-induced hemolysis was by TRF (89.734%) which was comparable to that of the standard (79.76 %) at 50 µg/mL. Since it was found that several chemicals that can release hydrolytic enzymes from lysosomes can also damage erythrocytes, Ajayi et al.[27] suggested that the membrane of an erythrocyte serves as a model for the membrane of a lysosome. So, since the above fractions were able to inhibit the activities of the hydrolytic enzymes against erythrocytic membranes, they can serve as a good source of anti-inflammatory drugs.
The FRF of Sida linifolia leaves showed the highest % inhibition (94.64%) on protein denaturation at 100 µg/mL. Compared to the standard (Aspirin) at 50 µg/mL, there was a significant increase in the fraction's capacity of inhibiting protein denaturation at this concentration. The TRF significantly inhibited protein denaturation with the highest % inhibition (77.02%) at 100 µg/mL, and this was comparable to that of the standard (79.76) at 50 µg/mL. The ARF at 800 µg/mL exhibited the highest (88.02%) inhibition against protein denaturation. Against protein denaturation, the activity of the FRF was the best, followed by the TRF and then the ARF. This is consistent with a study by Enechi et al.[28], which showed that compounds that may prevent protein denaturation are a potential target for the development of anti-inflammatory drugs, as this process is a well-established source of inflammation. Also, Yesmin et al.[29] elucidated the membrane-stabilizing mechanism of anti-inflammatory agents. According to a study by Agrawal & Paridhavi[30], albumin proteins that have been denatured produce antigens, which set off a type III hypersensitivity reaction that causes inflammation. Sen et al.'s research[31] supported the idea that the mechanism underlying protein denaturation is unpredictable and involves changes to hydrophobic, disulfide, and electrostatic hydrogen bonds. This is in line with earlier research conducted by Sangeetha & Vidhya[32], which explained how protein denaturation causes the creation of autoantigens in inflammatory illnesses such as rheumatoid arthritis, diabetes, and cancer. Dharmadeva et al.[33] suggested that inhibition of protein denaturation can attenuate inflammatory activities.
The FRF of Sida linifolia leaves had an anti-proteinase activity with the highest % inhibition (39.96%) at 800 µg/mL. The TRF of S. linifolia effectively inhibited proteinase activity significantly with the highest % inhibition (32.04%) at a level of 800 µg/mL. Also, at 800 µg/mL, the ARF had the highest anti-proteinase activity (39.85%). The fraction with the best anti-proteinase activity was FRF, followed by ARF and then TRF. The result revealed that the FRF, TRF, and ARF of Sida linifolia leaves were effective in inhibiting proteinase activities with increasing concentrations. This corresponds with a study by Enechi et al.[28] which also demonstrated that a plant extract exhibits significant anti-proteinase activity at different concentrations. According to research by Bermúdez-Humarán et al.[34], proteinases are involved in several immune system arms, participate in a variety of physiological and pathologic states, and are crucial in inflammation. Proteinase inhibition has been reviewed by Coppini et al.[35] as a therapeutic target in the management of many inflammatory disorders.
The FRF of Sida linifolia leaves was most effective (85.90%) at inhibiting PLA2 activity at the highest level of 800 µg/mL which could be compared to that of the standard (Prednisolone) at 50 µg/mL. The inhibitory effect of TRF against PLA2 activity was highest (70.63%) at 400 µg/mL. The ARF showed the highest (69.82%) inhibitory effect against PLA2 activity at 800 µg/mL. The TRF showed the highest inhibitory activity against PLA2 activity, followed by the FRF and then the ARF. The findings of this investigation is consistent with a study conducted by Olarenwaju et al.[36], which clarified the mechanism by which anti-inflammatory agents stabilize their membranes by blocking PLA2 activities, thereby impeding the synthesis and eicosanoids' release, which are implicated in the mechanisms of inflammation.
-
The ethanol leaf extract of Sida linifolia exhibited antimalarial potential by reducing the amount of the malarial parasite Plasmodium berghei in the blood and again it improved the blood cell counts and other related parameters in malaria-infected mice. The flavonoid-, terpenoid-, and alkaloid-rich fractions of S. linifolia exhibited characteristics that reduced inflammation by membrane stabilization, protecting protein denaturation, inhibiting proteinase and phospholipase A2 activities. The anti-inflammatory properties of S. linifolia leaf lie both in the polar (flavonoids and alkaloids), and non-polar (terpenoids) constituents of the plant. The extract and fractions have proven to be good sources of antimalarial and anti-inflammatory agents as advocated by traditional healers. The next step in the development of new drugs from these fractions, for synthetic chemists, is to isolate the active principles responsible for the said activities and possibly synthesize a new line of pharmaceuticals from them.
-
The authors confirm contribution to the paper as follows: study conception and design, supervision of the experiments: Nwankwo NE; experiments, draft manuscript preparation, statistical analysis and data interpretation: Ashiakpa NP. Both authors reviewed and approved the final version.
-
All data generated or analyzed during this study are included in this article.
-
The University of Nigeria, Nsukka staff provided support and solidarity during the experiment, which the authors appreciate. We greatly value the contributions made by the staff members at the other universities we visited for the study.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Nwankwo NE, Ashiakpa PN. 2024. Antimalarial potential of ethanol extract, and anti-inflammatory properties of flavonoid-, terpenoid-, and alkaloid-rich fractions of Sida linifolia L. Food Materials Research 4: e018 doi: 10.48130/fmr-0024-0009
Antimalarial potential of ethanol extract, and anti-inflammatory properties of flavonoid-, terpenoid-, and alkaloid-rich fractions of Sida linifolia L.
- Received: 28 December 2023
- Revised: 17 April 2024
- Accepted: 05 May 2024
- Published online: 04 July 2024
Abstract: Sida linifolia L. has been employed ethnomedicinally in the treatment of inflammatory diseases such as whitlow and malaria but is yet to be scientifically validated for use in the management of ailments. This investigation sought to assess the antimalarial potential of ethanol leaf extract as well as the in vitro anti-inflammatory qualities of flavonoid-, terpenoid- and alkaloid-rich leaf fractions of Sida linifolia L. The acute toxicity (LD50) study and the effect of the extract on parasitemia and hematological parameters were determined. In vitro, anti-inflammatory parameters were also determined. It was demonstrated that S. linifolia leaf extract was safe and significantly (p < 0.05) reduced parasitemia in malaria-infected mice. The extract also improved blood counts in mice infected with malaria. The activity of the alkaloid-rich fraction against hypotonicity-induced hemolysis was significantly (p < 0.05) higher than those of the terpenoid-rich and flavonoid-rich fractions. On protein denaturation, the activity of the flavonoid-rich fraction had significant (p < 0.05) improvement compared to those of the terpenoid-rich and alkaloid-rich fractions. The fraction with the best anti-proteinase activity was the flavonoid-rich which was better by a significant (p < 0.05) margin than those of alkaloid-rich and terpenoid-rich fractions. The terpenoid-rich fraction showed the highest inhibitory activity against PLA2 activity, followed by the flavonoid-rich and then the alkaloid-rich fractions. Crude leaf extract of S. linifolia proved potent against malaria, and the flavonoid-, terpenoid- and alkaloids-rich fractions exerted anti-inflammatory properties, justifying its use in traditional medicine in the treatment of malaria and other inflammatory diseases.
-
Key words:
- Phytoconstituents /
- Anti-inflammatory /
- Membrane stabilization /
- Hemolysis /
- Proteinase