-
A variety of factors, including meat resource scarcity, health concerns, environmental and animal welfare issues, have driven the emergence and development of meat alternatives[1]. Plant-based meat analogs are prepared from plant proteins, carbohydrates, oils, fragrances, gums and adhesives through a series of processing techniques[2]. At present, there are main meat substitutes that have a similar flavor, texture, and appearance to meat[3,4]. Plant-based meat analogs have become a rapidly growing area of research, with hundreds of articles published each year. It is necessary but challenging for researchers to stay on top of research trends and monitor the latest important advances. Although some literature reviews and meta-analyses on plant-based meat analogs have provided a lot of useful information and reliable findings[5,6], there are still some shortcomings. Systematic literature reviews are intended to provide a qualitative summary of progress on a particular research topic. They are limited by the knowledge and perspectives of the authors and cannot evaluate a large number of publications. A meta-analysis can summarize evidence from multiple, homogeneous studies to address a particular question. However, these two methods cannot present the current situation and emerging trend of knowledge structure in the whole research field. Therefore, a new method is needed to conduct a comprehensive and quantitative analysis of plant-based meat analogs. The method needs systematically summarize important advances, show current research hotspots, and propose future research directions.
Bibliometric analysis is a quantitative and objective analysis method based on thousands of publications. It uses mathematical and statistical methods to study the distribution structure, quantitative relations, and their change rules[7−9]. This can comprehensively present the research status and predict the development trend of the whole field. Moreover, bibliometric analysis can visualize information and intuitively present the characteristics of the research field[10,11]. The results can help researchers identify advances in the field, and research directions, and select collaborators or target journals[12]. Bibliometric analysis is an important method for identifying active researchers and potential collaborators, sorting out hot topics, describing dynamic trends, and identifying future research frontiers in specific fields. In recent years, bibliometric analysis has become more and more popular in food research[13,14]. However, there are few bibliometric analyses about plant-based meat analogs. With the increasing research on plant-based meat analogs, rigorous, in-depth, and useful bibliometric analysis is needed to help relevant scholars understand the global research development in this field and better grasp the latest research trends.
Therefore, this study conducted a bibliometric analysis of literature related to plant-based meat analogs published between January 2000 and October 2023. The research situation of plant-based meat analogs were summarized from the perspectives of publication time, number of national publications, research institutions, journals, keywords, and so on. The main objectives are: (1) to understand the development and evolution of historical research; (2) identify key contributors to the field of plant-based meat analogs, including countries, institutions and authors; (3) indicate the main published journals; (4) discuss current research hotspots; (5) predict the future research trend in this field.
-
PubMed is one of the commonly used databases for bibliometric analysis. Based on the PubMed database, this analysis was conducted on October 3, 2023. Literature on plant-based meat analogs published since 2000 were searched. The search terms were set to plant-based meat or plant meat to include all current descriptions of plant-based meat analogs such as plant-based meat analogs, plant-based meat analogs, plant-based meat substitutes, plant-based meat alternatives. The literature language was limited to English. The literature types were restricted to articles and reviews.
Statistical analysis
-
Software such as VOSviewer 1.6.16 (Leiden University, the Netherlands), CiteSpace V 5.8.R3 (Drexel University, the USA), Bibliometrix (University of Naples Federico II, Italy) as well as Citexs platform were integrated to complete literature data mining. Data were analyzed from the perspectives of countries, institutions, authors, journals, keywords, citation relationships, associated genes, and biological pathways. The general trend, distribution, and hot spot changes in the field were visualized. According to the input keywords, based on bibliographic coupling, the number of citations, impact factor (IF), publication time, and other conditions, we recommended the literature highly related to the keywords. The classic literature was further summarized based on the sharing relationship of recommended literature. The overlapped and co-cited parts of recommended literature and classical literature were defined as core literature. The BioBERT biomedical language representation model was used to mine and statistically analyze the disease entity words in the abstract of literature. Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were conducted by OmicsBean platform. Microsoft Office Excel 2019 software (Microsoft, Redmond, WA, USA) was used for descriptive statistical analysis, curve fitting, and to produce tables and figures. Select the best fitting model according to the size of the correlation coefficient (R2). The annual growth rate of the number of publications is calculated according to the following formula: Growth rate = [(number of publications in the next year − number of publications in the last year) / number of publications in the last year] × 100. The GraphPad Prism 9 software (Dotmatics, San Diego, CA, USA) was applied to make plot histograms and bubble diagrams.
-
The time distribution of literature publication generally shows the development characteristics of research field. In this study, 2,595 publications on plant-based meat analogs were retrieved. As shown in Fig. 1a, the number of publications each year showed an overall trend of rapid growth. At the early stage, the number of publications on plant-based meat analogs between 2000 and 2015 remained very small (no more than 90). However, there was a steady rise in the publications from 2015 to 2020. Since 2017, the number has exceeded 100. Annual publications increased dramatically from 2020 to 2023, of which 2021 has the fastest growth rate of 58.5%. Overall, more than 70% of the literature was published after 2015, and more than 50% was published after 2020. As of the search date (2023-10-03), 304 publications have been published worldwide in 2023, accounting for 11.7% of the total number of publications. The cumulative publication trend of plant meat analogs followed this formula: y = 68.665e0.1874x (R2 = 0.994, X is the year and Y is the cumulative number of publications). By fitting the growth curve, it was predicted that the cumulative number of publications in this field would exceed 3,500 by 2024. These results indicated that the research in this field was in a rapidly rising stage and was expected to continue to grow in a certain period.
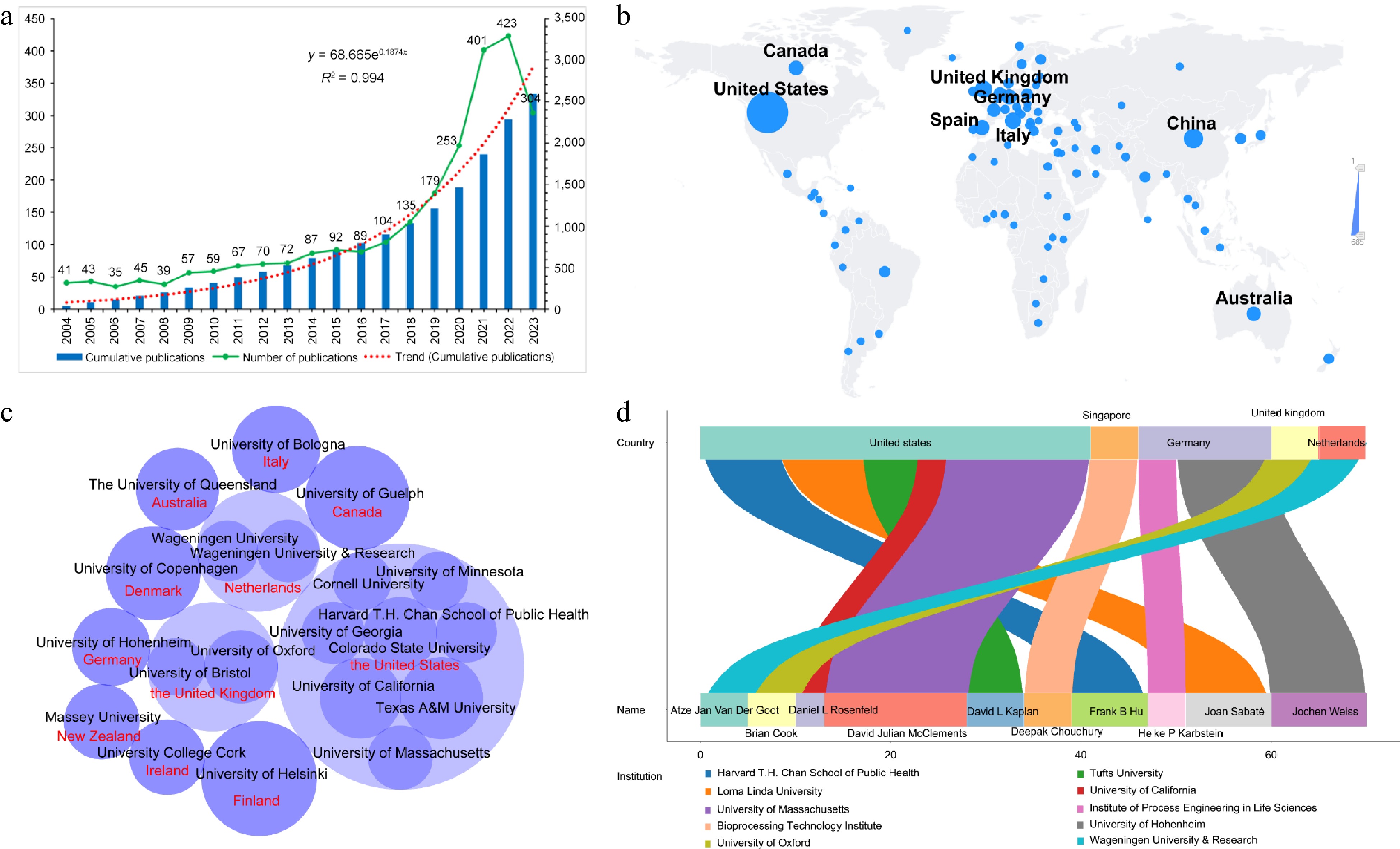
Figure 1.
Overview of research related to plant-based meat analogs from 2000 to 2023. (a) The specific number of annual publications; (b) Visualization world map of publications; (c) Distribution of core research institutions; (d) Active authors.
Most productive countries, institutions, and authors
-
The amount of literature can reflect the activity of scientific research. Therefore, the total number of documents published by one country is an important indicator of the output and productivity of this country. From 2000-01 to 2023-10, research on plant-based meat analogs has been carried out in hundreds of countries around the world. As shown in Fig. 1b, the United States had significantly more research publications than other countries. At present, a total of 685 papers have been published in the United States, accounting for 24.87% of the total. China and the United Kingdom ranked second and third, with 242 and 196 papers accounting for 8.79% and 7.12%, respectively. Countries such as Italy, Spain, Germany, Canada, and Australia also belonged to the list of countries with high literature production.
Further statistical analysis showed that the top 20 research institutions based on the number of published documents were distributed in 11 countries (Fig. 1c). Eight of these institutions belong to the United States, with two each in the United Kingdom and New Zealand. The University of Helsinki (Finland) and Texas A&M University (the United States) took the top two spots with 33 and 28 publications, respectively. The University of California (the United States) and the University of Guelph (Canada) followed, and both published 27 papers. Other institutions with more than 20 publications were the University of Oxford (the United Kingdom, 26), Colorado State University (the United States, 22) and the University of Copenhagen (Denmark, 22).
The statistical results of author publications showed that the top 10 authors in this field came from 10 research institutions in five countries (Fig. 1d). David Julian McClements at the University of Massachusetts in the United States currently published 15 papers in the field, which was the highest output of the author. In second place was Jochen Weiss at University of Hohenheim in Germany, who published 10 papers on the subject. Joan Sabaté and Frank B Hu at Loma Linda University and Harvard T.H. Chan School of Public Health in the United States followed, who respectively had nine and 10 publications. On the whole, the research of plant-based meat analogs has attracted global attention.
Most active journals and research area
-
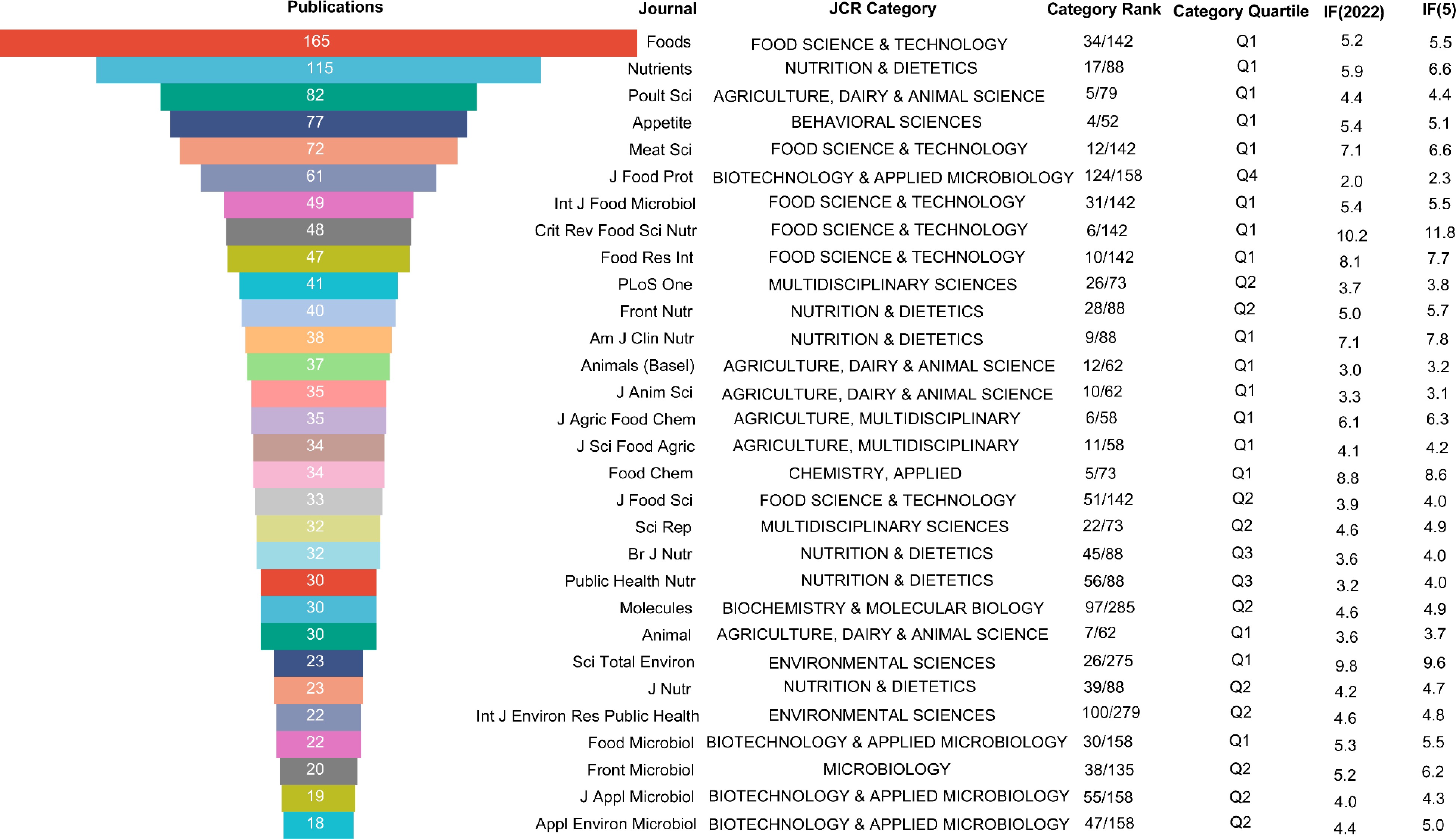
To comprehensively understand the relevant journals in this field, the information of the top 30 journals, including the JCR category, category rank, category quartile, impact factor in 2022 and the 5-year impact factor, were systematically collated. As shown in Fig. 2, research on plant-based meat analogs have been published in the fields of agricultural science, biochemistry, biotechnology, molecular biology, microbiology, food science, food chemistry, nutrition, and environmental science. Among them, the field of food science published the most papers, a total of 414. This was followed by nutrition and agricultural science, with 278 and 184 publications, respectively.
According to the JCR category, 17 of these 30 journals were Q1 partitions, accounting for 56.7% of the total. There were 10 journals in the Q2 division, accounting for 30% of the total. The journal that published the most literature on plant-based meat analogs was Foods, with a total of 165, which was significantly higher than other journals. The journal is ranked 34th in the food science and technology category and is located in the Q1 region. The journal's latest IF is 5.2 and its 5-year IF is 5.5. Foods was founded in 2012, covering a wide range of topics including food science and technology, physical and chemical properties of food, food safety, food microbiology, functional food and health. The annual publication volume of the journal has increased rapidly in recent years. The journal with the next largest amount of literature on plant-based meat analogs was Nutrients. The latest IF of the journal is 5.9 and its 5-year IF is 6.6. It ranks 17th in nutrition and was classified as area Q1. Other journals that published more than 40 papers included Poultry Science (82), Appetite (77), Meat Science (72), Journal of Food Protection (61), International Journal of Food Microbiology (49), Critical Reviews in Food Science and Nutrition (48), Food Research International (47), and PLoS One (41). Among them, Meat Science, Food Research International and Critical Reviews in Food Science and Nutrition rank 12th, 10th, and 6th respectively in food science and technology journals, occupying a very important position. Meat Science is an international journal that features comprehensive research on engineering-food technology. It was founded in 1977 by the publisher Elsevier. The latest IF and 5-year IF are 7.1 and 6.6, respectively. Food Research International is also published by Elsevier, covering the field of food science and technology. It has an IF of 8.1 for 2022. Critical Reviews in Food Science and Nutrition is an international journal featuring food science and nutrition research. It was first published in 1980 by Taylor & Francis Inc. The journal has a 5-year IF of 11.8. These popular and important journals deserve special attention from researchers involved in plant-based meat analogs to get the latest advances in the field.
Keywords and research topics
-
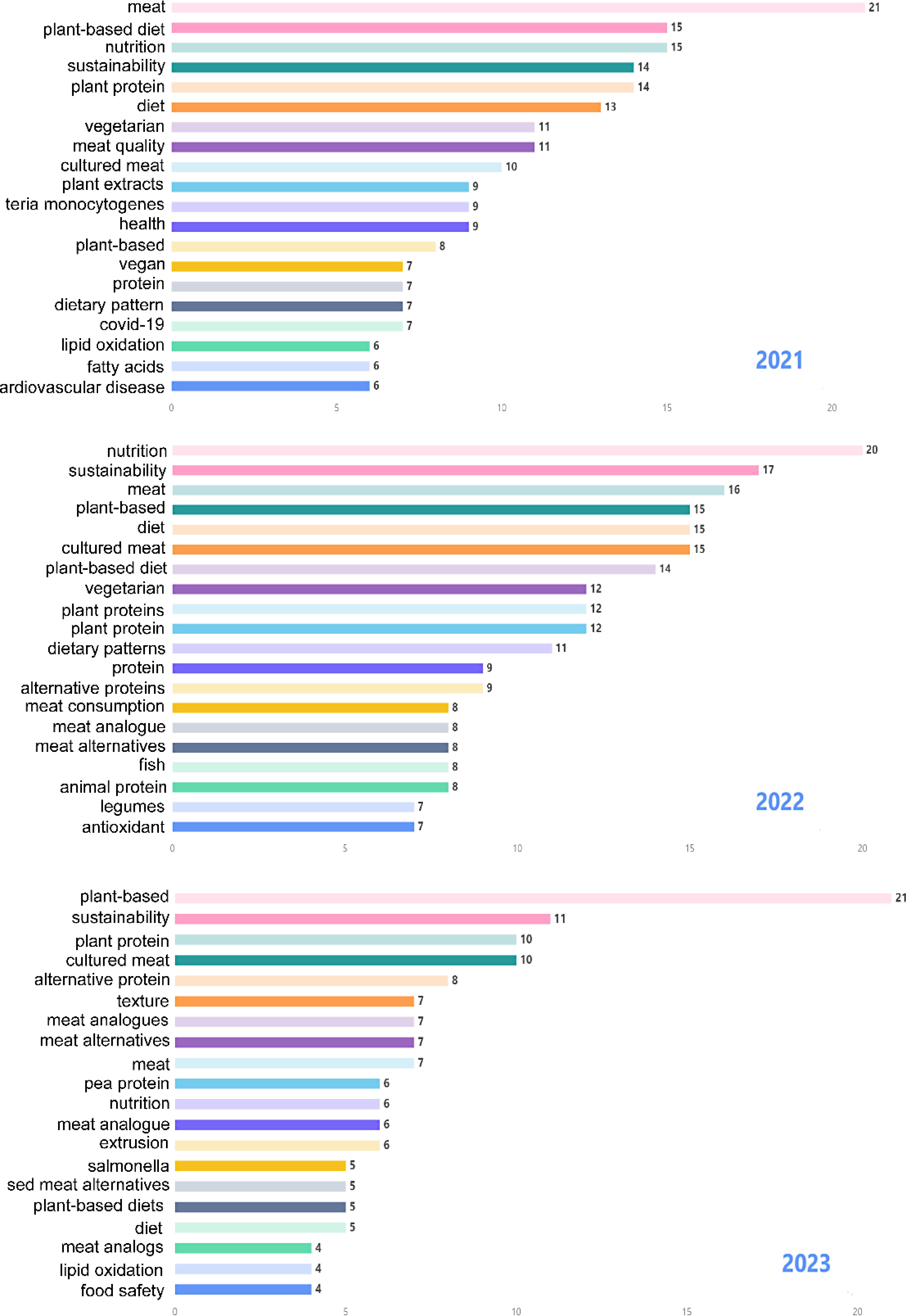
Keywords are usually the most representative terms used to explain the research topic. They are highly concise and generalized about the research purpose, research object, and research method. In order to explore the evolution trend and research hotspot of plant-based meat analogs, word frequency analysis of keywords was first performed. As shown in Fig. 3, meat appeared the most frequently, appearing 89 times in total. In addition, diet (76), sustainability (74), nutrition (71), plant-based (60), plant protein (54), vegetarian (52), cultured meat (46), meat quality (46), and protein (46) were all high-frequency words.
Then, 2000-01 to 2023-10 were divided into the following four periods: 2000.01 to 2014.12, 2015.01 to 2018.12, 2019.01 to 2020.12, and 2021.01 to 2023.10. The popularity ranking and ranking change of keyword word frequency in period were analyzed. As shown in Fig. 4, in the first stage, keywords directly related to plant-based meat analogs were not prominent. However, when it comes to the second stage, the keywords surrounding plant-based meat analogs gradually became clear and diversified. At this stage, the main keywords were allergenicity, alternative proteins, and flavor. In the third and fourth stages, the major keywords were cultured meat, plant-based meat analogs, animal welfare, and sustainability. These changes demonstrated that plant-based meat analogs have attracted the attention of more and more researchers.
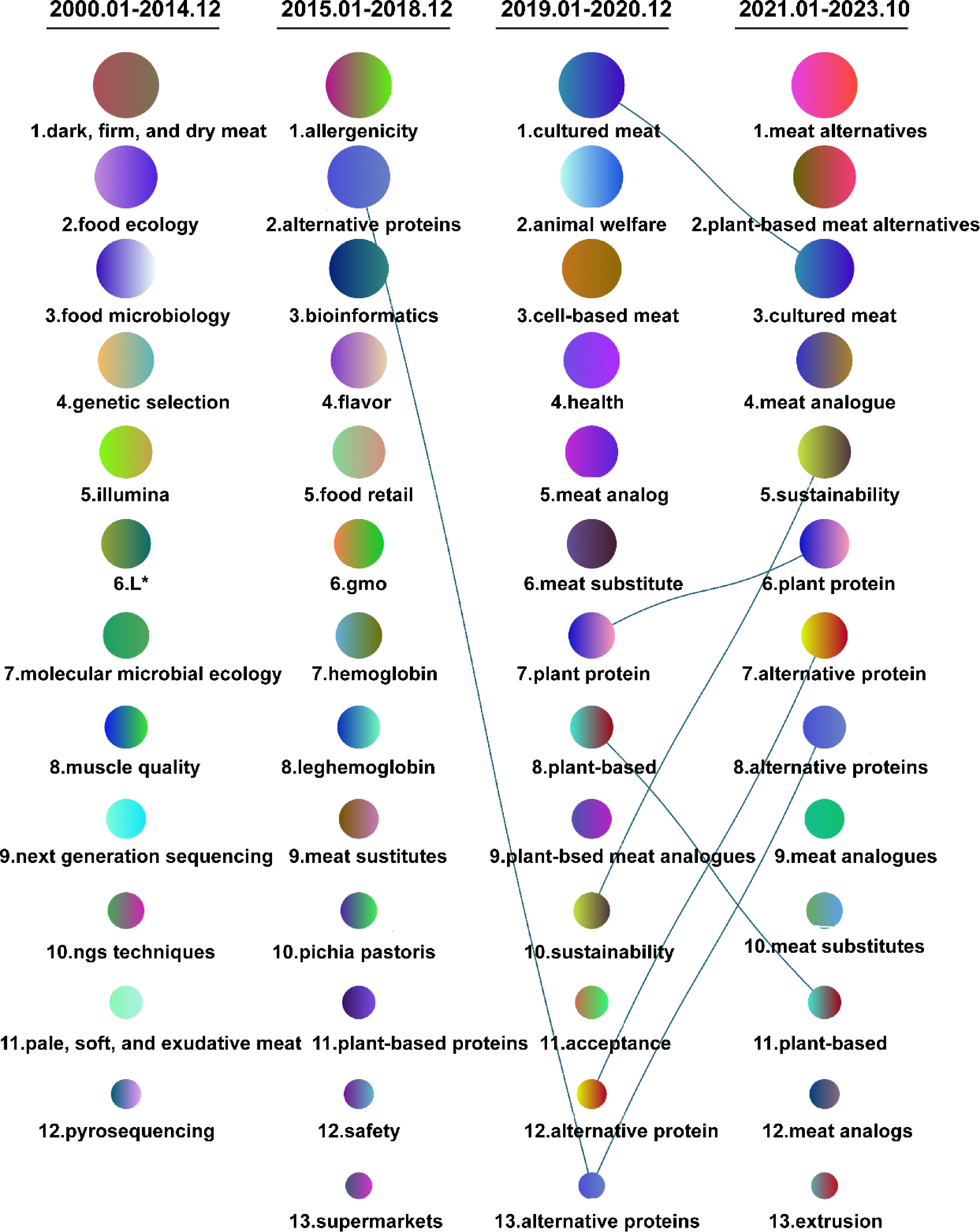
Figure 4.
Changes in keywords related to plant-based meat analogs over time. The larger the circular area, the higher the degree of attention of keywords.
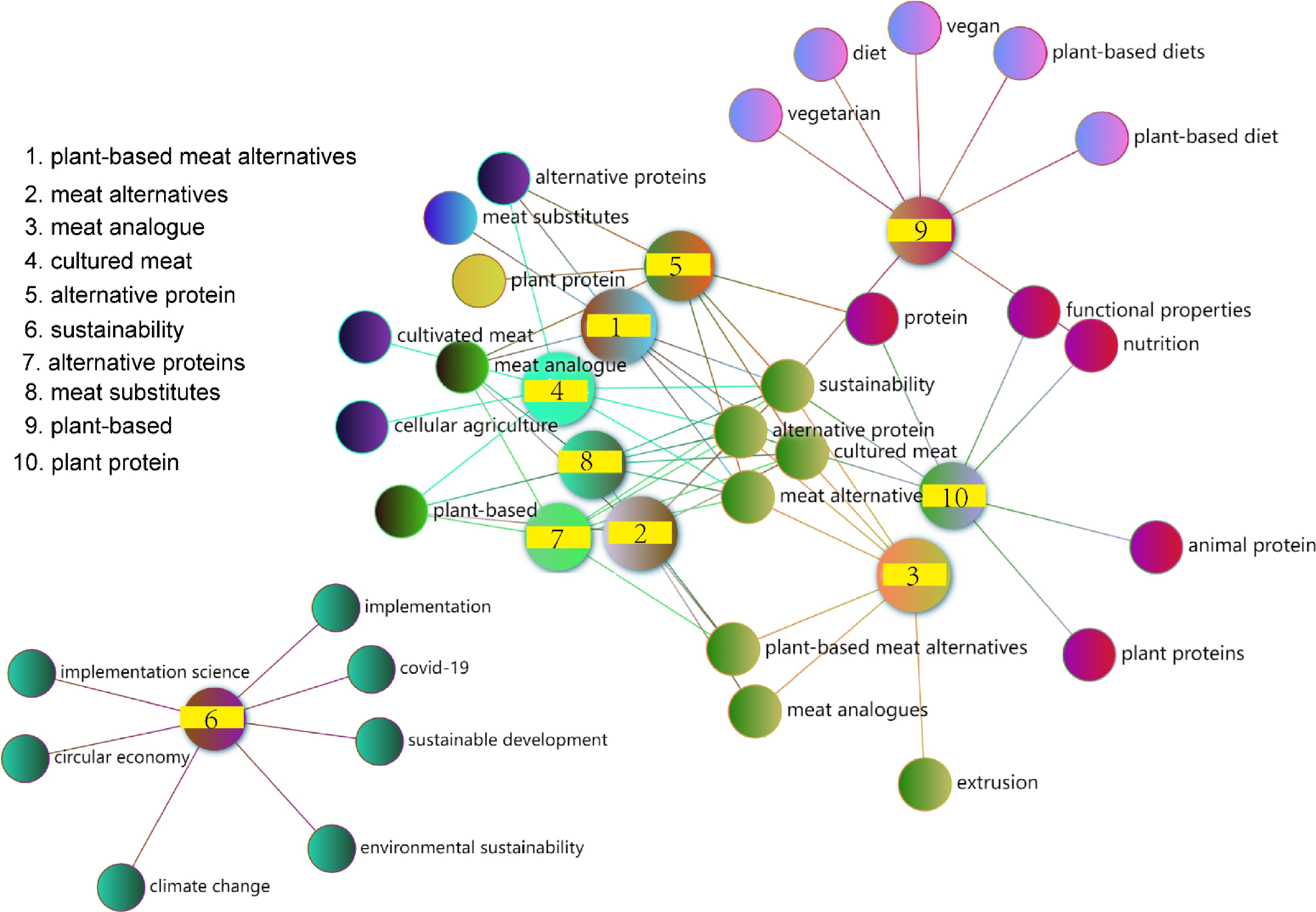
To better evaluate current research hotspots, we conducted a separate analysis of keyword frequency in the last 3 years. As shown in Fig. 5, the top five most frequent keywords in 2021 from high to low were meat, plant-based diet, nutrition, sustainability, and plant protein, while in 2022 the top 5 changed to nutrition, sustainability, meat, plant-based, and diet. So far, the prefix 'plant-based' has been the most frequent keyword in 2023, followed by sustainability, plant protein, cultured meat, and alternative protein. A co-occurrence analysis showed the interrelated knowledge network of the research topics in the three years. The results showed that current hot topics related to plant-based meat analogs include alternative proteins and their functional and nutritional characteristics, as well as sustainable development (Fig. 6).
Research status and frontiers
-
To have a more detailed and in-depth understanding of the current research status and future research direction of plant-based meat analogs, the citation relationships of current relevant papers in this field were analyzed (Fig. 7a, Supplemental Tables S1−S3). The recommended literature indicated the latest progress of research on plant-based meat analogs (Supplemental Table S1). The classic literature was groundbreaking research in the field, by analyzing these documents, researchers can quickly grasp the historical process of research on plant-based meat analogs (Supplemental Table S2). The core literature suggested the ongoing focus of researchers in the field (Supplemental Table S3). From the systematic analysis, it is clear that the idea of processing plant-based ingredients for protein foods is not a new concept, because many products, such as tofu and tempeh, have been consumed in Asia for centuries[15]. Differently, these products are mainly aimed at vegetarians or vegans and have not attracted global attention. To date, some emerging technologies, such as extrusion, have not been used to develop plant-based foods, making plant-based proteins possess meat-like structures[16,17]. These plant-based meat analogs, which have similar texture, flavor, and color to meat, become a hot topic in the food and research communities as one of the meat substitutes.
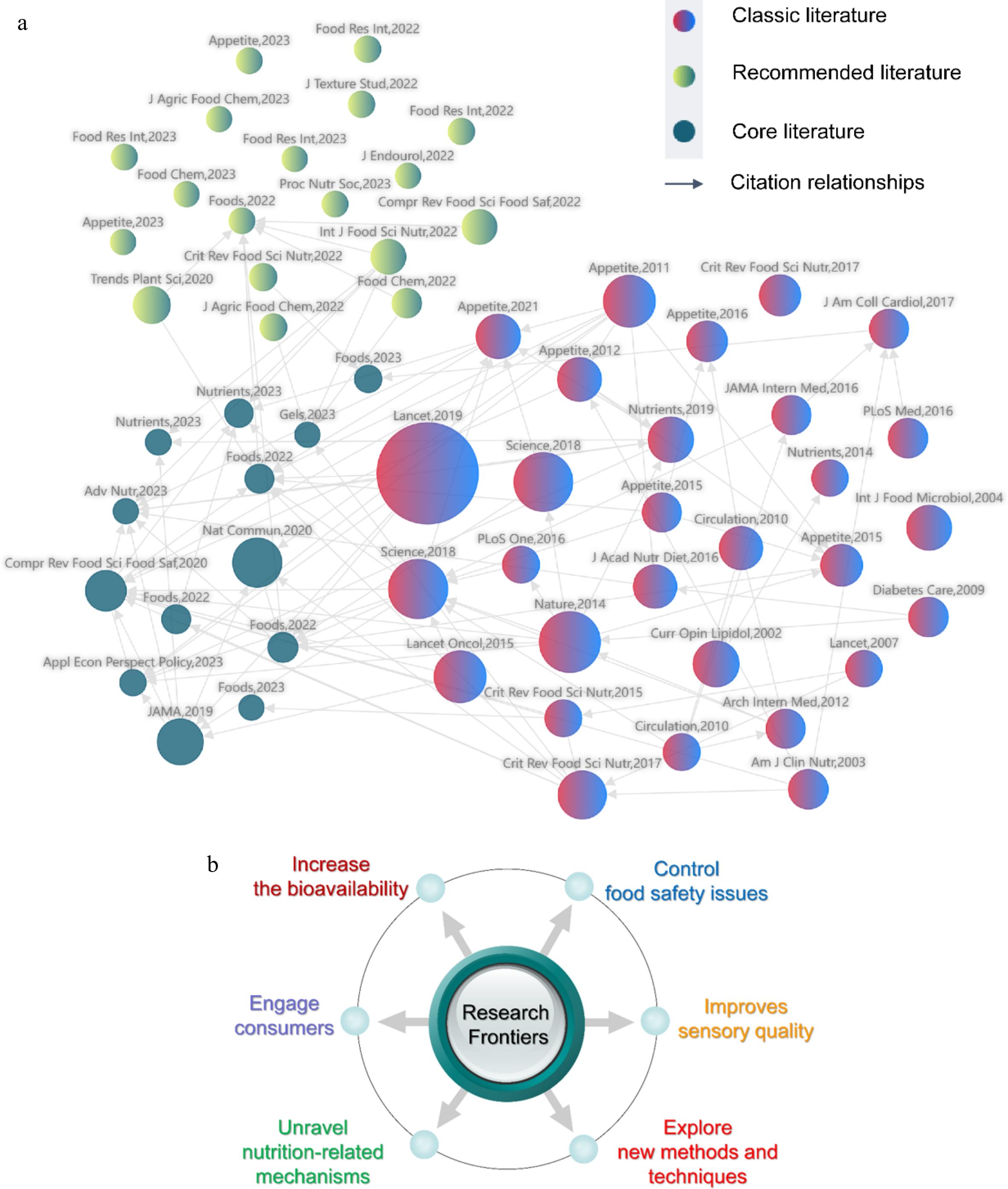
Figure 7.
Analysis of the research status and trends in the field of plant-based meat analogs. (a) Citation relationships between published papers; (b) Possible research trends. Each ball represents an article. The size of the sphere is positively correlated with the number of co-citations.
The rise of plant-based meat analogs
-
Meat is an important source of protein with high biological value. However, the modern relationship among meat, environment and health is very complex. With the rapid growth of the world population, the demand for meat is increasing, and the supply of meat is most likely to be insufficient[18,19]. Furthermore, increased awareness of sustainable development has made consumers gradually realize that animal rearing for meat production consumes a lot of resources and creates environmental problems such as greenhouse gas emissions[20,21]. In addition, meat production involves animal welfare issues[22]. What's more, several studies have linked excessive meat intake to chronic diseases such as obesity, diabetes, and cardiovascular disease[23−25]. These factors drive more and more people to look for meat alternatives. At the same time, some studies have shown that plant-based diets are more environmentally sustainable than diets rich in animal products. Vegetarians and vegans also have a lower risk of health conditions such as ischemic heart disease, type 2 diabetes, hypertension, and obesity[26−29]. Together, these circumstances have driven a global transition from animal protein to plant-based protein, further fueling the rise of plant-based meat analogs[30].
Research status of plant-based meat analogs
-
Some studies have focused on quality characteristics such as texture[31,32] and flavor[33,34] of plant-based meat analogs. With the increasing supply and sales, more and more people pay attention to the nutritional properties of plant-based meat analogs[35,36]. The composition of plant-based meat analogs is similar to that of real meat products, but the ingredients come from different sources. Plant-based meat analogs are made from various plant components through a series of processing processes such as extrusion, high temperature, and cooling. Many studies have pointed out that different processing may lead to changes in protein structure, and the changes will further affect its digestive characteristics[37−39]. However, both in vitro and in vivo studies have indicated that the digestion and absorption characteristics of proteins in plant-based meat analogs are not as good as those in meat[40,41]. How to improve the bioavailability of nutrients of plant-based meat analogs is a question worth considering.
Recently, a large number of studies have also pointed out that there were certain differences in the nutrient content between plant-based meat analogs and real meat[42,43]. Most plant-based meat analogs have lower protein, cholesterol, and vitamin B12 content but higher carbohydrate, dietary fiber, and sodium content than real meat. In addition, the fat content varies greatly among products. The differences in carbohydrate (dietary fiber, sugar, and starch), fat, and sodium content between real meat and plant-based meat analogs drew particular attention from the researchers[44,45]. Moreover, in order to simulate the special properties of meat, the formulation of plant-based meat analogs includes many food additives, such as colors, flavorings, and adhesives, in addition to purified or semi-purified plant proteins[46]. The use of refined raw materials and multiple ingredients has led to most current plant-based meat analogs being classified as ultra-processed products[47]. Consumption of ultra-processed foods may increase the associated risk of obesity, metabolic syndrome, and hypertension[48,49]. As a result, there is a growing awareness that plant-based meat analogs cannot be directly equated with plant-based foods and cannot be considered equally beneficial to health. Whether these plant-based meat analogs can be used as good alternatives to meat has aroused consideration[30,50].
Research frontiers of plant-based meat analogs
-
However, there are relatively few studies on the nutritional value and physiological effects of plant-based meat analogs. For the first time, Xie et al. directly took plant-based meat analogs as research objects in a mouse model to explore the differences between them and real meat in terms of gastrointestinal digestive function, appetite regulation, and liver metabolism[41,51,52]. They found that compared with real meat, plant-based meat analogs reduced gastrointestinal digestive function in mice by down-regulating the expression of gastrointestinal nitrogen nutrient sensors[41]. Specific digestive peptides and flavor substances in plant-based meat analogs increased the food intake of mice by altering the balance between appetite regulators[51]. This further affected lipid metabolism in mice, resulting in increased body weight and lipid accumulation[52]. These findings revealed the differences in nutritional function and related metabolic mechanisms between real meat and plant-based meat analogs, and provided a theoretical reference and scientific basis for a rational diet. Besides, recent studies have found that multiple plant-based meat analogue products were contaminated with one or mixtures of up to seven mycotoxins[53]. That suggests high risks for consumers. To better understand the risks associated with switching to a plant-based meat diet, future systematic studies of natural toxin contamination of plant-based meat alternatives are needed. Policymakers also need to consider dealing with these natural toxins in plant-based meat analogs to ensure consumer safety[54].
Notably, consumer acceptance of plant-based meat analogs and how to increase consumption and attract new consumers have long been the focus of academia, industry and business[50,55,56]. Consumer acceptance largely depends on attitudes and beliefs about meat substitutes and food neophobia[57]. In order to make meat alternatives more attractive to meat consumers, improving their sensory quality and the similarity to meat has been a common practice. At present, some effective techniques have been applied to the production of plant-based meat analogs. For example, fermentation is booming as an effective technology for improving the sensory, nutritional, and functional properties of plant-based meat analogs[58,59]. Also, machine learning-based methods have been proposed to predict the structural properties of meat analogs[60], which provides convenience for optimizing product development cycles and reducing costs.
In conclusion, although a lot of studies have been carried out on the quality characteristics of plant-based meat analogs, how to further improve the quality and attract more consumers is still a hot topic. Moreover, it is the future trend to systematically investigate and control the safety issues such as pollution of natural toxins, as well as explore the health effects and related mechanisms. More methods and techniques need to be explored and developed to optimize the quality and nutritional characteristics of plant-based meat analogs (Fig. 7b).
Associated genes and biological pathways
-
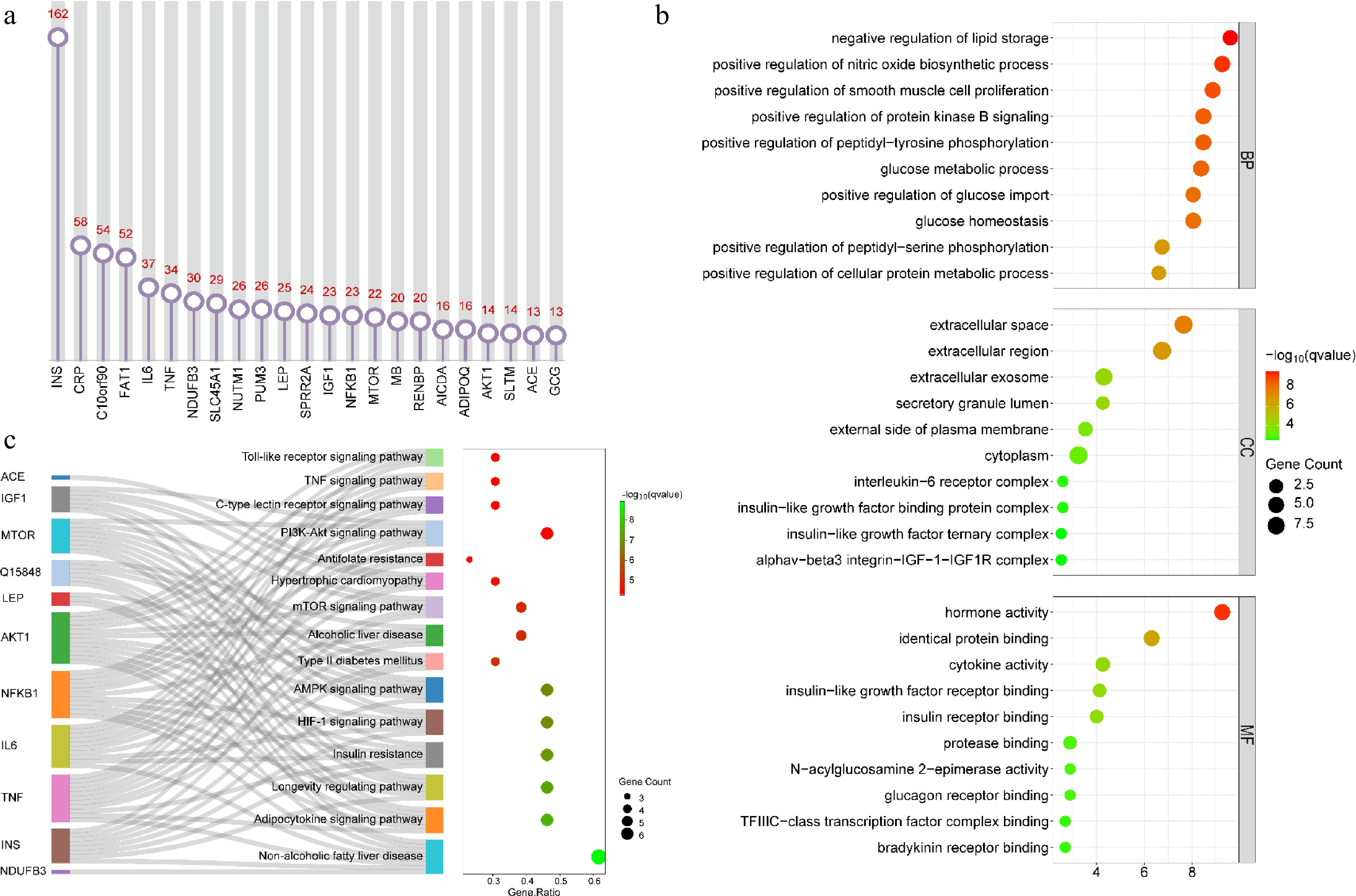
The genetic entity words in the abstract of 2595 publications were mined and analyzed using the BioBERT biomedical language representation model. As shown in Fig. 8, a total of 23 genes were discovered. Among them, the maximum number of literature involving INS was 162, and there were 58, 54 and 52 publications related to CRP, C10orf90 and FAT1, respectively. Other genes covered in at least 30 publications included IL6 (37), TNF (34), and NDUFB3 (30) (Fig. 8a). Enrichment analysis of all genes found that these genes were significantly enriched in terms of molecular function (MF), cellular component (CP) and biological process (BP). In terms of BP, these genes were mainly involved in peptidyl-serine/tyrosine phosphorylation, protein kinase B signaling, nitric oxide biosynthetic process, smooth muscle cell proliferation, and protein, glucose, and lipid metabolic process. In terms of CC, these genes were mainly concentrated in extracellular space, extracellular region, extracellular exosome, secretory granule lumen, external side of plasma membrane, cytoplasm, interleukin-6 receptor complex, insulin-like growth factor binding protein complex, alphav-beta3 integrin-IGF-1-IGF1R complex, and insulin-like growth factor ternary complex. In terms of MF, these genes mainly have hormone, cytokine, and N-acylglucosamine 2-epimerase activity, as well as identical protein, insulin-like growth factor receptor, insulin receptor, protease, glucagon receptor, TFIIIC-class transcription factor complex, and bradykinin receptor binding functions (Fig. 8b). KEGG pathway analysis showed that these genes were mainly involved in alcoholic/non-alcoholic fatty liver disease, type II diabetes mellitus, hypertrophic cardiomyopathy, adipocytokine, AMPK, mTOR, PI3K-Akt, C-type lectin receptor, TNF, toll-like receptor, HIF-1 signaling pathway, insulin resistance, antifolate resistance, and longevity regulating pathway (Fig. 8c). These results suggested that plant-based meat analogs were mainly involved in metabolically related pathways and diseases, providing important clues for future health research.
-
Plant-based meat analogs have been one of the hot topics in recent years. The present analysis summarized the current state of research, research hotspots, and future trends in the field from the micro level (authors, institutions, and keywords) to the macro level (world, country, and topic). This is of vital reference value for further research.
The number of annual publications can directly reflect the research scale and attention of a certain field, and predict its future development trend. Based on the PubMed database, the temporal distribution characteristics of the number of publications related to plant-based meat analogs were analyzed. The results showed that this field did not attract much attention from researchers before 2015. Since then, more and more attention and interest have been paid to this field. This may be related to the fact that the World Health Organization classified processed meat as 'carcinogenic to humans' and red meat as 'probably carcinogenic' in 2015[61]. Meat-related health concerns, coupled with a growing awareness of sustainability, have prompted the search for meat alternatives. In the following years, the market for plant-based meat analogs expanded[4,44,62], attracting more and more researchers. At present, plant-based meat products are not only favored by vegetarian consumers but also attract many meat lovers[63]. Under these circumstances, the research on plant-based meat analogs will continue to develop rapidly in the next few years and will gradually go deeper.
Research on plant-based meat analogs has been carried out in hundreds of countries around the world. Multidimensional analysis of research can help to understand the degree of academic support and recognition for the research field. This is also conducive to the harmonious development of the field and the cooperation between relevant institutions and authors. Journals are important carriers for displaying academic information and disseminating knowledge. However, few researchers have a comprehensive view of all relevant journals in a field. The hot journals not only represent the recognition and choice of most researchers, but are also an important way to obtain the hot research content in this field. The analysis of published journals can guide researchers to select key publications to read and understand the main research results and latest progress in this field. At the same time, it is also helpful for researchers to accurately locate their research results and quickly find suitable journals for submission.
Keyword and reference analysis are one of the important methods and indicators of bibliometrics. Based on keyword co-occurrence analysis and literature co-citation analysis, it can reveal the evolution process, main research direction, and hot frontier of a specific field[64,65]. Systematic analysis showed that plant-based meat analogs were widely accepted, in part because they were considered an environmentally friendly alternative to meat[1]. However, the situation of energy consumption, land occupation, and water resource utilization in the actual production process remains to be clarified. As an alternative to meat, plant-based meat analogs not only need to mimic the texture, color, and flavor of meat but also have considerable biological value. At present, a series of processing and ultra-long formulations have also caused nutritionists to worry about their nutritional functions. Some researchers have investigated the differences in nutritional function between plant-based meat analogs and real meat from the aspects of substance composition, nutrient content, and bioavailability[41,45]. But the health implications of these differences were poorly explored. The undigested portion of the diet goes into the large intestine and is used by gut microbiota. Different nutrient substrates will shape different intestinal microecology[38,66]. Gut microbiota is not only important to the health of the gut but also affects many aspects. A large number of studies have uncovered the bidirectional communication between gut microbiota and extra-intestinal organs such as the brain (gut-brain axis), liver (gut-liver axis), kidney (gut-kidney axis), heart (gut-heart axis), and so on[67−69]. Therefore, it is important to clarify the nutritional characteristics of plant-based meat analogs for scientific dietary guidance and maintenance health.
In addition, BioBERT is a language representation model for biomedical text mining and training. It has been proven to be very effective in many biomedical text-mining tasks. The important genes and key biological pathways were found through this method, which can provide important implications for the subsequent research of plant-based meat analogs in the field of nutrition. In short, the research of plant-based meat analogs has initially formed a diversified research perspective. However, it is still necessary to improve the acceptance of products and reveal their nutritional characteristics through multi-dimensional and in-depth research.
-
This work is the first bibliometric analysis to map and describe the knowledge landscape of plant-based meat-analogs related research from 2000−2023. The integration of various bibliometrics software and tools for analysis and visualization increases the richness of the results. However, there are certain limitations in this work. The literature search is only based on the PubMed database, and some literature could be omitted, which may introduce bias in paper selection and lead to the omission of important studies. Nevertheless, we believe our results remain a valid global research profile in the field of plant-based meat analogs. This work can provide an important reference for the further development of research on plant-based meat analogs.
-
The authors confirm contribution to the paper as follows: study conception: Xie Y, Li C; data collection and analysis: Xie Y, Cai L, Zhou G, Li C; draft manuscript preparation: Xie Y; manuscript revision: Cai L, Zhou G, Li C. All authors read and approved the final version.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This work was supported by Jiangsu Innovative Group of Meat Nutrition, Health and Biotechnology, and National Natural Science Foundation (grant number: 32072211).
-
The authors declare that they have no conflict of interest. Guanghong Zhou and Chunbao Li are the Editorial Board members of Food Materials Research who were blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of these Editorial Board members and their research groups.
- Supplemental Table S1 Recommended literature based on citations, impact factor, time of publication, and relevance to keywords.
- Supplemental Table S2 Classic literature screened based on the co-citation relationship of recommended literature.
- Supplemental Table S3 Core documents based on overlapping references between recommended and classic literature.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Xie Y, Cai L, Zhou G, Li C. 2024. Global research landscape and trends of plant-based meat analogs: a bibliometric analysis. Food Materials Research 4: e020 doi: 10.48130/fmr-0024-0011
Global research landscape and trends of plant-based meat analogs: a bibliometric analysis
- Received: 20 November 2023
- Revised: 25 March 2024
- Accepted: 28 May 2024
- Published online: 02 August 2024
Abstract: Plant-based meat analogs have become an important topic in recent years. To scientifically understand the research situation of plant-based meat analogs, we analyzed 2,595 publications from January 2000 to October 2023 by bibliometric method based on the PubMed database. The results showed a gradual rise in the number of annual publications, with the fastest growth rate of 58.5% in 2021. The country with the most publications was the United States (685, 24.87%), followed by China (242, 8.79%) and the United Kingdom (196, 7.12%). The University of Helsinki, Texas A&M University and the University of California were core research institutions. Popular and important journals were mainly Foods, Nutrients, Meat Science, Food Research International, and Critical Reviews in Food Science and Nutrition. Current research topics focused on alternative proteins and their functional and nutritional characteristics, as well as sustainable development. The research interests have gradually expanded from quality characteristics to nutritional characteristics. Further improving the quality, controlling natural toxin contamination, as well as systematically investigating the effects on health were future research trends. The effects of plant-based meat analogs on metabolic pathways and diseases were important clues in the study of nutritional health. This bibliometric analysis comprehensively and quantitatively presents the research landscape and hotspots, and further suggests future research directions. These findings can benefit researchers in selecting appropriate journals and finding potential collaborators to achieve in-depth research in this field.
-
Key words:
- Meat analogs /
- Bibliometric analysis /
- Research status /
- Research trends