-
Cultured meat is a rapidly developing meat production method that leverages the principles of muscle development and cell culture to produce meat products in laboratories or factories. It is one proposed way to address the environmental pressures faced by traditional animal husbandry[1,2]. Despite various advantages of cultured meat over conventional livestock farming, there are still technical hurdles in bringing cultured meat to the market, mainly including high-quality seed cells, cell expansion, and cell-to-meat formation[3,4].
Muscle stem cells (MuSCs), also called satellite cells (SCs), are essential for cultured meat production. MuSCs are a type of adult stem cell that resides under the basal lamina of the myofiber[5]. During muscle repair, they undergo symmetric and asymmetric divisions, proliferate, and differentiate into new muscle fibers or return to a resting state to maintain the stem cell pool[6]. In this process, MuSCs express specific myogenic transcription factors and regulatory factors, among which paired box 7 (PAX7) is a key marker necessary for maintaining stem cell characteristics and myogenic function[7]. MuSCs co-express PAX7 and myogenic differentiation (MYOD), indicating that the cells exit the resting state and begin to differentiate into muscle cells[8]. Subsequently, activated MuSCs express myogenic regulatory factors, MYOD and Myogenin, to promote myogenic differentiation. In the mature stage of differentiation, muscle cells elongate, align, and fuse, expressing the key protein myosin heavy chain (MYHC )[9]. In vitro, isolated MuSCs transit into myoblasts and subsequently differentiate into myofibers, a process extensively utilised in cultured meat[10−12].
Skeletal muscle is a complex tissue consisting of muscle fibres, MuSCs, and various cell types such as immune cells, fibro-adipogenic progenitors (FAP), and smooth muscle cells (SMCs). These cells form structures such as blood vessels and connective tissues, supporting muscle growth and function[13]. Muscle, fat, and collagen are essential components in meat[14]. In vitro, FAPs can differentiate into adipocytes and fibroblasts[15], under appropriate conditions, adipocytes form fat that provides cultured meat with flavour substances, juiciness, and mouthfeel[16,17]. Fibroblasts and SMCs can secrete collagen and other extracellular matrices, which provide nutrition, texture, and flavour to cultured meat products[18−22]. Therefore, MuSCs, FAPs, and SMCs all serve as indispensable seed cells for cultured meat production.
Existing single-cell transcriptomics on humans and mice[23] have revealed diverse cell types in human and mouse skeletal muscle[24,25]. However, there is a limited understanding of the muscle biology of agriculturally important species, like pigs. This lack of understanding restricts the knowledge of the initial cell types for cultured meat development, and the search for specific surface markers and purification methods[26−28]. There are various methods to obtain MuSCs from complex muscle tissue, including density gradient centrifugation[29], magnetically activated cell sorting (MACS)[30], and fluorescence-activated cell sorting (FACS)[31], with FACS being the most commonly used method. Ding et al.[37] successfully sorted porcine MuSCs by utilising cell surface antigens CD31, CD45, CD56, and CD29: CD31 is a marker of endothelial cells[32], CD45 marks hematopoietic cells[33], and CD56 and CD29 are expressed in MuSCs[34]. Porcine MuSCs obtained through this approach quickly lose PAX7 expression and myogenic differentiation ability during passaging. As demonstrated, CD29 is highly expressed in both MuSCs and FAPs[35]. Thus, the MuSCs obtained through Ding's sorting method tend to overproduce FAPs during extended passaging[36]. This outcome reduces the expansion efficiency of MuSCs and inhibits the ability of muscle cells to differentiate, fuse, and form myofibers[36]. Based on single-cell sequencing analysis, more than ten cell types in muscle tissue were identified, and markers for different cells were characterized[25]. Upon this, Messmer et al. screened out unique surface markers between FAPs and MuSCs, and they successfully isolated high-purity MuSCs, FAPs, and SMCs from bovine muscle tissue[36]. However, there is a lack of research on whether similar sorting strategies can be applied to pigs.
Here, we initially utilised the method as previously described (cell surface antigen combinations of CD31, CD45, CD56, and CD29)[37] to isolate MuSCs and found that the MuSCs exhibited decreased expression of PAX7 and impaired myogenic differentiation capabilities after passaging. Then, we implemented a novel strategy incorporating CD31, CD45, JAM1, integrin alpha5 (ITGA5), and integrin alpha7 (ITGA7)[36], which efficiently sorted muscle-derived cells into three distinct populations expressing markers specific to FAPs, MuSCs, and SMCs. The novel strategy yielded MuSCs with a nearly 20% higher PAX7 positivity rate and enhanced myogenic differentiation. After five passages, these MuSCs showed significantly elevated PAX7 expression and differentiation capacity compared to the previous method. Immunofluorescent staining and transcriptome analysis confirmed the effective separation of MuSCs, SMCs, and FAPs. Collectively, our method provides a means to isolate highly pure populations of FAPs, SMCs, and MuSCs from porcine muscle, which provides high-quality seed cells for the production of cultured meat.
-
Cells were isolated as described previously and adapted to pig tissues[37]. In brief, thigh and hip fresh muscle tissue was dissected and dissociated by dispase II (2 mg/mL, Roche, Cat# 4942078001) and collagenase (2 μg/mL, Roche, Mannheim, Germany, Cat# 11088866001) in DMEM (Invitrogen, Cat# C11995500BT) supplement with 3% penicillin-streptomycin (Gibco, 15140122, Carlsbad, CA, USA, Cat# 14140122). The sample was passed through a 100 μm cell strainer to remove any remaining clumps or debris, and then it was centrifuged to collect the cellular precipitates. Red blood lysis (Biosharp, Cat# BL503A) treated the precipitates. Final filtration with 40 μm cell strainer. The cells were resuspended with 1% BSA in PBS and stained with an antibody cocktail consisting of FITC conjugated CD31 (Bio-Rad, Richmond, CA, USA, Cat# MCA1097F), FITC conjugated CD45 (Bio-Rad, Richmond, CA, USA, Cat# MCA2220F), PE-Vio770 conjugated JAM (Miltenyi Biotec, Cat# 130-109-484), PE-conjugated ITGA5 (Miltenyi Biotec, Cat# 130-110-532), APC conjugated ITGA7 (Miltenyi Biotec, Cat# 130-123-833). Cell sorting was performed with a BD Influx cell sorter using 488, 561, and 633 nm lasers.
Cell culture and myogenic differentiation
-
Dishes (Corning, NY, USA, Cat# 430167) were coated with 0.025% rat tail collagen type I (Corning, Cat# 354236). FACS isolated cells were cultured on collagen-coated dishes in DMEM/F12 medium (Gibco, Cat# C11330500BT) supplemented with 15% fetal bovine serum (Gibco, Cat# A5669710), 5 ng/mL bFGF (GenScript, Cat# Z03166), 1% penicillin-streptomycin, and passaged every 3 d. For myogenic differentiation, purified cell types were plated onto 2% Matrigel (Corning, Cat# 356234) coated vessels at a density of 1.25 × 105 cm−2 in DMEM/F12 medium containing 15% fetal bovine serum, 5 ng/mL bFGF and 1% Penicillin-Streptomycin. After 12 h, the medium was switched to DMEM (Gibco, Shanghai, China, Cat# C11995500BT) with 2% horse serum (Hyclone, Logan, UT, USA, Cat# SH30074.02) and 1% penicillin-streptomycin. The pig cell was induced for 120 h of differentiation.
RNA-seq analysis
-
The cell samples were sent to BGI Co. Ltd. (Shenzhen, China) for RNA sequencing, each cell type had three replications. cDNA library construction and sequencing were performed after the high RNA integrity of each sample was confirmed using the Agilent 2100 Bioanalyzer prior. High-quality reads of pig samples were aligned to the SUS reference genome (Sscrofa11.1) and respectively using Bowtie2. The gene expression levels were normalised to fragments per kilobase of exon model per million mapped reads (FPKM) using RNA-seq by Expectation Maximization (RSEM).
Quantitative RT-PCR
-
Total RNA was extracted and purified using Fastpure Cell/Tissue Total RNA Isolation Kit (Vazyme, Cat# RC101), and its concentration and purity were measured using a NanoDrop spectrophotometer (Thermo Fisher Scientific). cDNA was synthesised using HiScript III RT SuperMix for qPCR (Vazyme, Cat# R323). RT-PCR was performed by using primers as specified in Table 1 and ChamQ SYBR qPCR Master Mix (Vazyme, Cat# Q311). The results were evaluated using the ΔΔCt method, and GAPDH was determined as an internal control.
Table 1. Primers for qPCR analysis.
Gene Primer sequence (5'-3') Accession no. PAX7 Forward GTGCCCTCAGTGAGTTCGATT XM_021095458.1 Reverse TCCAGACGGTTCCCTTTGTC CNN1 Forward CGTGCTATATAAGGGCCGGT XM_013987363.2 Reverse CGTCCATGAAGTTGTTGCCG PDGFRA Forward CCTACATCGGCGTCACCTAC NM_001315756.1 Reverse GGCAGAGGGATGATGTAGCC ITGA5 Forward CTTCAAACGCTCCCTCCCAT XM_001925252.7 Reverse AGCCTCCTCCCTGTCAGTAG ITGA7 Forward AGACGGCTTCCCAGACATTG XM_021091233.1 Reverse AATGGTTCCCATCCACGTCC MYOG Forward AACCCCACTTCTATGACGGG NM_001012406.1 Reverse TTATCTTCCAGGGGCACTCG MYHC Forward CCGTGCTCCGTCTTCTTTCC NM_001104951.2 Reverse CGCTCCTTCTCTGACTTGCG GAPDH Forward GTCGGAGTGAACGGATTTGGC NM_001206359.1 Reverse CTTGCCGTGGGTGGAATCAT Western blot
-
Whole-cell lysates were prepared using RIPA buffer (Byotime, Cat# P0013B) complemented with PMSF (Beyotime, Cat# ST506). Protein concentrations were determined by a BCA protein assay kit (Thermo, Cat# 23225), and lysates were run on 4%−20% precast polyacrylamide gels (Genscript, Nanjing, China, Cat# M00625) and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked for 2 h with 5% non-fat dry milk in TBST, then incubated with primary antibodies: PAX7 (1:50, DSHB, Cat# AB528428), CNN1 (1:1,000, Abcam, Cat# AB46794), PDGFRA (1:1,000, Abcam, Cat# AB203491), MYOG (1:1,000, BD pharming, Cat# 556358), MYHC (1:1,000, Abcam, CAT# ab37484), GAPDH (1:5,000, Millipore, Darmstadt, Germany, CAT# MAB374) at 4 °C. The next day, membranes were incubated using secondary antibodies HRP-conjugated goat anti-Rabbit IgG (Cwbiotech, Nanjing, China, Cat# CW0103S) or goat anti-mouse IgG (Cwbiotech, Cat# CW2333S) that were diluted at 1:2,000. Protein bands were detected using SuperSignalTM West Pico Chemiluminescent Substrate (Thermo, Carlsbad, CA, USA, Cat# 34580) under ImageQuant 4000 (General Electric, Boston, NY, USA). The software ImageJ was used to analyse grayscale values.
Immunofluorescent staining
-
Cells were cultured on Nunc Glass Base Dish (Thermo, Cat# 150680) and carefully washed with 1 × PBS then fixed with 4% paraformaldehyde (PFA) (Beyotime, Nanjing, China, Cat# P0099) overnight at 4 °C. Permeabilization was performed with 0.5% Triton X-100 in PBS for 30 min and cells were blocked with 5% BSA in PBS at room temperature for 30 min. Primary antibodies PAX7, CNN1, PDGFRA, MYOG, and MYHC were incubated on cells at 4 °C overnight. Secondary antibodies: Alexa Fluor 594 goat anti-mouse IgG (H + L) (Invitrogen, Cat# A11005) or Alexa Fluor 488 goat anti-rabbit IgG (H + L) (Invitrogen, Carlsbad, CA, USA, Cat# A11034) (1:500) were incubated for 1 h. Cells were counterstained with VECTASHIELD mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA, Cat# H-1200), which were photographed under a fluorescence microscope (Leica, TCS SP8 X, Watzlar, Germany). The fusion index was calculated as the percentage of nuclei within the myotube with at least three nuclei to the total nuclei.
Statistical analysis
-
The data is presented as the mean ± SD and analysed using GraphPad Prism V9. For comparisons of two groups, a two-tailed Students' t-test was used. For comparisons of three or more than three groups, a one-way ANOVA with Fisher's protected least significant difference (PLSD) was used. Statistical significance was defined as p < 0.05, p < 0.01, p < 0.001, or p < 0.0001. Experiments were repeated in triplicate for each experimental condition (n ≥ 3).
-
According to Ding's method[37], mononuclear cells were obtained from the muscle tissue of 7-day-old piglets through enzymatic digestion. Fluorescent antibodies CD31, CD45, CD56, and CD29 were used to stain the cells, followed by FACS, demarcating the CD31−/CD45−/CD29+/CD56+ cell population as MuSCs (Fig. 1a). PAX7, a specific marker for myogenic stem cells, was used to indicate MuSCs (Fig. 1b). The PAX7 positive rate in the unsorted cells was approximately 40%, while the CD29+/CD56+ cell population was about 85% (Fig. 1b). Subsequently, the porcine MuSCs were passaged to P5, and the PAX7 gene expression in P5 cells decreased to 16.7% of P1 (Fig. 1c). Upon myogenic differentiation, the myotube fusion rate of P5 MuSCs significantly reduced to about 33% of P1 (Fig. 1e, f). These results indicate that the porcine MuSCs obtained via Ding's method fail to maintain PAX7 expression and myogenic differentiation ability during passaging.
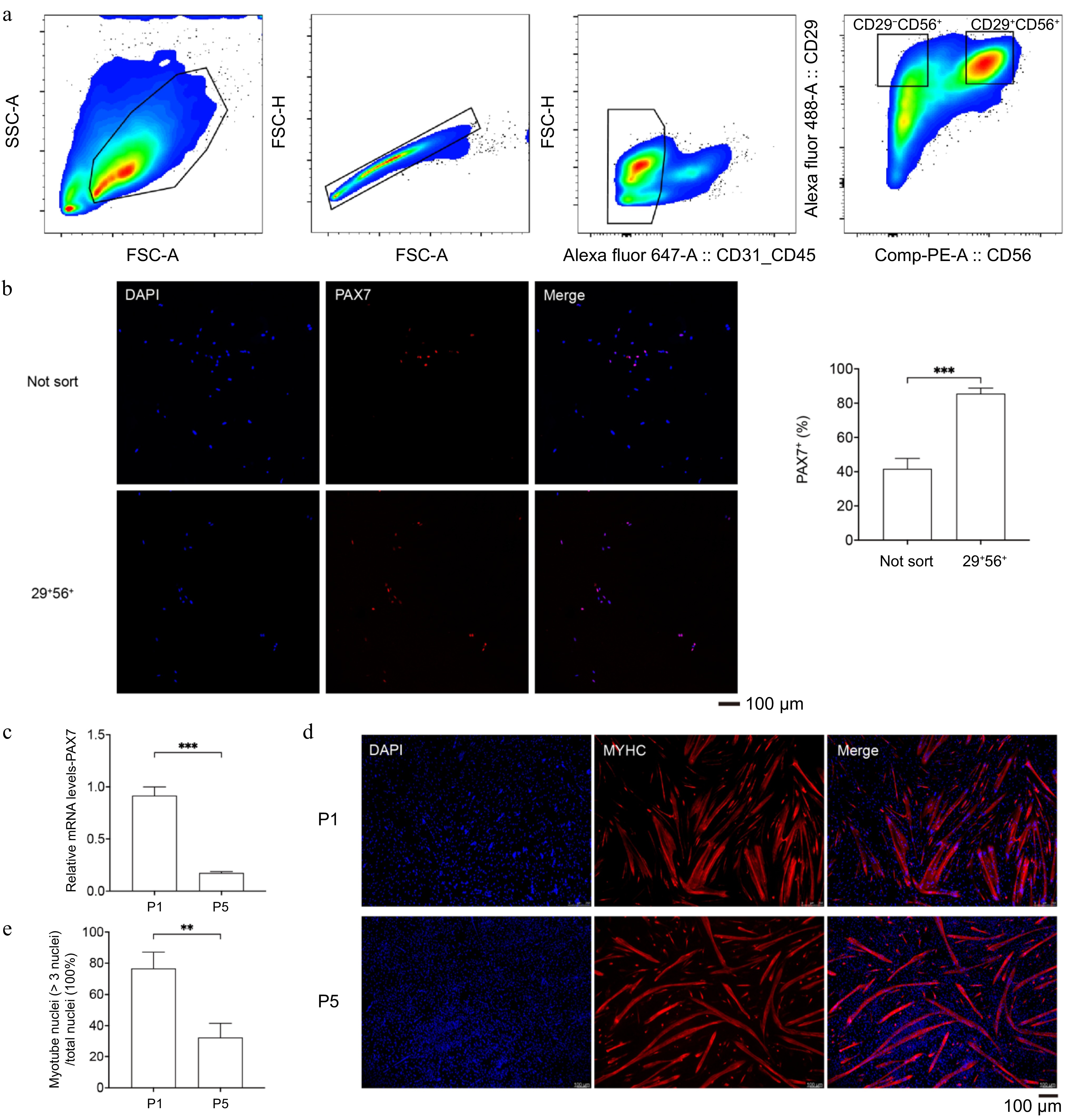
Figure 1.
The MuSCs sorting and the decrease in myogenic characteristics during passaging. (a) Representative contour plots of flow cytometry, showing gating strategies for cell type purification. (b) Immunofluorescence staining of PAX7 (red) in unsorted and newly sorted cells (left); DAPI-stained cell nuclei (blue); Scale bar = 100 μm; Positive cell percentage (right). (c) qPCR analysis of PAX7 expression in P1 and P5 porcine MuSCs. (d) Immunofluorescence staining of MYHC (red) after induced differentiation of P1 and P5 MuSCs; DAPI-stained cell nuclei (blue); Scale bar = 100 μm. (e) Myotube fusion rate. p-value: ** < 0.01, *** < 0.001.
Novel strategy for sorting three types of cells with higher purity from porcine muscle tissue
-
We stained the cells using a combination of fluorescent antibodies containing CD31/CD45/JAM/ITGA5/ITGA7[36,37]. Initially, we gated the CD31−/CD45−/JAM1− population to exclude endothelial cells, blood cells, and other contaminating cells (Fig. 2a). Subsequently, the cell population was further divided into three subgroups: ITGA5+/ITGA7− (5+7−), ITGA5+/ITGA7+ (5+7+), and ITGA5−/ITGA7+ (5−7+) (Fig. 2a). The proportions of each cell group were 13%, 2.5%, and 41.5%, respectively (Fig. 2a). Upon culturing, the three groups exhibited significantly different morphologies: the 5+7− group had a spindle shape, the 5−7+ group was more rounded and spherical, and the 5+7+ group was flatter and larger (Fig. 2b). Compared to the two groups identified in the previous sorting strategy—MuSCs identified as CD29+/CD56+ and FAPs identified as CD29+/CD56−—the advanced sorting strategy yielded more uniform morphological characteristics (Fig. 2b).
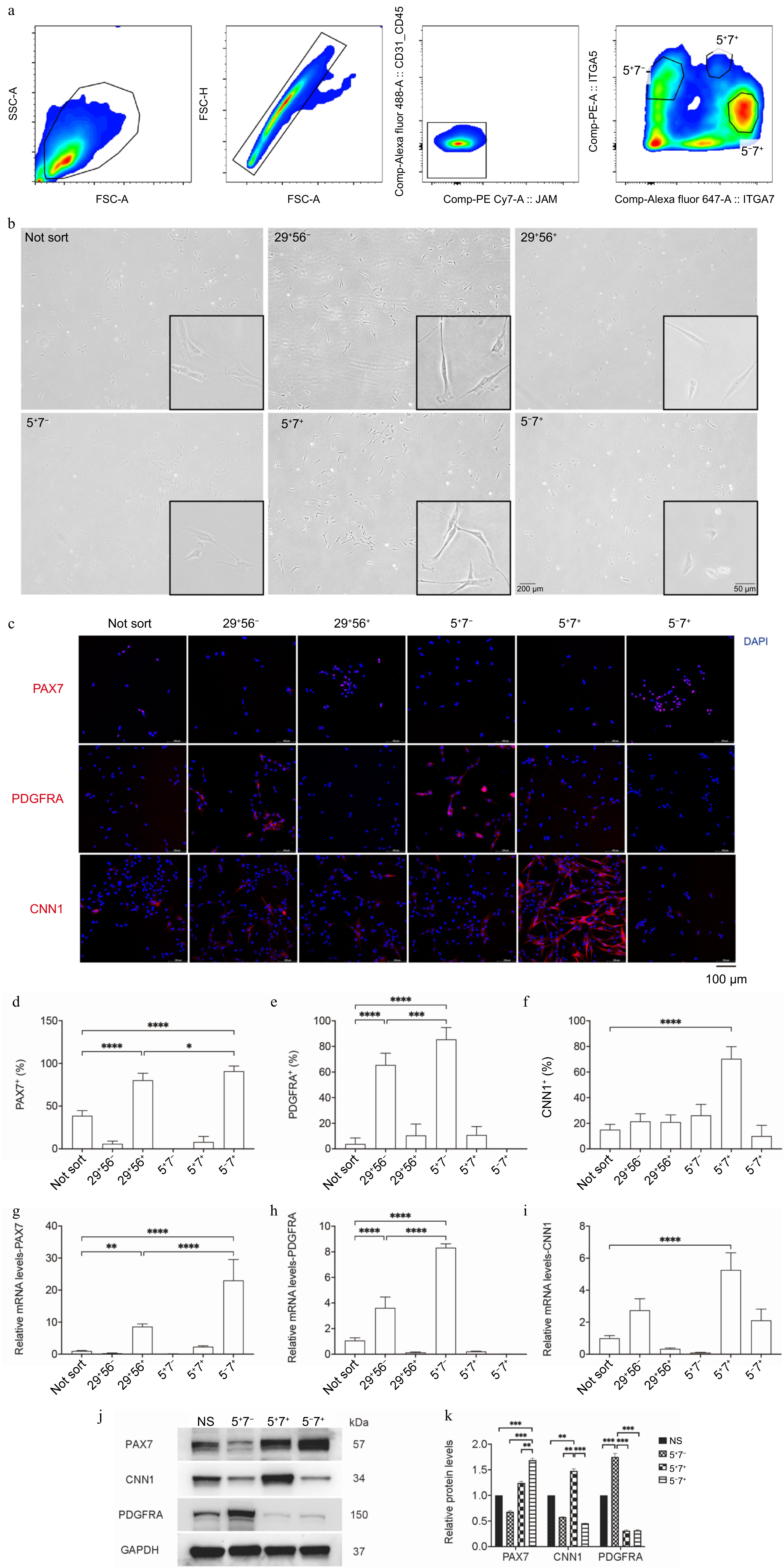
Figure 2.
Probing porcine muscle tissue cells using flow cytometry and assessing specific markers. (a) Representative contour plots from flow cytometry, showcasing gating strategies for cell type purification. (b) Photomicrographs of the purified cell types under brightfield conditions. (c) Immunofluorescence staining for PDGFRα, Pax7, and CNN1 (in red) in the purified cell types, with DAPI-stained cell nuclei (in blue); scale bar = 100 μm. (d)−(f) The percentage of positive cells among the purified cell types. (g)−(i) qPCR analysis of PAX7, CNN1, and PDGFRA expression. (j), (k) Western blot analysis of PAX7, CNN1, and PDGFRA. The relative protein levels of PAX7, CNN1, and PDGFRA were normalized to GAPDH using ImageJ software. Error bars represent the standard deviation (SD). p-value: * < 0.05, ** < 0.01, *** < 0.001, **** < 0.0001.
Next, we analyzed the expression levels of specific markers in the three cell groups: PAX7, a specific marker for MuSCs, and CNN1, which is highly expressed in SMCs[38], and PDGFRA, a marker for FAPs[39]. The immunofluorescence staining indicated that the PAX7 positive rate in the 5−7+ cell group reached over 90%, 10% higher than the CD29+/CD56+ cell group (Fig. 2c, d). The PDGFRA positive rate in the 5+7− group reached 90%, which increased by about 15% in comparison with the CD29+/CD56− cell group (Fig. 2c, e). The CNN1 positive rate in the 5+7+ group reached 75%, significantly higher than other groups (Fig. 2c, f). Subsequently, the gene expression levels of specific markers in distinct groups were measured. The results showed that PAX7 was highly expressed in the 5−7+ group, approximately 2-fold than that of the CD29+/CD56+ group (Fig. 2g), and PDGFRA was highly expressed in the 5+7− group (Fig. 2h), and CNN1 exhibited the highest expression in the 5+7+ group (Fig. 2i). Further, Western blot was committed and the results were consistent with the above trends achieved in immunofluorescence and qPCR analysis (Fig. 2j, k). Taken together, these results indicate that the three cell groups obtained by the novel sorting strategy correspond well to FAPs, SMCs, and MuSCs with significantly improved cell purity compared to the previous approach.
Transcriptome sequencing identifies the identity of three cell groups
-
We performed transcriptome sequencing on the three groups of cells sorted using the new strategy to verify their cell types further. Principal component analysis (PCA) separated the cells into three distinct groups (Fig. 3a). By analyzing the specific gene sets of three cell populations[24,25], we found that the 5−7+ group highly expressed markers characteristic of MuSCs, the 5+7+ group highly expressed markers characteristic of SMCs, and the 5+7− group highly expressed markers characteristic of FAPs (Fig. 3b). In addition, ITGA5 was found to be highly expressed in FAPs and SMCs, with significantly lower expression in SCs. At the same time, ITGA7 showed the highest expression in MuSCs, significantly decreased in SMCs, and was almost undetectable in FAPs (Fig. 3c). Gene set enrichment analysis (GSEA) indicated that the 5−7+ group was significantly enriched in myogenesis-related gene sets associated with skeletal muscle development (Fig. 3d). The Venn diagram shows that the number of intersection genes between the differential genes of group 5−7+ compared to group 5+7− and the differential genes of group 5−7+ compared to group 5+7+ is 5,262. These genes were mainly enriched in biological processes related to skeletal muscle development and skeletal muscle cell differentiation (Fig. 3f). Through transcription factor enrichment analysis, related genes were mainly enriched in the Paired box domain (including PAX3, PAX7, etc.) and the basic helix-loop-helix (bHLH) family (including MYF5, MYOD, etc.) terms (Fig. 3g). The transcriptome results further confirmed that the novel strategy effectively categorizes porcine muscle tissue cells into three distinct groups: SCs, SMCs, and FAPs, corresponding to the 5−7+, 5+7+, and 5+7− groups, respectively.
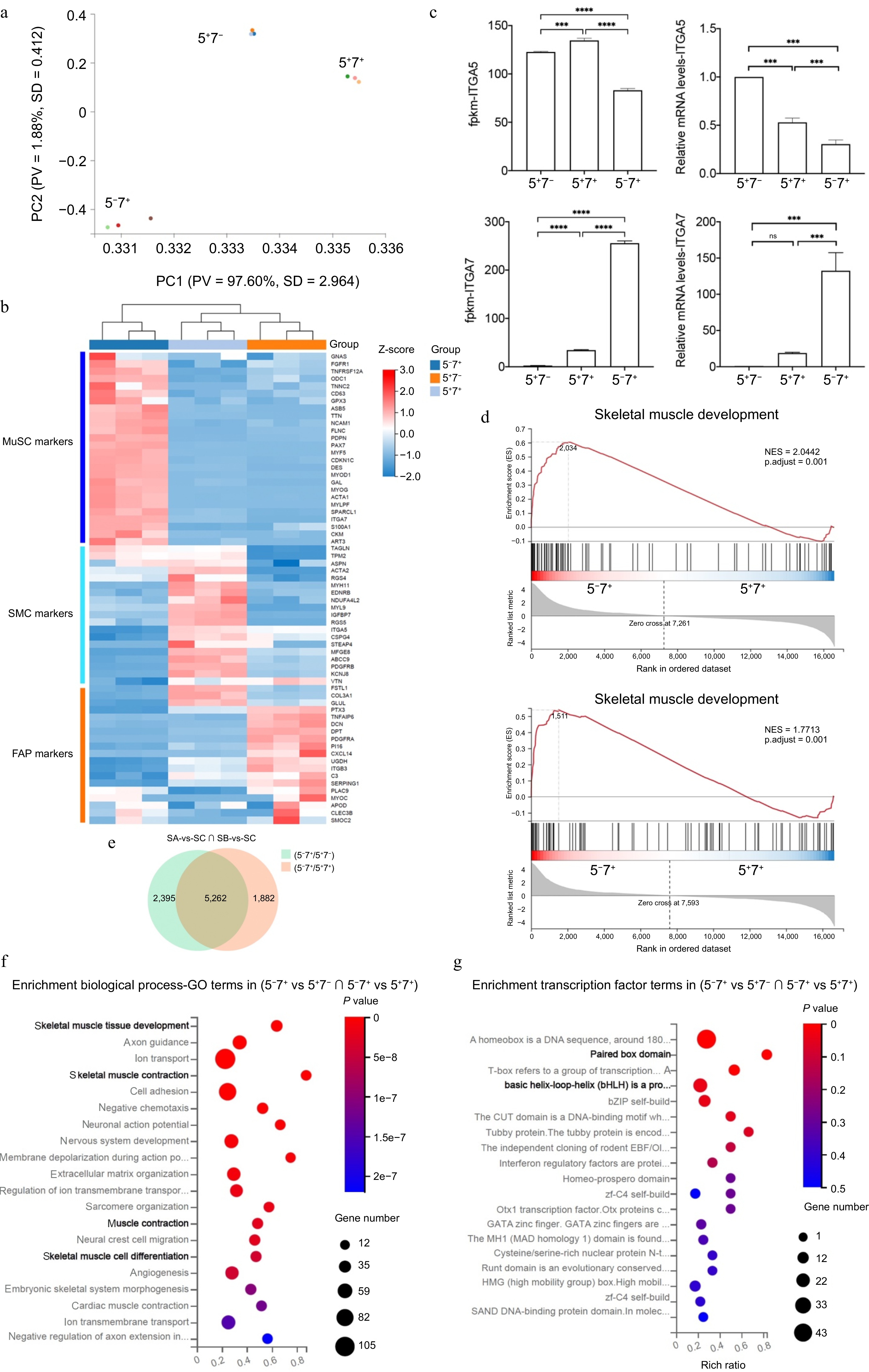
Figure 3.
Transcriptome analysis of three types of cells. (a) PCA of RNA-seq using all gene read counts. N = 3 cell lines per group. (b) Heatmap of different cell type markers across the three populations. (c) Expression levels of ITGA5 and ITGA7 in the three cell types. (d) Gene Set Enrichment Analysis (GSEA) for the cell populations. (e) Venn diagram depicting the intersection of comparisons between Group A and Group B, Group A, and Group C. Fold change > 1, p-value < 0.05. (f) GO analysis of biological processes is based on (e). (g) Enrichment analysis of transcription factors based on (e). Error bars represent the standard deviation (SD). p-value: *** < 0.001, **** < 0.0001. Ns, no statistical significance (p > 0.05).
The novel strategy yields MuSCs with enhanced myogenic characteristics
-
The foundation of cultured meat production lies in the ability of MuSCs to differentiate into myofibers when induced under appropriate conditions. Distinct cell populations were induced to differentiate under low-serum conditions. As evidenced by MYHC immunofluorescence staining[40], the fusion rate of the 5−7+ cells was approximately 90%, significantly higher than the 35% of unsorted cells (NS) and the 61% of the 29+/56+ group (Fig. 4a, b). Consistently, the MYHC gene and protein expression level in the 5−7+ group was about 1.5 times that of the 29+/56+ group (Fig. 4c−e). These data suggest that the novel strategy obtains MuSCs with significantly improved myogenic potential.
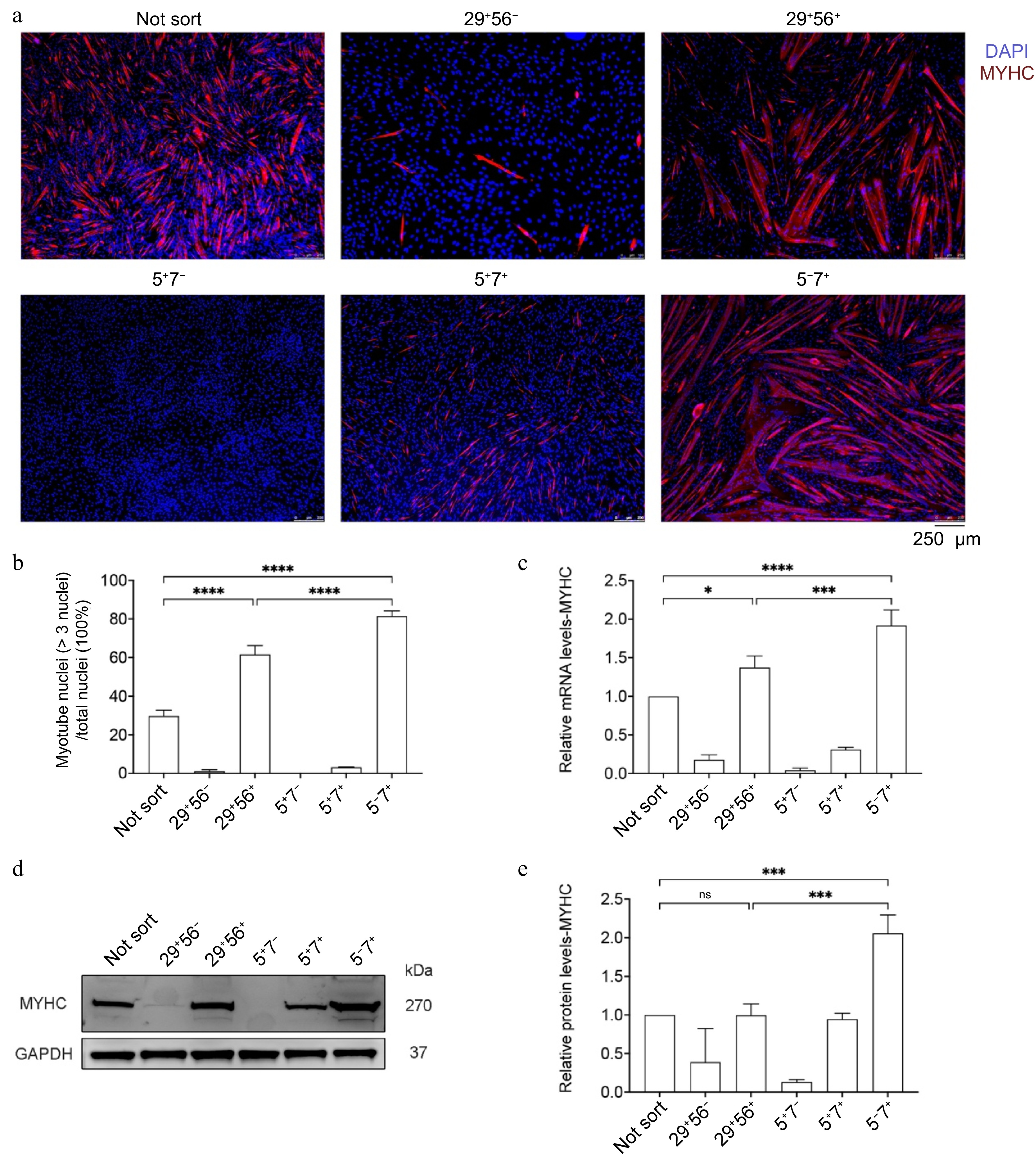
Figure 4.
Differentiation capacity of various cell types. (a) Bright-field images and MYHC immunofluorescence staining of the newly sorted cell populations induced to differentiate for 72 h. Scale bar, 250 μm. (b) Myotube fusion rate. (c) qPCR for MYHC indicating myogenic genes, N = 4. (d) Western blot detection of MYHC protein expression. (e) with quantitative results, N = 3. Error bars indicate the standard deviation (SD). p-value: * < 0.05, *** < 0.001, **** < 0.0001. Ns, no statistical significance (p > 0.05).
To further investigate the changes in MuSCs during continuous passaging, the stemness of MuSCs from passages P2 to P5 was measured. The PAX7 gene expression level in 5−7+ P2 cells was about ten times that of the CD29+/CD56+ group (Fig. 5a). Though the expression of PAX7 gradually decreased in both types of cells during culture, the CD29+/CD56+ group hardly expressed PAX7 by passage P4, whereas the 5−7+ group maintained a high level of PAX7 expression even by P5 (Fig. 5a). Meanwhile, the FAP marker PDGFRA expression level in the CD29+/CD56+ P2 cells was about three times that of the 5−7+ group and further upregulated during passaging (Fig. 5b). In contrast, PDGFRA expression in the 5−7+ group remained low during expansion. In addition, there was no significant difference in CNN1 expression level between the two at P2, but at P5, the CD29+/CD56+ group's CNN1 expression was significantly elevated than that of the 5−7+ group (Fig. 5c). Furthermore, the myogenic differentiation of MuSCs from P3 to P5 was induced. Figure 5d and e show that the fusion rate of the 5−7+ group cells from P3 and P5 was significantly enhanced than that of the CD29+/CD56+ cells, approximately 2.5 times. These results indicate that the MuSCs obtained by the new strategy have an increased purity during proliferation and exhibit improved myogenic capacity during differentiation.
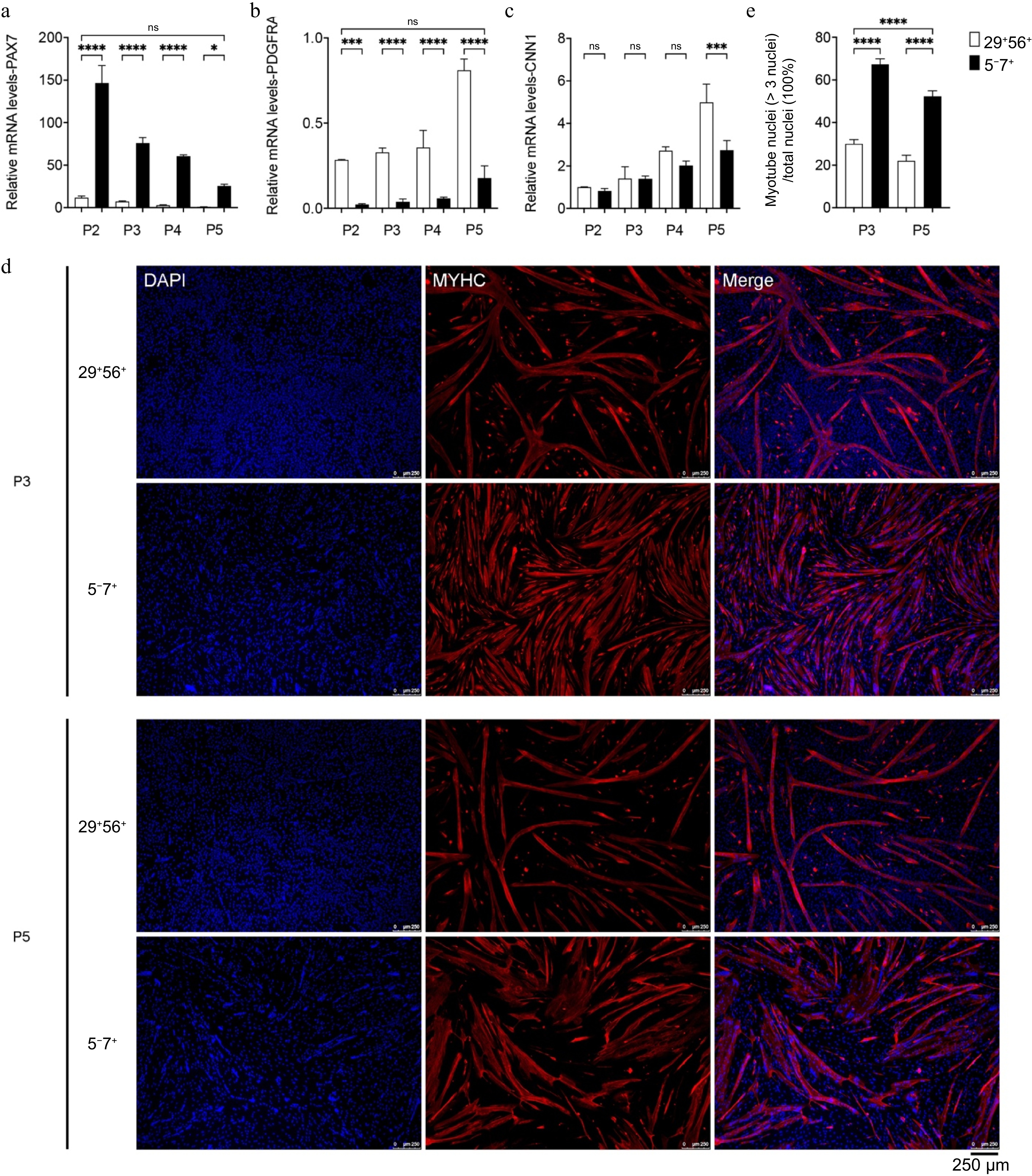
Figure 5.
The impact of passaging on cell purity and myogenic differentiation capacity. (a)−(c) qPCR analysis of the expression of PAX7, CNN1, and PDGFRA. (d) Immunofluorescence staining of MYHC (red) in MuSCs of P3 and P5 after induction of differentiation; DAPI-stained cell nuclei (blue); scale bar = 250 μm. (e) Myotube fusion rate (right). p-value: * < 0.05, *** < 0.001, **** < 0.0001. Ns, no statistical significance (p > 0.05).
-
Muscle tissue is the most commonly consumed livestock tissue by humans, providing essential nutrients such as protein, vitamins, and minerals[41] and serves as a vital source of energy and necessary nutrients. However, the environmental pressures brought about by traditional animal husbandry have increasingly raised concerns. Exploring new meat production approaches to alleviate these pressures significantly impacts meat consumption. Cultured meat has emerged as a novel meat production method in recent years, drawing attention to its clean production process and the possibility of precise control. It encompasses the culture of various products, including muscle[42,43], adipose[3,44], and connective tissues.
Stem cell types used to produce cultured meat include MuSCs, mesenchymal stem cells, and pluripotent stem cells[45−47]. As unipotent stem cells, MuSCs have the advantage of being easily induced to differentiate into myofibers, whereas other stem cells require complex regulation to commit myogenesis. Thus, high-quality MuSCs are crucial for researching and producing cultured meat. MuSCs originate from muscle tissue, which is highly complex due to the interaction of various cell types, such as FAPs, SMCs, and immune cells[48,49]. Ding et al.[37] successfully isolated highly purified porcine MuSCs using CD31, CD45, CD56, and CD29 surface markers. However, MuSCs obtained using this strategy were shown to decrease in purity and stemness during passaging, the results consistent with us and FAPs being the main contaminating cells, which could negatively impact the expansion of the ideal cell type. Messmer et al. used a single-cell sequencing approach over time to characterize cellular heterogeneity within muscle-derived cell cultures to reveal the specific cell population in muscle tissue. They found that ITGA5, as a negative selection marker for MuSCs, is highly expressed in FAPs, while CD29, as an SC marker in the previous strategy[50], is highly expressed in both MuSCs and FAPs. As well, ITGA7 is significantly expressed in MuSCs and nearly expressed in FAPs. Moreover, ITGA7 showed a higher expression difference in MuSCs compared to FAPs than CD56. The expression patterns of ITGA5 and ITGA7 in the two cell populations may correlate with their functional roles. The proliferation and differentiation of FAPs require the engagement of ECM signals recognized by ITGA5[51]. ITGA7 is crucial to participate in cellular plasticity, maintaining muscle development integrity, and preserving cytoarchitecture in skeletal myoblasts, cardiac, and SMCs[52,53]. Then, they established a new strategy for the FACS of bovine MuSCs. Based on this, we tested this new strategy for sorting mononuclear cells from pig muscle and obtained three cell populations, and confirmed their identities as MuSCs, FAPs, and SMCs through transcriptome analysis and immunofluorescence staining, consistent with the results of Messmer et al.
MuSCs (MuSCs) terminally differentiate into multinucleated myofibers, marked by MYHC protein expression[5], which is pivotal for the nutritional quality of cultured meat[54]. The 5−7+ group, sorted using our novel strategy, shows superior myogenic differentiation compared to the CD29+CD56+ group, highlighting its suitability as seed cells for cultured meat production. Additionally, fibro-adipogenic progenitors (FAPs) and smooth muscle cells (SMCs) contribute to cultured meat by generating adipose tissue and secreting collagen, respectively, thus enhancing flavor, nutritional value, and texture[21,35]. Future culturing methods could involve separate or co-cultured approaches to replicate traditional meat characteristics and improve production efficiency[55,56]. We successfully isolated MuSCs and distinct PDGFRA+ and CNN1+ cell populations, identified as FAPs and SMCs by transcriptome analysis, for potential use in cultured meat. However, their performance needs further evaluation. Flow cytometry revealed incomplete separation of muscle tissue cells into three distinct groups, with only MuSCs forming a core cluster, suggesting limitations in sorting efficiency. The availability of porcine-specific ITGA5 and ITGA7 antibodies may enhance sorting in the future.
In conclusion, we have tested the efficacy of CD31, CD45, JAM1, ITGA5, and ITGA7 as a cell surface antigen combination for the FACS of cells derived from porcine muscle tissue. Our results confirm that this method can effectively separate high-purity MuSCs, FAPs, and SMCs from porcine muscle tissue-derived cells. This approach establishes a foundation for supplying premium seed cells for researching and producing cultured meat products.
The funding for this study was provided by the Nanjing Major Science and Technology Special (Comprehensive Category) Project (Grant No. 202309014), the Jiangsu Agricultural Science and Technology Innovation Fund [Grant No. CX(24)1019], the National Natural Science Foundation of China for Young Scientists (Grant No. 32101991), the Fundamental Research Funds for the Central Universities (Grant Nos KYT2023003; KYT2023004; KYT2024003), Jiangsu Synergetic Innovation Center of Meat Processing and Quality Control, and the State Key Laboratory of Meat Quality Control and Cultured Meat Development, the National Natural Science Foundation of China (Grant No. 32272468), and the Postgraduate Research & PracticeInnovation Program of Jiangsu Province (Grant No. KYCX23_0769).
-
The porcine and pig muscle tissues used in this study were derived from a week-old pig approved by the Animal Ethics Committee, Nanjing Agricultural University, China (Approval Code: IACUC2020172; Approval Date: 2020).
-
The authors confirm contribution to the paper as follows: study conception and design: Ding S, Hu Z; data collection: Hu Z, Liu Z; analysis and interpretation of results: Hu Z, Liu Z; draft manuscript preparation: Guo R, Ding S, Liu Z. Supervision, project administration, funding acquisition, resources: Zhou G, Guo R, Ding S. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated or analyzed during the current study are available from the corresponding author on request.
-
The authors declare that there are no conflicts of interest regarding the publication of this manuscript. However, for the sake of transparency, we acknowledge that S.J. Ding, who is a corresponding author of this paper, is a co-founder of Joes Future Food Technology Co. Ltd. This relationship does not affect the integrity of the research presented herein, as the study was conducted independently and without influence from Joes Future Food Technology Co. Ltd. All data and conclusions presented in this study are based solely on objective analysis and were not influenced by any commercial interests.
-
# Authors contributed equally: Zenan Hu, Zheng Liu
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Hu Z, Liu Z, Guo R, Ding S, Zhou G. 2025. Isolation and purification of different high-purity cell populations from pig muscle tissue. Food Materials Research 5: e002 doi: 10.48130/fmr-0025-0001
Isolation and purification of different high-purity cell populations from pig muscle tissue
- Received: 14 October 2024
- Revised: 20 December 2024
- Accepted: 30 December 2024
- Published online: 28 February 2025
Abstract: Cultured meat technology is an emerging approach to meat production that generates edible meat tissue by cultivating animal-derived stem cells. Muscle stem cells (MuSCs) are essential seed cells for cultured meat production. However, due to the complexity of muscle tissue, obtaining highly pure MuSCs and maintaining their purity during passaging remains a significant challenge. Our research addressed the issue by reevaluating the cell sorting strategy for porcine MuSCs and other cell types. A new combination of markers—CD31, CD45, JAM1, ITGA5, and ITGA7—were introduced here, sorting the muscle mononuclear cells into three distinct groups. Immunofluorescence staining and RNA-sequencing indicated three distinct cell types—MuSCs, smooth muscle cells (SMCs), and fibro-adipogenic progenitors (FAPs)—each displayed high expression levels of their characteristic markers. Additionally, after successive passaging, MuSCs obtained through this refined approach exhibited higher cell purity and improved myogenic properties compared to previous methods. Overall, this study presents a method for simultaneously obtaining MuSCs, SMCs, and FAPs with high purity from porcine muscle tissue, providing a high-quality source of seed cells for cultured meat production.












