-
Microorganisms are developing multidrug resistance (MDR) for many antibiotic and medicinally important compounds due to improper usage. This is causing a major threat to society, the economy, and public health[1]. Therefore, alternate sources of drugs are being searched which could be suitable for the microbe's MDR properties[2]. There have been documented efforts in research to identify new, powerful natural or synthetic antimicrobial agents to combat MDR[3,4]. Recently, it was shown that synthetic cationic and hydrophobic peptides with N-terminal labels, which are linked to the human cathelicidin LL-37 peptide, are effective against both Gram-negative and Gram-positive infections[5]. Plant-based secondary metabolites are gaining more attention among natural or synthetic antimicrobial agents. Plants contain biologically active metabolites, known as phytochemicals, that can be extracted from various plant parts, including the leaves, barks, seeds, flowers, and roots, and can be subsequently employed as sources of antimicrobial compounds[6,7]. The extraction of these compounds can be affected by both the solvent selected for extraction as well as by the extraction method followed. Similarly, the selection of mobile phase solvents for separating phytochemicals from the extract is also very important. Therefore, optimization of extraction method conditions and mobile phase combinations is required to separate and identify these complex plant-based bioactive secondary metabolites from crude extracts of medicinal plants[8].
Taxus wallichiana (Zucc.) is a significant evergreen tree of medicinal importance, it is being used to extract the medicine taxol from its bark, and because of that it has drawn a lot of attention. Generally, it can be grown in temperate regions of the Indian Himalayan region (IHR) between 1,800 and 3,300 m above sea level[9]. The Unani and Ayurvedic medical systems have both looked into the medicinal applications of this species. T. wallichiana crude extracts also include bioactive metabolites, frequently with potential antioxidant effects. T. wallichiana is renowned for its numerous ethno-medical applications[10]. Asthma and bronchial problems are managed with its needle paste[11]. Additionally, Himalayan tribal people use tea made from the needles, stem, and bark of Himalayan yew to treat colds, coughs, and hypertension. It has been found that lignan compounds from T. baccata heartwood exhibit both antibacterial and cytotoxic properties against a panel of oncology cell lines[12]. T. wallichiana plant part extracts have been researched mainly for their potential for different types of cancer treatment and with little focus on their antibacterial activities[10,13]. The antimicrobial potential of the Taxus wallichiana plant's needles, bark, and stem have been reported, and according to qualitative assessments, actinobacteria, fungi, and bacteria (both Gram-positive and Gram-negative) were all inhibited by the crude extracts of the plant. The initial findings of the quantitative estimations made to the minimum inhibitory concentration also provided support for it[14−16]. A detailed study is required for the identification of antimicrobial compounds present in T. wallichiana plant part extracts.
The goal of the current work is to separate and collect the fractions of T. wallichiana- plant parts extracts for screening and detection of antimicrobial compounds and then characterize the antimicrobial compounds for their identification to better understand their biochemical makeup.
-
Plant samples (needle, bark, and stem) were collected from the Jageshwar area, District Almora (29°35'−29°39' N and 79°59'−79°53' E) of Uttarakhand, India. A collection of plant samples were submitted to the herbarium records of G.B. Pant National Institute of Himalayan Environment, Kosi-Katarmal, Almora, Uttarakhand, India (Voucher number: GBPI 5050), and the plant was identified as an evergreen woody tree of Taxacease family, it was identified by Dr. K. Chandra Sekar working as Scientist 'F' at the Institute. The gathered plant samples were ground into a fine powder for the next experiments after being cleaned and allowed to air dry.
Chemicals and solvents
-
HPLC-grade ethanol, methanol, acetone, chloroform, ethyl acetate (E. acetate), hexane, and acetic acid were aquired from Merck, India. Additionally, tryptone yeast extract broth, potato dextrose broth, agar, formic acid, benzene, and toluene were obtained from Hi-media, India. Furthermore, arachidic acid, behenic acid, palmitic acid, stearic acid, myo-inositol, hexadecane, timolol, nicotinamide, procainamide, and cinchonine were purchased from Sigma Aldrich, India. Lastly, the silica gel (mesh 60−120) and TLC plates (Silica gel 60 F254) were procured from Merck Germany.
Microorganisms
-
Two Gram-positive bacteria, Bacillus subtilis (NRRL B30408) and B. megaterium (MCC3124). Two Gram-negative bacteria, Escherichia coli, and Serratia marcescens (MTCC4822). Two Actinobacteria, Nocardia tenirefensis (MCC2012), and Streptomyces sp. (MCC2003). Five fungi, i.e., Paecilomyces variotii (ITCC3710), Aspergillus niger (ITCC2546), Fusarium oxysporum (ITCC4219), F. solani (ITCC 5017), and Trametes hirsuta (MTCC11397) were used in this study. The microbiology lab of the Institute G.B. Pant National Institute of Himalayan Environment in Almora, Uttarakhand, constructed a microbial culture collection from which these test microorganisms were collected.
Extraction using the maceration method
-
Two grams of plant material (needle, bark, and stem individually) were combined in a 1:5 ratio (dry powder : solvents) with methanol, ethanol, and ethyl acetate. Using parafilm, the conical flask's mouth was sealed. Using a Remi rotary shaker set to 160 rpm for 48 h at room temperature, samples were macerated. Each solvent extract was vacuum-dried at 35−40 °C using a vacuum oven (Narang Scientific Works, New Delhi, India, Model-257) following extraction. The dried extract was then dissolved in 2 mL of the appropriate solvent, kept apart, and used later at 4 °C.
Qualitative and quantitative phytochemical analysis
-
Various qualitative tests were performed for phytochemical screening following the method described by Gul et al.[17]. All the plant parts extracts were evaluated for total phenolic content, total flavonoid content, and total tannin content, with slight modification (concerning extract volume) in methods as described by Kumaran & Karunakaran[18], Quettier et al.[19], and Nwinuka et al.[20], respectively. The results of total phenolic content are expressed in terms of mg gallic acid equivalent per g dry weight of extract (GAE mg/g dw), flavonoid concentration in terms of mg quercetin equivalent per g dry weight of extract (QE mg/g dw), and tannin content in terms of mg tannic acid equivalent per g dry weight of extract (mg/g dw). The standard equations derived from the calibration curves were utilized for quantification.
Preliminary screening of antimicrobial activity in different plant parts i.e., stem needles, and bark
-
Agar plate-based bioassays employing the disc diffusion method were carried out to estimate the antibacterial potential of T. wallichiana plant part extracts qualitatively. Fungal culture suspensions were made in Potato Dextrose (PD) agar, whilst bacterial and actinobacterial culture suspensions were made on Tryptone Yeast extract (TYE) agar. With the use of a glass spreader, 100 μL of each test organism (separately) was evenly distributed on the corresponding agar surface (TYE agar plates for bacteria and actinobacteria, and PD agar plates for fungus). With the use of sterile forceps, sterile 5 mm filter paper (Whatman No. 1) discs were put over the agar surface. The agar disc was covered with 15 μL of extract. After that, the plates were incubated at 25 °C. Based on the zone of inhibition (mm) measurements after 24 h for bacteria and 120 h for actinobacteria and fungus, the findings were recorded. Each experiment was carried out in triplicate.
Determination of antimicrobial compounds in the crude needle extracts
Sequential extraction for TLC bio-autography
-
Ten grams of powdered dried needles were macerated in a 1:5 (w/v) mixture of hexane, chloroform, ethyl acetate, methanol and water. Hexane, chloroform, ethyl acetate, methanol, and water were used in increasing order of solvent polarity during the maceration of the same sample. Each solvent extract was vacuum dried after extraction using a vacuum oven (Narang Scientific Works, New-Delhi, India, Model-257) at 35 to 40 °C. The dried extract was then individually dissolved in 2 mL of each solvent.
Separation and identification of antimicrobial compounds
-
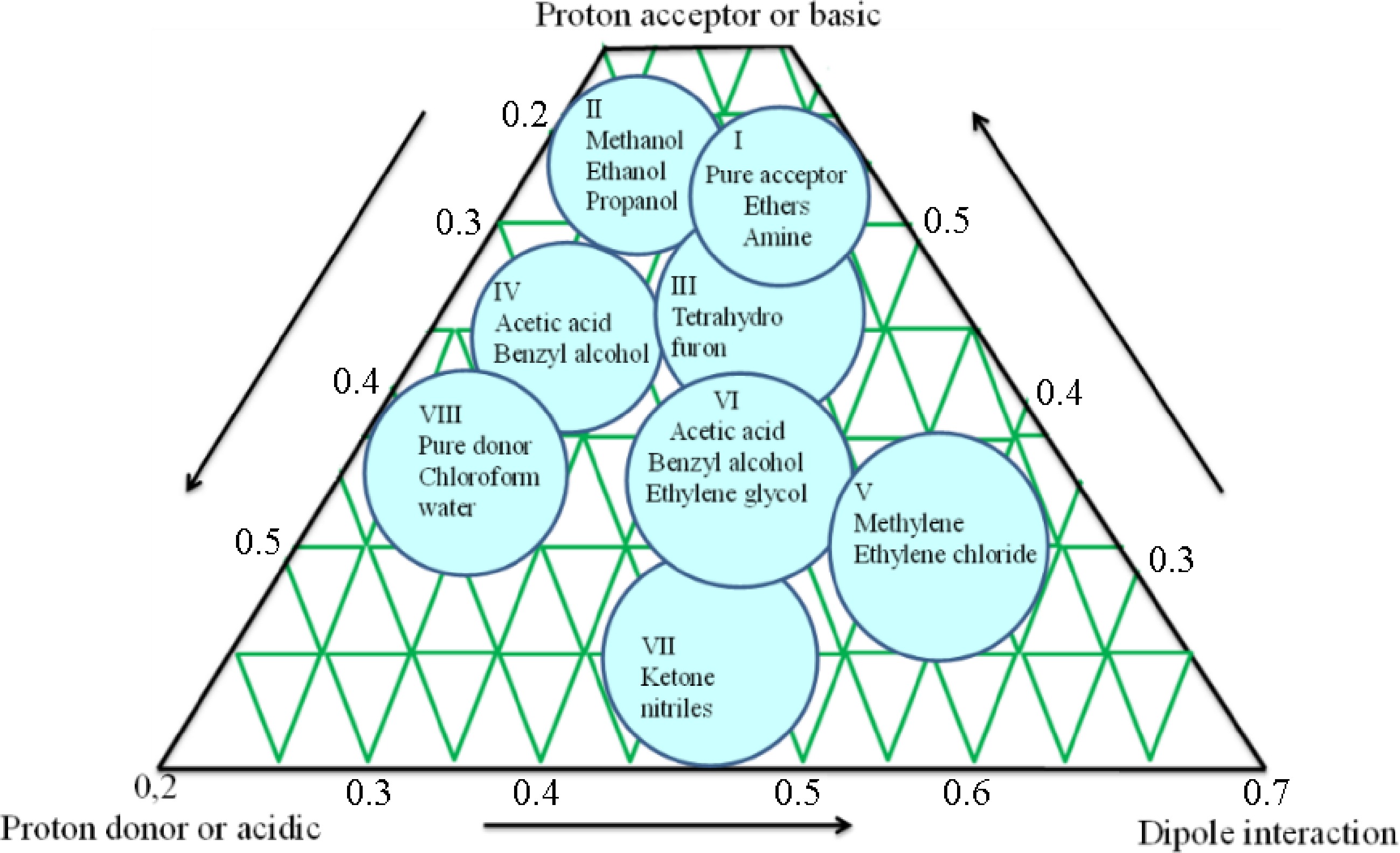
Column and thin layer chromatography (TLC) extracts with antimicrobial activity were further used to isolate and identify antimicrobial compounds. The selectivity triangle approach, created by Snyder, was used to optimize the mobile phase for the chromatographic apparatus[21]. Through column chromatography, extracts with antibacterial activity were purified using a glass column (32 cm) filled with silica gel (60−120 mesh). Hexane was used to charge the silica bed before a 1 mL sample was put into the column and 10 different mobile phase combinations listed in Table 1 were used to elute the sample. After collecting the eluted fractions, TLC-bioautography was used to further separate the fractions that have antibacterial activity.
Table 1. Solvent strength of mobile phase used for separation of compounds using column chromatography.
Solvent strength Mobile phase I (%) Hexane (100) − 0 Hexane (90) E. acetate (10) 0.44 Hexane (80) E. acetate (20) 0.88 Hexane (70) E. acetate (30) 1.32 Hexane (60) E. acetate (40) 1.76 Hexane (50) E. acetate (50) 2.2 Hexane (40) E. acetate (60) 2.64 Hexane (30) E. acetate (70) 3.08 Hexane (20) E. acetate (80) 3.52 Hexane (10) E. acetate (90) 3.96 Mobile phase II (%) E. acetate (100) − 4.4 E. acetate (90) Methanol (10) 4.47 E. acetate (80) Methanol (20) 4.54 E. acetate (70) Methanol (30) 4.61 E. acetate (60) Methanol (40) 4.68 E. acetate (50) Methanol (50) 4.75 E. acetate (40) Methanol (60) 4.82 E. acetate (30) Methanol (70) 4.89 E. acetate (20) Methanol (80) 4.96 E. acetate (10) Methanol (90) 5.03 Mobile phase III (%) Acetic acid (0.1) Methanol (99.9) 5.1 Based on the selectivity triangle, a TLC plate (silica gel 60 F254, Merck, Germany) was used to separate the fraction with antibacterial activity using various combinations of mobile phases (Table 2). The TLC plates were examined for antimicrobial activity, and any areas with potential for antimicrobial activity were scraped off and dissolved in ethyl acetate. Finally, utilizing the solvent systems listed in Table 3, column chromatography was performed once more to further purify fractions to isolate molecules responsible for antibacterial activity. Afterward, the above-mentioned procedure for testing the antimicrobial activity was used for testing the antimicrobial properties of the fractions and was evaluated using a qualitative test (the disc diffusion assay) and a quantitative assessment (the minimum inhibitory concentration, or MIC). The same mobile phase from which the fractions were taken was utilized as a control.
Table 2. Solvent strength of mobile phase used for separation of compounds using thin layer chromatography.
Code Mobile phase Ratio Solvent strength Separation Spot Rf value M1 Chloroform : E. acetate : Formic acid 5:4:1 4.4 Yes 2 0.6, 0.7 M2 E. acetate : Methanol : Benzene 2:0.5:2.5 3.6 Yes 3 0.3, 0.6, 0.68 M3 Ethanol : Chloroform 1:1 4.2 Yes 4 0.4, 0.56, 0.64, 0.83 M4 Toluene : E. acetate : Formic acid 6:4:0.5 3.2 Yes 5 0.25, 0.38, 0.43, 0.64 and 0.81 M5 Hexane : Acetone : Toluene : Ethanol 10:7:7:6 5.2 No No No M6 Chloroform : Acetonitrile 7:3 4.6 Yes 3 0.77, 0.8, 0.85 M7 Chloroform : Methanol 7:1 3.9 Yes 8 0.67, 0.15, 0.19, 0.32, 0.40, 0.45,0.62, 0.75 M8 E. acetate : 2-Propanol 95:5 4.3 No No No M9 Chloroform : E. acetate : Methanol 25:20:5 4.3 Yes 5 0.25,0.4, 0.54, 0.68, 0.76 M10 Chloroform : Formic acid : Acetonitrile 6:0.5:3.5 3.3 No No No Table 3. Solvent strength of mobile phase used for separation of compounds present in spot 2 through thin layer chromatography and column chromatography.
Code Mobile phase Ratio Solvent strength M11 Hexane : E. acetate 7:3 1.32 M12 Toluene : Methanol 8:2 2.94 M13 Methanol : Chloroform 1:1 4.6 M14 DMF : DCM : Acetonitrile 5:1:4 5.83 M15 Acetic acid : Methanol : Water 5:2.5:2.5 6.77 Determination of antimicrobial compounds in the selected rich fractions
Gas chromatography and mass spectrometry (GC/MS)
-
With the use of the Shimadzu GC/MS-QP2010 ultra, certain fractions were examined. The eluted components' electron impact (EI) mass spectrum was captured using a 10 μL injection volume. The column's (DB35-MS Capillary non-polar column with dimensions of 30 mm × 0.25 mm ID × 0.25 μm film) initial temperature was maintained at 50 °C for 2 min, after which it was raised to 250 °C at a rate of 7 °C/min for a hold time of 3 min, and then to 280 °C for 18 min at a rate of 10 °C/min. The carrier gas (Helium) flow rate was held constant at 1.21 mL/min. The mass range of 50−1,000 Da has been scanned. NIST 14 and Willey 8 library spectra tools were used to identify the chemical components.
Liquid chromatography and mass spectrometry (LC/MS)
-
Using LC/MS (Waters, MS Synaptic GZ HDMS LC-MS UPLC H-Class) with a C18 (1.7 μm) column, injection volume of 20 μL, and 0.1% formic acid: acetonitrile: methanol (20:30:50 v/v/v; isocratic mode) as mobile phase, the non-volatile bioactive chemicals included in the extract were identified. The column was kept at a constant temperature of 35 °C. The chromatogram and mass spectrum analysis were done and the results were compared to the reference mass spectrum found in the Metlin library and the online tool Massbank.
FTIR (Fourier Transform Infrared Spectroscopy)
-
The Agilent-Cary 630 FT-IR spectrometer was used to record the FTIR spectra. Using Microlab FTIR software, 20−40 μL of samples were used for FTIR analysis spanning the frequency range of 650−4,000 cm−1 at 8 cm−1 intervals. Based on the wave number, the spectrum was further examined for the presence of potential functional groups.
Antimicrobial effects of recognized compounds and their detection in needle extract
-
Following the identification of antimicrobial compounds, all compounds were procured from Sigma, India, and their antimicrobial activity was assessed through the utilization of the technique delineated in an earlier section. Subsequently, the fatty acids in the needle methanolic extracts are measured. Gas chromatography (Chemito GC, Ceres 800 plus) with a Flame Ionization Detector (FID) was used to evaluate the fatty acids. A BP20 column (30 m × 0.25 mm) in splitless mode was used for the analysis, and 10 μL of the extract was injected. The stationary phase film thickness was 0.25 mm. The temperatures of the injector and detector were adjusted to 250 and 290 °C, respectively. The flow rate of the carrier gas, helium, was kept constant at 0.75 mL/min. The oven was preheated at 60 °C, then raised to 260 °C at a pace of 3 °C per min, and left there for 10 min. By comparing the retention durations of the fatty acids to a variety of fatty acid standards, the fatty acids were found.
Using RP-HPLC (Shimadzu LC solution, Japan) and a PDA (Photodiode Array) detector, non-volatile chemicals were examined. Using a C18 reverse-phase column, the active metabolites in methanolic needle extracts were measured. Every sample had an injection volume of 20 μL, and the mobile phase was operated in gradient mode. The following compounds' wavelengths and mobile phase compositions were measured: procainamide (275 nm, methanol : water), nicotinamide (275 nm, 0.1 % TFA in water : acetonitrile : methanol), myoinositol (280 nm, methanol : 0.02 M H2SO4), and cinchonine (260 nm, acetonitrile : methanol : phosphoric acid), with a flow rate of 1 mL/min for all the compounds, except 1.2 mL/min for nicotinamide, and 0.6 mL/min for myoinositol.
Statistical analysis
-
Data were collected from all trials in triplicate and were expressed as a mean with standard deviation (SD). GraphPad, Prisma 8 software was used to create the heatmap.
-
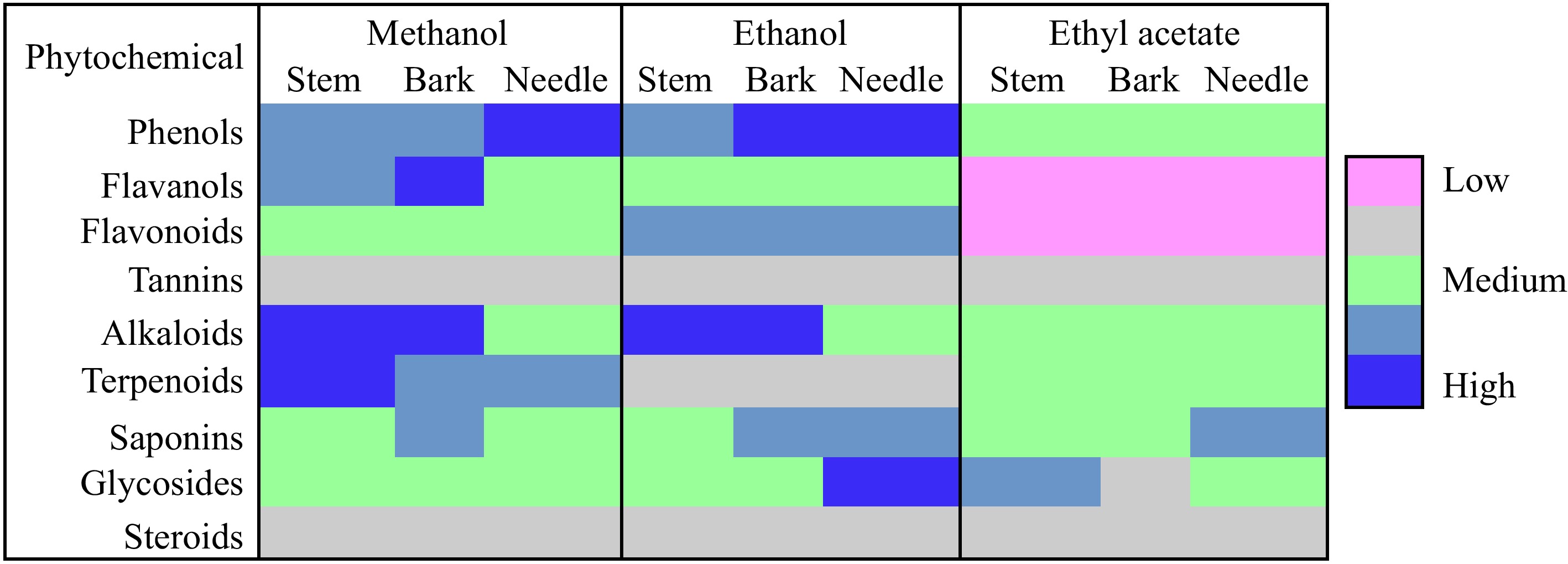
The extract yield was higher in the methanolic extract (15%) followed by ethanol (12%) and ethyl acetate (9%). The qualitative analysis of T. wallichiana needle, stem, and bark shows the presence of phytochemical substances such as phenol, flavanol, flavonoid, tannin, saponin, terpenoid, glycoside, and steroid (Fig. 1). Total phenolic, flavonoids and tannin content were higher in ethanolic extracts of needles (82.20 ± 0.51 mg GAE/g (dw), 79.72 ± 0.69 mg QRE/g (dw), 12.98 ± 0.34 mg TAE/g (dw)) and methanol (81.37 ± 0.85 mg GAE/g (dw), 54.10 ± 0.58 mg QRE/g (dw), 10.52 ± 0.29 mg TAE/g (dw)) extracts, respectively. In stem, phenolic (52.57 ± 0.39 mg GAE/g (dw)) and tannin (9.09 ± 0.11 mg TAE/g (dw)) content was higher in methanolic extracts while flavonoids content was higher in ethanolic extracts (38.41±0.68 mg QRE/g (dw)). Likewise for bark, phenolic (68.52 ± 0.72 mg GAE/g (dw)) content, flavonoids (61.57 ± 1.09 mg QRE/g (dw)), and tannin (13.05 ± 0.18 mg TAE/g (dw)) content were higher in ethyl acetate extract. Results are shown in Fig. 2.
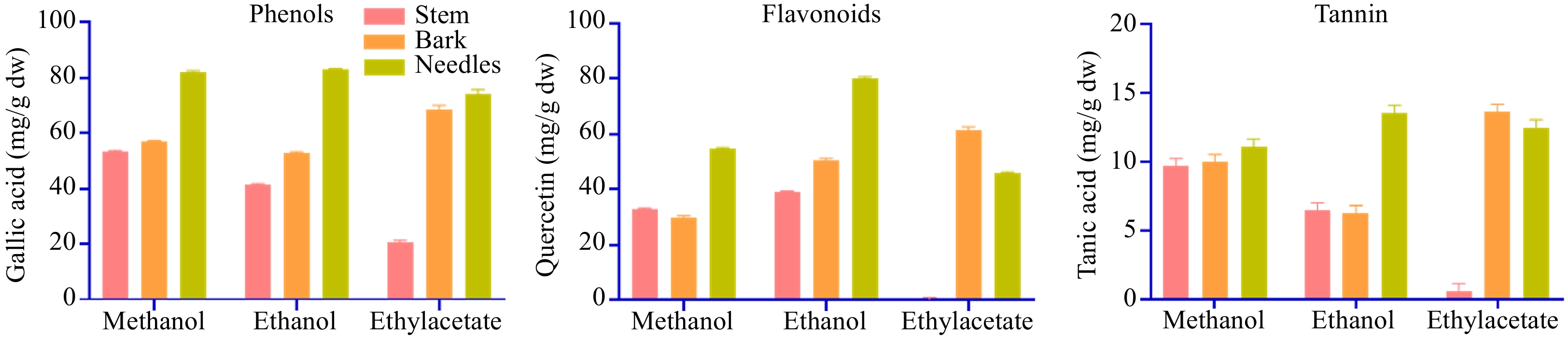
Figure 2.
Total phenolic content, total flavonoids content, and total tannin content of stem, bark, and needle extracts of T. wallichiana.
Antimicrobial potential of needle, bark, and stem
-
Using extracts from T. wallichiana needle, bark, and stem a preliminary test was conducted to determine which plant part would be most effective for the isolation and identification of antimicrobial metabolites. It's interesting to note that all plant part extracts exhibited antimicrobial action against the three types of microorganisms i.e., fungi, actinobacteria, and bacteria. T. wallichiana extracts; were found more effective against Bacillus species in terms of inhibition, and were less effective against E. coli. Between two tested Bacillus species, ethanol needle extract demonstrated greater inhibition for B. subtilis, whereas needle methanol extract demonstrated greater inhibition for B. megaterium. Likewise, needle and bark methanolic extracts showed higher antimicrobial activity for S. marcescens, needle and bark ethanolic extracts for P. chlororaphis, and needle methanol and ethanol extract for E. coli. Methanolic extracts of needles and bark showed significant inhibition against actinobacteria also i.e., N. tenirefensis, and Streptomyces sp., Fig. 3 displays the zone of inhibition (mm) results. T. wallichiana parts extracts showed antifungal activity against five fungus species i.e., P. variotii, T. hirsuta, F. oxysporum, A. niger, F. solani., and P. variotii showed the highest zone of inhibition with needle ethanolic extracts, T. hirsuta with bark ethanolic extract, F. oxysporum, and A. niger with needle methanol, and F. solani with needle and bark ethyl acetate extracts. Overall, the extent of the zone of inhibition for ethanolic and methanolic plant component extracts demonstrated strong antibacterial activity (Fig. 3).
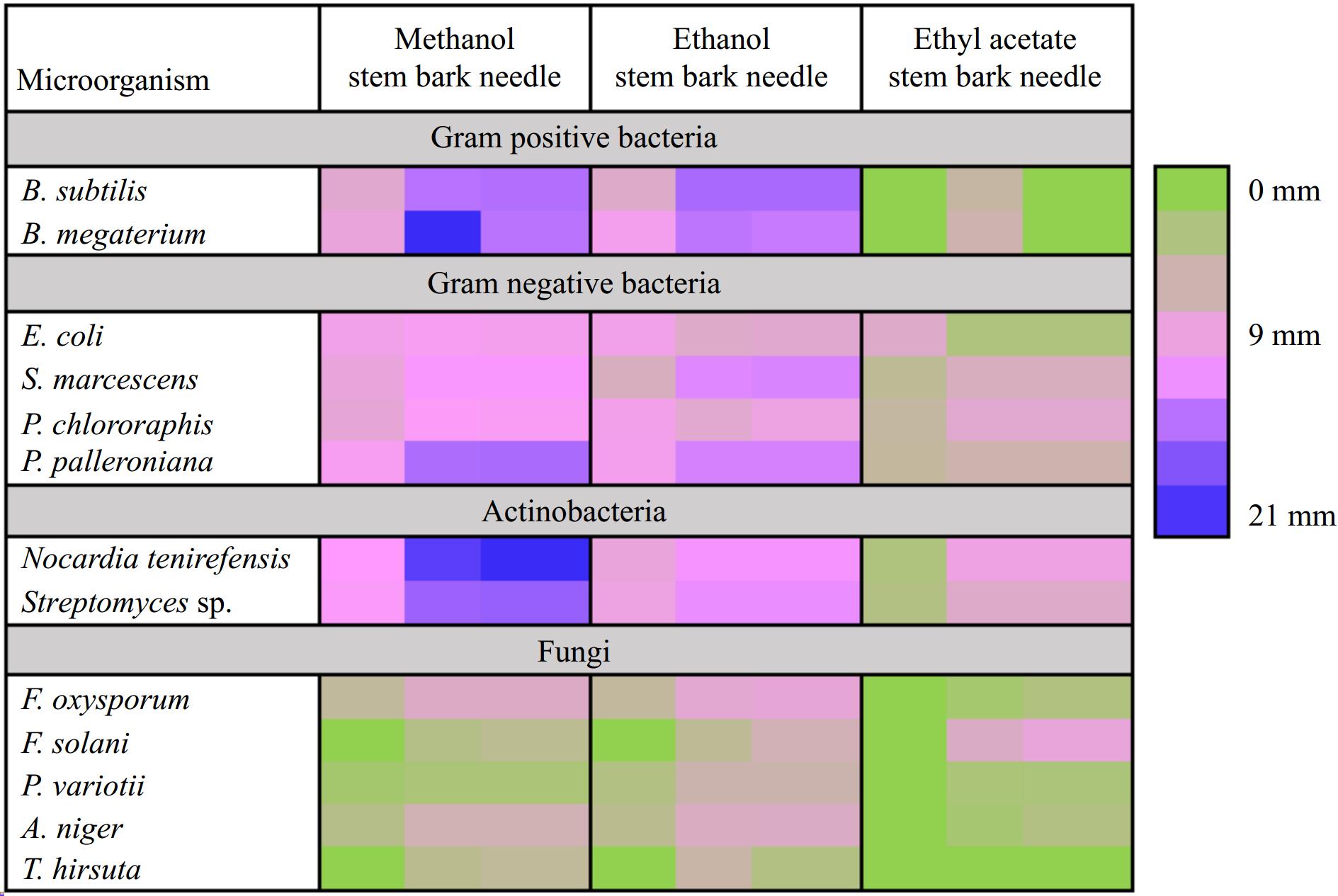
Figure 3.
Heatmap showing preliminary screening of antimicrobial potential (zone of inhibition (mm)) of needle, stem, and bark of T. wallichiana.
Screening for the best extracts for further purification studies
-
Several combinations of mobile phases were tried using the selectivity triangle approach for TLC, column chromatography, and bioautography to separate antimicrobial chemicals in needle fractions. Column chromatography and TLC have been used to identify and further purify a large number of bioactive fractions. The most often used techniques are thin-layer chromatography (TLC) and column chromatography because they are affordable, convenient, and available in a variety of stationary phases[22].
Based on the results of the preliminary test of phytochemical and antimicrobial assay, needles were selected for further studies of antimicrobial compound isolation and characterization. There are several bioactive secondary metabolites from various classes included in the plant's crude organic extracts. When extracting desired compounds, it is beneficial to use multiple solvents sequentially, starting from those with low polarity and gradually increasing to high polarity. This approach helps prevent overlapping similar types of secondary metabolites in the desired extracts. Based on that in the current study, utilized hexane, chloroform, ethyl acetate, methanol, and water were utilized. It was found that only the extracts from methanol and ethyl acetate (ME & EAE) displayed antimicrobial activity, while the hexane, chloroform, and aqueous extracts exhibited no such activity.
Based on the results of antimicrobial activity ME & EAE were selected for further fractionation using TLC and column chromatography. It is essential to fully understand the nature of the existing secondary metabolites in the extract before developing the mobile phase for column and thin-layer chromatography. In this context, Gas Chromatography-Mass Spectrometry (GC-MS) was conducted for the extracts (ME & EAE) exhibiting antimicrobial activity. The extract's mass spectra revealed the presence of carboxylic sugars, fatty acids, phenols, sterols, and alkanes. Benzene, propanol, gamma-sitosterol, phenols, and benzoic acid were prevalent in both extracts. The findings mentioned have been previously documented in the essential oil of Nasturtium officinale[23] and in the leaf extract of Adiantum capillus[24].
Mobile phase designing for column chromatography
-
The secondary metabolites identified in the crude extract were then utilized to create the mobile phases for column chromatography, which was utilized to separate the secondary metabolites further, using a selectivity triangle. In the present study, optimization of the mobile phase was done through a selectivity triangle. It ultimately identifies and quantifies the blend of intermolecular interactions that occur between solutes and solvents/phases[25]. The basic principle of the selectivity triangle is shown in Fig. 4 and details on the first set of mobile phases are shown in Table 1.
Bioassay-guided fractionation and screening for antimicrobial activity
-
Ten-bed volumes of mobile phase (details are given in Table 1) were passed through the column containing methanol and ethyl acetate extracts. Collected fractions were subjected to testing for antimicrobial activity. The methanol extract fractions exhibited selective antibacterial activity, while the ethyl acetate extract fractions demonstrated robust antimicrobial and antifungal activity. Following the results, the ethyl acetate extract fractions were chosen for further bioautography. A schematic diagram explaining the results is shown in Fig. 5.
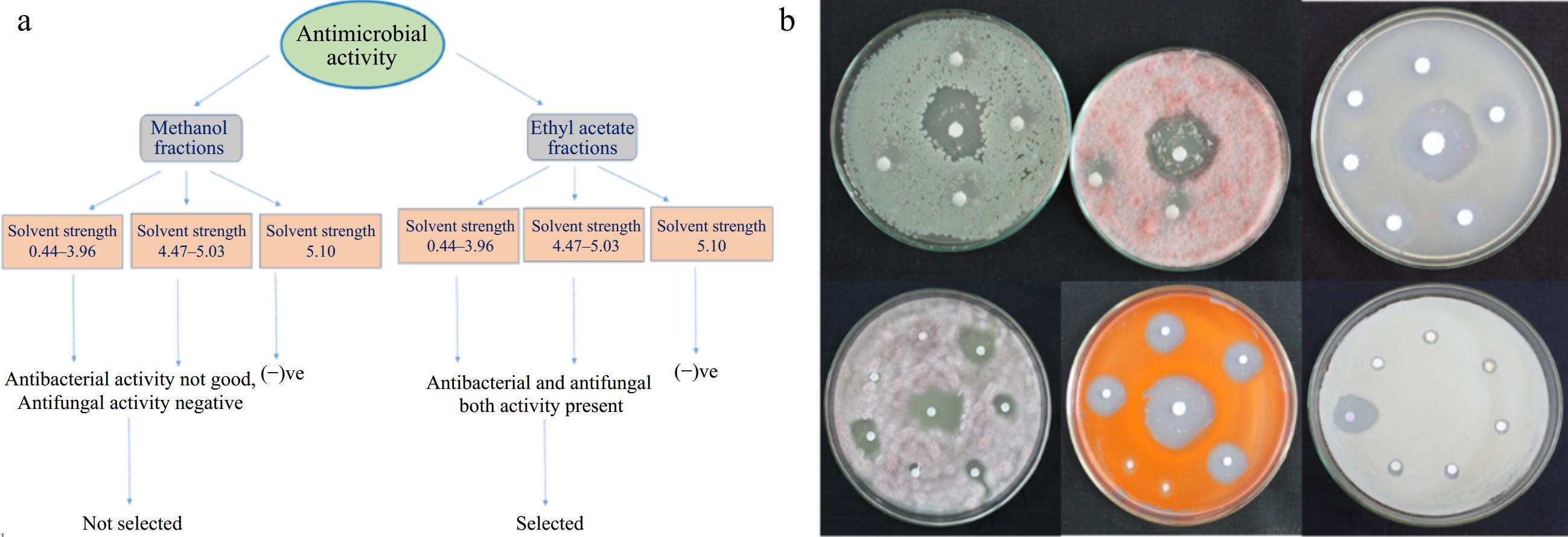
Figure 5.
(a) Schematic representation of different subfractions collected from fractionation of methanol and ethyl acetate fraction of T. wallichiana needle. (b) Antimicrobial activity of collected fractions of ethyl acetate extract through column chromatography.
TLC-bioautography
-
Isolated fractions with antimicrobial activity were further subjected to separation of antimicrobial compounds using a bioautographic technique[26−28]. With the help of a selectivity triangle, 10 different mobile phases were designed for the separation of compounds through TLC (Table 2). From the 10 mobile phases, three did not show any separation. The mobile phases, which had shown an RF value between 0.2−0.8 cm, were considered as good (Table 2).
Seven mobile phases M1, M2, M3, M4, M6, M7, and M9 (Table 2) were found suitable for the separation of compounds present in the fraction having antimicrobial activity. Based on Rf values, mobile phase M3 and M4 were used for further study. During bio autography out of four spots, three spots showed antimicrobial activity. Spot 1, 2, and 3 have antimicrobial activity. Spot 1 and 3 have selective antibacterial activity and spot 2 has antibacterial, antiactinobacterial, and antifungal activity. Therefore, spot 2 was selected for further separations and detection using column chromatography and thin-layer chromatography. However, spot 2 did not separate through M3 and M4. Hence, another set of mobile phases was designed with the help of a selectivity triangle to improve the separation process. The solvent strength of the new mobile phase is given in Table 3. The schematic diagram is shown in Fig. 6a.
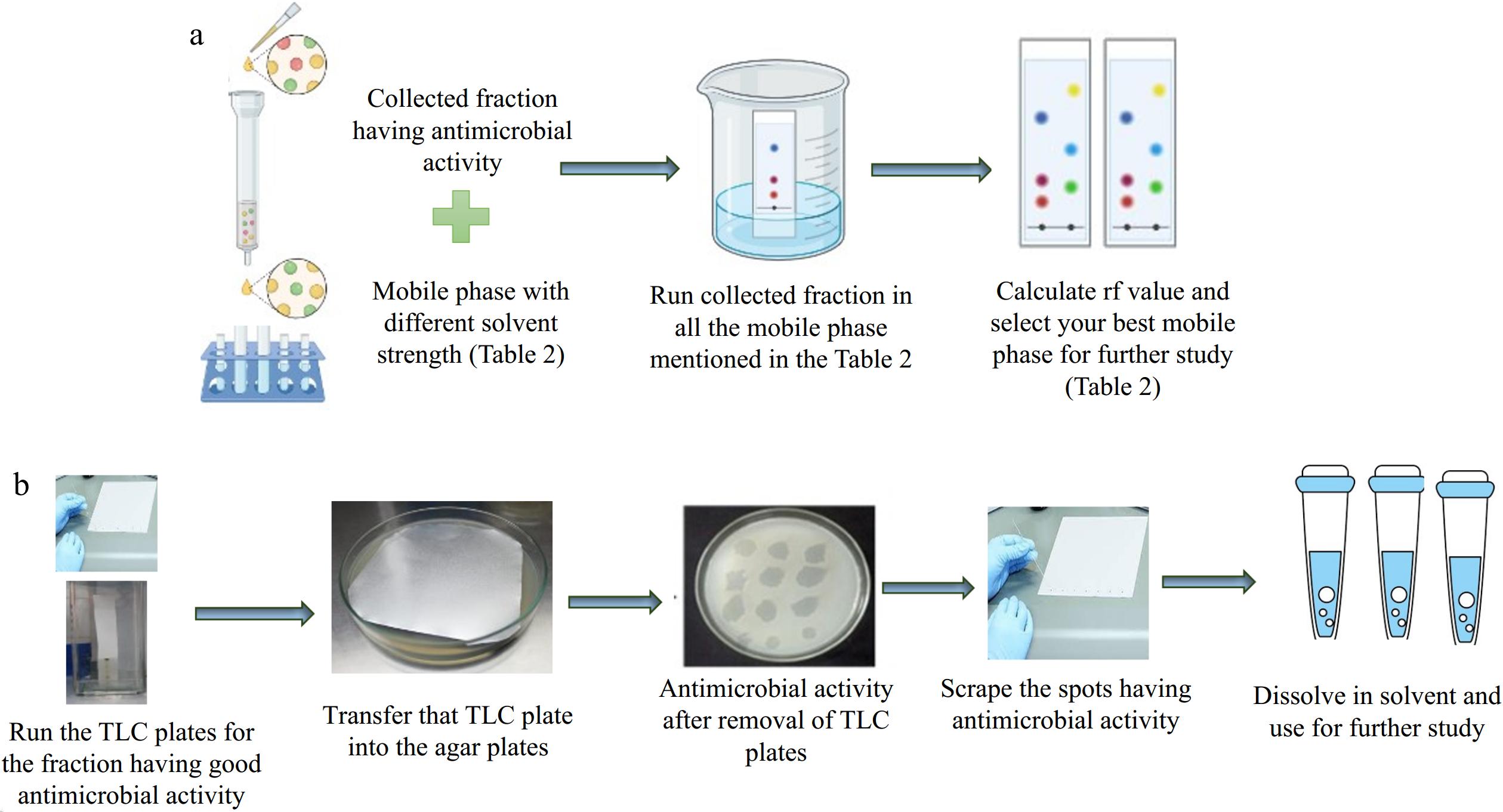
Figure 6.
(a) Schematic diagram for finalizing the mobile phase for the TLC. (b) Schematic diagram for the TLC-bioautography.
Screening for the best fractions having potential antimicrobial activity
-
Ten-bed volumes were passed through the column packed with spot 2. Fractions were collected through six different mobile phases, and afterward, all the fraction was then tested for their antimicrobial activity. After this exercise, three rich fractions (FA, FB, and FC) were selected based on their antimicrobial activity against all three tested groups including bacteria, actinobacteria, and fungi. The schematic diagram is shown in Fig. 6b and the results on the zone of inhibition are shown in the heatmap of Fig. 7a.
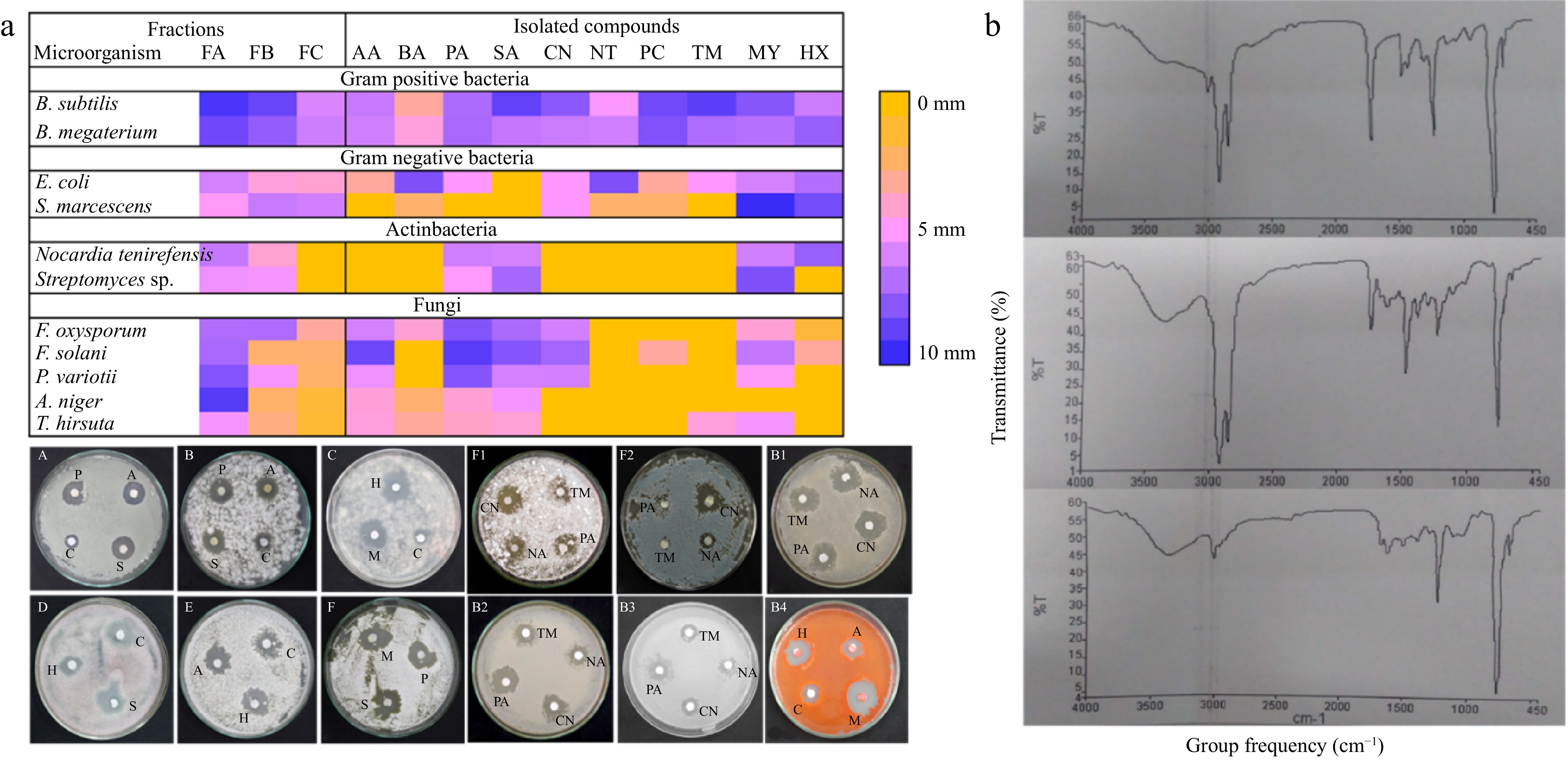
Figure 7.
(a) Heatmap showing antimicrobial potential (zone of inhibition (mm)) of final fraction and isolated compounds having antimicrobial activity. PA= palmitic acid, SA= stearic acid, AA= arachidic acid, MY = myoinositol, and HA = hexadecane, PA = procainamide, CN = cinchonine, NA = nicotinamide, TM = timolol. B1) B. megaterium, B2) B. Subtilis, B3) E. coli; and B4) S. marcescens. (A and F2) A. niger, B and C P. variotii, D) F. oxysporum E and F) T. hirsuta. B) FTIR spectra of rich fraction FA, FB, and FC of T. wallichiana needles.
Description of antimicrobial compounds
-
All of the fractions were subjected to the identification and characterization of antimicrobial compounds after the final three rich fractions (FA, FB, and FC) with antibacterial activity were chosen. Rich fractions (FA, FB, and FC) were subjected to GCMS (for volatile chemicals), LCMS (for non-volatile compounds), and FTIR (for functional group) analyses to characterize antimicrobial substances. A list of identified compounds through LCMS and GCMS are given in Table 4. Compounds like benzoic acid, hexadecenoic acid, or palmitic acid were found in all three fractions according to GCMS analysis. Fatty acids, aromatic carboxylic acids, aliphatic hydrocarbons, and sesquiterpenoid alcohols made up the bulk of the compounds that were found. Likewise, the existence of many categories of substances, such as alkaloids, vitamins, flavones, quinones, carboxylic acid, lipids, etc., was revealed by LCMS analysis.
Table 4. A list of identified compounds in fractions showed antimicrobial activity.
Fraction (FA) compounds Fraction (FB) compounds Fraction (FC) compounds GC-MS 1,6-Octadine-3-ol Benzoic acid Benzenepropanol Benzoic acid 4-Tetradecene Megastigmatrienone Benzene, 1-methoxy 4-(2-propenyl) 2,4-Ditert-butylphenol Ar-tumerone Phenol, 2-methyl-5-(1-methylethyl) 9-Octadecanoic acid Mome-inositol 1-Tridecene Hexadecane Hexadecanoic acid Pentadecane 1,4-Dimethyl-2 phenoxybenzene 9-Octadecenoic acid Succinic acid E-15-Heptadecenal Eicosanoic acid E-14-Hexadecenal Benzene dicarboxylic acid Di-n-octyl phthalate Neophytadiene Hexadecanoic acid Hexadecanoic acid 1-Nonadecene 1-Nonadecene Ecosanoic acid Docosanoic acid Octacosanol 1-Tetradecanol Phenol, 2,4-bis(1-phenylethyl) Benzene dicarboxylic acid Di-n-octyl phthalate LC-MS 1-aminocyclopropane-1-carbocyclic acid Nicotinamide Nicotinamide Methyl methanethiosulfonate Cinchonine Dodecylsulfonylacetic acid Methionyl-Glycin Ranitidine Diphenoxylic acid Ergothioneine Psoralenol Cinchonine Procainamide Squamocin B Ergothioneine Alcoifosfamide Trimethaphan Timolol Timolol Boviquinone Trimethaphan Lobaric acid Frangulanine To find out what functional groups were present in the identified compounds in the purified fraction, FTIR analysis was performed. Based on the fingerprint region (1,500–4,000 cm−1) and functional groups, FTIR spectra were analyzed. Figure 7b displays the FTIR spectrum. The functional groups of the compounds identified by GC-MS and LC-MS were compared with the FTIR spectra. All of the fractions share bands between 1,000 and 1,275 cm−1 (C-O/C-N str) between 3,000 and 3,100 cm−1 (C-H str in sp2 hybridized carbon, such as alkene = C-H str or aromatic C-H str). The bands at 3,362 cm−1, which are common between FB and FC, and the bands between 2,800 and 3,000 cm−1 (C-H str in sp3 hybridized carbon, such as methyl and methylene C-H str), which were common between FA and FB, suggested that N-H stretching may have occurred based on the band's shape, while broadness suggested the possibility of a carboxylic group or ester. Additionally, the tiny hump at 3,362 cm−1 suggested that FB and FC may include secondary or tertiary amides. In FA and FB, the band about 2,850 suggested the presence of methyl and methylene C-H str; in fraction FC, this was less evident. The only bands found in FC were around 3,017 cm−1 and the band at 1,675.95 cm−1, which are typically caused by the alkenyl C=C stretch/amide and the aromatic C-H stretch, respectively.
Antimicrobial compounds in needle-rich fractions (FA, FB, and FC)
-
After all the biochemical characterization i.e., GC-MS, LC-MS, and FTIR, antimicrobial compounds were identified in all three fractions (Table 5). Ten compounds i.e., arachidic acid, behenic acid, palmitic acid, and stearic acid (fatty acid); vitamins (nicotinamide); alkaloids (cinchonine, timolol); amino benzamides (procainamide); carbocyclic sugars (myoinositol); and alkane hydrocarbons (hexadecane) were identified among all the compounds in the three rich fractions (FA, FB, and FC) that had antibacterial activity. To assess the antibacterial activity of the standard compounds against all the specified microorganisms, plate-based bioassays and minimum inhibitory concentration (MIC) were employed. Results in detail are given in Fig. 7a. Identified fatty acids were found potential antibacterial and antifungal agents. Likewise in some previous reports also using bioassay-guided fractionation, antibacterial fatty acids have been extracted from a variety of plants. Yff et al.[29] isolated palmitic acid from Pentanisia prunelloides which was effective towards bacteriological contagions. Palmitic and stearic acids are reported in Labisia pumila leaves with antibacterial activities[30,31].
Table 5. List of compounds identified in the separated fraction (spot 2) having antimicrobial potential.
S. No. Compounds Formula Molar mass (g/mol) Classification Concentration in needles (mg/g (dw)) GC-FID analysis 1 Arachidic acid C20H40O2 312.53 Saturated fatty acid 22.94 ± 0.09 2 Behenic acid C22H44O2 340.58 Saturated fatty acid 31.04 ± 0.05 3 Palmitic acid C16H32O2 256.43 Saturated fatty acid 16.81 ± 0.03 4 Stearic acid C18H36O2 284.48 Saturated fatty acid 20.10 ± 0.06 RP-HPLC-PDA analysis 5 Cinchonine C19H22N2O 294.17 Alkaloid 3.75 ± 1.21 6 Nicotinamide C6H6N2O 122.12 Vitamin 21.14 ± 0.53 7 Procainamide C13H21N3O 235.325 aminobenzamides 14.61 ± 0.71 8 Timolol C13H24N4O3S 316.421 Alkaloid 6.31 ± 0.54 9 Myoinositol C6H12O6 180.16 carbocyclic sugar 1.45 ± 1.01 10 Hexadecane C16H34 226.41 alkane hydrocarbon − Shafaghat[32] has reported the antibacterial potential of fatty acids derived from the various parts of the plant Hypericum scabrum (flower, leaf, stem, and seed). Cerdeiras et al.[33] reported the antibacterial activity of stearic acid, present in the aerial sections of Ibicella lutea. In addition to fatty acids, six more compounds i.e., myoinositol, hexadecane, chinchonine, timolol, procainamide, and nicotinamide were present as per MS data and these compounds also showed antibacterial properties along with antifungal activities. These substances have specific antifungal action in addition to strong antibacterial activity. Comparably, reports of myoinositol's antibacterial properties have been made[34,35]. Hexadecane from Allium nigrum has also been shown to have antibacterial activity[36]. Finally, through this piece of work, a separate fraction of T. wallichiana needle extract was obtained with known compounds, which have shown good antimicrobial activity with selected microbial species. This can be further utilized for in-depth studies with other types of microbes. After testing for antimicrobial activity, the compounds in the needle extracts were quantified using GC and HPLC alongside their standard compounds. The results of the quantification are given in Table 5.
-
Due to the increasing demand for T. wallichiana bark, there has been a continuous decline in the availability of raw materials sourced from its natural habitats. Consequently, there is a notable scarcity of information concerning various aspects such as the phytochemical composition and antimicrobial properties of T. wallichiana. In the current research, a thorough examination was conducted, emphasizing the diversity of phytochemicals, antimicrobial functions, and selection of suitable solvents for the extraction of bioactive components responsible for antimicrobial activity. Needles showed the highest antimicrobial potential among the stem, bark, and needles of T. wallichiana. Based on the preliminary data, rich-fractions of needles were subjected to LC-MS, GC-MS, and FTIR analyses, and a total of 10 compounds with antimicrobial potential i.e., myoinositol, hexadecane, cinchonine, procainamide, nicotinamide, palmitic acid, stearic acid, arachidic acid, behenic acid, and timolol have been identified from the needles. It is also suggested that focusing on utilizing the needles, rather than the bark and stem is optimal for maximizing their antimicrobial potential. Notably, T. wallichiana, an evergreen tree, has needles with a lifespan of approximately three and a half months, underscoring the importance of promoting needle usage for sustainable extraction of taxol and other potential biologically active secondary metabolites.
-
The authors confirm contribution to the paper as follows: study conception and design: Pandey A, Agnihotri V; experiments conduction, data interpretation, draft manuscript writing: Adhikari P; manuscript editing & finalization: Pandey A, Agnihotri V. All authors reviewed the results and approved the final version of the manuscript.
-
The data that support the findings of this study are available on request from the corresponding author.
The authors are grateful to Director GBP-NIHE Almora, Uttarakhand, India, for extending facilities. The first author gratefully acknowledges the support from the National Mission on Himalayan Studies (NMHS-H-JRF-006) (Ministry of Environment, Forest & Climate Change, Govt. of India, New Delhi) for financial support.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Adhikari P, Agnihotri V, Pandey A. 2024. Strategic engineering for detecting antimicrobial compounds from Taxus wallichiana Zucc. (Himalayan yew). Medicinal Plant Biology 3: e018 doi: 10.48130/mpb-0024-0020
Strategic engineering for detecting antimicrobial compounds from Taxus wallichiana Zucc. (Himalayan yew)
- Received: 30 April 2024
- Revised: 25 July 2024
- Accepted: 26 July 2024
- Published online: 12 September 2024
Abstract: Taxus wallichiana Zucc. (Himalayan yew) has been well-documented for containing therapeutically significant active ingredients. Its bark contains pharmaceutically important compounds i.e., taxol and its derivatives which are well known for their anticancer potential. However, T. wallichiana has received limited attention for its equally significant antimicrobial properties. Keeping this background in view, T. wallichiana was selected for the detailed investigation of antimicrobial activities, and isolation and characterization of secondary metabolites responsible for antimicrobial activity in different plant parts i.e., needle, bark, and stem extracts. In plate-based bioassays, plants exhibited antimicrobial action against the three main categories of microorganisms (fungi, bacteria, and actinobacteria). Based on the preliminary antimicrobial study, methanol and ethyl acetate extracts, were selected for further experiments. The bioautographic technique was used for identification, and the mobile phase was optimized with the help of a selectivity triangle. After continuous column and thin-layer chromatography, fractions were identified as having good antifungal, antibacterial, and antiactinobacterial activity. These fractions were selected for further characterization using techniques like GC-MS/LC-MS, and FTIR. These analyses support the identification of several fatty acids, including arachidic acid, behenic acid, palmitic acid, and stearic acid; vitamins (nicotinamide); alkaloids (cinchonine, timolol); amino benzamides (procainamide); carbocyclic sugars (myoinositol); and alkane hydrocarbons (hexadecane), which have antimicrobial activity in T. wallichiana needles. The information gathered from this study will help modern medicine make new drug discoveries that combine different active ingredients from medicinal plants to treat a wide range of ailments.