-
Flower color is one of the most important components in floral traits that help higher plants attract pollinators for successful fertilization. There are abundant flower color variations among species, cultivars, and hybrid progenies, in which the white flowers, commonly as a symbol of purity and holiness, play important roles in ceremonial events and ikebana. It is well known that anthocyanins are the core pigments to generate different degrees of red, violet, or blue flowers[1,2]. Comparatively, there were only some colourless co-pigments like flavanones, flavones and flavonols found in the white flowers, such as that seen in the white petals of cornflower, Scutellaria baicalensis, and Cymbidium ensifolium[3−5]. Namely, anthocyanin absence leads to the white color formation in most higher plants.
Anthocyanin, a subclass of flavonoids, is an important secondary metabolite catalyzed by a series of enzymes[6,7]. Initially, chalcone synthase (CHS) catalyzes the tetrahydroxychalcone synthesis from 4-coumaroyl CoA and malonyl CoA, which was rapidly isomerized to the naringenin by chalcone isomerase (CHI). Then flavanone 3-hydroxylase (F3H) catalyzes the hydroxylation at its 3-position to generate dihydrokaempferol (DHK), which can be further catalyzed by the flavonoid 3'-hydroxylase (F3'H) and flavonoid 3'5'-hydroxylase (F3'5'H) to yield dihydroquercetin (DHQ) and dihydromyricetin (DHM), respectively. DHK, DHQ, and DHM are further converted to pelargonidin, cyanidin, and delphinidin by dihydroflavonol 4-reductase (DFR) and anthocyanidin synthase (ANS), respectively. Finally, sugar molecules and acyl groups are attached to the anthocyanidins by both glycosyltransferase (GT) and acyltransferase (AT). Therefore, any block in the structural genes will trigger anthocyanin deficiency. For example, down-regulation of the early biosynthetic genes, such as CHS and F3H, led to the white flower phenotypes in Ipomoea purpurea, Torenia fournieri, Dahlia variabilis, gentian, and C. kanran[8−13]. Moreover, deficiency of the late biosynthetic genes (DFR, ANS and GT, etc.) also blocked anthocyanin accumulation and facilitated white flower formation, such as the cases in Salvia miltiorrhiza, Aquilegia vulgaris, strawberry, and Iris bulleyana[14−17].
Anthocyanin biosynthetic genes are under the control of a regulatory complex composed of MYB, bHLH, and WD40[18,19]. MYB transcription factors play crucial roles in this process. The mutation of R2R3 MYB transcription activators resulted in the evolutionary transitions to white flowers in Antirrhinum and Petunia[20,21], while R3 MYBs usually function as transcription inhibitors involved in down-regulating multiple anthocyanin biosynthetic genes, leading to anthocyanin absence in higher plant organs[22−25]. In addition, bHLHs of the IIIf subgroup are involved in anthocyanin biosynthesis, which are further divided into two distinct clades, namely bHLH1 and bHLH2 genes[26]. Usually, the bHLH2 plays essential roles in anthocyanin biosynthesis, and its mutation often results in anthocyanin decrease and color fading[27−30]. Moreover, the bHLH1 proteins in Arabidopsis thaliana and Antirrhinum majus also directly regulate anthocyanin biosynthesis[31−33].
Our previous study revealed that the transcripts of structural genes involved in anthocyanin biosynthesis, e.g., CcF3H, CcF3'H, and CcDFR were nearly undetectable in the white cornflower petals lacking anthocyanin accumulation[3], which was possibly caused by the upstream transcription factors (TFs). Besides, two TFs, CcMYB6-1, and CcbHLH1, were identified as activators synergistically regulating anthocyanin biosynthesis in cornflower[34]. In the present study, the anthocyanin accumulation in both vegetative and reproductive organs in two cornflower cultivars were first monitored and it clarified that anthocyanins were completely absent in the white cornflower. To further explore the molecular mechanism underlying the white color transition in cornflower, the transcript abundances of both structural genes and transcription factors involved in anthocyanin biosynthesis were detected, and the full-length sequence of CcMYB6-1 and CcbHLH1 were isolated, followed by the multi-sequence alignment and phylogenetic analysis. Subsequently, the genomic sequence of CcbHLH1 was isolated to elucidate its truncation reason. Furthermore, the subcellular localization analysis, yeast-two hybrid, bimolecular luminescence complementary assay, dual-luciferase assay, as well as the transient over-expression in tobacco leaves were conducted to explore the functional changes after CcbHLH1 truncation. The findings will enhance our understanding of the regulation mechanism of anthocyanin biosynthesis in cornflower, and provide new insights on the white color transition in higher plants.
-
Two Centaurea cyanus cultivars with blue and white petals were used in this study, namely, 'Dwarf Tom Pouce Blue' (DTPB) and 'Dwarf Tom Pouce White' (DTPW) (Fig. 1a). The seeds were purchased from the Outsidepride Seed Source, LLC. Their seeds were sown in an equal volume mixture of peat and vermiculite, then placed in phytotron under 23 °C and 16 h/8 h (light/dark) conditions. After one month of cultivation, the seedlings began to blossom, and capitula were divided into four developmental stages based on our previous publication[3] (Fig. 1b). Briefly, the buds in stage one (S1), stage two (S2), and stage three (S3) were uncolored, less than 50% pigmented, and fully pigmented, respectively, while the petals in stage four (S4) were opened and fully pigmented (Fig. 1c). The roots, stems, leaves, sepals, and petals (S1−S4) were collected into 2 mL RNAase-free tubes, rapidly precooled in liquid nitrogen and stored at −80 °C before use.

Figure 1.
Different cultivars and developmental stages of cornflower. (a) Two cornflower cultivars with white and blue petals in DTPW and DTPB, respectively. (b) The capitula were classified into four developmental stages, bar = 1 cm. (c) The pigmentation pattern of cornflower petals in different developmental stages, bar = 1 cm.
Gene isolation and qRT-PCR analysis
-
Total RNAs were extracted from petals of DTPW and DTPB, respectively, followed by the first-strand cDNA generation using M-MLV reverse transcriptase (Promega, Germany). Polymerase chain reaction was conducted to amplify the full or partial-length of CcMYB6-1 and CcbHLH1 in the two cornflower cultivars. The 3′-rapid amplification of cDNA ends (3'-RACE) was performed to isolate the CcbHLH1# in DTPW, followed by verification of the obtained sequence using the high-fidelity enzyme of KOD-201 (TOYOBO, Japan). To further explore the expression characteristics of structural and regulatory genes, the qRT-PCR was performed in roots, stems, leaves, sepals, and petals of both DTPW and DTPB using the TB Green® Premix Ex Taq™ II (Takara, Japan). Conflower actin (KY621346) was used as the reference gene. All the primers were designed based on the transcriptome database[34] and listed in Supplementary Table S1.
DNA extraction and isolation of genomic CcbHLH1
-
The fresh leaves were cut from DTPB in the vegetative period, precooled in liquid nitrogen, and ground into a powder. Then the whole-genome DNA was extracted using the super plant genomic DNA kit according to the instructions (TIANGEN, China). To further explore the genomic structure of CcbHLH1, the PrimeSTAR® Max DNA polymerase (Takara, Japan) was used to amplify its genomic sequence. Furthermore, the purified PCR products were ligated into the pCE3 blunt vector, followed by the transformation into Escherichia coli DH5α and sequencing. All the primers are listed in Supplementary Table S1.
Phylogenetic analysis and sequence alignment
-
A maximum likelihood phylogenetic tree was constructed by the Jones-Taylor-Thornton (JTT) model using MEGA11. Deduced protein sequences were firstly aligned using MUSCLE, followed by the phylogeny test with 1,000 bootstrap replications. Besides, gaps or missing data were treated as complete deletions. DNAMAN software was applied to obtain the multi-sequence alignment result.
Subcellular localization of key genes
-
The pCAMBIA vector carrying an EGFP was digested with NcoI and SpeI, followed by the ligation with CcMYB6-1, CcbHLH1 and its mutant without stop codons using homologous recombination. The empty and recombinant plasmids were transformed into Agrobacterium tumefaciens GV3101, respectively. GV3101s were resuspended in the liquid mixture containing 10 mM MgCl2, 10 mM MES, and 200 μM acetosyringone, then were adjusted to the OD600 of 1, followed by the infiltration into Nicotiana benthamiana leaves using needless injections. Before observation, the leaves were immersed in the DAPI (10 μg/mL) for 30 min. Finally, a laser scanning confocal microscope (Leica TCS SP8, Wetzlar, Germany) was used to obtain the fluorescence signal.
Yeast two-hybrid assay
-
The restriction endonucleases EcoRI and BamHI (New England Biolabs) were used to linearize pGBKT7 and pGADT7 empty vectors. The whole length of CcMYB6-1 was cloned into pGADT7 to form AD-CcMYB6-1 recombinant, while CcbHLH1 and its mutant were cloned into pGBKT7 to construct BD-CcbHLH1 and BD-CcbHLH1# recombinants. Then, these empty vector and/or recombinant plasmids were co-transformed into Y2H strain by use of yeastmaker™ yeast transformation system 2 (Clontech, USA) and plated on the SD/-Leu/-Trp solid medium, followed by incubation upside down at 30 °C for three days. The matchmaker™ insert check PCR mix 2 (Takara, Japan) was used to ensure the successful insertion of target genes into the yeast strain, followed by the evaluation on the SD/-Trp-Leu-His-Ade medium supplemented with X-α-Gal and 3-amino-1,2,4-triazole (3AT).
Bimolecular luminescence complementary assay
-
The bimolecular luminescence complementary assay was further conducted to get more evidence of protein-protein interaction between CcMYB6-1, and CcbHLH1, or CcbHLH1#. The empty pCAMBIA1300-cluc (cLUC) and pCAMBIA1300-nluc (nLUC) plasmids were digested with KpnI and SalI for linearization. The full-length sequence of CcMYB6-1 was ligated into linearized cLUC, while CcbHLH1 and CcbHLH1# without stop codons were cloned into linearized nLUC, respectively. Subsequently, the empty or recombinant plasmids were transformed into A. tumefaciens GV3101 severally, followed by the infiltration of GV3101s of different combinations into N. benthamiana leaves at an equal volume ratio of cLUC and nLUC. After three days of co-culture in the dark, the D-luciferin potassium salt solution was sprayed on the leaf abaxial surface, followed by the observation of fluorescence signals using a molecular imaging system (LB983 NightOwl II).
Dual luciferase assay
-
The promoter region of CcDFR, a key structural gene catalyzing anthocyanin biosynthesis, was isolated as in our previous publication[34]. Restriction endonucleases KpnI and BamHI (New England Biolabs) were used to digest pGreenII 0800-LUC empty vector in rCutSmart buffer at 37 °C for 15 min, then CcDFR promoter of 1,510 bp was cloned into the linearized pGreenII 0800-LUC by homologous recombination. The full-length sequences of CcMYB6-1, CcbHLH1 and CcbHLH1# were recombined into pGreenII 62-SK vector. All the recombinants were individually transformed into A. tumefaciens GV3101 and verified as a positive clone by polymerase chain reaction. Subsequently, a total of six groups including SK, SK + CcMYB6-1, SK + CcbHLH1, SK + CcbHLH1#, CcMYB6-1 + CcbHLH1, and CcMYB6-1 + CcbHLH1# were individually mixed with LUC-DFRpro at a ratio of 10:1 (v:v). The GV3101s containing different recombinants were infiltrated into N. benthamiana leaves using a needless injector. After three days of cultivation, the infiltrated leaves were painted with a layer of D-luciferin potassium salt liquid containing 0.1% Triton-x-100, and then photographed using the molecular imaging system (LB983 NightOwl II). Moreover, the firefly luciferase and Renilla luciferase were extracted following the instructions of Dual-Luciferase® Reporter Assay System E1910 (Promega, USA), and detected by EnVision (PerkinElmer, USA).
Transient expression in tobacco leaves
-
To explore the functional changes before and after CcbHLH1 mutation, N. benthamiana were used for transient expression. GV3101s containing mixed plasmids including SK + CcMYB6-1, SK + CcbHLH1, SK + CcbHLH1#, CcMYB6-1 + CcbHLH1, or CcMYB6-1 + CcbHLH1# at a ratio of 1:1 (v:v) was infiltrated into leaves using a needleless injector, followed by cultivation in the dark for three days and photographed after nine days. About 0.1 g infiltrated tobacco leaves were weighed and stored at −80 °C before use.
Semiquantitative analysis of anthocyanin
-
Cyanidin-3-O-glucoside (Cy3G) was used to obtain the regression equation (Y = 2.9301X, R2 = 0.9959) at 525 nm. About 0.1 g of fresh samples were accurately weighed, ground into a powder using a grinding machine, and extracted with 1 mL mixture of methanol : H2O : formic acid : trifluoroacetic acid = 70:27:2:1 (v : v : v : v) at 4 °C overnight, followed by centrifugation at 12,000 rpm for two minutes. As for leaves, stems and sepals, 500 μL chloroform was added to remove chlorophyll before centrifugation. The liquid supernatant was transferred into new tubes for anthocyanin detection using a spectrophotometer.
Statistical analysis
-
Data processing was conducted by use of Excel 2021, and SPSS 20.0 was performed to obtain the significant difference analysis using Ducan's multiple test at 1% level. Origin 2021 was used for visualizing the figures.
-
The previous research on cornflower has clarified that there were pelargonidin derivatives in the pink and red petals as well as cyanidin derivatives in the blue, mauve, and black petals, while no anthocyanin was detected in the white petals using the ultra-performance liquid chromatography coupled with photodiode array and tandem mass spectrometry[3]. Here, we further attempted to detect the anthocyanins in root, stem, leaf, and sepal, besides, the dynamic changes of anthocyanin content were also traced among four developmental stages of petals (Supplementary Fig. S1). There was no anthocyanin in the DTPW petals, which was consistent with our previous results[3], while the vegetative organs showed no anthocyanin accumulation, either (Fig. 2a & b), suggesting anthocyanins were completely absent in DTPW. Comparatively, the sepals accumulated trace anthocyanins and the anthocyanin contents in petals continuously increased with flower development and peaked at stage four (Fig. 2a & b), suggesting anthocyanins accumulated in both sepals and petals of DTPB.
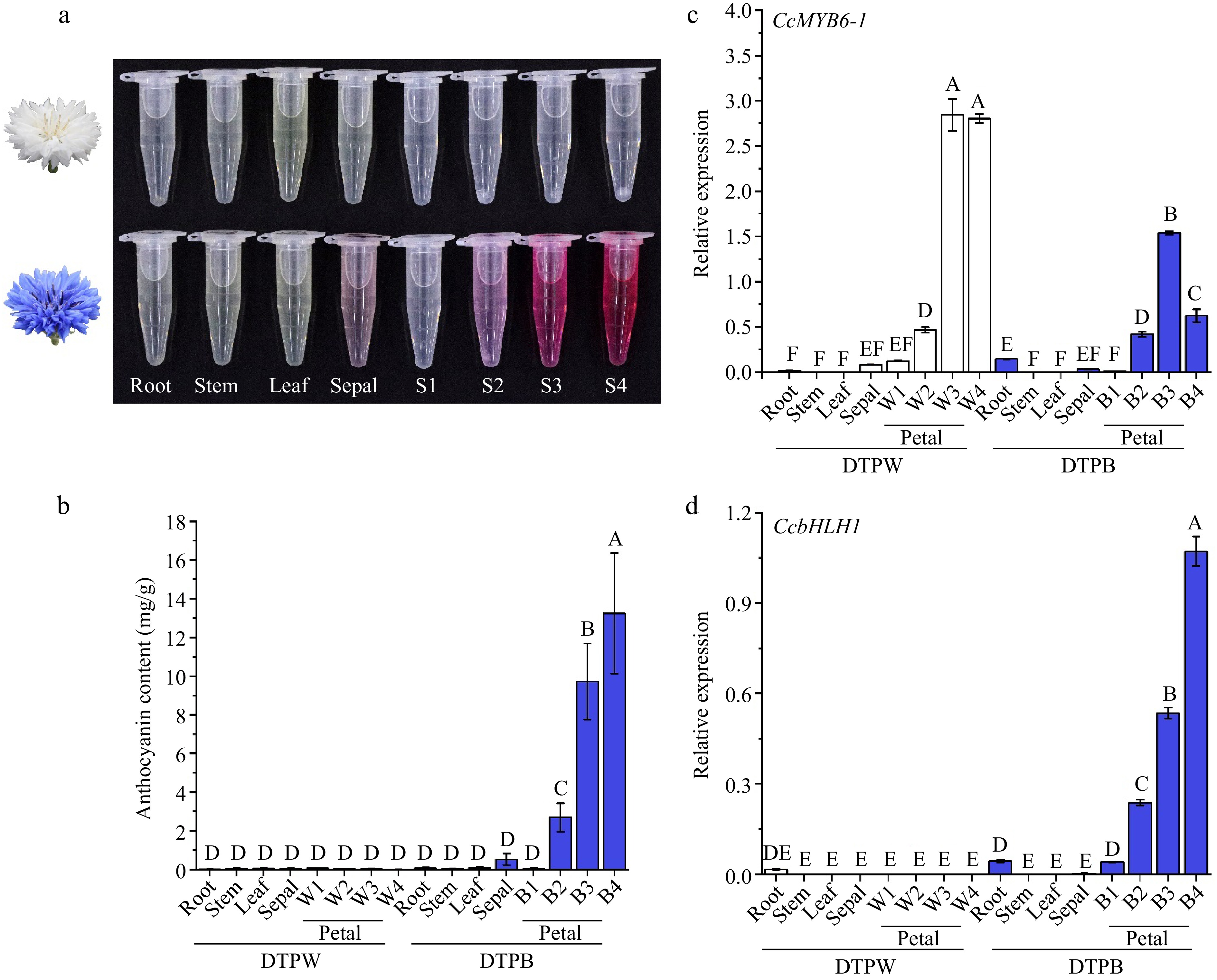
Figure 2.
Anthocyanins showed specific accumulation in different cultivars and organs of cornflower. (a) The anthocyanin extracts of distinct organs from DTPW and DTPB. (b) Anthocyanin content in different cultivars and organs. Error bars were the S.E. of four biological replicates with each from three individual plantlets. (c), (d) The spatio and temporal expression patterns of CcMYB6-1 and CcbHLH1 in DTPW and DTPB. Error bars were the S.E. of three technical replicates. Different capital letters indicate significant difference at 1% level by Duncan's multiple test.
The expression pattern of structural genes was further analyzed including CcF3H, CcF3'H, CcDFR, CcANS, CcGT, and CcAT in the spatio levels to clarify the potential mechanism of anthocyanin absence in DTPW (Supplementary Fig. S2). Notably, the CcDFR was specifically expressed in the reproductive period. All the biosynthetic genes in the blue petals were significantly higher expressed than others, while the white petals only showed a tiny amount of gene expression, which were consistent with our previous publication[3]. These results suggested that the down-regulating of biosynthetic gene expression accounted for the anthocyanin absence in DTPW. Our previous research identified two transcription factors (TFs) positively regulating anthocyanin biosynthesis in cornflower, namely CcMYB6-1 and CcbHLH1[34]. The qRT-PCR results revealed that the white petals showed significantly higher expression of CcMYB6-1 (Fig. 2c), while the CcbHLH1 transcript was nearly undetectable in DTPW (Fig. 2d), indicating CcbHLH1 may be responsible for the down-regulating of biosynthetic genes in DTPW.
A spontaneous mutation of CcbHLH1 occurs in the white cornflower petals
-
Further, an attempt was made to isolate the full-length sequences of those two TFs using the cDNA library constructed from the petals of both DTPW and DTPB. Firstly, a polymerase chain reaction was performed to amplify the whole length of two TFs using primers based on the published sequence information in the blue cornflower cultivar DTPB. The agarose gel electrophoresis showed that CcMYB6-1 could be successfully amplified in DTPW (Supplementary Fig. S3a), and its sequence contained the whole R2 and R3 domains, 100% consistent with that in DTPB (Fig. 3a), suggesting CcMYB6-1 was not the main cause for anthocyanin absence in DTPW. Then, three pairs of primers were designed to amplify different fragments of CcbHLH1 (Supplementary Fig. S3b), and the agarose gel electrophoresis showed that there were clear bands of fragment 1 and fragment 2, however, fragment 3 could only be amplified in DTPB (Supplementary Fig. S3c), suggesting its full-length was possibly missing in the white petals. Then 3' RACE was performed to obtain the whole length of CcbHLH1 in DTPW. Finally, a sequence with 1,125 base pairs was joined and further verified by amplification using high-fidelity enzyme. The multisequence alignment analysis showed that the truncated CcbHLH1 in DTPW lost partial WD activation domain, the whole basic helix-loop-helix domain in the N-terminal, and the aspartokinase, chorismite mutase, TyrA (ACT)-like domain in the C-terminal (Fig. 3b). For convenience, the truncated CcbHLH1 was renamed as CcbHLH1# in the following assays. These results revealed that a naturally spontaneous mutation of CcbHLH1 occurred in DTPW, which may account for its anthocyanin absence.
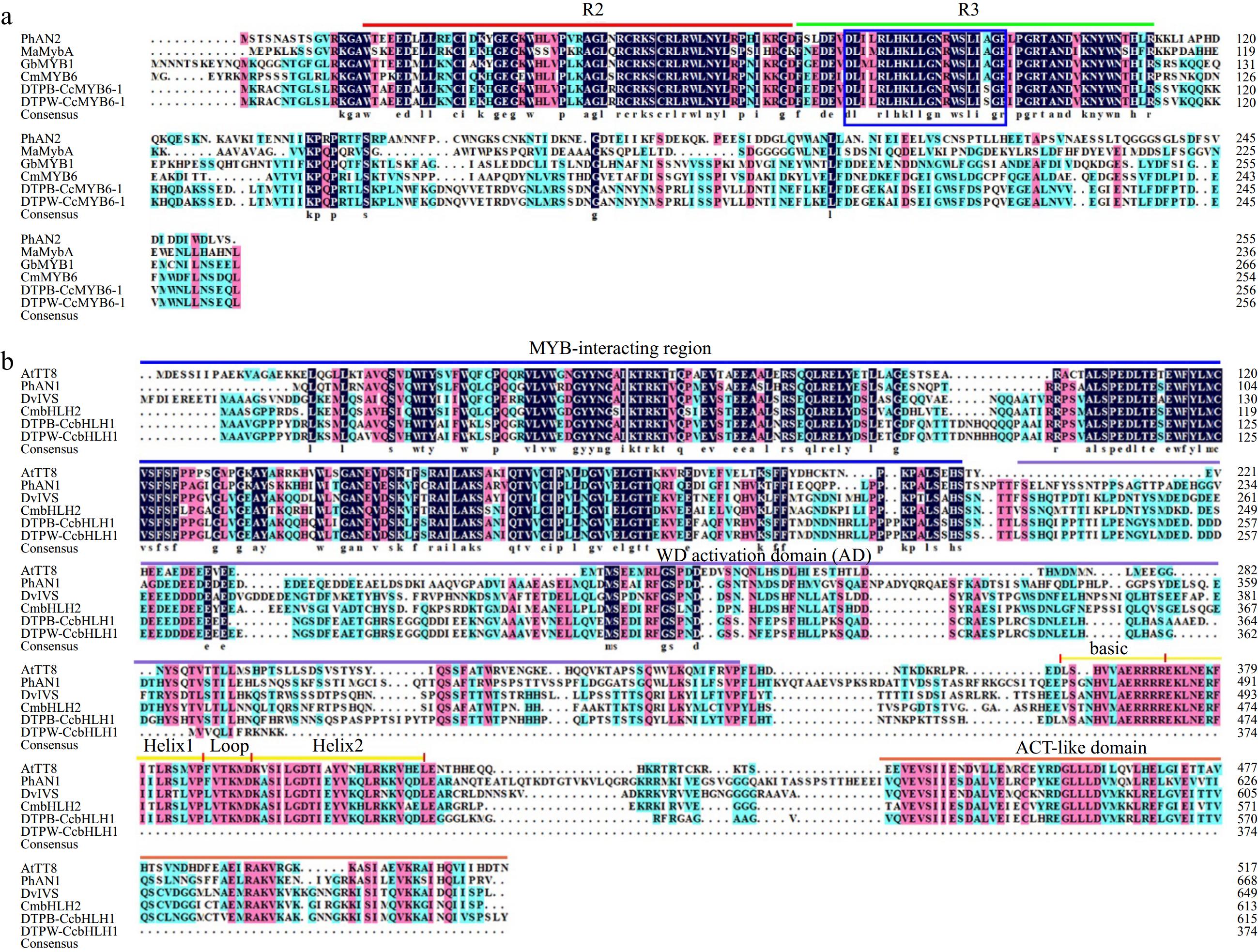
Figure 3.
Amino acid sequence alignment of MYBs and bHLHs regulating anthocyanin biosynthesis in different species. (a) Protein sequence alignment of MYBs with the conserved R2 and R3 domains marked in red and green lines, respectively. The blue rectangle indicated conserved motif of [D/E]LX2[K/R]X3LX6LX3R. PhAN2 (P. × hybrida, AAF66727), MaMybA (Muscari armeniacum, AVD68967), GbMYB1 (Gynura bicolor, BAJ17661), CmMYB6 (Chrysanthemum × morifolium, AKP06190). (b) Protein sequence alignment of bHLHs. The MYB-interacting region, WD activation, bHLH, and ACT-like domains were marked with lines in different colors. AtTT8 (A. thaliana, CAC14865), PhAN1 (P. × hybrida, AAG25927), DvIVS (D. variabilis, BAJ33515), and CmbHLH2 (C. × morifolium, ALR72603).
Alternative splicing is responsible for the truncation of CcbHLH1
-
The qRT-PCR results revealed that CcbHLH1# was specifically expressed in the petals, and its abundance in the white petals was significantly higher than that in the blue petals (p < 0.01) (Fig. 4a). To further explore the molecular mechanism underlying the truncation of CcbHLH1, its genomic sequence was isolated in DTPB, which contained four exons and three introns (Fig. 4b). The mature mRNA of CcbHLH1 consisted of four complete exons, carrying the key basic, helix-loop-helix domain (Fig. 4b). Comparatively, the CcbHLH1# transcript consisted of exons 1, 2, and partial retention of intron 2, losing the key bHLH domain (Fig. 4b). Furthermore, phylogenetic analysis of the predicted amino acid sequences of CcbHLH1 and CcbHLH1# with other bHLHs that regulate flavonoid biosynthesis in other species revealed they belong to the bHLH2 clade within the IIIf subgroup (Fig. 4c).
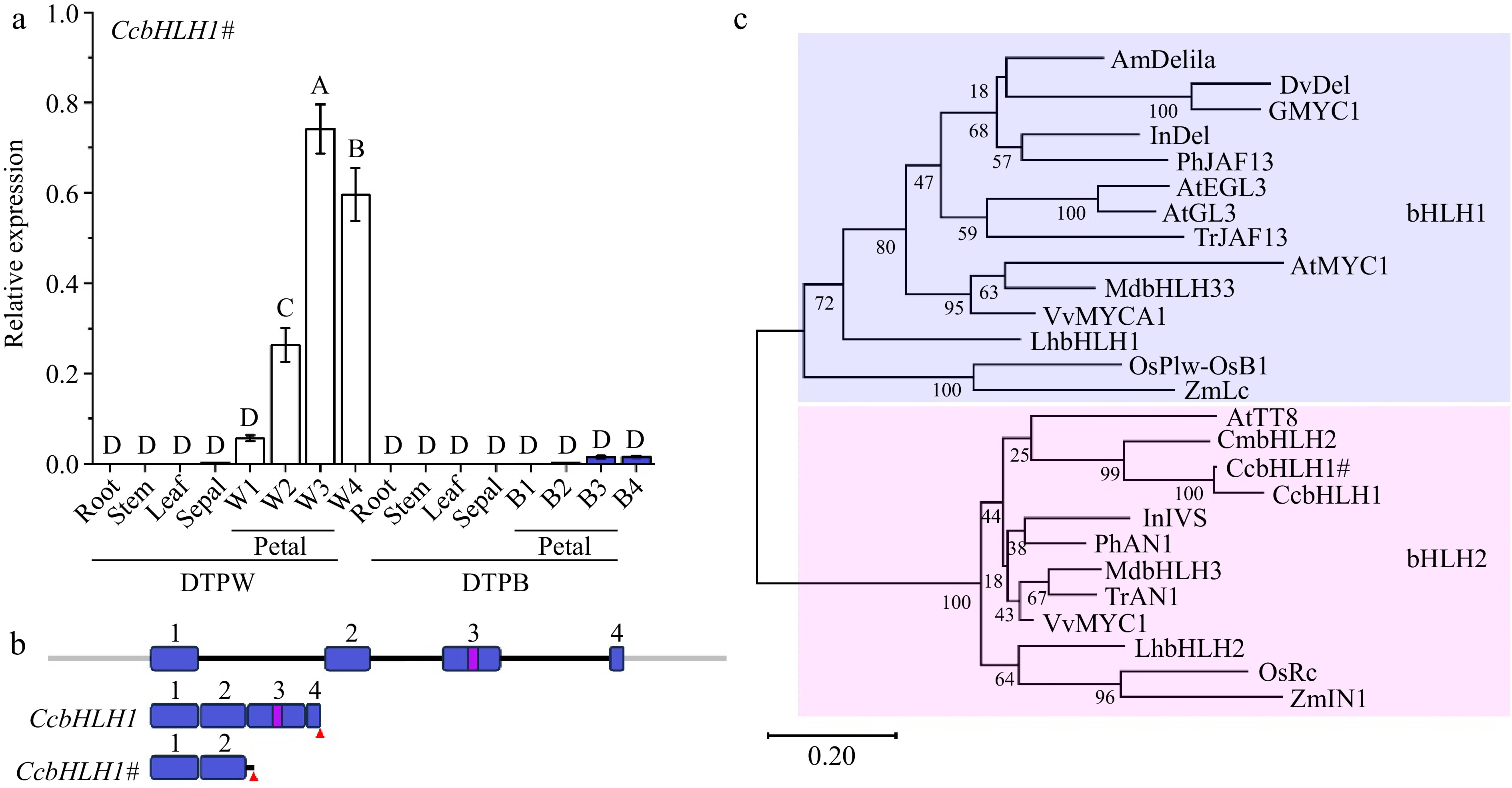
Figure 4.
Molecular and phylogenetic analysis of CcbHLH1 and CcbHLH1#. (a) The spatio and temporal expression patterns of CcbHLH1# in DTPW and DTPB. Error bars were the S.E. of three technical replicates. Different capital letters indicate significant difference at 1% level by Duncan's multiple test. (b) Structure of CcbHLH1 and CcbHLH1# genes. Exons are numbered in blue rectangles, introns are indicated by black lines, the bHLH domain is indicated in a violet rectangle, and the red triangle represents stop codon. (c) Phylogenetic tree of subgroup IIIf bHLHs. AmDelila (A. majus, AAA32663.1), DvDel (D. pinnata, BAJ33516.1), GMYC1 (Gerbera hybrid, CAA07615.1), InDel (I. nil, XP_019171149.1), PhJAF13 (P. × hybrida, AAC39455.1), AtEGL3 (A. thaliana, OAP12509.1), AtGL3 (A. thaliana, NP_680372.1), TrJAF13 (Trifolium repens, AIT76563.1), AtMYC1 (A. thaliana, NP_001154194), MdbHLH3 (Malus domestica, ADL36597.1), MdbHLH33 (M. domestica, ABB84474.1), VvMYCA1 (Vitis vinifera, NP_001267954.1), LhbHLH1 (Lilium hybrida, BAE20057.1), LhbHLH2 (L. hybrida, BAE20058.1), OsPlw-OsB1 (Oryza sativa, BAB64301.1), ZmLc (Zea mays, AAA33504.1), AtTT8 (A. thaliana, Q9FT81), CmbHLH2 (C. × morifolium, ALR72603.1), InIVS (I. nil, XP_019197480.1), PhAN1 (P. × hybrida, AAG25928.1), TrAN1 (T. repens, AIT76559.1), VvMYC1 (V. vinifera, NP_001268182.1), OsRc (O. sativa, ADK36625.1), ZmIN1 (Z. mays, AAB03841.1).
The subcellular localization analysis of CcbHLH1
-
To further clarify if the functional position changed after CcbHLH1 truncation, the subcellular analysis was conducted using GFP as a reporter gene, whose transcription was activated by the constitutive CaMV 35S promoter. The CcMYB6-1, CcbHLH1, and CcbHLH1# without stop codons were successfully fused with GFP. There were strong GFP fluorescence signals widely found in the whole cell transformed with an empty vector. Comparatively, the GFP fluorescence signals of CcMYB6-1, and CcbHLH1 were mainly focused in the nucleus (Fig. 5), which was consistent with our previous identification that CcMYB6-1 and CcbHLH1 functioned as transcription factors synergistically involved in regulating anthocyanin biosynthesis in cornflower[34]. Similarly, the green fluorescence signals of CcbHLH1# were also observed in the nucleus (Fig. 5), suggesting the loss of multi-domains didn't change its functional position.
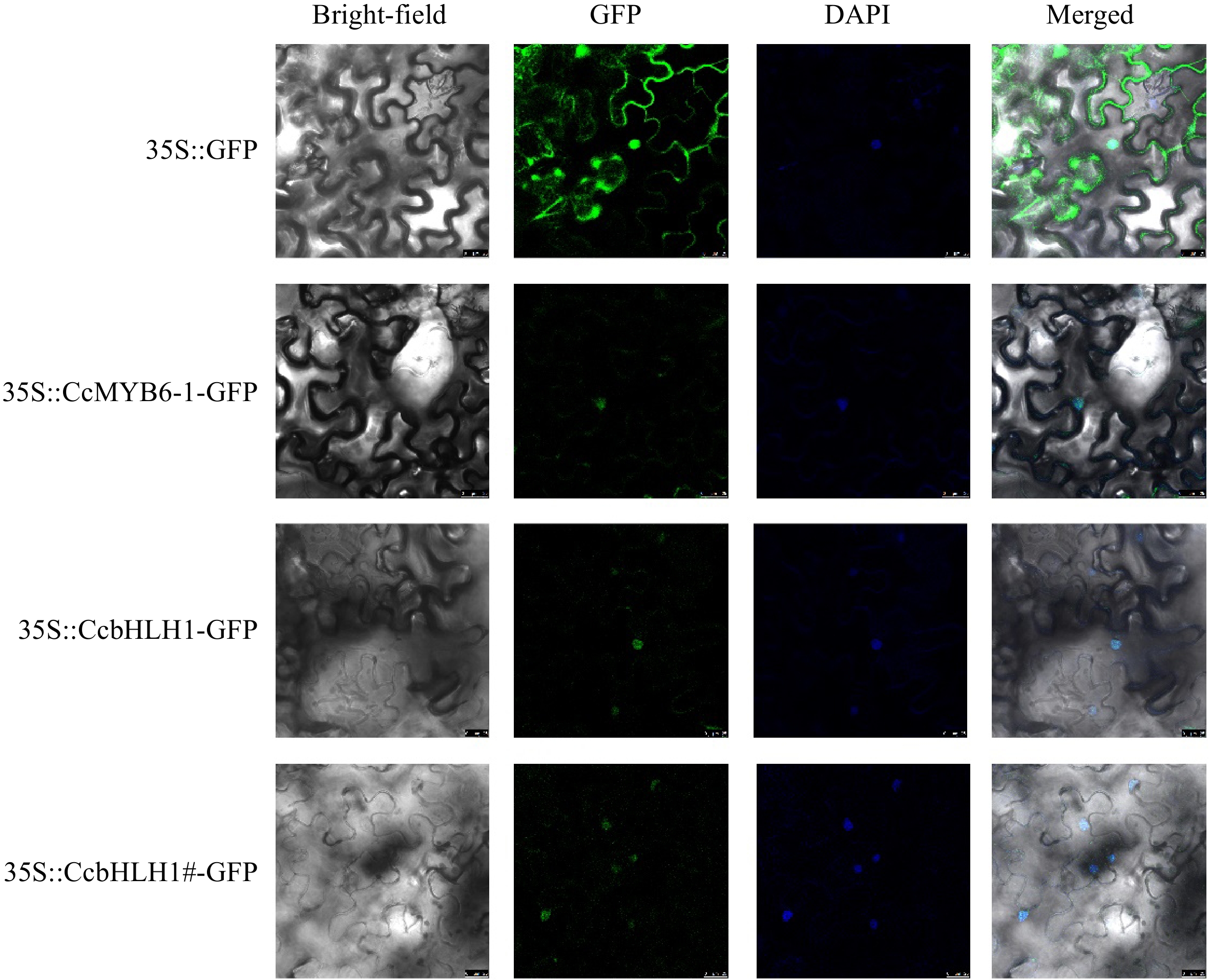
Figure 5.
The subcellular localization analysis of CcMYB6-1, CcbHLH1, and CcbHLH1#. DAPI was used as a nuclear-localized marker. Photos were captured two days after infiltration.
CcbHLH1# remains the protein-protein interaction with CcMYB6-1
-
The yeast two-hybrid assay was conducted to explore whether the protein-protein interaction relationship changed between the truncated CcbHLH1 and CcMYB6-1. Yeast transformed with pGBKT7-53 and pGADT7-T as well as pGBKT7-lam and pGADT7-T were designed for the positive and negative control, respectively. A total of five combinations were performed, and all of them could grow well on the SD/-Trp-Leu plate, suggesting all the target plasmids were successfully transformed into the Y2HGold yeast strain (Fig. 6a). The following observation found that yeast transformed with BD-CcbHLH1/CcbHLH1# and empty AD or empty BD and AD-CcMYB6-1 could not grow on the SD/-Trp-Leu-His-Ade+X-α-gal + 3AT plate, similar as the negative control. Comparatively, yeast co-transformed with CcMYB6-1 and CcbHLH1 or CcbHLH1# grew well and exhibited significant blue color on the same medium, similar as the positive control (Fig. 6a). Moreover, the bimolecular luminescence complementary assay was also conducted to obtain more evidence. There was no fluorescence signal detected in the combinations of nLUC + cLUC, nLUC + cLUC-CcMYB6-1, and nLUC-CcbHLH1/CcbHLH1# + cLUC, while significantly stronger fluorescence signals were found both in the nLUC-CcbHLH1 + cLUC-CcMYB6-1 and nLUC-CcbHLH1# + cLUC-CcMYB6-1, further verifying the protein-protein interaction between CcbHLH1/CcbHLH1# and CcMYB6-1 (Fig. 6b). These results indicated that the truncation of CcbHLH1 didn't change its protein interaction with CcMYB6-1.
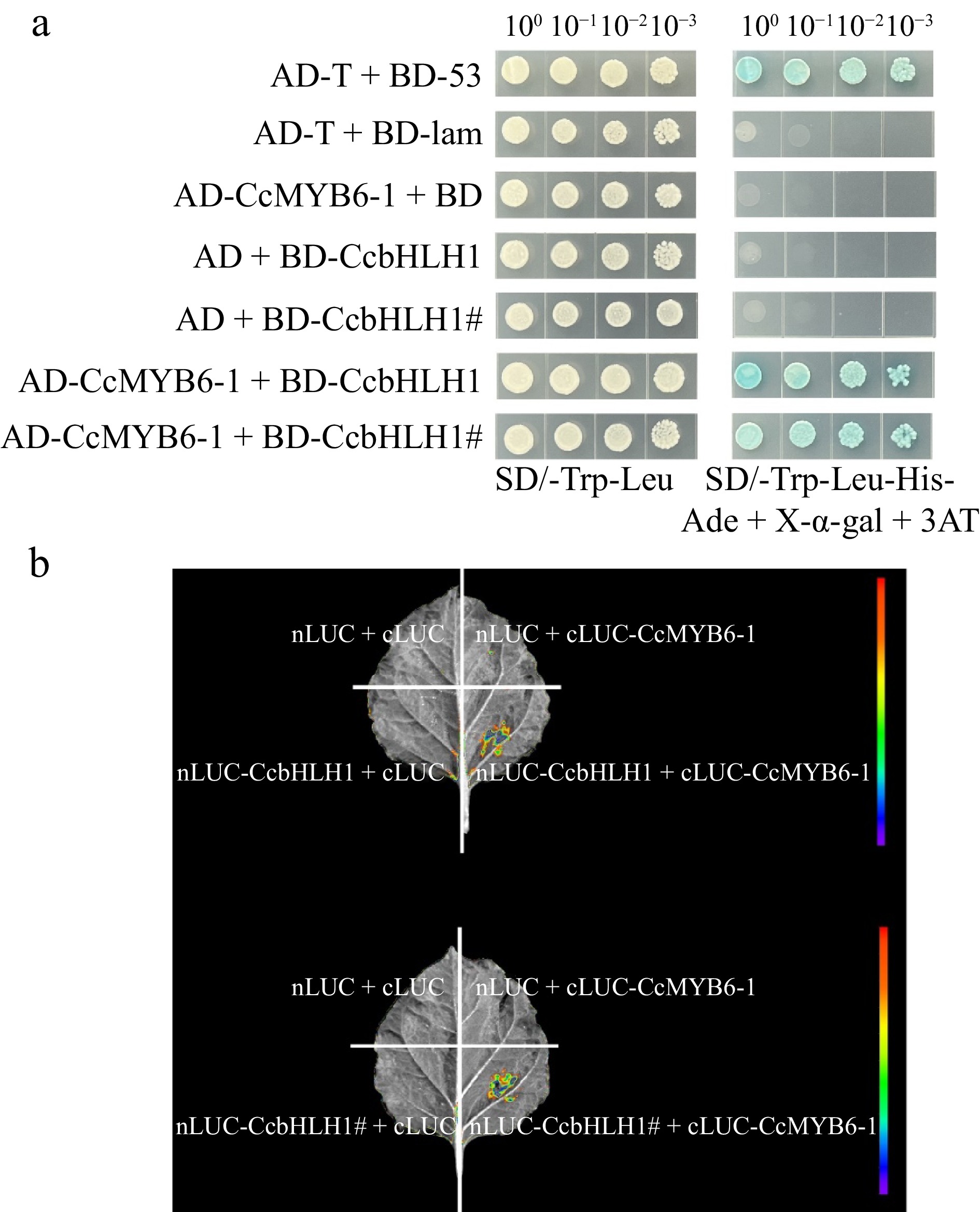
Figure 6.
Protein-protein interaction before and after CcbHLH1 mutation with CcMYB6-1. (a) Y2H strain transformed with targeted plasmids were plated on the SD/-Trp-Leu (left) and SD/-Trp-Leu-His-Ade + X-α-gal + 3AT (right) solid medium. (b) The firefly fluorescence of tobacco leaves injected with different combinations of targeted genes.
CcbHLH1# loses the ability to enhance the trans-activation of CcDFR promoter
-
A previous study indicated that CcMYB6-1 could trans-activate the promoters of structural genes involved in anthocyanin biosynthesis and this trans-activation was significantly enhanced when co-expressed with CcbHLH1[34]. The late anthocyanin biosynthetic gene CcDFR was then chosen to clarify whether the mutational CcbHLH1 could retain the ability to enhance gene expression. Firstly, tobacco leaves were infiltrated with CcbHLH1, CcbHLH1#, and CcMYB6-1, respectively, followed by the visualization of firefly fluorescence using a molecular imaging system. The fluorescence signal in CcMYB6-1 was significantly stronger than that in the CcbHLH1/CcbHLH1#, suggesting CcbHLH1 or its mutant alone could not trans-activate the CcDFR promoter (Supplementary Fig. S4). Then CcbHLH1 and CcMYB6-1 were co-expressed and more stronger fluorescence signal was obtained, however, the signal in CcbHLH1# + CcMYB6-1 was similar as that in the SK + CcMYB6-1, suggesting CcbHLH1# could not enhance the trans-activation of the CcDFR promoter (Fig. 7a). Moreover, the dual luciferase assay was conducted to obtain more concrete statistics. The value of LUC/REN in SK was set as 1 for convenience. The combinations of SK + CcbHLH1/CcbHLH1# could not up-regulate the activity of CcDFR promoter, which was consistent with the molecular imaging results. Comparatively, co-transformed CcMYB6-1 and CcbHLH1 significantly enhanced the activity of the CcDFR promoter with 12.9-fold induction, while there was no significant difference between CcMYB6-1 + CcbHLH1# and SK + CcMYB6-1 (p < 0.01) (Fig. 7b). These results indicated that the truncated CcbHLH1 lost the ability to enhance the trans-activation of CcDFR promoter, which possibly was the main reason for the anthocyanin absence in DTPW.
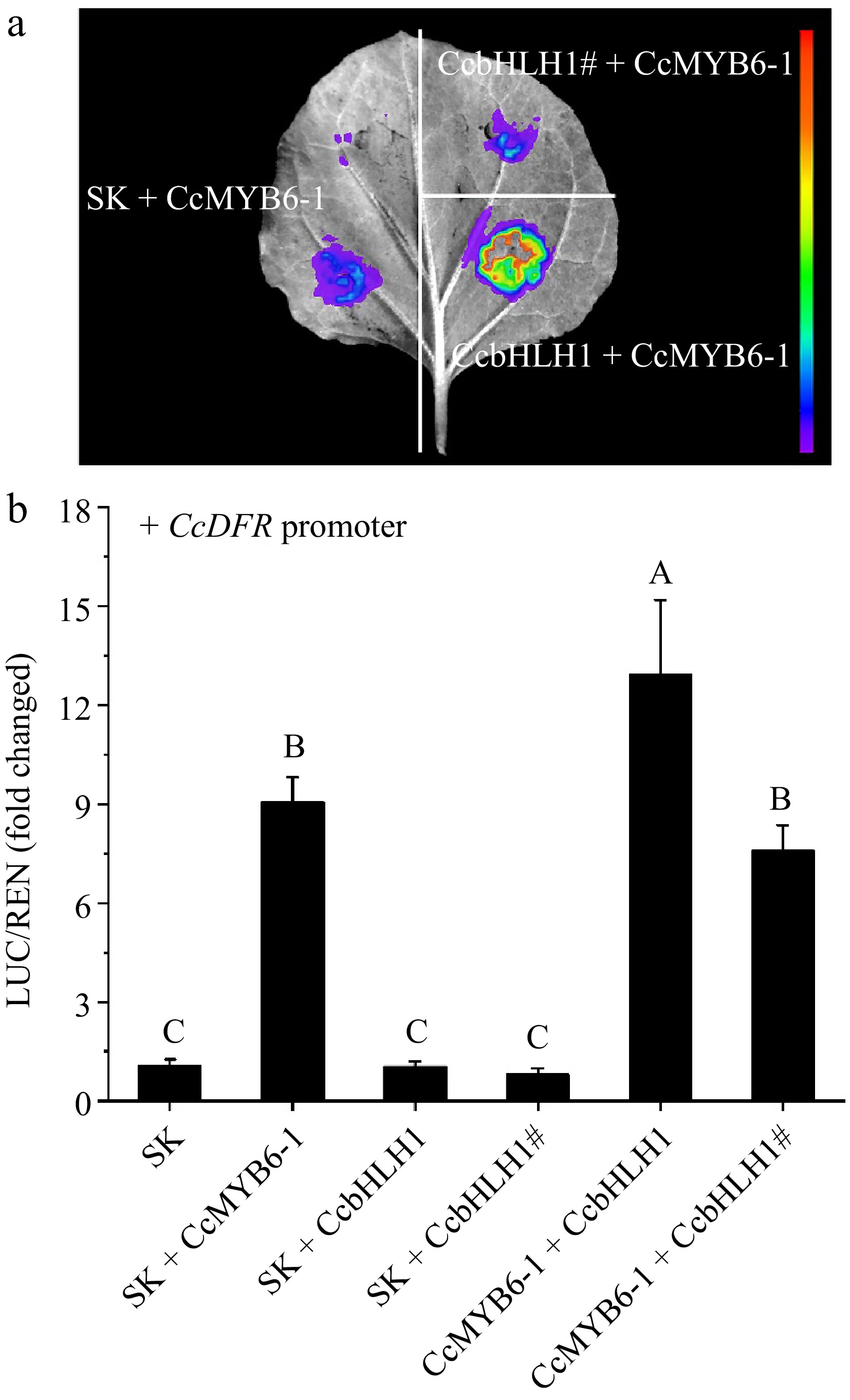
Figure 7.
Changes of trans-activation of CcDFR promoter before and after CcbHLH1 mutation. (a) The firefly fluorescence captured by a molecular imaging system after three days infiltration. (b) In vivo interactions between CcMYB6-1, CcbHLH1, and its mutant CcbHLH1# on the CcDFR promoter. Error bars were the S.E. of three biological replicates with each from at least two individual leaves. Different capital letters indicate significant difference at 1% level by Duncan's multiple test.
Anthocyanin content in tobacco leaves decreases after CcbHLH1 mutation
-
Due to the lack of a stable genetic transformation system, the transient over-expression in tobacco leaves were conducted to clarify the functional changes after CcbHLH1 mutation. A total of five combinations were designed, including SK + CcMYB6-1, SK + CcbHLH1, SK + CcbHLH1#, CcMYB6-1 + CcbHLH1, and CcMYB6-1 + CcbHLH1#. After 9 d of co-cultivation, tobacco leaves infiltrated with CcMYB6-1 turned green to red, in which the CcMYB6-1 + CcbHLH1 group exhibited the darkest red, followed by CcMYB6-1 + CcbHLH1#, and SK + CcMYB6-1 groups. On the contrary, tobacco leaves infiltrated with SK + CcbHLH1 or SK + CcbHLH1# retained green (Fig. 8a). These results indicated that only CcMYB6-1 expressed can the tobacco leaves turn red, which was consistent with our previous research[34]. Notably, the leaves exhibited pale red rather than darker red after CcbHLH1 mutation, suggesting its function of synergistically regulating anthocyanin biosynthesis with CcMYB6-1 was also missing. Furthermore, the semi-quantitative interpretation using Cy3G as a standard was performed to obtain more direct evidence. There was no anthocyanin detected in SK + CcbHLH1 and SK + CcbHLH1# groups, while the anthocyanin content in CcMYB6-1 + CcbHLH1 was 10.8 mg/g (fresh weight, FW), significantly higher than that in other combinations. Notably, there was no significant difference between SK + CcMYB6-1 and CcMYB6-1 + CcbHLH1# (p < 0.01) (Fig. 8b), suggesting the truncated CcbHLH1 didn't play a role in stimulating anthocyanin biosynthesis together with CcMYB6-1. These results revealed that the loss of the conserved multi-domains of CcbHLH1 led to the loss of the ability to up-regulate anthocyanin biosynthesis with CcMYB6-1, which can account for the anthocyanin absence in the white petals of cornflower.
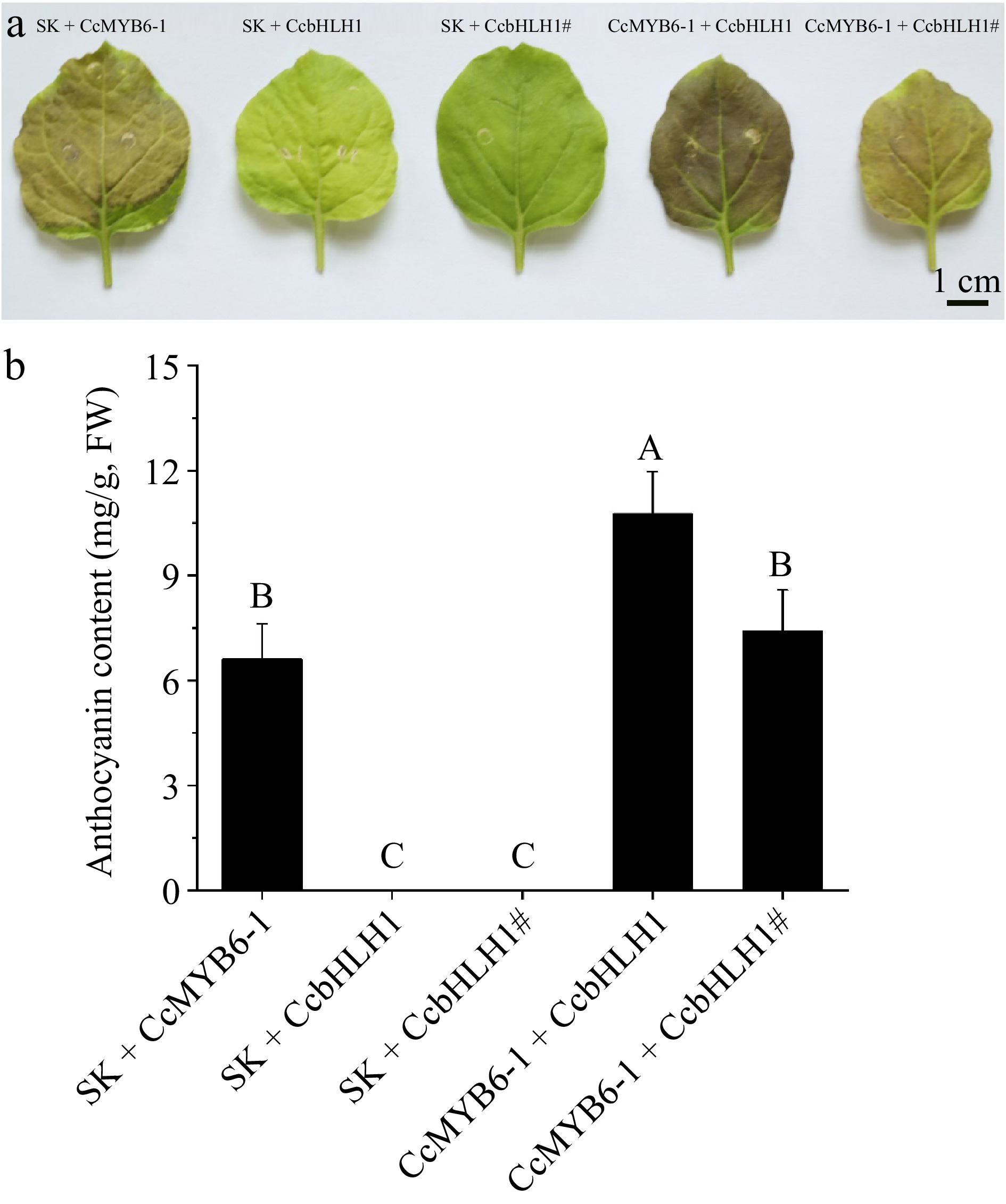
Figure 8.
Transient over-expression in tobacco leaves. (a) Tobacco leaves infiltrated with five different combinations including SK + CcMYB6-1, SK + CcbHLH1, SK + CcbHLH1#, CcMYB6-1 + CcbHLH1, and CcMYB6-1 + CcbHLH1#. The photo was captured 9 d after infiltration. (b) The semi-quantification of anthocyanin accumulating in tobacco leaves. Error bars were the S.E. of four biological replicates with each from at least two individual leaves. Different capital letters indicate significant difference at 1% level by Duncan's multiple test.
-
Cornflower is favored by its exquisite capitulum and abundant flower color variation thereby is widely used in garden design, cut flowers, and food decoration. In our previous study, six cornflower cultivars with pure colors were collected. The UPLC-MS/MS analysis revealed that pelargonidin and cyanidin derivatives were the main anthocyanins accumulating in the pink/red and blue/mauve/black petals, respectively, while there was no anthocyanin detected in the white cornflower petals, which only accumulated apigenin derivatives[3]. Here, anthocyanins were further monitored in both vegetative and reproductive organs of cornflower (Fig. 2). There was no anthocyanin detected in roots, stems, and leaves, suggesting the anthocyanin deficiency in vegetative organs of cornflower. Notably, anthocyanins accumulated slightly in the sepals, and increased with petal development in DTPB, while no anthocyanin was found in both sepals and petals of DTPW, indicating its complete anthocyanin absence, which was further explained by the block of structural genes including CcF3'H, CcDFR, CcGT, and CcAT (Supplementary Fig. S2).
To date, CcMYB6-1 and CcbHLH1 have been functionally characterized as positive regulators involved in the anthocyanin biosynthesis in cornflower. Considering that MYBs mutation usually leads to the anthocyanin deficiency and white phenotype in Raphanus sativus taproots, grapes, strawberries, and citrus[35−38], we focused on the gene expression and sequence isolation of CcMYB6-1. Unexpectedly, the transcript level of CcMYB6-1 was significantly higher in DTPW petals (p < 0.01, Fig. 2), and its full-length sequence in DTPW was entirely consistent with that in DTPB (Fig. 3), which was distinct from the CgsMYB12 mutation caused by a 1-bp deletion in the white basal region of Clarkia gracilis[39], the FaMYB10 mutation caused by an AG insertion in the white-fleshed strawberry[37], as well as the LsTT2 mutation in the white seeds of lettuce[40]. Notably, CcbHLH1 transcript was undetectable in DTPW (Fig. 2), consistent with the absence of structural gene expression and anthocyanin accumulation. The following gene isolation and multi-sequence alignment showed that CcbHLH1 in the white cornflower was spontaneously truncated to 1,125 bp, losing the basic helix-loop-helix domain in the N-terminal and the ACT-like domain in the C-terminal (Fig. 3), two necessary domains in regulatory activity, the mutation of which usually results in anthocyanin decrease[41,42].
Generally, the base deletion or insertion, transposon insertion, and alternative splicing will give rise to the premature stop codon and frameshift, finally leading to the truncation of coding genes and anthocyanin loss[43−48]. In this study, the truncation of CcbHLH1 is the result of alternative splicing by comparing the genome sequence with two transcripts (Fig. 4). The truncated CcbHLH1# protein is still localized in the nucleus and could interact with CcMYB6-1 (Figs 5 & 6), suggesting the loss of necessary domains doesn't change its functional position and interaction relationship, which may be explained by the invariant MYB-interaction region in the N-terminal[18]. The following dual-luciferase assay revealed that CcbHLH1 alone couldn't stimulate the trans-activity of the CcDFR promoter, suggesting its function in a CcMYB6-1-dependent way (Fig. 7), which was different from petunia anthocyanin1, also a bHLH2 clade TF, that directly activates the expression of the dfrA gene[27]. However, the enhanced trans-activation of CcDFR promoter and anthocyanin biosynthesis ability when co-expressed with CcMYB6-1 disappeared after CcbHLH1 truncation (Figs 7 & 8), suggesting the loss of basic helix-loop-helix domain and ACT-like domain contributed to the regulatory inactivation, similar to the cases in chrysanthemum, tomato and petunia[29,49,50].
A possible explanation is that the truncated TFs function as inhibitors involved in either competing with the functional protein for activation sites of structural genes or the formation of effective MYB-bHLH-WD40 protein complex, thus significantly affects anthocyanin levels. In maize, the dominant mutant C1-I, a truncated MYB transcription factor acts as anthocyanin inhibitor by competing C1 for activator sites of the biosynthetic genes like R1-nj[51]. Truncated MYBs or bHLHs usually lose the original ability to form the MBW complex thus leading to the anthocyanin decrease and color fading like chrysanthemum, radish, and wheat[29,52,53]. Differently, the truncated CcbHLH1 of cornflower retains its interaction with CcMYB6-1, but this CcbHLH1#-CcMYB6-1 complex can't effectively enhance the trans-activity of CcDFR promoter and anthocyanin accumulation in tobacco leaves like CcbHLH1-CcMYB6-1 complex (Figs 7 & 8), suggesting that the truncated variant inhibits anthocyanin biosynthesis by forming a dysfunctional complex.
Alternative splicing plays a key regulatory role in anthocyanin accumulation, leading to the color variation of higher plant organs. The anthocyanin-free phenotype of the eggplant efc1 mutant is caused by the retention of the second intron in DFR by improper splicing[54]. In tomato and Brassica napus, alternative splicing brings the truncated R2R3-MYB protein without the key R3 domain, resulting in the non-interaction with bHLH transcription factor, and finally leading to anthocyanin loss[55,56]. Interestingly, there are three kinds of splicing variants of bHLH2 in chrysanthemum, namely, the activator CmbHLH2 with complete domains and the strongest regulatory effect, the activator CmbHLH2.1 with 26 amino acids difference at the C-terminal and less regulatory effect, as well as the dysfunctional CmbHLH2short with only partial MIR domain at the N-terminal[24,29,30], suggesting the more domains lost, the more functions disappear. The missing multi-domains in cornflower CcbHLH1# is also caused by alternative splicing, leading to the complete anthocyanin loss in DTPW. Together, these findings reveal alternative splicing may play a potential role in modulating anthocyanin biosynthesis in cornflower. The regulatory mechanism underlying alternative splicing remains to be seen in the near future.
-
The transcription activator in cornflower, CcbHLH1, was truncated to 1,125 bp because of alternative splicing, losing multi-domains at the C-terminal. The mutated CcbHLH1 protein could interact with CcMYB6-1, but lose the ability to trans-activate promoter activity of anthocyanin biosynthetic genes and to induce anthocyanin biosynthesis thereby, which resulted in the complete anthocyanin absence in the white cornflower. These obtained results provide insights into the molecular mechanism underlying the white color transition in higher plants.
This research was supported by the National Natural Science Foundation, China (No. 32101579 and 32171849), the Shandong Province Natural Science Foundation, China (No. ZR2021QC143) and Shandong students' project for innovation training (S202210452029).
-
The authors confirm contribution to the paper as follows: study conception and design: Deng C, Dai S; data collection and analysis: Deng C, Zheng X, Wang J, Li Y, Li J, Lu M, Gao R, Ji C, Hao Q; draft manuscript preparation: Deng C, Zheng X, Wang J, Li Y, Li J, Lu M, Gao R, Ji C, Hao Q. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article and its supplementary information files.
-
The authors declare that they have no conflict of interest.
- Supplementary Table S1 Primer information used in the present study.
- Supplementary Fig. S1 The spectrum feature of extracts from different tissues of two cornflower cultivars detected by spectrophotometer.
- Supplementary Fig. S2 The spatio expression pattern of anthocyanin biosynthetic genes in different cornflower cultivars. Different capital letters indicate significant difference at 1% level by Duncan's multiple test.
- Supplementary Fig. S3 The amplification of CcMYB6-1 and CcbHLH1 in DTPB and DTPW. (a) Agarose gel electrophoresis of the full-length sequence of CcMYB6-1; (b) Schematic diagram of the distribution of designed primers to amplify CcbHLH1 fragments; (c) Agarose gel electrophoresis of the partial fragments of CcbHLH1.
- Supplementary Fig. S4 The fluorescence signal in tobacco leaf infiltrated with CcMYB6-1, CcbHLH1 or CcbHLH1#.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Deng C, Zheng X, Wang J, Li Y, Li J, et al. 2024. Alternative splicing of activator CcbHLH1 gene accounts for anthocyanin absence in white cornflower. Ornamental Plant Research 4: e029 doi: 10.48130/opr-0024-0028
Alternative splicing of activator CcbHLH1 gene accounts for anthocyanin absence in white cornflower
- Received: 05 March 2024
- Revised: 10 September 2024
- Accepted: 21 October 2024
- Published online: 21 November 2024
Abstract: Anthocyanins play crucial roles in conferring multicolored characteristics to higher plant organs. Previous studies have investigated the absence of anthocyanin accumulation in white cornflower petals, where the anthocyanin biosynthesis pathway was obstructed by CcF3H. However, the genetic regulation mechanism underlying anthocyanin absence remained unclear. In the present study, the full-length sequences of two transcription activators CcMYB6-1 and CcbHLH1 were isolated in white cornflower petals. The multi-sequence alignment analysis revealed that there was no difference in the CcMYB6-1 sequence between the blue and white petals, while CcbHLH1 was truncated to a length of 1,125 bp on account of alternative splicing, resulting in a frame-shift mutation and premature codon, leading to the loss of basic, helix-loop-helix domains. The truncated CcbHLH1 could still interact with CcMYB6-1 as demonstrated by yeast-two hybrid and bimolecular luminescence complementary assays but lost the ability to enhance the trans-activation of CcDFR promoter using dual-luciferase assay. Transient over-expression of mutated CcbHLH1 in tobacco leaves resulted in a significant decrease in anthocyanin production. These results suggested the alternative splicing of CcbHLH1 caused the incapacity of trans-activating the anthocyanin biosynthetic pathway together with CcMYB6-1, finally leading to anthocyanin absence in white cornflower. The present findings further shed light on the genetic regulation mechanism of anthocyanin biosynthesis in cornflower and enrich the knowledge underlying the white color transition in higher plants.
-
Key words:
- Alternative splicing /
- Anthocyanin absence /
- Cornflower /
- CcbHLH1













