-
Transplant production in the greenhouse is a well-known technique for commercial high-value vegetable production[1]. The use of soilless mix for transplant production is popular among commercial greenhouse growers as it provides optimum growing conditions and promotes growth[2]. Generally, peat moss is used in soilless potting mixes in commercial greenhouses[3]. However, excessive use of peat is creating environmental issues of imbalance in carbon budgeting[4]. After the disturbance due to peat extraction, peat lands which act as carbon sinks, turn into a source of carbon dioxide, releasing it into the atmosphere. This is why the negative carbon footprint of peat mining demands the need for other alternative sustainable substrate media for the horticulture industry[4−6].
Several soilless alternative media have been tested including coconut coir, sawdust, bark, compost, and biochar[3]. Among them, the combination of biochar-compost to replace peat has been given importance because of their combined effect in creating a conducive growth media environment and promoting plant growth[7−9]. Álvarez et al.[3] reported that biochar and compost can be used as a part of the growing medium to partially substitute peat. It is known that once applied, biochar can be effective for more than 100 years in field soil conditions[10] but short-term studies in soilless media in the greenhouse can explain the immediate effect of biochar[11]. A study suggests that the 25% pelletized biochar inclusion in sphagnum peat moss improved media hydraulic properties at high matric potentials while increasing pH in the forest seedling production system[12].
Previous studies support the idea that biochar inclusion in a soilless production system improves the physicochemical properties of the media but full replacement of peat with a greater proportion of biochar may show negative effects[13]. Venkataramani et al.[8] reported that soilless peat media amended with different types and proportions of biochar-compost can enhance the concentration of some essential macronutrients like P, Ca, and S in the media. It is believed that these two components amend the soilless media and promote plant productivity. It is important to see the effect of biochar and compost combination on seedling productivity. The transplant productivity also depends upon the healthy root system of the transplants[14]. A healthy root system helps to establish the transplant in the growing media which is dependent upon the media properties[15]. The development of the root system directly influences the ability of transplants to obtain nutrients from the media[16]. While the interest is growing in biochar-compost combination use in soilless media, their effect on seedling establishment has been yet under-explained.
Cucumber (Cucumis sativus L.) is one of the important vegetable crops that is largely consumed in the US. According to the report, the import of fresh cucumbers for salads or for snacking has increased between 1970−2020 reflecting that domestic production cannot fulfill the increasing demand of greenhouse-produced cucumbers[17]. One of the major problems with less cucumber production in the greenhouse could be the lack of selection of appropriate media composition and healthy transplant production in greenhouse industries. Proper selection of soilless media is the base for the successful production of cucumber or any transplanted vegetables.
Nair & Carpenter[15] reported that studies are concentrated on the production of mature plants by transplanting into a biochar-amended medium; however, there is a knowledge gap in the direct seeding of vegetable crops in the biochar-amended with compost medium. It is equally important to understand the effect of different amendments like biochar and compost inclusion on germination[15] and the quality of seedlings produced. Hence, there is a need for studies to evaluate biochar of different types with a proper combination with other media components such as compost for healthy cucumber transplant production.
Many studies have reported the effect of biochar-compost inclusion in soilless media on plants' performance but changes in the physical and chemical properties of the media are still overlooked. Venkataramani et al.[8] tried to expand knowledge on the successful production of cucumber in biochar-compost amended soilless media but lacked how such integration can influence the seedling phase of the plant. Also, how peat replacement with biochar-compost can bring change in the roots of the seedling plants has to be understood well before using these components in media on a large scale. Hence, the objective of this study was to compare the combinations of three biochar types at different proportions with cotton-burr-compost in the standard growing media, and quantify their effect on media physicochemical properties, cucumber germination, and shoot and root growth of cucumber seedlings.
-
Two study trials were conducted at Horticulture Gardens and Greenhouse Complex, Texas Tech University, Lubbock, TX, USA (lat. 33°35'2.72'' N, long. 101°53'12.95'' W). Trial 1 was conducted from 12 February 2022 to 12 March 2022, while Trial 2 was conducted from 30 March 2022 to 30 April 2022. The greenhouse was east-west oriented, and most of the sunlight was allowed to be transmitted inside the greenhouse. Both experiments were conducted in a greenhouse where temperatures of 30 °C during the day and 25 °C at night were maintained throughout the experiment period. No additional lighting was provided.
The slicing type of cucumber cultivar 'Picolino' (Johnny's Selected Seeds, ME, USA) was used for the trials. Seven different treatments were prepared using different volume combinations of peat, perlite, vermiculite, cotton-burr-compost, hardwood biochar, softwood biochar, and hemp biochar[8]. Control had peat:perlite:vermiculite (50:25:25, %v/v). The peat had 85% peat moss and 15% perlite (BM6 Berger, Saint-Modeste, QC, USA), which was combined with perlite and vermiculite to make standard peat media. In the remaining six treatments, peat was either partially [12.5% (v/v) biochar and 12.5% (v/v) compost (Partial hardwood: PHW, Partial softwood: PSW, and Partial hemp: PH)] or completely [25% (v/v) biochar and 25% (v/v) compost (Full hardwood: FHW, Full softwood: FSW, and Full hemp: FH)] replaced by biochar-compost mixture. Cotton-burr-compost was purchased from Back to Nature (Slaton, TX, USA). Hardwood (HW) and softwood (SW) biochars were obtained from Wakefield Agricultural Carbon LLC (Columbia, MO, USA). Hemp biochar was manually prepared by burning hemp residues in an oxygen-limited 208-liter capacity steel drum for 24 h. The physicochemical properties of hardwood and softwood biochar have already been reported in Singh et al.[18]. No fertilizers were added for seedling growth in the greenhouse. Seedlings were grown in 6-cell trays where each cell was 3.8 cm2 (length × breadth) and 5.7 cm deep (9Greenbox, CA, USA). The treatments were randomized in a Randomized Complete Block Design with three replications. Each 6-cell tray was a replication leading to a total of 18 seedlings per treatment. The seedlings were watered daily with tap water in the greenhouse, and the experiment was terminated 30 d after the sowing of seeds.
pH and Electrical Conductivity (EC)
-
The pH and EC of the media were measured by a handheld Orion Star A325 pH/Conductivity Portable Multiparameter Meter (ThermoScientific, MA, USA) only at the beginning of the experiment. Four samples were collected for each treatment media which were mixed, and the measurements were done by following the protocol previously described by Singh et al.[18].
Water retention capacity and thermal conductivity of media
-
Four core samples were collected using 5 cm × 5 cm stainless steel cores (AMS, American Falls, ID, USA) with the help of a core sampler for each media treatment. The samples were subjected to different potential pressures (-ve) [0 (saturation), 0.1, 0.3, 1, 10, and 15 bar] in a pressure plate apparatus (Soil moisture Equipment Corp., Goleta, CA, USA) to obtain a water retention curve. Porosity and plant available water (PAW) were calculated as described by Singh et al.[18]. The thermal conductivity of the samples was recorded simultaneously at different pressure by using the KD2Pro Thermal Conductivity Meter (TR-1 sensor, Edaphic Scientific, Australia).
Germination and seedling height
-
The germination count was made when the plumule structure appeared above the media surface. The germination count was recorded at 4, 5, 6, 7, and 15 Days After Planting (DAP) in Trial 1 and at 5, 7, 8, 9, 11, and 13 DAP in Trial 2. Seedling height was measured from the base of the seedling to the tip from three random seedlings from each 6-cell trays of each treatment after 11, 15, 19, 25, and 31 DAP in Trial 1 and 13, 18, 21, 26, and 30 DAP in Trial 2.
Shoot and root growth parameters
-
Two random seedlings from each replication of each treatment were selected after 32 DAP in Trial 1 and 31 DAP in Trial 2. The selected seedlings were separated into shoots and roots. The shoot length and root length were measured with the help of a measuring ruler and the elongation ratio or shoot:root ratio (cm/cm) was calculated by dividing shoot length by root length[19]. The leaves were separated from the seedling stem and the number of leaves was counted, and the leaf area was measured using a benchtop leaf area meter (LI-3100C Area Meter, Lincoln, NE, USA). The fresh shoot weight was measured using a balance scale.
Root parameters
-
Each root sample was washed placing them over the fine-mesh sieve strainer to avoid root escaping. Forceps were used to remove any attached soilless particles in the roots. The clean root samples were stored in 50 ml falcon tubes filled with 40 mm of deionized water. The root samples were scanned by a scanner (EPSON V800; Reagent Instruments Inc.) and analyzed for different root parameters including root length density (RLD), root surface area density (RSAD), and root fineness classification (% of total root length of each diameter class) using WinRHIZO Pro version 2016a software (Regent Instruments Inc., Canada). Four diameter classes a) 0–0.5 mm, b) 0.5–1.0 mm, and c) 1.0−1.5 mm d) > 1.5 mm were categorized for the total root length and % of the total root length for each diameter classes were calculated[20].
Statistical analysis
-
The data were analyzed using R version 3.5.2 with the Agricolae package 1.3–5 to evaluate the effect of each treatment on the measured parameters. Data for each trial were analyzed separately. The values presented in tables and graphs are the average values for each media treatment. Analysis of variance (ANOVA) was performed, and the Duncan Multiple Range Test (DMRT) test at a 5% level of significance was used for the mean separation. SigmaPlot version 14 (Systat Software, San Jose, CA, USA) was used for preparing graphs and figures.
-
The pH of control was acidic (5.2), whereas biochar-amended media increased the pH to 7.8−9.4 (Table 1). The highest pH was recorded in FH. The pH of biochar-compost amended media was increased by 50%–73% than the control and was comparatively higher in full biochar-compost compared to partial biochar-compost replacement media (FHW vs. PHW, FSW vs. PSW, FH vs. PH). This shows that the amount of biochar in the media can affect the pH of the media. Comparing different types of biochar-compost integrations, hemp biochar-compost treatment maintained the most basic nature of the media compared to hardwood and softwood biochar-compost amendments in both replacement rates. This indicates that the feedstock source of the biochar can also influence the pH of the media. The result suggests that biochar as a liming agent prevents acidity in the growing media. The results can be supported by the study of Martins et al.[21] which demonstrated that biochar pH was 8.76 whereas the compost pH was near to neutral (7.91). Similar results were obtained in previous studies which reported that the biochar itself is basic in nature which increases the pH of the substrate[22−24]. The buffering capacity of the biochar can be attributed to the negative charge on the surface of the biochar which prevents rapid change in pH[25]. This indicates that biochar contributes to maintaining pH which depends upon the proportion as well as the feedstock source of the biochar in the substrate media.
Table 1. The pH and EC of different media treatments used in greenhouse cucumber experiments at Lubbock, TX, USA in 2022.
Soilless media type pH EC (dS/m) C 5.2 0.178 PHW 7.9 0.378 PSW 7.8 0.38 PH 8.7 0.74 FHW 9.0 0.592 FSW 8.3 0.612 FH 9.4 1.078 C: Control, FHW: Full hardwood, FSW: Full softwood, FH: Full hemp, PHW: Partial hardwood, PSW: Partial softwood, PH: Partial hemp. The EC of the control was 0.178 dS/m which was very low compared to biochar-compost media types. The highest EC was reported in FH i.e., 1.078 dS/m. This shows that EC was increased 2–6 times by the biochar-compost amendment compared to the control. Comparing partial and full replacement treatments, the full replacement treatments (FHW, FSW, FH) had higher EC compared to partial replacement treatments (PHW, PSW, PH). Similar to pH, hemp biochar-compost treatment had higher EC values both in partial as well as full replacements compared to hardwood or softwood biochar-compost treatments. Huang & Gu[25] suggested that the increase in EC due to biochar inclusion in container substrate can be due to the high EC of the biochar. The high EC of the substrate can also be due to the high pH of the media component[26]. The high EC of the media except the control could be due to the combined effect of biochar and compost. Similarly, Venkataramani et al.[8] reported that the EC of the media was increased with an addition of both biochar and compost amendments compared to standard peat media. In contrast, Martins et al.[21] suggested that compost is responsible for increasing the EC of the media than biochar. This is because biochar mixes had low cation exchange capacity (CEC) and low EC because of poor extractable macronutrients than the compost. The EC varied from 0.378−1.078 dS/m in biochar-compost amendment treatments which falls under the normal range (< 1.2 dS/m) of the EC requirement for cucumber seedlings[27]. This indicates that biochar and compost combination maintain pH and EC in the normal range and encourages partial availability of nutrients for growing seedlings and, hence, ameliorating the media.
Water retention capacity and thermal conductivity of media
-
The effect of the biochar-compost inclusion in the media on water retention capacity and thermal conductivity over different pressure bars (-ve) is presented in Figs 1 & 2, respectively. Biochar-compost amendment improved the hydro-physical properties of soilless media. In Trial 1, PH maintained comparatively greater volumetric water content over different pressure regimes compared to other treatments although the difference was not significant (Fig. 1a). But in Trial 2, FH maintained a noticeable improvement in water retention capacity over different pressure regimes (Fig. 1b). Both FH and PH in both trials had greater water retention capacity. Kim et al.[28] reported that water content in the media was increased because of the improvement in pore space for water storage in biochar-amended growing media. We also noticed that biochar-compost amended media improved porosity and enhanced the plant’s available water. Porosity was 3% greater in PH in Trial 1, and 3% and 1.5% more in PHW and FH, respectively in Trial 2 compared to the control (data not shown). Plant available water (PAW) was 21%, 14%, 8% and 2% greater in PH, FSW, FH and PSW, respectively in Trial 1, and 16%, 12%, 9%, 7% and 1% greater in PH, FSW, PHW, FH and PSW, respectively in Trail 2 compared to the control (data not shown). However, other biochar-compost amended media did not improve porosity and PAW, which implies that change in hydro-physical properties depends on peat and biochar-compost properties as well as their proportion in the media. Similarly, Nieto et al.[29] reported that the hydro-physical properties are influenced by the types and amounts of different components of the media.
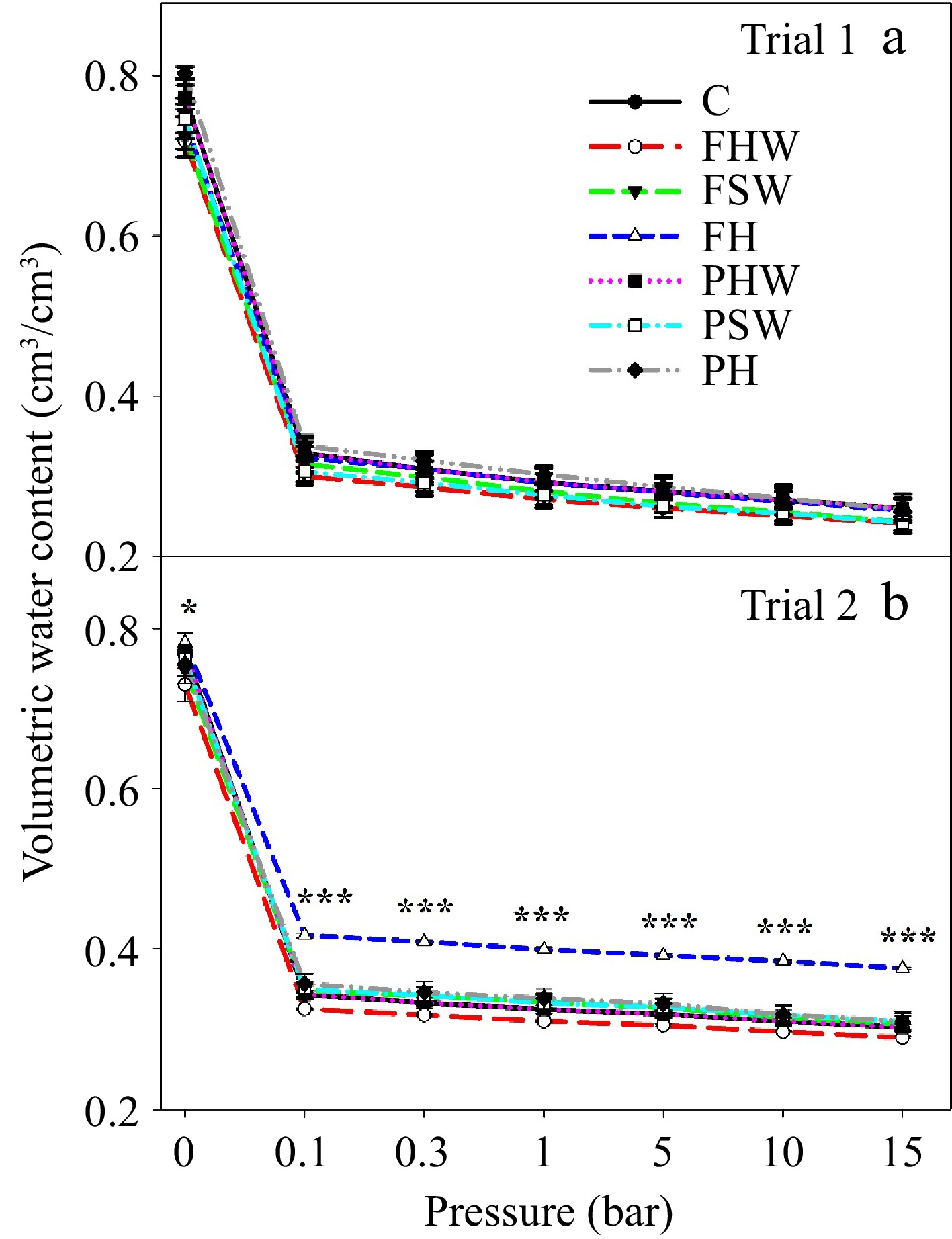
Figure 1.
Water retention curve of different media in (a) Trial 1 and (b) Trial 2. The pressures applied (-ve) were 0, 0.1, 0.3, 1, 5, 10, and 15 bar. Bars in the graph indicate the standard error. * and *** indicate significant differences at a p ≤ 0.05 and p ≤ 0.001 level of significance, respectively. C: Control, FHW: Full hardwood, FSW: Full softwood, FH: Full hemp, PHW: Partial hardwood, PSW: Partial softwood, PH: Partial hemp.
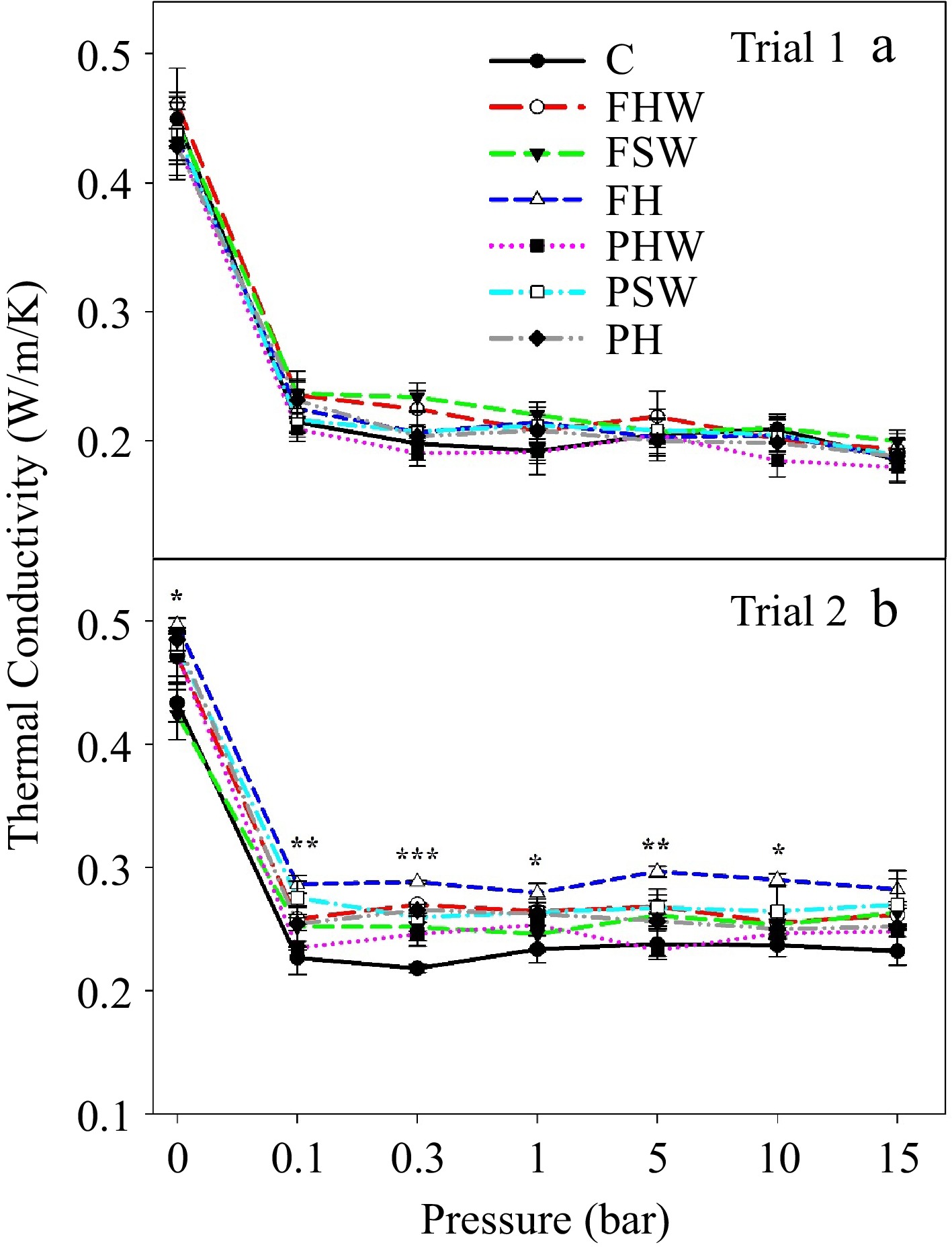
Figure 2.
Thermal conductivity (W/m/K) of different media in (a) Trial 1 and (b) Trial 2. The pressures applied (-ve) were 0, 0.1, 0.3, 1, 5, 10, and 15 bar. Bars in the graph indicate the standard error and *, **, *** indicate significant differences at a p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001 level of significance, respectively. C: Control, FHW: Full hardwood, FSW: Full softwood, FH: Full hemp, PHW: Partial hardwood, PSW: Partial softwood, PH: Partial hemp.
The thermal conductivity of the media depends upon the amount of perlite and water content in it[30,31]. Javid[31] reported that thermal conductivity improves substantially when perlite is present because it establishes a strong relationship with heat transfer in the medium. In this study, the difference in the thermal property (conductivity) was solely for water content in the media because perlite was present in every treatment (Fig. 2a & b). The greater the water content in the media, the higher will be the thermal conductivity because water is a better medium for heat transfer than air[30]. That is why there is a sharp increase in thermal conductivity in FH in Trial 2 (Fig. 2b) when there is a high volume of water present in the media throughout the pressure bars (Fig. 1b). Thermal conductivity can be an important factor for soilless substrate vegetable production as it maintains the media temperature and helps in root growth and development. The temperature of the media environment can be a prime factor for germination and root system development. It can be of more importance during the winter season for protected agriculture, where growers had to spend lots of money maintaining an optimum environment to promote seedling growth. The high thermal conductivity facilitates heat transfer, which in the short term improves water and nutrient uptake by reducing water viscosity and by increasing membrane permeability[32]. Considering this, most of the biochar-compost amended treatments, especially FH can be of choice that not only holds water for a longer time but also improves the thermal properties of media for optimum seedling growth.
Germination and seedling height
-
In both trials, biochar-compost-amended media accelerated the germination process compared to the control (Fig. 3). In Trial 1, FH, PH, and PSW had all the germination (18 seeds) at 6 DAP whereas FHW, PH, and PSW had full germination after 9 DAP in Trial 2. Except for others, PHW had only 17 seeds germinated in Trial 1 at 11 DAP (Fig. 3a) whereas FH, FSW, and, PHW had only 17 seeds germinated in Trail 2 (Fig. 3b). It is evident that the control achieved full germination only at 11 DAP in both trials. Also, looking at the trend in both trials, FH and PH tend to have a faster germination rate than other treatments. Phosphorus (P), a macronutrient responsible for seed germination is found in higher concentrations in hemp biochar-compost amended media than in other biochar-compost treatments[8]. It may also be due to the higher water retention capacity of hemp biochar amended media (Fig. 1), which could have helped in faster germination. It has been well-reported that biochar can enhance the germination of different vegetable crops including tomato[11,33], pepper[11,15,34], cucumber[35] and lettuce[36]. However, there are some reports that show biochar did not influence or negatively influenced the germination of some crops[37−39]. This difference in germination using different biochar amendments is because of the difference in concentration of nutrients present in the biochar, which is largely affected by the source of biochar[40,41].
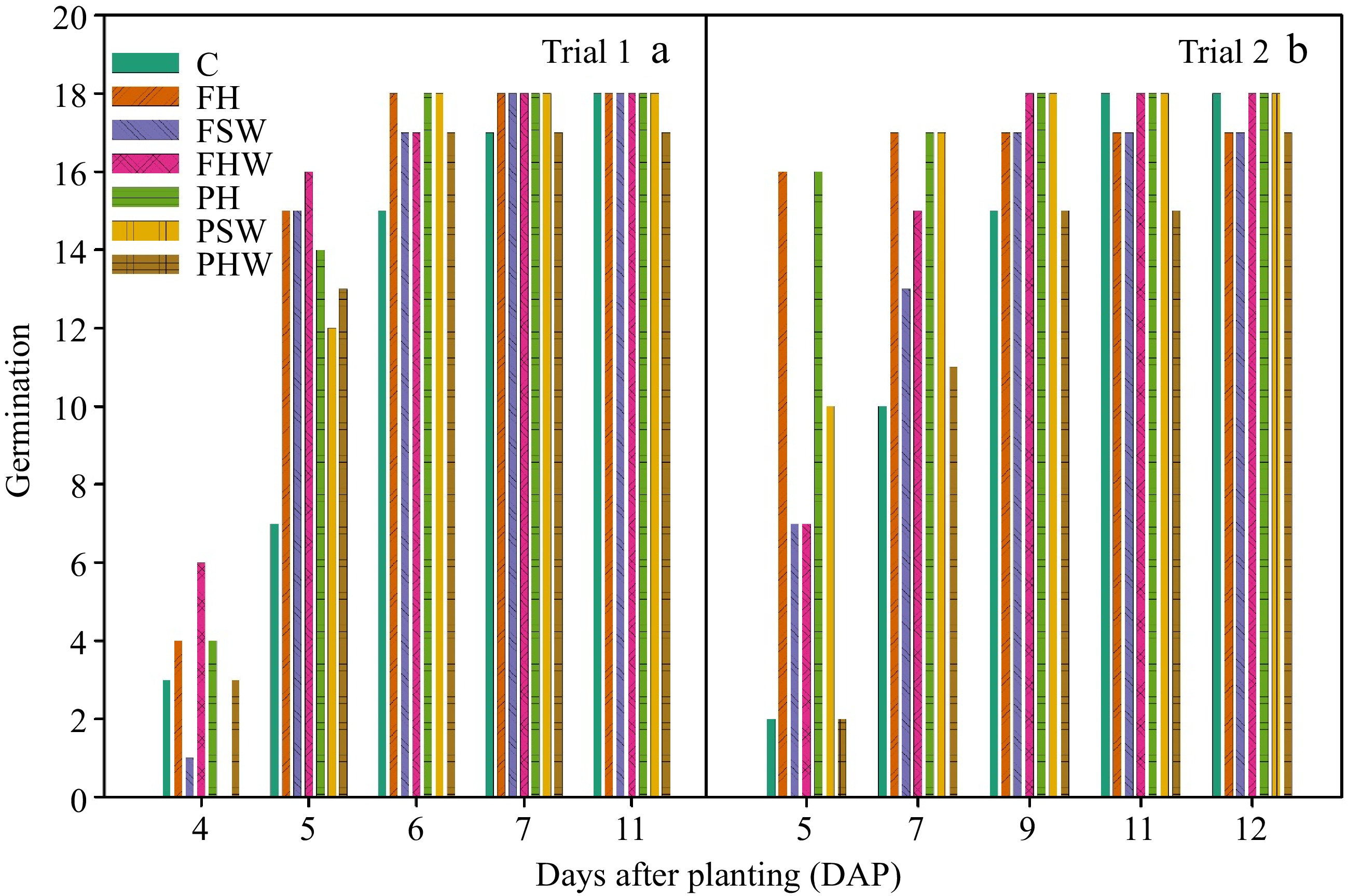
Figure 3.
Germination counts of cucumber seeds in (a) Trial 1 and (b) Trial 2. Germination count was recorded at 4, 5, 6, 7, and 11 DAP in Trial 1 and 5, 7, 9, 11, and 12 DAP in Trial 2. C: Control, FHW: Full hardwood, FSW: Full softwood, FH: Full hemp, PHW: Partial hardwood, PSW: Partial softwood, PH: Partial hemp.
The significant difference in height among the different media started from the very beginning (Fig. 4). FSW media constantly maintained a greater seedling height in both trials. In Trial 1, FSW had the highest seedling height of 8.5 cm after 31 DAP (Fig. 4a); whereas, in Trail 2, it had a 5.9 cm seedling height after 30 DAP (Fig. 4b). Comparing full and partial replacement treatments, FSW had significantly taller seedlings than FHW and FH, and PSW and PHW had significantly taller seedlings compared to PH in Trial 1. FSW and PHW produced significantly taller seedlings compared to PSW and FHW, respectively. In Trial 2, FSW had significantly taller seedlings compared to FHW and FH, but the height was comparable among PSW, PHW, and PH. It was only FSW that had significantly taller seedlings than PSW among full and partial replacement treatments. This finding is in accordance with the previous study of Venkataramani et al.[8], where authors reported that softwood biochar amended with compost can accelerate the height of cucumber seedlings compared to other biochar types. Control had the poorest growth and had a height of 4.3 cm and 3.16 cm in Trial 1 and Trial 2, respectively. The poor growth in the control can be attributed to low pH and EC, where these were higher in other biochar-compost amended treatments (Table 1). The low pH impacts the seedlings growth by limiting the availability of essential nutrients and increasing the toxicity of aluminum (Al) and manganese (Mn). The low EC indicates ion imbalance and less dissolved salts including essential nutrients. It may also be because the control had relatively poor water holding capacity (Fig. 1) and lower thermal conductivity (Fig. 2). This indicates that control media tends to dry up quickly which reduced the growth of cucumber seedlings. The results are supported by previous studies where seedling height was enhanced by biochar amendment compared to un-amended media[42,43]. Another reason for the increased height in cucumber seedlings in biochar-compost amended media was the cotton-bur compost present in the media. The compost might have provided some nutrients to the seedlings in a manner that stimulated growth in all treatments except control. A similar result was obtained by Tosca et al.[44] where Photinia sps growth was accelerated in biochar cum compost media compared to control. The growth of ornamental crops like Syngonium podophyllum was also improved through the inclusion of biochar or biochar-compost mixture in peat substrate[45]. The seedling height has an important role for transplant nursery growers[15], and it is better to have a vigorous seedling transplant than a poor stunted one for sustainable crop production.
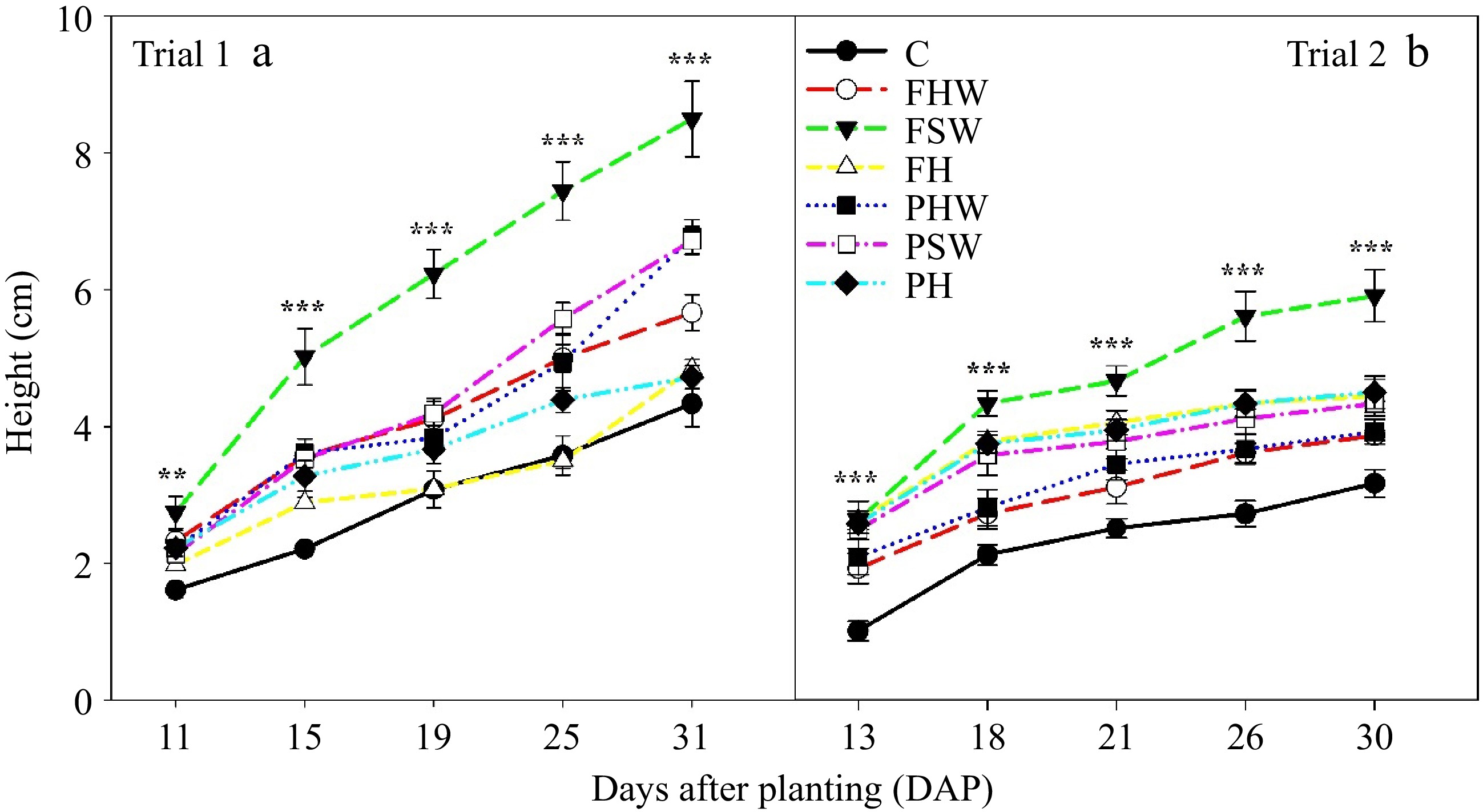
Figure 4.
Cucumber seedling height in (a) Trial 1 and (b) Trial 2. Bars in the graph indicate the standard error. ** and *** indicate significant differences at a p ≤ 0.01 and p ≤ 0.001 level of significance, respectively. C: Control, FHW: Full hardwood, FSW: Full softwood, FH: Full hemp, PHW: Partial hardwood, PSW: Partial softwood, PH: Partial hemp.
Shoot and root growth parameters
-
The different media showed significant effects on fresh shoot weight, the number of leaves, leaf area, and shoot:root ratio of cucumber seedlings (Table 2). Fresh shoot weight, number of leaves, and leaf area were significantly higher in FSW compared to other full, and partial replacement treatments (Table 2). In Trial 1, control had 82%, 35%, 82%, and 45 % less fresh shoot weight, number of leaves, leaf area, and shoot: root ratio, respectively compared to FSW. In Trial 2, fresh shoot weight, number of leaves, leaf area, and shoot: root ratio reduced by 84%, 31%, 85%, and 47%, respectively in control compared to FSW. FSW only had significantly greater fresh shoot weight, number of leaves and leaf area compared to other full replacement treatments (FHW and FH) as well as partial replacements treatments (PSW, PHW and PH) in both trials. Similar results were obtained by Venkataramani et al.[8] on the dry biomass of cucumber plants where softwood biochar-compost amended treatments contributed to higher vegetative growth of cucumber plants compared to other treatments. The greater fresh weight, number of leaves, leaf area, and shoot:root ratio in FSW can be due to greater seedling height compared to other treatments (Fig. 4). The results show that biochar when used as a component of the media with compost can produce a healthier and more vigorous transplant compared to control. This claim is supported by Parkash & Singh[46] and Regmi et al.[47] who reported that biochar has a larger surface area which tends to allow more water and nutrient retention leading to vigorous young growing plants. Another study by Dispenza et al.[22] reported that conifer biochar in potting mixture media increased shoot weight, leaf number, and leaf area in Euphorbia × lomi plant. Due to the presence of humic acid in the compost, seedlings are protected through osmoregulation and ion-homeostasis against potential salt stress caused by biochar and compost leading to healthy growth of the seedlings[21,48,49].
Table 2. Effect of different media types on fresh shoot weight (g), number of leaves, leaf area, and shoot:root ratio of cucumber seedlings in Trial 1 and Trial 2 in greenhouse experiment at Lubbock, TX, USA in 2022.
Trial Media Fresh shoot weight (g) No. of leaves Leaf area (cm2) Shoot:Root Trial 1 C 0.82c* 3.00d 11.88c 0.30c FHW 1.85bc 4.16ab 23.26bc 0.41bc FSW 4.77a 4.66a 69.07a 0.55a FH 1.54bc 3.66bc 17.97bc 0.35c PHW 1.68bc 4.00b 22.48bc 0.48ab PSW 2.15b 4.00b 30.29b 0.39bc PH 1.38bc 3.16cd 16.70bc 0.37bc Trial 2 C 0.56b 3.00b 8.36b 0.19d FHW 1.26b 3.00b 16.07b 0.30bc FSW 3.52a 4.33a 54.60a 0.36ab FH 1.55b 3.16b 17.08b 0.41a PHW 1.16b 3.00b 16.77b 0.26cd PSW 1.40b 3.16b 22.02b 0.34abc PH 1.20b 2.83b 15.85b 0.33bc The values represent the mean value of individual treatment in each of the trials. * The different letters in a column and s indicate a significant difference at p ≤ 0.05. C: Control, FHW: Full hardwood, FSW: Full softwood, FH: Full hemp, PHW: Partial hardwood, PSW: Partial softwood, PH: Partial hemp. RLD significantly varied among the media treatments (Table 3). In Trial 1, RLD increased by 37%, 38%, 21%, and 8% in FSW, FH, PHW, PSW, respectively; whereas, in Trial 2, it increased by 43%, 3%, 17%, 14% in FSW, PHW, PSW, PH, respectively compared to control. RLD decreased by 7% and 12% in FHW and PH, respectively in Trial 1 whereas it decreased by 8% and 26% in FHW and FH, respectively in Trial 2 compared to control. FSW and FH had significantly higher RLD compared to PSW and PH, respectively but PHW increased RLD significantly than FHW in Trial 1. FSW had significantly higher RLD than PSW but contrarily PH increased RLD more than FH in Trial 2 while others remained comparable. RSAD was also affected by various media types (Table 3). In Trial 1, RSAD increased by 38%, 46%, and 24% in FSW, FH, and PHW, respectively, and decreased by 3%, 15%, and 1% in FHW, PSW, and PH, respectively compared to control. However, all the biochar amended media enhanced RSAD in Trial 2 by 19%, 55%, 9%, 23%, 35%, and 28% in FHW, FSW, FH, PHW, PSW, and PH respectively, compared to control. Similar to RLD, FSW and FH had significantly higher RSAD compared to PSW and PH, respectively, and PHW increased RSAD significantly than FHW in Trial 1. A significant increase in RSAD was obtained in FSW compared to PHW in Trial 2. Comparing different types of biochar-compost treatments, softwood biochar- and hemp biochar-compost amended media in full replacement increased RLD and RSAD in Trial 1, but only softwood biochar full replacement outnumbered the other two full replacements in Trial 2. PHW had significantly higher RLD than PH, and significantly greater RSAD than PSW and PH in Trial 1. No significant difference was observed among partial replacements for RLD and RSAD in Trial 2. This suggests that roots are sensitive to media components which also affect other physical properties of the media. The space for root growth is limited in soilless culture, and hence seedling tends to increase root density[50]. This increased root density indicates more oxygen and nutrient consumption per unit volume at the root zone. In such limited rooting volume, the seedling tends to utilize the available resources like space, nutrients, and water[32,51]. In this study, the greater RLD and RSAD in biochar-compost amended treatments indicate that this combination promotes root growth compared to the peat-dominated control treatment. Root growth is crucial for transplants because transplanting shock may occur with poor root structure, and later reduce the overall performance of the crop[14]. The roots are important for anchorage, water, and nutrient uptake throughout the growth stages of the plant[52,53]. The increase in roots biomass suggests that the transplants produced in the amendment treatments will help in successful acclimatization and adaptability after transplanting.
Table 3. Effect of different media types on root length density (RLD), root surface area density (RSAD), and root classification categories (0.0−0.5 mm, 0.5−1.0 mm, 1.0−1.5 mm, > 1.5 mm) of cucumber seedlings in Trial 1 and Trial 2 in greenhouse experiment at Lubbock, TX, USA in 2022.
Trial Media RLD
(cm/
cm3)RSAD
(cm2/
cm3)Root classification category (% of total root length per diameter) 0.0−0.5
mm0.5−1.0
mm1−1.5
mm> 1.5
mmTrial 1 C 6.08c* 0.76c 77.22b 18.52ab 2.59d 1.66cd FHW 5.69c 0.74c 77.21b 17.47ab 2.43de 2.88ab FSW 9.69a 1.22ab 76.59bc 18.04ab 3.35bc 2.00c FH 9.88a 1.41a 73.87cd 18.20ab 4.38a 3.54a PHW 8.17ab 1.00b 78.09b 16.60b 3.13cd 2.16bc PSW 6.62bc 0.66c 84.82a 12.23c 1.73e 1.20d PH 5.38c 0.75c 73.35d 19.79a 3.98ab 2.86ab Trial 2 C 6.58bc 0.71d 83.57a 12.67d 2.05d 1.69ab FHW 6.04bc 0.88bcd 70.04cd 24.25a 3.83ab 1.86ab FSW 11.48a 1.58a 75.58b 18.04c 3.96ab 2.40a FH 5.21c 0.78cd 69.18d 24.07a 4.46a 2.28a PHW 6.80bc 0.92bcd 73.75bc 21.70ab 3.09bc 1.44b PSW 7.97b 1.09b 75.17b 19.91bc 3.24bc 1.65ab PH 7.65b 0.99bc 75.50b 20.46bc 2.72cd 1.30b The values represent the mean value of individual treatment in each of the trials. * The different letters in a column and s indicate a significant difference at p ≤ 0.05. C: Control, FHW: Full hardwood, FSW: Full softwood, FH: Full hemp, PHW: Partial hardwood, PSW: Partial softwood, PH: Partial hemp. The distribution of roots among different diameter classes also varied among media treatments (Table 3). Although there was some variation in diameter classes (% of total root length per diameter) in Trial 1 and Trial 2, but it is noticeable that the greatest share in root diameter classes was 73.35% to 84.82% in 0.0–0.5 mm followed by 12.23% to 19.79% in 0.5–1.0 mm, 1.73% to 4.38% in 1.0–1.5 mm, and 1.20% to 3.54% in > 1.5 mm. This indicates that cucumber seedling has more proportion of fine roots with a diameter of 0.0–0.5 mm which can penetrate the tiny pores of media for extracting water and nutrients as well as for developing a robust root system. Judd et al.[52] suggest that plants grown in soilless substrates tend to develop finer roots. A similar proportion of roots was found by Parkash et al.[20] in cucumbers in field conditions. This implies that cucumber plants tend to develop fine roots in soilless media as well as in field soil conditions, and extracts water and nutrients for developing plants. Another, interesting finding of this experiment was to record the highest root distribution in FH among all the classes (> 0.5 mm) in both trials. PH also contributed a similar proportion in Trial 1 only. This may be attributed to higher pH and EC, and greater water retaining capacity of the hemp biochar-cotton compost amendment in the media compared to other treatments. This is in agreement with the statement by Balliu et al.[32] and Jones[54] that the physical and chemical properties of the media alter nutrient and water availability which in turn affect root growth. Thermal property (temperature) can also influence root growth. Balliu et al.[32] reported the vital role of root zone temperature in enhancing the root density of lateral roots of peas. This suggests how roots interact with the complex media environment and alter root morphology depending on water and nutrient availability and thermal property. Bláha[55] even reports the importance of root growth by stating a conception that a 1% change in root system can bring a 2% change in crop yield which was further highlighted by Balliu et al.[32]. All this signifies the importance of the study of root system architecture that will explain its adaptation to the growing environment and its contribution to crop growth and performance.
-
This experiment aimed to prepare a suitable substrate combination by adding different biochar types with cotton burr compost at different proportions to see their effect on the seedling performance of cucumber. Biochar-compost amended treatments acted as a liming agent that prevented the media from acidity and improved electrical conductivity. Among the treatments, full hemp biochar-compost treatment (FH) had the largest increase in pH and EC. FH significantly increased the volumetric water content and thermal properties of the media, indicating its potential to retain more water in the media and maintain warmness around the growing roots. Germination was accelerated by the biochar-compost amendment, which can be profitable for industries that require a supply of a large number of transplants for commercial production. Full softwood biochar-compost treatment (FSW) increased shoot as well as root growth of cucumber seedlings. This study suggests that biochar-compost can replace peat either partially or even completely and improve properties for vigorous seedling production.
-
The authors confirm contribution to the paper as follows: conceptualization: Kafle A, Singh M, Singh S; methodology: Kafle A, Singh M, Venkataramani S; data collection: Kafle A, Venkataramani S; data curation: Kafle A; software and analysis: Kafle A; writing-original draft preparation: Kafle A; supervision: Singh S; review and editing: Deb S, Singh S, Saini R. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
This project did not receive any external funding. We would like to acknowledge the Horticulture Gardens and Greenhouse Complex, Department of Plant and Soil Science, Texas Tech University for providing all the necessary facilities and support during the study period. We also thank Preetaman Bajwa for assisting in the experiment.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Kafle A, Singh S, Singh M, Venkataramani S, Saini R, et al. 2024. Effect of biochar-compost amendment on soilless media properties and cucumber seedling establishment. Technology in Horticulture 4: e001 doi: 10.48130/tihort-0023-0029
Effect of biochar-compost amendment on soilless media properties and cucumber seedling establishment
- Received: 01 November 2023
- Revised: 05 December 2023
- Accepted: 22 December 2023
- Published online: 12 January 2024
Abstract: The interest in replacing peat with biochar in soilless substrate media is increasing however, the proportion of biochar inclusion in the media which could improve the media properties as well as the seedling performance of vegetables is still unknown. Therefore, the aim of the current study was to test different biochar types at different proportions with cotton burr-compost in the growing media on hydro-physicochemical properties of media, germination, and shoot and root growth of cucumber seedlings. Two trials were conducted in 2022 using cv 'Picolino' in Randomized Complete Block Design with three replications. Control included peat:perlite:vermiculite at 50:25:25 %v/v. Other treatments were prepared to replace peat either partially [12.5% (v/v) biochar and 12.5% (v/v) compost (Partial hardwood: PHW, Partial softwood: PSW, and Partial hemp: PH)] or completely [25% (v/v) biochar and 25% (v/v) compost (Full hardwood: FHW, Full softwood: FSW, and Full hemp: FH)]. Biochar-compost inclusion increased the pH and EC of the medium. Water retention capacity and thermal conductivity of the medium were found to be improved in hemp biochar-compost treatment. FSW increased fresh shoot weight, the number of leaves, leaf area, and shoot:root ratio by 83%, 33%, 84%, and 46%, respectively compared to control. Root length density and root surface area density increased by 40% and 47%, respectively in FSW compared to control. Most of the biochar-compost amended media performed better for the cucumber seedling production compared to control showing a possibility of replacing the peat in the media for sustainable transplant production.
-
Key words:
- Peat /
- Transplants /
- Root architecture /
- Leaf area












