-
Microgreens are leafy greens consisting of fully developed cotyledons and the first true leaves. They are currently in high demand in diverse population settings. Consumers in urban areas are looking for local fresh produce to avoid the nutritional loss often associated with long transport and storage of produce[1]. Leafy greens can also be grown in a space station to supplement a space crew's diet[2−4]. Microgreens are different from sprouts because microgreens are grown under light in comparison to sprouts grown under dark conditions[5]. They are typically harvested between 7 and 20 d after gemination. Researchers have provided information on the nutritional quality of microgreens based on various cultivars and plant cultivation conditions[6]. In comparison to mature plants, microgreens are rich in phytochemicals, vitamins, and mineral elements (macro-elements: Ca, Mg, P, and micro-elements: Fe, Zn, K, Se, Cu and Mn)[7−9]. Sun et al.[10] found the nutrient levels up to nine times higher in microgreens compared to mature greens. In addition, high levels of nitrate are present in certain microgreens although in lower levels than baby leaves[11].
The phytochemical composition and antioxidant capacity especially varies in the Brassicaceae family[12−15]. Glucosinolates are unique secondary metabolites mainly found in Brassica vegetables and beneficial for human health[16]. Polyphenols act as reducing agents, hydrogen donating antioxidants and singlet oxygen quenchers most often in response to biotic or abiotic stress[17,18]. Polyphenols are distributed in different plant tissues and organs at different concentrations. The most widespread groups of polyphenols in Brassica species are the flavonoids (mainly flavonols but also anthocyanins). The anthocyanins are an important group of plant pigments with multifunctional components of food due to their antioxidant activity and other beneficial biological properties. Despite the microgreens small size, they have high levels of health beneficial secondary metabolites which contribute to their value in the market[19−23].
The progression through the initial stages of post-germination in plant ontogeny has a substantial impact on inducing notable changes in both the physical and chemical composition, as well as the phytochemical content of plants[12,24,25]. Determining the optimal harvest stage for microgreens involves a meticulous balance, considering the maximization of yield, ensuring swift crop turnover, and achieving elevated sensory and functional quality. However, there is limited information on the optimal harvest time in terms of both high microgreen yield and good quality. Kyriacou et al.[17] specifically characterized microgreens as greens that are harvested when the first pair of true leaves appears, and cotyledons are fully expanded and still turgid. Nevertheless, there is variability in the reported developmental stages at harvest across different studies, with some suggesting harvest at the cotyledonary stage[26,27], others at the emergence of the first true leaf[12,28,29], and still others at the second true leaf[27,30−32]. Beyond the evident shift in fresh biomass during successive ontogenic stages, previous research has highlighted noteworthy alterations in secondary plant metabolites and mineral content, which are integral components contributing to the functional qualities of vegetables[33]. In the present study, two Brassicaceae species broccoli (Brassica oleracea var. italica Plenck) and radish (Raphanus sativus L.) were grown in the controlled growth chamber and harvested at different times after first true leaf emergence. The microgreen yield, nutritional quality and shelf-life were evaluated to determine the best harvest time.
-
Organic broccoli cultivar 'Di Cicco' and radish cultivar 'Rambo' seeds were purchased from True Leaf Market Seed Company (Salt Lake City, UT, USA). The experiment was carried out at the US department of agriculture (USDA)-Agricultural Research Center (ARS) (Food Quality Laboratory), Beltsville, Maryland, USA, in a controlled growth chamber (GC-20 Bigfoot series, Biochambers, Winnipeg, MB, Canada). The average 1,000 seed weight for the broccoli and radish was 3.15 g and 8.13 g. The germination rate was above 90% for both cultivars. Seeds were sown at a density of ~66,000 and 26,000 seeds/m2 for broccoli and radish, respectively on plastic trays (W × L × D: 25 cm × 52 cm × 6 cm) filled with a BioStrate™ felt hydroponic growing mats (Grow-Tech, Inc, Brentwood, TN, USA). A tray was used to grow two replicates for a total of two trays for each experimental unit. Seeds germinated in darkness at 25 °C and 90% relative humidity (RH) for 4 d. Daily irrigation of ~0.4 L water was manually applied to each tray. Deionized water (pH 5.8) was used to keep the mats moist throughout the experiment. On the fifth day after sowing, the seedlings were grown in hydroponic conditions at 25 °C and RH at 65/75% accordingly under a 16/8 h photoperiod regime delivered by Light Emitting Diode (LED) panels (K5 Series XL750, Kind LED, Santa Rosa, CA, USA). Photosynthetic photon flux density at the canopy level was maintained at 200 ± 10 mmol/m2/s of white light. The plants were fertilized by plant fertilizer (General Hydroponics, Inc; FloraGro 2-1-6 fertilizer, Sebastopol, CA, USA) by hand spray at day 7 after sowing the seeds. Daily rotation of tray positions maintained an equal distribution of light and humidity over the chamber growth surface. The first true leaf of broccoli and radish emerged on day 10 and day 7 respectively. Then the microgreens were harvested at days 11, 12, 13 and 14 for broccoli or days 8, 9, 10, and 11 for radish. Microgreens were harvested by hand with sterilized scissors. For each harvest day, three replicates were harvested. The length of ten randomly picked hypocotyls were measured for hypocotyl lengths for each replicate. The experiments were repeated three times.
Evaluation of microgreen decay
-
On harvest day, 10 g samples of each microgreen species microgreens were harvested and stored in food-grade linear low-density polyethylene (LLDPE) bags. The LLDPE bags were 16 cm × 12 cm size (8.0 g per bag) and 51 μm thickness. The samples were then stored in three replications at 4 °C (in the dark) and RH 40%. For decay assessment, microgreens were visually inspected on days 4, 8, 12, and 16 of storage. The evaluation system was modified from Rennie et al.[34] based on the physical condition of the microgreens (Table 1). The extent of decay was recorded as a percentage of the total. The rating scale ranged from 1% (no decay) to 100% (degraded product). The higher the percentage, the more decayed plants. A marketability threshold was set at 50% on the visual quality scale. A rating of above 50% designated an unmarketable product.
Table 1. Microgreen decay evaluation scale based on overall visual analysis modified from Rennie et al.[34].
Score Description Visual quality 0% Essentially free from defects, freshly harvested Excellent < 10% Minor defects, not objectionable Good < 25% Moderately objectionable defects Fair > 50% Excessive defects, not saleable, marketability threshold Poor 100% Unusable, degraded product Very poor Evaluation of leakage exudate
-
On harvest day and each day of decay analysis (4, 8, 12, and 16), 10 g per replicate of harvested microgreens were weighed and placed in 100 mL of deionized water (DI) for 30 min. An electrical conductivity reading of the water was taken using the Oakton WD-35419-20 Eutech series benchtop meter (Cole-Parmer, Vernon Hills, Illinois, USA). Microgreens were stored in plastic containers and frozen at −80 °C. Percent leakage was obtained by dividing measured leakage by total leakage.
Chlorophyll analysis
-
One gram of each fresh broccoli sample and radish sample separately was added to 40 mL of 80% (v/v) acetone diluted in deionized water (DI) water and kept in darkness at room temperature (RT) until all the chlorophyll was cleared from the tissue. Absorbance of each sample was read at 663 nm and 646 nm using 1-mL cuvettes and a Bio-Rad SmartSpec™ plus UV–Vis spectrophotometer (Hercules, CA, USA). Total chlorophyll concentrations (mg/g F.W.) were calculated from absorbances (Abs) as described by Lichtenthaler & Buschmann[35].
Phenolics, glucosinolates, anthocyanins, and antioxidant activity analysis
-
Four grams of fresh microgreens sample were ground in a pestle and mortar using liquid nitrogen. Total glucosinolate content was evaluated by spectrophotometric estimation of Mawlong et al.[36]. For glucosinolate extractions, 0.1 g of ground fresh sample was added to 2-ml of 80% methanol in a 2-mL vial. Samples were incubated overnight or at least 12 h at RT. Tubes were centrifuged at 10,000 g for 4 min and supernatants were collected. One hundred µL of supernatant was added to a glass test tube with 300 µL water and 3 mL of 2 mM sodium tetrachloropalladate. Samples were incubated at RT for 1 h in the dark. Each sample (250 µL) was measured in triplicate in a multi-mode detection microplate reader, BioTek, Synergy™2 (Shoreline, WA, USA) at 425 nm. The glucosinolate concentration is calculated using the formula 1.40 + 118.86 × observation data (O.D.) at 425 nm.
For phenolic and anthocyanin analyses, 2.5 g of ground frozen sample was added to 5 mL of extraction buffer comprising formic acid, methanol and water in a 3:60:37 ratio, respectively. The samples were stored in a 50-mL falcon tube at 4 °C overnight. The following day, the samples were centrifuged at 20,000 g for 20 min at 4 °C. The supernatant was collected and divided for phenolics and anthocyanin analysis. One 300 µL sample was used to determine total phenolics and two 600 µL individual samples were used to determine total anthocyanins as described below.
Total phenolic content (TPC) was determined using the modified colorimetric Folin–Ciocalteu method[37]. For phenolic analysis, the 300 µL of supernatant sample and 300 µL of 0.2 M Folin–Ciocalteu reagent, and 2.1 mL water were added in a glass culture tube and mixed briefly. Then 75% sodium carbonate was added in a glass culture tube and samples were incubated at room temperature for 2 h in the dark. Each sample (250 µL) was measured in triplicates in a multi-mode detection microplate reader (BioTek, Synergy™, USA) at 765 nm. The total phenolic concentration is calculated using the formula ((O.D. 765 × 315.16 × 51) × 0.001). The same procedure was applied for standard solutions of different concentrations of gallic acid. The phenolic content was expressed as gallic acid (GA) equivalents.
Total anthocyanin content was measured according to the pH differential method in Giusti & Wrolstad[38] by two buffer systems: 0.4 M sodium acetate (pH = 4.5) and 0.025 M potassium chloride (pH = 1). For anthocyanin analysis, 600 µL supernatant sample and 2.4 mL of 0.025 M potassium chloride pH 1 were added in a glass culture tube. In addition, another 600 µL supernatant sample and 2.4 mL of 0.025 M potassium chloride pH 4.5 were also added to a separate glass culture tube. Samples were incubated at room temperature in the dark for 15 min. Each sample (250 µL) was measured in triplicate in a multi-mode detection microplate reader (BioTek, Synergy™) at 510 and 700 nm. The following formula was used to calculate total anthocyanins in mg/kg units: [(O.D. 510 pH 1 – O.D. 700 nm pH 1) – (O.D. 510 nm pH 4.5 – O.D. 700 nm pH 4.5) × 449.2 × 255 × 1,000] / 26,900.
The ferric reduction capacity of the tissues was determined using the ferric reducing antioxidant power (FRAP) assay based on Benzie & Strain[39]. The FRAP reagent was made from 300 mmol/L acetate buffer (pH 3.6) and 10 mmol 2,4,6-Tris(2-pyridyl)-1,3,5-triazine (TPTZ) in a 40 mmol/L HCl solution with 20 mmol/L FeCl3 in 10:1:1 ratio. 100 μl of the samples and 100 μl of the different concentrations of standard solutions (Trolox) were mixed with 900 μL FRAP reagent separately. Then, the samples and the standard were read at 595 nm with a microplate reader (BioTek, Synergy™). The standard Trolox curve was made with a concentration range of 10–800 μmol Trolox equivalents (TE). The total antioxidant activity was expressed as mg TE/100 g FW.
Data analysis
-
Quantitative measurements were analyzed in three replications per harvest day. Data was subjected to ordinary one-way ANOVA with Tukey's multiple comparisons test in GraphPad Software, LLC. Two-way ANOVA with Tukey's multiple comparisons test was performed for tissue decay after storage days. Two-way ANOVA with Tukey's multiple comparisons test was performed for tissue leakage after storage days. In all ANOVA tests, each p-value was adjusted to account for multiple comparisons, and this was reported as adj p-value. The asterisks in graphs represent statistical significance based on adj p-value.
Statistical regression for correlation of postharvest quality parameters was performed in Microsoft Excel. The statistical regression was performed separately for broccoli and radish microgreens. The correlation coefficient (r) values were shown. The asterisks in tables represent statistical significance between correlation coefficients as p-value equal to or less than 0.05.
-
Under our indoor growth conditions, we observed the first true leaf emergence in a few broccoli plants on day 10 post seed sowing. Thus, broccoli microgreens were harvested on days 11, 12, 13, and 14 because harvest day 11 is the start of the first true leaf emergence in about 10% of plants. On harvest day 13, there were about 75% of plants with emergence of first true leaf. There was no significant difference in the number of true leaves between harvest days 13 and 14. There was a higher microgreen yield (fresh weight) when comparing yield harvested on day 12 to day 11. The yield of broccoli microgreens was significantly higher (adj p <0.0001) when harvested on days 13 and 14 compared to on day 11 or 12 (Fig. 1c). The hypocotyl length was also significantly taller (adj p < 0.0001) for plants harvested at days 14 compared to days 11 and 12 (Fig. 1d). The harvest day positively correlated with the yield (correlation coefficient r = 0.946) and hypocotyl length (r = 0.868) (Table 2). The microgreen yield also positively correlated with the hypocotyl length (r = 0.872).
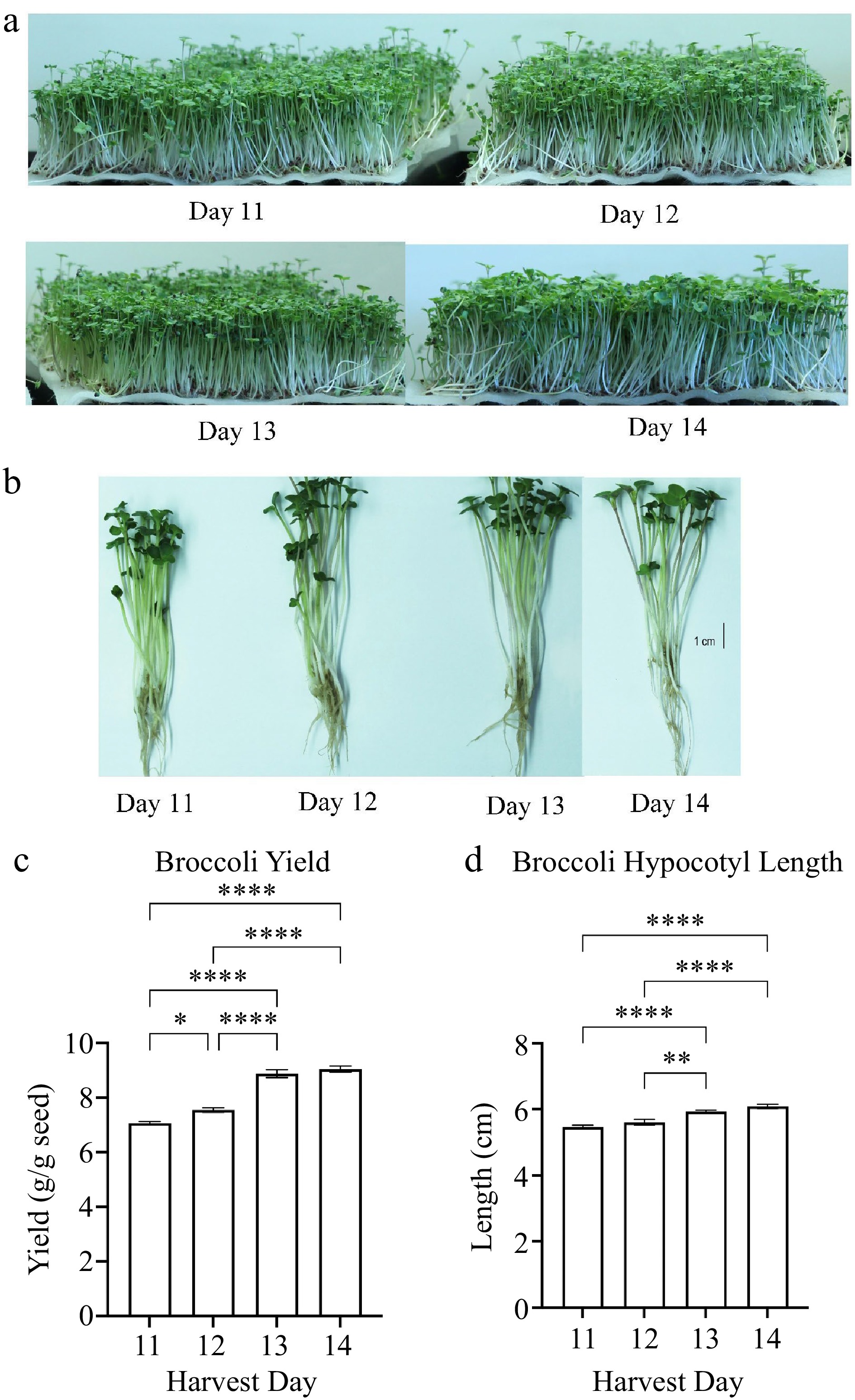
Figure 1.
(a), (b) Broccoli plants images and measurements of microgreen (c) yield and (d) plant height. Seedlings of broccoli cultivar Di Cicco grown hydroponically in BioStrate™ growing mats for 11 to 14 d post sowing. Microgreen yield and height were measured at harvest days 11 to 14. Bars represent standard error of the means. Asterisks indicate significant differences in yield or height between harvest days. * Indicates adjusted (adj) p-value = 0.0485; ** indicates adj p-value = 0.0043; **** indicates adj p-value < 0.0001.
Table 2. Correlation for broccoli microgreen quality traits.
Harvest day Plant yield Plant hypocotyl length Total chlorophyll Total
phenolicsTotal glucosinolates Total antioxidant activity Tissue decay after 16 storage days Tissue leakage after 16 storage days Harvest day 1.000 Plant yield 0.946**** 1.000 Plant hypocotyl length 0.868** 0.872** 1.000 Total chlorophyll 0.917**** 0.930**** 0.841** 1.000 Total phenolics −0.276 −0.286 −0.303 −0.343 1.000 Total glucosinolates −0.457 −0.461 −0.334 −0.421 0.350 1.000 Total antioxidant activity 0.735* 0.639* 0.731* 0.574* 0.073 0.053 1.000 Tissue decay after 16 storage days −0.789* −0.904**** −0.750* −0.794* 0.294 0.305 0.459 1.000 Tissue leakage after 16 storage days 0.151 0.320 0.313 0.097 0.202 0.040 0.413 0.334 1.000 The correlation coefficient (r) values are shown for comparisons of broccoli microgreens quality traits. The asterisks represent significance in r. * indicates 0.0021 < p-value ≤ 0.05; ** indicates 0.0002 < p-value ≤ 0.0021; *** indicates 0.0001 < p-value ≤ 0.0002; **** indicates p-value < 0.0001. To determine whether other vegetables had the same trend, we did a similar test on radish. Radish microgreens were harvested at the start of about 10% first true leaf emergence initiating on day 8 post sowing (Fig. 2a & b). On harvest day 10, there were about 75% of plants with emergence of first true leaf. The yield of radish microgreens was significantly higher (0.0002 < adj p-value ≤ 0.0021) when harvested at harvest days 10 and 11 compared to harvest days 8 and 9 post sowing (Fig. 2c). There was a positive correlation between harvest day and plant yield (r = 0.875) (Table 3). The hypocotyl length for radish microgreens was not affected by harvest day after first true leaf emergence (Fig. 2d).
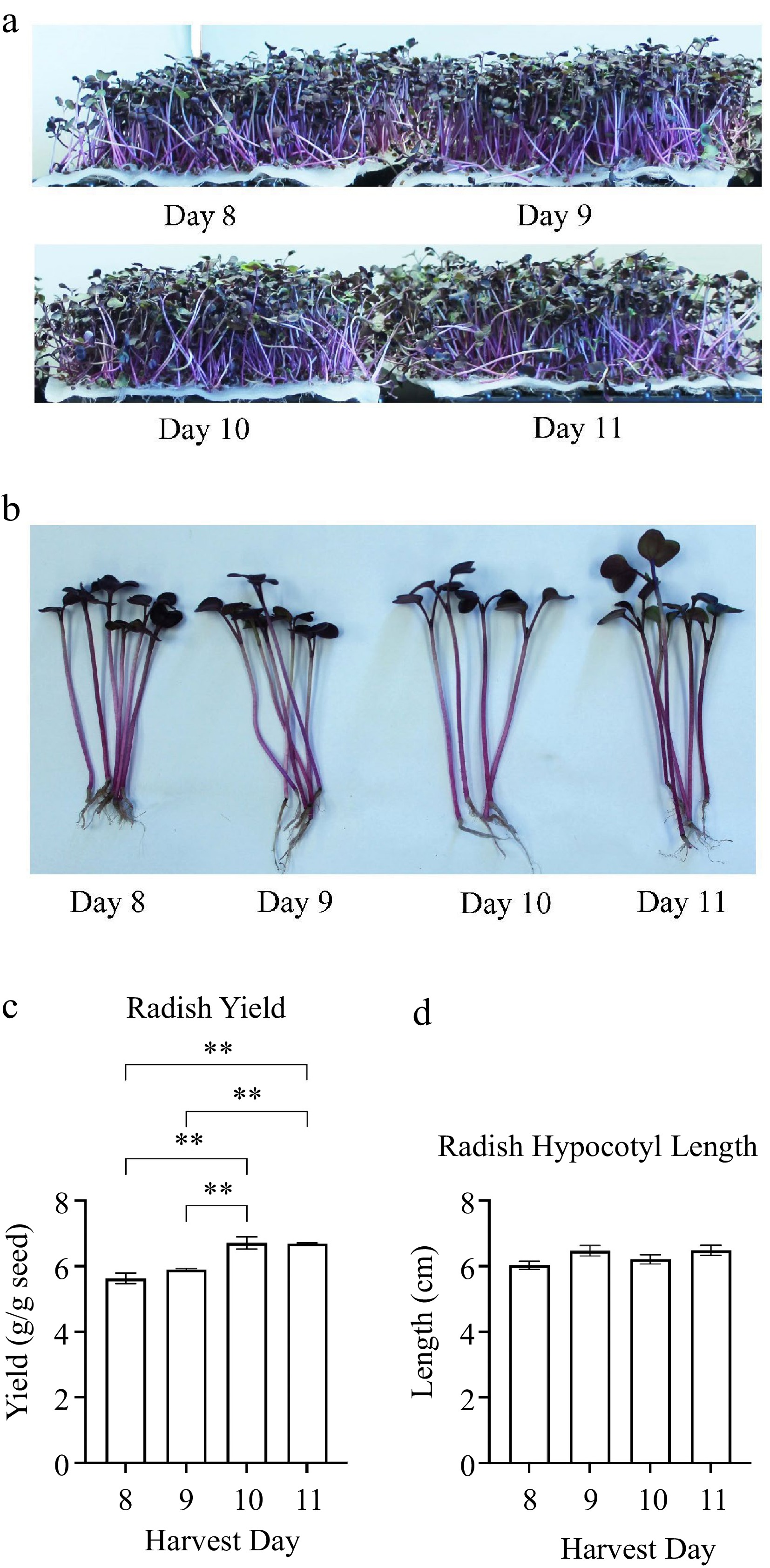
Figure 2.
(a), (b) Radish plant images and measurements of (c) yield and (d) plant height. Radish cultivar Rambo were grown hydroponically in BioStrate™ growing mats for 8 to 11 days post sowing. Radish microgreens' yield and height were measured on harvest days 8 to 11. Bars represent standard error of the means. Asterisks indicate significant differences between harvest days. ** Indicates 0.0002 < adj p-value ≤ 0.0021.
Table 3. Correlation for radish microgreens quality traits.
Harvest day Plant yield Plant hypocotyl length Total chlorophyll Total anthocyanins Total phenolics Total glucosinolates Total antioxidant activity Tissue decay after 16 storage days Tissue leakage after 16 storage days Harvest day 1.000 Plant yield 0.875** 1.000 Plant hypocotyl length 0.295 0.156 1.000 Total chlorophyll 0.025 0.026 0.331 1.000 Total anthocyanins −0.659* −0.391 −0.262 0.256 1.000 Total phenolics 0.293 0.490 0.237 0.107 −0.362 1.000 Total glucosinolates −0.267 −0.232 −0.014 0.085 0.488 −0.423 1.000 Total antioxidant activity 0.324 0.395 0.048 0.273 0.195 0.392 0.290 1.000 Tissue decay after 16 storage days −0.673* −0.724* −0.048 0.193 0.165 −0.317 0.030 0.411 1.000 Tissue leakage after 16 storage days 0.216 0.395 0.048 0.335 0.195 0.392 0.290 0.324 0.411 1.000 The correlation coefficient (r) values are shown for comparisons of radish microgreens quality traits. The asterisks represent significance in r, * indicates 0.0021 < p-value ≤ 0.05; ** indicates 0.0002 < p-value ≤ 0.0021. Effect of harvest day on total chlorophyll, phenolics, glucosinolates, anthocyanins, and antioxidant activity
-
The broccoli total chlorophyll content was significantly higher on harvest day 13 (0.75 mg/g FW) compared to harvest days 11 (0.62 mg/g FW) and 12 (0.66 mg/g FW) (Fig. 3a). The total chlorophyll content was highest on harvest day 14 (0.77 mg/g FW) (Fig. 3a). The harvest day positively correlated with the total chlorophyll content (r = 0.917) (Table 2). The plant yield and hypocotyl length also positively correlated with the total chlorophyll content (Table 2). However, the total glucosinolates in broccoli decreased on harvest days 12 (40.44 µmole/g), 13 (40.67 µmole/g), and 14 (40.84 µmole/g) compared to harvest day 11 (41.57 µmole/g) (Fig. 3b). There was not a significant change in total phenolics in broccoli for harvest days 11 to 14 (Fig. 3c). Neither phenolics nor glucosinolates correlated with the physiological or other nutrient qualities of broccoli microgreens (Table 2). The total antioxidant activity levels were the lowest at harvest day 14 (1.5 mg TE/100 g FW) compared to harvest day 11 (2.0 mg TE/100 g FW), harvest day 12 (2.1 mg TE/100 g FW), and harvest day 13 (1.9 mg TE/ 100 g FW) (Fig. 3d). The total antioxidant activity was comparatively similar between harvest days 11 to 13 in broccoli (Fig. 3d).
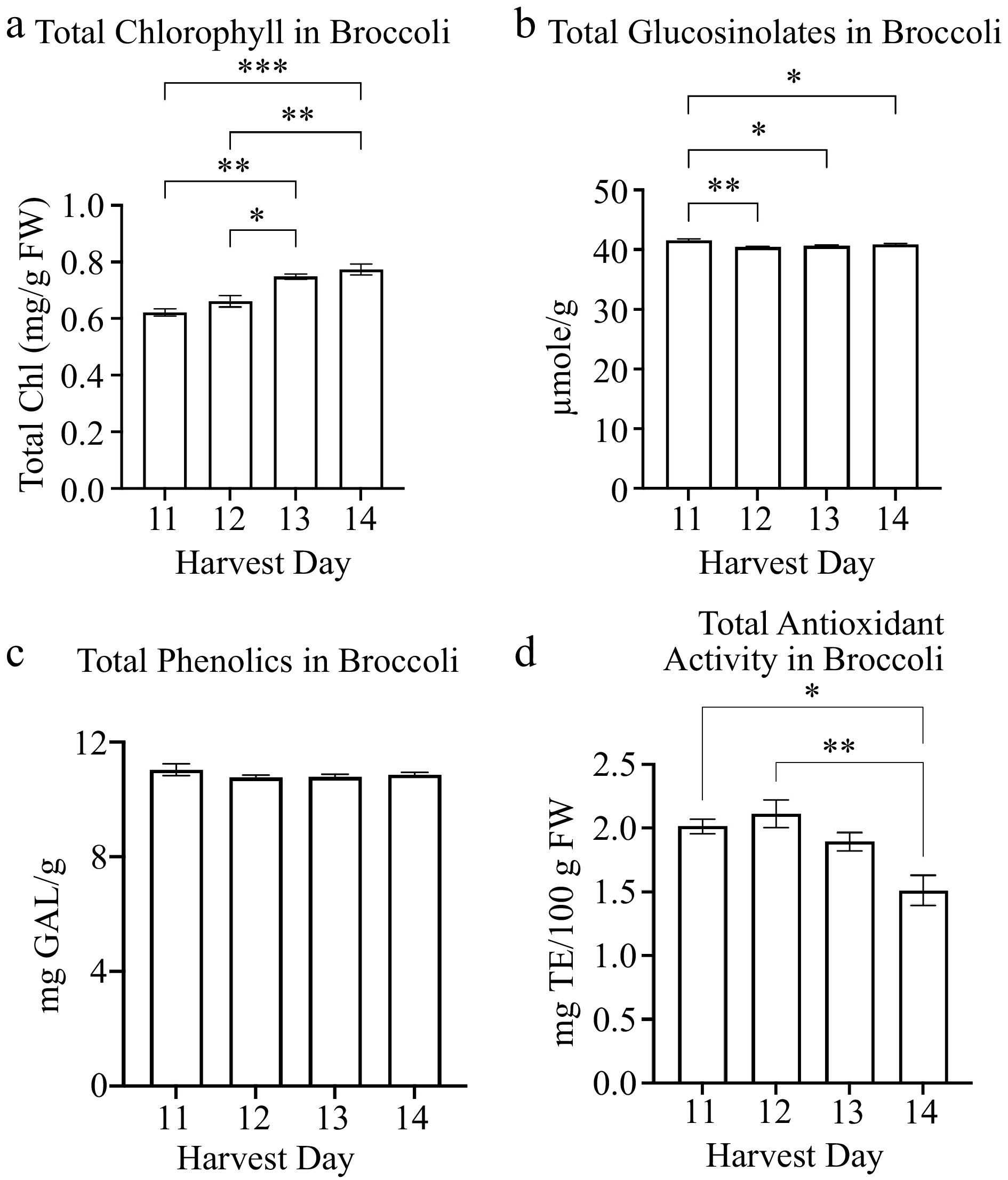
Figure 3.
Concentrations of broccoli microgreens (a) total chlorophyll and secondary metabolites: (b) glucosinolates, (c) phenolics and (d) antioxidant activity in broccoli microgreens for several harvest days is shown. Bars represent standard error of the means. Asterisks indicate significant differences in total chlorophyll or glucosinolates between harvest days. * Indicates 0.0021 < adj p-value ≤ 0.05; ** indicates 0.0002 < adj p-value ≤ 0.0021; *** indicates 0.0001 < adj p-value ≤ 0.0002.
The total chlorophyll content was similar between harvest days 8 to 11 in radish (Fig. 4a, Table 3). The total glucosinolates and phenolics were comparatively similar between harvest days 8 to 11 in radish (Fig. 4b & c, Table 3). The radish total antioxidant activity was significantly higher on harvest day 10 (4.2 mg TE/100 g FW) compared to harvest day 8 (3.02 mg TE/100 g FW), 9 (3.8 mg TE/100 g FW), and 11 (3.2 mg TE/100 g FW) (Fig. 4d). The total antioxidant activity was lowest on harvest day 8 (3.02 mg TE/100 g FW) (Fig. 4d). The harvest day positively increased the total antioxidant activity during microgreen growth from day 8 to 10. However, the total antioxidant activity in radish decreased on harvest day 11 (Fig. 4d). An additional antioxidant measured in radish was total anthocyanin content. There was a negative correlation between total anthocyanins and harvest day (r = −0.659) (Table 3). The anthocyanin levels were the lowest at harvest day 11 (4,300 mg/kg) compared to harvest day 8 (5,562 mg/kg), 9 (5,748 mg/kg), and 10 (5,322 mg/kg) (Fig. 4e).
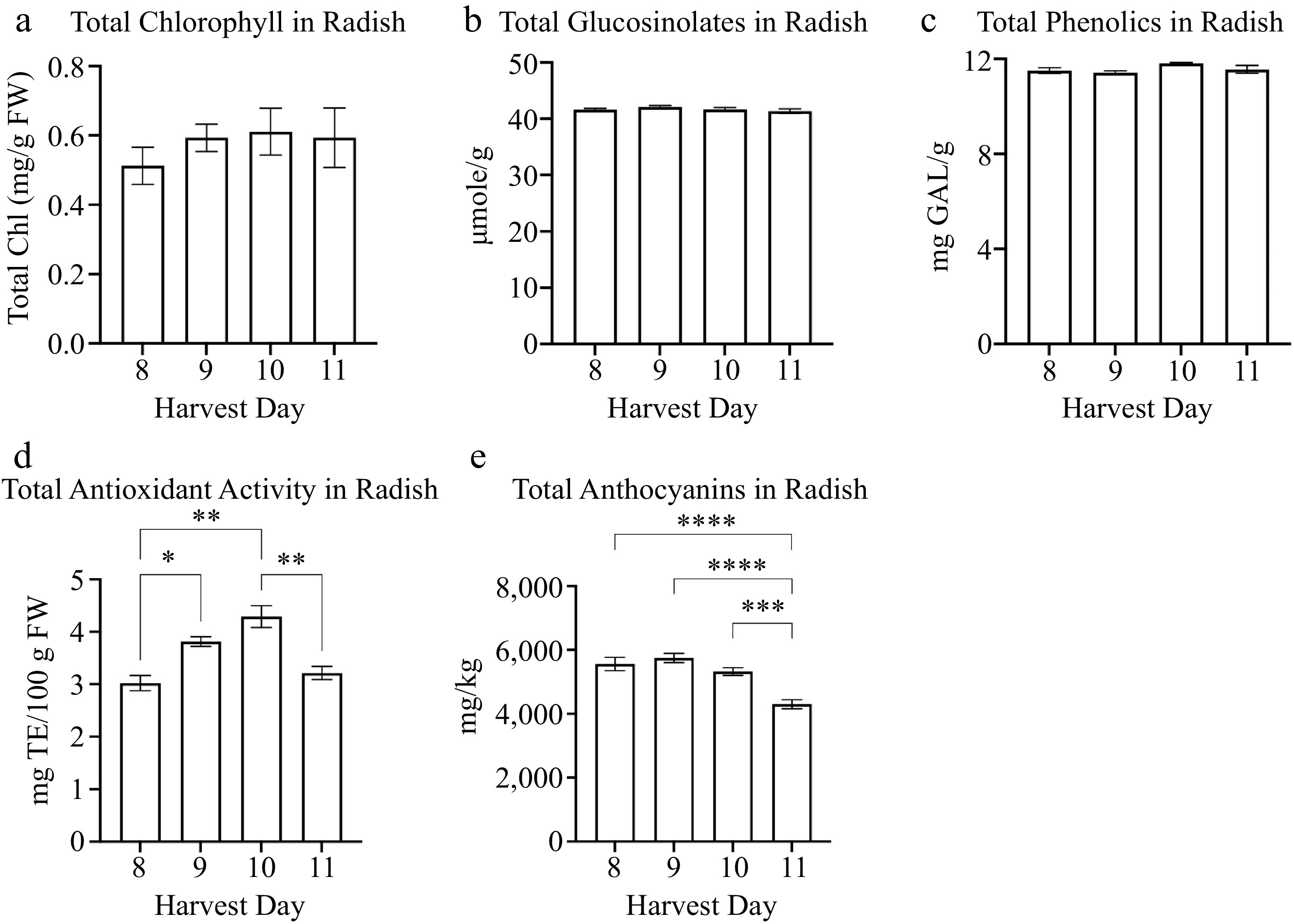
Figure 4.
Concentrations of radish microgreens (a) total chlorophyll and secondary metabolites: (a) glucosinolates, (b) phenolics, (c) antioxidant activity, and (d) anthocyanins for radish microgreens at several harvest days is shown. Bars represent standard error of the means. Asterisks indicate significant differences in total anthocyanins between harvest days. * Indicates 0.0021 < adj p-value ≤ 0.05; ** indicates 0.0002 < adj p-value ≤ 0.0021; *** indicates 0.0001 < adj p-value ≤ 0.0002; **** indicates adj p-value < 0.0001.
Effect of harvest day on extent of decay of broccoli and radish microgreens
-
The extent of broccoli and radish decay (Fig. 5) was observed on storage days 4, 8, 12 and 16 of storage at 4 °C. The difference in decay between broccoli samples harvested at different ages was visible by the 8th day of storage (Fig. 5a). Differences in decay between radish samples harvested at different ages were apparent by the 12th day of storage (Fig. 5c). At storage day 16, the highest incidence of decay for both broccoli and radish microgreens occurred in plants harvested at the stage when the first true leaf had emerged in about 10% of the plants (harvest day 11 for broccoli and harvest day 8 for radish). The lowest incidence of decay occurred in plants harvested when about 75% of the first true leaves had emerged, i.e., on harvest day 13 for broccoli and harvest day 10 for radish. The extent of microgreen decay after 16 d of storage negatively correlated with harvest day (r = −0.7889 in broccoli) (Table 2), (r = −0.673 in radish) (Table 3). The incidence of decay in radish after 16 storage days negatively correlated with the yield (r = −0.724) (Table 3). In comparison, the incidence of decay in broccoli negatively correlated with microgreen yield, hypocotyl length, and total chlorophyll content (Table 2). The tissue leakage is variable for both broccoli and radish at storage days 0 to 16 for all harvest days (Fig. 5b & d).
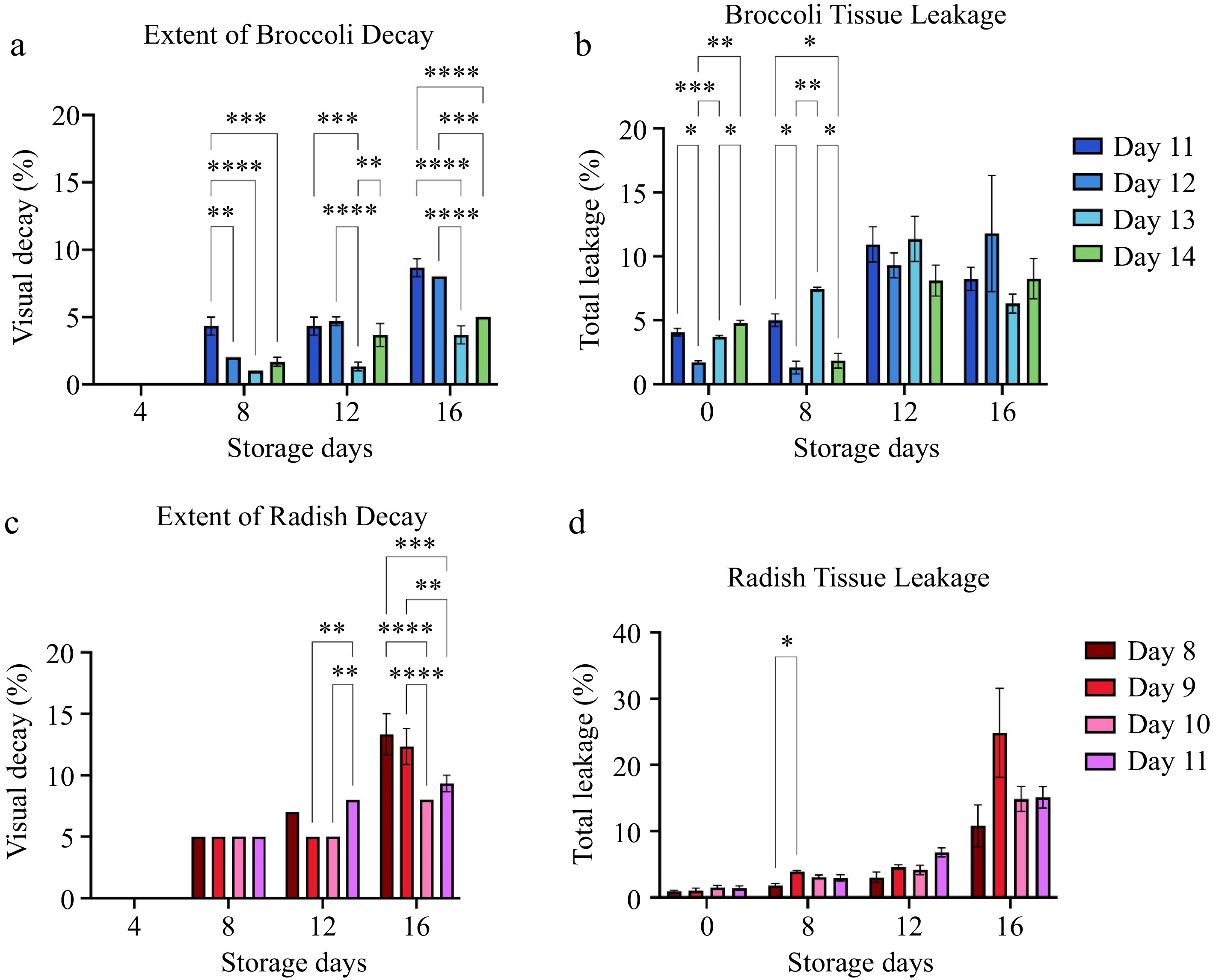
Figure 5.
The extent of decay and tissue leakage for (a), (b) broccoli and (c), (d) radish microgreens at several storage days is shown. Bars represent standard error of the means. Asterisks indicate significant differences in total anthocyanins between harvest days. * Indicates 0.0021 < adj p-value ≤ 0.05; ** Indicates 0.0002 < adj p-value ≤ 0.0021; *** indicates 0.0001 < adj p-value ≤ 0.0002; **** indicates adj p-value < 0.0001.
-
The ideal harvest time for microgreens should strike a balance between achieving high yield, rapid crop turnover, and maintaining superior quality. Much of the existing research on microgreens has no clarity regarding plant growth stage to harvest. In their review of microgreens, Kyriacou et al.[17] specified microgreens as greens harvested when the first pair of true leaves emerges, and cotyledons are fully expanded and still turgid. However, the developmental stage at harvest varies among different studies, with some researchers advocating for harvest at the cotyledonary stage[26,27], others at the emergence of the first true leaf[12,28,29], or at the second true leaf[27,30−32].
In this study, we demonstrated that harvest day 13 is the optimal time for 'Di Cicco' broccoli microgreens based on higher yield, chlorophyll content, and lower decay at storage day 16. The optimal time to harvest Rambo radish microgreens was at harvest day 10 based on higher yield and lower decay at storage day 16 (Figs 2 & 5). Both day 13 for broccoli and day 10 for radish were at the stage when about 75% first true leaf emerged. Furthermore, both broccoli and radish microgreens at this stage exhibited the lowest decay and the longest shelf life (Tables 2 & 3). However, when microgreens were harvested after this stage (day 13 and day 10 for broccoli and radish microgreens), the yield did not increase significantly, while the quality and shelf life of broccoli and radish microgreens decreased. This could result from the depletion of the reserved nutrients in the cotyledons because we did not provide much additional plant nutrients during hydroponic culture. Hanley et al.[40] studied the seedling growth pattern of pea without adding any nutrients and found the relative growth rate decreased between 11 and 13 days after emergence. The slow growth is correlated with the reduction of nutrients such as nitrogen, phosphorus and potassium in the cotyledons. Hence, it will be interesting to investigate whether applying plant nutrients will affect the optimal harvest time of microgreens.
The total chlorophyll in broccoli showed a significant positive correlation with the harvest day, plant yield, and hypocotyl length. The higher chlorophyll content in broccoli may play a role in light harvesting for photosynthesis and other plant metabolic pathways essential for nutrient acquisition. As shown in previous research, the plant photosynthesis rate correlates with increased chlorophyll and increased biomass in plants[41]. Although the parameters of nutrient quality and plant physiology (yield and height) did not show a difference between harvest days 13 and 14, there is lower decay for harvest day 13. There was not a larger leaf size in true leaves at harvest day 14 compared to day 13. The same comparison can be made for radish 'Rambo' microgreens harvest days 10 and 11, and there was less decay for harvest day 10 microgreens. Hence, harvest day 13 broccoli and harvest day 10 radish had the longest shelf life. The observed significant variability across growth stages also extends to the concentration of chlorophyll in microgreens. This aligns with similar findings that emphasized a substantial interaction between growth stages and chlorophyll pigment concentration, as demonstrated in prior research involving red and green butterhead lettuce cultivars harvested at different times, covering both microgreens and mature growth stages[24]. Notably, studies conducted by Klopsch et al.[26] and Heinze et al.[42] revealed a significant increase in chlorophyll levels from cotyledonary microgreens to the mature-leaf stage. This suggests that the brief ontogenic interval from an earlier harvest time to a later harvest time may introduce variations in chlorophyll levels, crucial components of the developing photosynthetic apparatus synthesized in response to evolving light-harvesting requirements[43].
Interestingly, there were slightly lower glucosinolates in harvest days 13 and 14 in broccoli and there were comparatively similar phenolic levels between harvest days 11 to 14. In radish, there were similar phenolic and glucosinolate levels between harvest days 8 to 11. The current study indicated that the harvest time was not potentially a significant source of variation in glucosinolates and phenolic content.
The total anthocyanin contents decreased in radish at later harvest days. A decrease in anthocyanins can be due to a dilution of anthocyanins after cotyledons and leaf expansion[44]. Kyriacou et al.[17] reached the conclusion that the developmental changes in red genotypes were inconsistent. In Komatsuna, anthocyanin concentration increased during the transition when the second true leaf emerged. In contrast, in Pak Choi, maximal concentrations were observed when the first true leaf appeared. This inconsistency highlights that, beyond the evident disparity in the relative abundance of anthocyanins, the variation with developmental stage is primarily genotype-specific. Consequently, determining the optimal harvest stage based on visual quality must be tailored to each specific genotype, emphasizing the need for a case-by-case approach.
The radical-quenching antioxidant activity can significantly differ among microgreen varieties, especially between red and green types, with red microgreens generally demonstrating greater antioxidant capacity[45]. This suggests that including red microgreens in the human diet is expected to help counteract the effects of oxidative stress. This observation aligns with earlier research by Brazaitytė et al. [46] and is further substantiated by our current study, where the purple radish microgreens exhibited higher antioxidant capacity compared to the green broccoli microgreens (Figs 3 & 4). Additionally, the antioxidant characteristics of microgreens seem to vary not just based on genotype but also with growth stage. In the case of radish, the total antioxidant activity was notably higher on harvest day 10, which also coincided with the optimal harvest day, resulting in the least decay after storage.
-
Microgreens are usually harvested after first true leaf emergence. However, the harvest time varied by difference researchers and growers. In this study, optimal harvest time for broccoli and radish under hydroponic culture were identified as day 13 for broccoli and day 10 for radish microgreens, characterized by approximately 75% first true leaf emergence. Microgreens harvested on these days exhibited higher overall yields, increased chlorophyll content in broccoli, and lower decay compared to other harvest days. In terms of nutritional quality, total phenolic and glucosinolate content in radish and broccoli mcirogreens showed minimal variation across harvest times. Instead, delaying radish harvest to day 11 significantly reduced anthocyanin content and antioxidant activity. Similarly, delaying broccoli harvest to day 14 significantly decreased antioxidant activity. While determining the optimal harvest stage should account for genotype-specific yield and nutritional quality considerations, harvesting on day 13 for broccoli and day 10 for radish is recommended for growers in hydroponic growth conditions.
-
The authors confirm contribution to the paper as follows: conception and experiment design: Yang T, Ortiz I, Zhu X, Shakoomahally S; data collection: Ortiz I, Zhu X, Shakoomahally S; analysis and interpretation of results: Ortiz I, Zhu X, Shakoomahally S, Wu W, Kunle-Rabiu O, Turner E, Yang T; draft manuscript preparation: Ortiz I, Shakoomahally S, Wu W, Yang T. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This study was supported by USDA-ARS National Program 306 (No. 8042-43000-016-00D), USDA NE1836 and USDA-ARS/NASA MOU No. 58-8042-1-003.
-
The authors declare that they have no conflict of interest. Tianbao Yang is the Editorial Board member of Technology in Horticulture who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and the research groups.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Ortiz I, Zhu X, Shakoomahally S, Wu W, Kunle-Rabiu O, et al. 2024. Effects of harvest day after first true leaf emergence of broccoli and radish microgreen yield and quality. Technology in Horticulture 4: e003 doi: 10.48130/tihort-0023-0031
Effects of harvest day after first true leaf emergence of broccoli and radish microgreen yield and quality
- Received: 03 October 2023
- Revised: 20 December 2023
- Accepted: 26 December 2023
- Published online: 02 February 2024
Abstract: Microgreens are 7−20-d old young vegetables with rich nutrition. In commercial settings, their harvest time varies after first true leaf emerges. To determine the optimal harvest time, the microgreen yield and quality of broccoli (Brassica oleracea var. italica Plenck) and radish (Rhaphanus sativus L.), were analyzed after different harvest times. Under hydroponic culture at 25 °C and 16/8 h photoperiod, the first true leaf of broccoli and radish emerged on day 10 and day 7 respectively. Broccoli harvested at day 13 and radish harvested at day 10, showed significantly highest yield and chlorophyll content compared to that of plants harvested on any other day. The microgreen yield was 20% higher when broccoli and radish were harvested at day 13 and day 10 compared to those harvested on earlier days. The overall visual quality of microgreens stored at 4 °C was evaluated for the extent of decay. There was less visible decay for broccoli harvested at day 13 and radish harvested at day 10. Regarding nutrient quality, there was no significant difference for glucosinolates and total phenolics among different harvested days. However, anthocyanins were decreased significantly for radish harvested at day 11. Overall, the results suggest that the best harvest time for radish and broccoli microgreens is when about 75% true leaves emerge. This research will provide microgreen growers a basis for determining the optimal harvest time of microgreens.
-
Key words:
- Food Quality /
- Postharvest /
- Phenolics /
- Shelf life /
- Harvest Time /
- Broccoli /
- Radish /
- Glucosinolate












