-
Bananas (Genus Musa, family Musacaea) are herbaceous perennial monocots grown in more than 150 countries worldwide[1]. In the Philippines, banana accounts for around 17.2% of the total agricultural exports[2]. Cavendish bananas remain the primary cultivar grown commercially, accounting for 53.2% of the total production in the Philippines, followed by Lakatan (16.8%) [1] and Cardaba (14%)[2]. Commercial bananas, including the Cavendish group, are generally seedless and sterile[3]. Large-scale propagation of banana is therefore highly dependent on using vegetative planting materials- sword suckers, rhizomes, and bits- that potentially carry disease-causing microorganisms[4]. Throughout the years, various methods have been explored for banana production via plant tissue culture. The process allows the propagation of thousands of plantlets from a small amount of planting material. Shoot tip cultures and sword suckers are used primarily for in vitro propagation of true-to-type and disease-free plantlets. However, increased production of off-types has been observed using these methods[5].
Somatic embryogenesis is another important means of plant production. It is defined as the asexual reproduction of plants from somatic embryos[6]. The success of the technology relies on the potential of cells for totipotency: the ability of a single cell to divide and undergo differentiation[7]. Somatic embryo formation is based on dedifferentiation in plants and the plants ability to reinitiate cell division. Somatic embryos may be induced using direct or indirect methods. Indirect embryogenesis, unlike direct, involves an intermediate callus phase from organized tissues[8]. Studies have reported the establishment of direct somatic embryogenesis, but low plant conversion rates were observed (for example, Remakanthan et al.[9]). Panis et al. reported direct somatic embryogenesis from protoplast cultures[10]. Recently, the use of shoot-tip cultures has been reported[9]. Here, the indirect production of somatic embryos from callus cultures were the focus.
Somatic embryogenesis was first described in carrot (Daucus carota) cells in culture[11]. Although initially investigated for micropropagation of plants, somatic embryogenesis is also utilized for gene expression programs and genetic transformation to improve quality and disease resistance[12]. Genetic transformation using somatic embryos has been proven to minimize the formation of chimeric plantlets[13]. In plant breeding, somatic embryogenesis shortens the breeding cycle[14]. The protocol is also primarily used for cryopreservation of Musa germplasms[15].
Several cultivars of banana, especially those belonging to the Cavendish subgroup, have been propagated from somatic embryogenesis (Table 1). The protocol for somatic embryogenesis in bananas is standardized using different types of explants. However, low embryo germination and plant conversion rates remain a concern[13]. Other issues include the labor-intensive optimization of culture medium, high production costs, and the formation of off-types[16]. Nevertheless, somatic embryogenesis has been exploited to generate planting materials that are of value and disease-free. Several of these methods have been scaled up to commercial laboratories and some for the protection and preservation of commercial banana cultivars that are under threat of extinction[17,18]. Studies have reported the use of somatic embryogenesis in banana but few have focused on the different culture conditions for growth. This review explored the different culture conditions used for somatic embryogenesis in banana and some of their advantages and constraints.
Table 1. Cultivars with successful embryogenic callus (EC) and cell suspension (ECS) protocols.
Cultivar Genetic group Explants used EC ECS Ref. Calcutta 4 AA Scalps
Axillary budsx x [110] Lakatan AA Shoot tips x x [30] Highgate AAA Scalps x [47] Yangambi km5 AAA Immature flowers x [90] Williams AAA Scalps
Immature flowersx x [47,109,16,
92,113]Grand Nain AAA Scalps
Immature flowers
Shoot tipsx x [37,47,111,
112,39,92,9]Nanicão AAA Leaf sheath disks x [27] Gros Michel AAA Immature flowers x x [90,113] Lady finger AAB Scalps x [47] Prata AAB Scalps x [47,114] Saba ABB Immature flowers
Scalpsx x [106,115] Cardaba ABB Scalps, shoot tips x [106,116,117] Bluggoe ABB Shoot tips
Scalpsx x [26,118] -
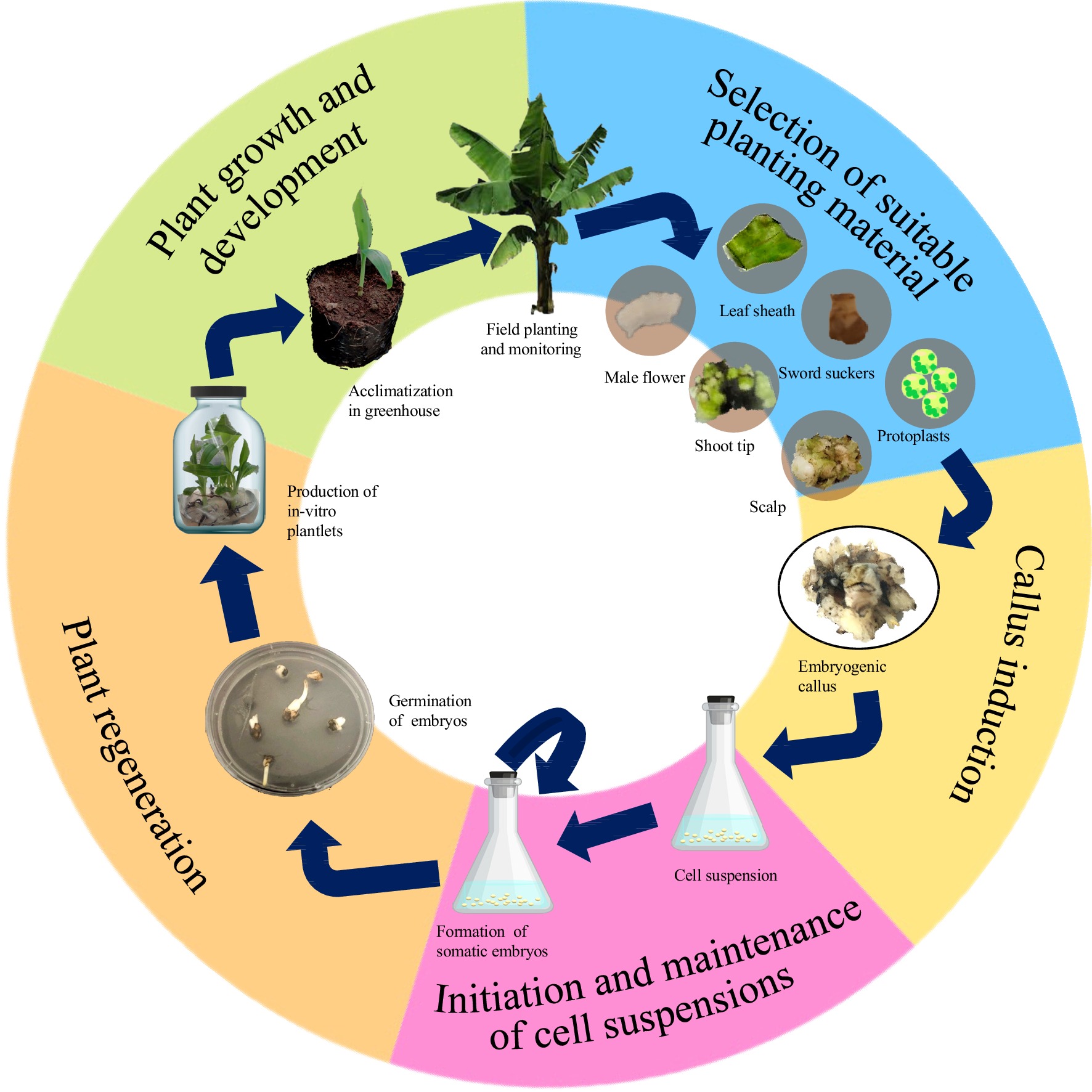
Somatic embryogenesis is an elaborate and complex process involving the production of a whole new plant from unorganized cells. The process is generally comprised of five stages: selection of suitable explant, production of embryogenic callus, development of somatic embryos from cell suspensions, regeneration of viable cells into plantlets, and field monitoring of acclimatized plants (Fig. 1). Each developmental stage requires different nutritional and environmental conditions for growth and is controlled by several factors including endogenous hormones, proteins, and transcription factors[19].
-
The quality and volume of embryogenic callus are crucial for implementing the subsequent steps in somatic embryogenesis[16]. A callus is a mass of unorganized cells naturally found in plants that form in response to stress and wounding[6]. Callus formation in plants is highly controlled by abiotic (light condition, pH and osmotic pressure, sugar content) and biotic (explant age and size, genotype, phytohormones) stimuli[20]. Callus formation differs between monocots and dicots and between diploid and triploid species[21,22]. Pathogen infection also leads to callus formation in plants through auxin and cytokinin production[23].
Callus forms may vary from one set-up to another and can be differentiated based on macroscopic characteristics[20]. Generally, four types of calli can be observed in banana cultures: white and compact (Fig. 2a), clear and friable (Fig. 2b), yellow nodular (Fig. 2c), and ideal callus with translucent proembryos (Fig. 2d). Out of these four, only the ideal callus with translucent proembryos can regenerate and develop into a whole new plant[20]. The translucent proembryos contain differentiated and competent cells that enable plant organogenesis and regeneration[24]. Meanwhile, the white and compact, clear and friable, and yellow nodular calli are all non-embryogenic and non-regenerative types that may be used for further biotechnological studies such as metabolite production and cell suspensions[24,25]. In some cases, shoots and roots may form alongside these non-embryogenic calli that also have the potential to develop into new plants[20].

Figure 2.
Types of callus formed in banana: (a) white and compact (non-embryogenic), (b) clear and friable, (c) non-embryogenic yellow nodular, and (d) ideal callus with translucent proembryos.
Scalps (meristematic tissues with cauliflower-like structure) and immature flowers (male and female inflorescence) are the two most commonly used explants in banana[16]. However, shoot-tips[26], leaf sheaths[27], sword suckers[28], and protoplasts[29] from tissue-cultured plantlets have also been reported. Callus induction may take from 8 weeks to 8 months, depending on the type of explant used. The formation of callus cultures from scalps take the longest, with 6 months average induction time[16]. Induction of embryogenic callus in 12 weeks has been observed from shoot tips[30], sword suckers[28], and immature flowers[20,31]. Callus induction from protoplast cultures are initiated in about three weeks[3,32]. However, it is usually derived from established cell suspensions[29].
Somatic embryogenesis relies on the exogenous application of auxins and cytokinins to promote in-vitro callus induction in plants[16,33]. The combination of callus induction hormones differs from the type of explant used (Table 2). Commonly used auxins for callus initiation are 2,4-dichloro phenoxy acetic acid (2,4-D), indoleacetic acid (IAA), naphthalene acetic acid (NAA), 3,6 dichloro-2 methoxybenzoic acid (Dicamba) and picloram. These may be prepared with cytokinins such as kinetin (KIN), 6-benzyl amino purine (BAP), and zeatin. Brassinosteroids and abscisic acid (ABA) also induce callus formation in some plant species[34,35]. Thidiazuron (TDZ), a hormone with both cytokinin and auxin effects on plants, was also found to induce callus formation in banana[36].
Table 2. Synthetic hormones commonly used for embryogenic callus induction in Musa spp.
Explant used Hormones tested (mg/L) Ref. 2,4-D IAA NAA KIN 2iP BAP TDZ 4-CPPU ZEATIN Picloram Dicamba Immature flowers 2−6 1 1 [107,121,16,111,
92,82,119,120,
122,52,123,39]2−9 [62,37,125,16,112,13] 2 [126] 2 0.5−1 [111] 1 0.22 [54] Scalps 1 0.22 [106,118,127,115,109,
114,54,113,110,48]2−2.9 2.2−3.2 [128] 6.4 [128] 5.7 [128] Shoot tips 0.05 1 [9] 0.1−4 [9] Leaf sheaths & rhizomes 6.63 [129] Protoplasts 2 [29] Leaf sheath disks 1.1 6.64 [22] 100 100 [27] Sword suckers 0.5-2 0.5 [28] Optimum hormone levels for callus induction in banana vary from one genotype to another. For auxins, concentrations range from 0.2 to 4 mg/L when used alongside cytokinins and 4 to 9 mg/L if treated alone. Cytokinins, at 0.5 to 1.0 mg/L, are combined with auxins for callus induction. In addition, culture additives such as amino acids (e.g. proline, glutamine, methionine, tryptophan), sugars (e.g., sucrose, maltose, myo-inositol), and vitamins (e.g., biotin) also support callus induction in banana[22,37−40].
Light exposure also affects callus formation in banana. In numerous studies, callus formation was frequently performed under dark conditions. One study found that light exposure is positively correlated with tissue browning due to increased physiological activity[22]. Hence, the dark treatment seems to prevent necrosis caused by photooxidative stress[41]. Color change of medium is also frequently encountered and can be resolved using gerlite as a gelling agent[22]. Blackening or browning of tissues due to the wounding of explant can be minimized by subculture every two weeks[16]. The addition of antioxidants such as ascorbic acid[42], citric acid[43], cysteine[44], activated charcoal[43], polyvinylpyrrolidone (PVP)[45], potassium citrate, and citrate[46] have been proven to prevent explant browning in banana. It is challenging to optimize culture conditions and culture medium composition due to the extremely low amount of good embryogenic material available for use. Usually, young banana suspensions require a high inoculum density and frequent transfer to a new medium (every three to seven days) during the first few months[47]. In Grand Nain, only 3% to 10% of embryogenic calli (EC) were formed from scalps and 8% from immature flowers[16]. But for other species, % EC can reach up to 97%[48]. The embryogenic potential of callus is also expected to decrease over long periods of incubation[9,21].
-
Somatic embryos are clones of the parent material formed in response to the changing culture conditions of the explant[49]. Unlike sexual structures (zygotic embryos), somatic embryos form in response to the drastic reduction of auxin levels after exposure to callus cultures[7]. Somatic embryos possess a bipolar structure that allows the formation of both apical and radical meristems where shoot and root structures initiate, respectively[13]. Depending on the cultivar, embryos generally form in 3 to 8 months[47].
Complex processes are known to affect somatic embryogenesis in banana. Kumaravel et al. have characterized 25 endogenous proteins in banana associated with somatic embryo formation[40]. Several studies have further explored the involvement of genetic transcription factors in growth[21,50−52]. The addition of cytokinins, alternation of physiological state (pH), and heat shock are known drivers of somatic embryogenesis[21,53]. Reduction of MS salts to half strength and exposure to dark conditions to reduce osmotic pressure and prevent phenolic oxidation, respectively have also been frequently performed in established ECS protocols but the underlying principle remains poorly understood[47,48,54]. In cassava (Manihot esculenta), the use of half and quarter-strength MS resulted in enhanced viability and formation of somatic embryos compared to full-strength MS medium[55]. On the other hand, Groll and co-workers reported a higher formation of mature somatic embryos in full-strength MS[56].
There are four main stages in the formation of somatic embryos- globule stage, oblong stage, heart stage, and torpedo stage- a developmental process shared with zygotic embryos that can be differentiated through distinct cell shape formation[12,57,58]. The first stage, the globular stage, is achieved through the establishment of embryogenic cell suspensions (ECS). Banana ECS protocols vary with the explant used for callus formation (Table 3) and are established by transferring the embryogenic callus into a (liquid) medium with reduced auxin levels or callus-induction medium devoid of agar; most with added amino acids (e.g. L-glutamine and malt extract) that function for metabolism and protein synthesis (Table 4)[13,16,59,60]. For instance, L-glutamine and proline were found to enhance the plant regeneration efficiency of banana (Musa acuminata cv. Berangan)[61]. Scalp-derived ECS utilizes a uniform concentration of exogenous growth regulators (e.g., 2,4-D and zeatin) during induction and multiplication phases[16]. For the immature flower method, somatic embryo expression is enhanced by reducing auxin concentration[59,60,62]. The continued presence of auxin drives the synthesis of gene products necessary to complete the globular stage through increased DNA demethylation[63,64].
Table 3. Culture media used for formation of somatic embryos in banana.
Components MA2 ZZ1 M2b ECS1 BM2 SK4 SS2 IM1 Macro-elements MS 1/2MS 1/2MS 1/2MS MS MS MS SH Microelements MS MS MS MS MS MS MS SH Vitamins MA MS Dhed'a MS MS MS MS MS FeEDTA + − − − − − − − 2,4-D (mg/L) 1 1 1 1 Picloram 0.1 1 Zeatin (mg/L) 0.219 0.219 0.219 BAP (mg/L) 0.05 Coconut water (%) 10 Biotin (mg/L) 1 1 Casein hydrolysate 200 Ascorbic acid (mg/L) 10 10 10 Malt extract (mg/L) 100 100 100 100 100 Amino acids Glutamine
100 g/LGlutamine
100 mg/L
Proline
4 mg/LGlutamine
100 mg/LGlutamine
100 mg/LGlutamine
100 mg/LSugar Saccharose
45 g/LSucrose
30 g/LSucrose
20 g/LSucrose
30 g/LSucrose
45 g/LSucrose
30 g/LSucrose
30 g/LSucrose
30 g/LpH 5.3 5.8 5.8 5.8 5.3 5.8 5.8 5.8 Cultivars tested Grand Nain, Tropical, Rasthali, Somrani monthan,
High gate, Williams,
Gros Michel, Lady
finger, PrataMas, Bluggoe,
Saba, CardabaCalcutta 4 Rajeli Dwarf Brazilian Grand Nain Grand Nain, Ardhapuri, Basrai, Shrimanti, Mutheli, Lalkela and
Safed VelchiRef. [39,107,90,131,52] [39,54,113,48] [118,130,115] [110] [82] [121] [9] [111] Ma2, M2b, BM2, SK4, IM1-immature flower method; ECS1, ZZI-scalps method; SS2-split shoot tips. Table 4. Culture media used for somatic embryo maturation in banana.
Components MA3 RD1 BM3 SK8 MM SS3 IM2 M3b Macro-elements SH 1/2MS SH 1/2MS SH MS SH MS Microelements SH MS SH MS MS MS SH MS Vitamins MA MS MS MS MS MS MS MS FeEDTA + − − − − − − − 2,4-D (mg/L) 1 BAP (mg/L) 5 0.05 0.05 IAA (mg/L) 0.2 NAA (mg/L) 0.2 0.2 Zeatin (mg/L) 0.05 0.05 Kinetin (mg/L) 0.1 0.1 2iP (mg/L) 0.2 Picloram (mg/L) 0.1 Myo-inositol (mg/L) 100 100 Biotin (mg/L) 1 Ascorbic acid (mg/L) 10 Malt extract (mg/L) 100 100 100 100 Amino acids Glutamine
100 mg/L
Proline
230 mg/LGlutamine
100 mg/LGlutamine
100 mg/LSugar Saccharose
45 g/LSucrose
30 g/LSucrose
45 g/LSucrose
30 g/LSaccharose
45 g/LSucrose
30 g/LSucrose
30 g/LSucrose
30 g/LGelling agent (g/L) Phytagel
4 g/LGelrite
3 g/LGelrite
2 g/LPhytagel
2.6 g/LGelrite
2 g/LGelrite
3 g/L− − pH 5.8 5.8 5.8 5.8 5.8 5.8 5.8 5.8 Cultivars tested Grand Nain, Gros Michel, Williams Grand Nain, Calcutta 4,
Somrani monthan,
High gate, Williams,
Lady finger, PrataRajeli, Grand
Nain, TropicalDwarf Brazilian Grand Nain; Rasthali Grand Nain Grand Nain, Ardhapuri, Basrai, Shrimanti,
Mutheli, Lalkela,
Safed VelchiBluggoe, Saba,
CardabaRef. [39,107,125] [47,110,54,113] [82,123,124] [121,132] [125,16,52] [9] [111] [118,115] Ma3, BM3, SK8, MM , IM2, M3b-immature flower method; RDI-scalps method; SS3-split shoot tips. At the globular stage, the pro-embryos also contain other mRNAs and proteins that generally inhibit the continuation of embryogenesis[11]. The removal of auxin is believed to result in the inactivation of these genes necessary to enter the next embryogenic growth stage[50]. Guzzo et al. proposed a model linking auxin response, asymmetric division, and totipotency: upon environmental stimuli, cells can be made morpho-genetically totipotent in response to auxin if the cells contain inducible receptors to complete embryogenesis; but only organogenesis or unorganized proliferation will occur otherwise[65]. Cytokinins, in minute concentrations, may also affect the sensitivity of somatic embryogenesis and cell division, but their molecular basis remains unknown[66,67].
The globular embryo then enters the oblong stage, signaling the shift from isodiametric to bilaterally symmetrical growth, followed by the beginning of the heart stage[68]. This globular-to-heart embryot ransition is pronounced by the outgrowth of the two cotyledons, hypocotyl elongation, and radicle initiation[11]. Finally, the embryo enters the torpedo stage, a stage with a distinct increase in size, before reaching full maturity[68]. Sometimes, immature embryos formed from callus cultures may undergo differentiation, and this can be prevented through high osmotic pressure and the addition of abscisic acid[21]. Removal of bigger aggregates of, more developed, somatic embryos is recommended because they have the tendency to accumulate starch and produce high amounts of polyphenols[47].
Water stress is one of the most important factors for somatic embryo maturation[69]. During maturation, embryos undergo gradual loss of water and initiate desiccation tolerance to survive[70−72]. Available ECS protocols regulate water availability to the developing somatic embryos through high concentrations of gelling gum or overlaid filter paper[13]. Studies suggest the involvement of early response to dehydration proteins (ERDs) in embryo maturation[73]. Oxygen availability and pH of the culture medium also affect embryo maturation. High levels of oxygen have been shown to promote somatic embryo multiplication while low levels result in histodifferentiation[74]. The optimum pH for embryo development is pH 5.8, but relatively lower quality and irregular embryos may also form at pH 4.5-5.5 and at pH 6.0 to 7.0[75−77].
-
The germination of the somatic embryo into normal shoots, termed regeneration, is achieved primarily on culture medium in a genotype-dependent manner. Plants derived from embryogenic cell suspensions (ECS), called emblings, are highly dependent on ECS density and quality[50]. High cell density (105 cells/mL) is for embryogenic cell clusters formation from and lower cell density (2 × 104 cells/mL) for embryo development originating from embryogenic cells[78,79]. Embling conversion rates vary within banana genotypes. For instance, 13% in the edible (AA) Pisang Mas and 13% to 25% for Grand Nain of the Cavendish subgroup (AAA)[80]. High regeneration rates (90% to 95%) from ECS cultures have been recorded for some triploid and diploid species such as cv. Dwarf Brasilian (AAB) and M. a. ssp. malaccensis (AA), both of which passed through a differentiation–maturation phase[13,81].
Most commonly, BAP, at 0.2 to 3 mg/L concentrations, is used for plant regeneration[47,54,82]. Sometimes, BAP is complemented with other cytokinins (at 0.2 to 0.5 mg/L) for embryo germination (Table 5). These are supplemented with antioxidants such as activated charcoal and ascorbic acid to prevent browning and further support the regeneration of tissues[83]. Kumaravel and co-workers further investigated different concentrations of NAA (2.68, 5.37, and 10.74 μM) for the regeneration of banana somatic embryos with three (100 and 200 μM) and methionine (335.09, 670.19, and 1 mM) as additives[40]. They also tested various concentrations of CaCl2 (5, 10, and 15 mM) and gibberellic acid (GA3) (1.44, 2.88, and 5.77 μM) with 11.41 μM IAA and 2.21 μM BAP. In 'Grand Nain', media supplemented with 5.37 μM NAA + 1.44 μM GA3 showed the highest regeneration efficiency (91.0%). The lowest regeneration was recorded in the medium supplemented with 1 mM methionine in 'Rasthali', whereas 'Grand Nain' media with 200 μM showed the least germination. It was found that in 'Grand Nain', an increased concentration of IAA recorded the highest regeneration (24.28%), but relatively lower (showed 18.96%) in 'Red Banana' in kinetin-supplemented media. These results demonstrate that in banana, regeneration is not only genome-dependent but also cultivar-dependent. The observed overexpression of IAA monooxygenase in the emblings also showed that tryptophan-dependent auxin biosynthesis plays a key role in somatic embryo formation. El-Kereamy et al. previously reported the overexpression of these proteins in rice resulted in enhanced shoot formation due to increased biosynthesis of GA and cytokinin, whereas Patterson et al. reported the role of germination-related proteins for root hormone regulation in Arabidopsis[84,85]. These results suggested that the endogenous hormones stimulated the formation of pro-embryonic roots and shoots of somatic embryos. Furthermore, scientists discovered important genes affecting the morphogenesis of somatic embryos. Boutilier and co-workers described the role of the BABY BOOM1 (BBM1) gene for morphogenesis in coffee (Coffea canephora) embryogenesis, while the LEAFY COTYLEDON1 (LEC1) and WUSCHEL-RELATED HOMEOBOX4 (WOX4) genes are crucial in the initial phase of cell differentiation[86−89]. Elhiti and co-workers further identified 12 candidate genes that play key roles in the early stages of somatic embryogenesis[6]. According to their study, epigenetic regulation occurs among the candidate genes involved.
Table 5. Culture conditions used for plant regeneration from somatic embryos of banana.
Components MA4 RD2 BM5 SK10 M4 SS4 IM3 SB4 Macro-elements MS 1/2 MS SH MS MS MS 1/2 MS MS Microelements MS MS SH MS MS MS MS MS Vitamins Morel MS MS Morel Morel Morel MS Morel FeEDTA + − − − − − − + IAA (mg/L) 2.0 2 BAP (mg/L) 0.5 0.227 0.5 2 0.4 NAA (mg/L) 0.5 Zeatin (mg/L) 2 Myo-inositol (mg/L) 100 Ascorbic acid (mg/L) 10 Activated charcoal (%) 0.5 Lactose (g/L) 0.1 Sugar Saccharose
30 g/LSucrose
30 g/LSucrose
30 g/LSucrose
30 g/LSaccharose
30 g/LSucrose
30 g/LSucrose
30 g/LSucrose
30 g/LGelling agent (g/L) Phytagel
3 g/LGelrite
3 g/LGelrite
3 g/LPhytagel
2.6 g/LGelrite
2 g/LGelrite
3 g/LGelrite
2 g/LGelrite
2 g/LpH 5.8 5.8 5.8 5.8 5.7 5.8 5.8 5.8 Cultivars tested Grand Nain, Tropical, Rasthali, Calcutta 4 Somrani monthan,
High gate, Williams,
Lady finger, PrataRajeli Dwarf Brazilian Mas Grand Nain Grand Nain, Ardhapuri,Basrai, Shrimanti, Mutheli, Lalkela and
Safed VelchiBluggoe, Saba,
CardabaRef. [39,107,125,110,52,123] [47,54,113,48] [82] [121,130] [13] [9] [111] [118,115] Ma4, BM5, SK10, IM3, SB4-immature flower method; RD2-scalps method; SS4-split shoot tips. -
A common method for quantitative and qualitative assessment of callus induction is obtaining the percent formation of ideal callus (IC) calculated using the formula: %IC = the number of IC/number of inoculated explants. The %IC values obtained for 'Grand Nain' range between 3% to 10%, using the scalping method, and 8% on average, using the immature flower method[16]. But a higher callus induction percentage of 70% has been reported using sword suckers[28]. Qualitative assessment of IC can be performed by physical examination of the type of callus formed as previously mentioned above (Fig. 2).
Formation and maintenance of somatic embryos
-
According to Strosse and co-workers, the quality of an embryogenic cell suspension (ECS) can be primarily assessed according to the number of embryos/mL of plated cells[16]. It can be conveniently applied for analysis since it only requires a very small aliquot (1 mL) of the cell material[47]. The number of embryos/mL can yield between 100 to 300,000[60,90]. But only one out of two to one out of five embryonic calli will lead to a good quality ECS, characterized by bright to light yellow color with a high proportion of homogeneous embryogenic cell aggregates[91]. On the other hand, pale white suspensions are indicative of a high proportion of starch-rich and non-regenerable cells[16].
ECS establishment can further be measured using the formula: % of ECS initiated = number of ECSs/number of IC placed in liquid medium or by counting the number of embryos formed per IC[13,39,92]. A cell viability test using fluorescein diacetate (FDA) is usually accompanied to determine ECS quality[93]. To perform the FDA test, add a few drops of fluorescein diacetate (FDA) stock (−20 °C, dissolved in acetone water) to distilled water until a blue shine is observed. Add 1 to 2 drops of this diluted stock to a suspension sample. Viable tissue fluorescence is brightly green when observed under ultra-violet light. Somatic embryos with an FDA score over 80% are considered to be viable and acceptable for regeneration.
ECS quality declines with increased subcultures[18]. Subsequently, higher rates of subculture result in an increased probability of contamination and a decreased growth rate, regeneration capacity, and higher risk of somaclonal variation[13]. The increased contamination and regeneration can be owed to the fast-growing, dense, and starch-rich cells taking over the cultures[91]. To reduce these problems, cryopreservation protocols have been developed which allow the storage of ECSs for longer periods[10]. In addition, early detection of undesirable genetic variation in suspensions can be assessed using the flow cytometry method[94].
Plant regeneration
-
The regeneration rate of somatic embryos often describes the success of a somatic embryogenesis protocol. Hence, proper evaluation of a regeneration process is crucial for somatic embryogenesis. Strosse and co-workers suggested the following criteria for evaluation: % of germination (number of plantlets obtained/number of embryos in medium) and regeneration capacity (Regeneration capacity = number of in vitro plants produced/mL of plated cells)[16]. According to their study, the regeneration capacity of an ECS may further be assessed using the following morphometric assays: total weight of the regenerated embryos, the average number of green shoots 1.5 months after shoot emergence, and the average amount of rooted shoots 1.5 to 2 months after root initiation. The settled cell volume (SCV) (precipitation by gravity forces), packed cell volume (PCV) (precipitation by centrifugation), and fresh and dry weights were also described as determinants of regeneration capacity and growth rate.
Subculture of regenerants (somaclones) is an important part of the regeneration stage to prevent the production of somaclonal variants[95]. The required number of cycles for the subculture of regenerated embryos (clones) depends on the genotype but usually ranges from 2 to 10 cycles[13]. The subcultured clones are then transferred to a rooting medium followed by acclimatization under greenhouse conditions before planting in the field[90]. Regenerated plantlets should be 6−8 cm tall before transplanting in the greenhouse[96]. High relative humidity (> 80%) and a temperature ranging from 19 to 30 °C are also required for growth under greenhouse conditions[97].
-
Somatic embryogenesis (SE) is essential in the development of in vitro regeneration systems which are critical steps for the development of resistant varieties[98]. Despite extensive studies in SE, low embryo regeneration rates, and somaclonal variation continue to be the bottlenecks of SE procedures in various banana embryogenic systems[90]. In 'Grand Nain', regeneration values reach as low as 8% under optimal conditions and less than 1% under non-optimal conditions[99]. Embryogenic responses of over 30% could be obtained, from scalps, for some plantain types and cooking bananas[47]. Recently, Youssef and co-workers recorded a high regeneration rate (80%) of 'Grand Nain' from male flower buds[92].
The in vitro culture environment, the type (and concentration) of plant growth regulators (PGRs), the plant's genetic background and the number and duration of subcultures can also affect the properties of plants regenerated by somatic embryos, contributing to the generation of genetic and epigenetic variation[100]. This variation is apparent in the culture's phenotype, more popularly known as somaclonal variation was thought to be a pre-existent genetic variation in the explant due to changes in chromosome structure, chromosome numbers such as polyploidy and aneuploidy, or induced during in vitro culture[101−104]. These genetic variations may be detected based on plant morphology (e.g. plant height, size, and number of hands) and using advanced DNA markers (e.g. ISSR, SSR, RAPD, SNP)[105].
Dhed'a observed 5%−10% abnormal somatic embryos recovered from a 'Bluggoe' (ABB, cooking banana) suspension derived from the scalp with only one off-type (0.7%) found with phenotypic changes[106]. Grapin and co-workers reported 16%−22% somaclonal variants regenerated from a 'French Sombre' (AAB, plantain) male flower-derived suspension[90]. Côte and co-workers reported 'variegated' plants with 'double' leaves (two parts coalescing at the central vein) in 'Grand Nain' plants due to somaclonal variation[107]. But all 500 tested plants showed later an agronomical behavior similar to that of plants produced by in vitro budding method. Contrastingly, Uma and co-workers evaluated genetic fidelity in banana cv. 'Grand Nain' and 'Rasthali' were produced from embryogenic cell suspensions using ISSR markers[3]. The overall variation was found to be 3.34% and 2.09%, respectively. Field evaluation further showed no negative effects of vegetative and yield, with no off-types produced.
Somaclonal variation in banana has been reported to be associated with long-term cultures or cultures that involve a callus phase or high rates of multiplication treatments[96,108]. The decline in the regeneration capacity of ECS cultures has also been associated with cytogenetic instabilities in triploid (AAA, genome) Cavendish bananas, off-type regenerants from long-term Bluggoe suspension cultures (ABB, cooking banana), and the subsequent loss of regeneration potential[13,95,100]. For example, a four-year-old Three Han Planty' (AAB, plantain) suspension was found to have very high regeneration potential with normal ploidy levels, but a nine-year-old 'Bluggoe' (ABB, cooking banana) suspension was found to lack 4−5 chromosomes[47].
-
This paper reviews the current protocols used for somatic embryogenesis in banana, with a focus on the commercial Cavendish group. Due to the various factors affecting somatic embryogenesis and the laborious aspect of optimization, protocols are usually standardized based on the explant source. Much attention was given to the alteration of culture media conditions such as the concentration of plant growth regulators and additives for the formation of desirable clones. However, the particular effect of these alterations on the genetic aspect and the formation of somaclonal variants is lacking. Understanding the physiological, biochemical, and molecular processes involved in each stage of growth is therefore essential for the proper optimization of somatic embryogenesis protocols. For example, determining the sensitivity of clones to changes in the exogenous hormone application, the subsequent levels of endogenous hormones, and gene regulation which miRNA-mediated gene silencing can offer. Functional characterization of key genes involved during somatic embryogenesis may lead to an enhanced understanding of the totipotency of plant cells and provide approaches to improve the efficiency of the process.
-
The authors confirm contribution to the paper as follows: topic conception: Cruz MA, Alcasid C, Silvosa-Millado CS, Balendres MA; data collection: Cruz MA; data curation: Cruz MA; formal analysis: Cruz MA, Alcasid C, Silvosa-Millado CS, Balendres MA; writing - original draft: Cruz MA; writing - review & editing: Alcasid C, Silvosa-Millado CS, Balendres MA; supervision: Balendres MA. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
-
The authors thank the Department of Agriculture-Bureau of Agricultural Research and the University of the Philippines Los Baños.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Cruz MA, Alcasid C, Silvosa-Millado CS, Balendres MA. 2024. Culture conditions for somatic embryogenesis in banana: brief review of the current practices, advantages, and constraints. Technology in Horticulture 4: e016 doi: 10.48130/tihort-0024-0013
Culture conditions for somatic embryogenesis in banana: brief review of the current practices, advantages, and constraints
- Received: 08 March 2024
- Revised: 12 June 2024
- Accepted: 17 June 2024
- Published online: 16 July 2024
Abstract: Banana (Musa spp.) is a high-value cash crop that serves as a staple food across Asia. However, numerous pests and diseases challenge the global production of bananas. The advent of advanced molecular technologies, such as plant tissue culture, played a pivotal role in banana production with enhanced physiology, morphology, and disease resistance. Since then, researchers and agricultural industries' interest has shifted to using plant tissue culture for the large-scale production of bananas. The production of somatic embryos from plant tissues, termed somatic embryogenesis (SE), is often utilized as an asexual means of reproducing banana plantlets with uniform genotypic characteristics. Various studies have also demonstrated the function of somatic embryogenesis for genetic transformation studies. However, the efficiency of SE protocols differs from one genotype to another. It is affected by several factors, including the type of explant, culture media, plant growth regulators, and environmental conditions. This review will summarize the current methodologies for performing SE in banana. In addition, the advantages and constraints of performing SE protocols were discussed.
-
Key words:
- Callus induction /
- Tissue culture condition /
- Musa /
- Micropropagation /
- Plant growth regulators