-
Pecan (Carya illinoinensis (Wangenh.) K. Koch) is a tree species with a large geographic range endemic to North America, producing a drupe containing an edible seed or nut. This nut is not only a popular snack but also a key ingredient in various culinary dishes, contributing significantly to the US agricultural economy. The pecan industry is valued at approximately USD
${\$} $ Over the years, the focus of genetic improvement in pecans has primarily centered on the grafted scion, as the challenges of clonal propagation for rootstocks have limited options. However, the selection of appropriate rootstocks is crucial, as it significantly influences the health, growth, phenology, and productivity of the grafted trees[4−11]. In optimal conditions, pecan yields can reach up to 2,000 pounds per acre[6,12]. This productivity highlights the importance of ongoing research and horticultural practices aimed at enhancing nut size, disease resistance, and overall yield, ensuring the long-term viability of the pecan industry. Pecan growers and the nursery industry commonly use open-pollinated seeds from regionally adapted cultivars for rootstock production. Seedstock selection influences valuable characteristics such as improved germination, vigor, budbreak timing, and/or abiotic stress tolerance[13−15]. Factors influencing the choice of seedstock include seed availability, seed fill, seed size, seedling vigor, seedling uniformity, and seedling root characteristics[6,11,16]. Generally, well-filled seeds are considered essential for good germination, with round seeds being preferred over long seeds due to improved performance in some mechanical planters[6]. Smaller seeds are often preferred when purchasing seeds due to the greater quantity per kilogram, maximizing potential seedling production[12]. One of the long-term goals of the USDA ARS Pecan Breeding Program is to improve regionally adapted pecan rootstocks through seedstock selection.
Pecan trees, native to temperate regions, exhibit a lower cold tolerance compared to other hickory species in the Carya genus. This characteristic can complicate the seed germination process, as breaking seed dormancy is crucial for overcoming the physiological and environmental barriers that inhibit germination[17−21]. Some pecan varieties have characteristics such as a thick shell, strong shell suture, or specific stratification preference, leading to challenges such as slow or uneven germination and weak seedling growth.
To enhance germination rates and improve seedling vigor, cold stratification—a process where seeds are exposed to cold, moist conditions for a specified period — is commonly employed in pecan nurseries. This method mimics natural winter conditions, promoting biochemical changes within the seeds that facilitate germination[4,11,22]. However, the specific effects of cold stratification on seedling growth remain poorly quantified, with limited research available on optimal conditions and outcomes. In contrast, freezing seeds before germination has been a practice aimed at killing pests like the pecan weevil[23,24]. This method, while effective for pest control, differs fundamentally from stratification. Freezing may halt all seed metabolic processes, potentially leading to reduced viability, whereas stratification enhances germination by preparing the seed for growth. Understanding these processes is vital for optimizing pecan cultivation and ensuring robust seedling development.
The USDA Pecan Breeding & Genetics Program conducted a complex seed germination experiment to investigate the effect of low temperatures on seed germination and seedling growth in the nursery. In this study, open-pollinated seeds from 12 seedstocks originating from different geographical locations were treated in combinations of freezing and stratified treatments. Non-frozen, non-stratified seeds were used as the control.
-
In this study, 12 seedstocks originating from different locations ranging from Santa Catarina, San Luis Potosí, Mexico, to Peruque, Missouri, United States of America (Table 1), were used to test the effects of freezing and stratification treatments on seed germination and seedling growth. The seedstocks can be grouped by eastern, southern, western, and northern provenances based on their geographical origins. These trees were either planted on their roots or grafted 20 or more years ago (Table 1) and maintained in the USDA-ARS National Collection of Genetic Resources for Pecans and Hickories (Carya) orchards in Brownwood and Somerville, TX, USA.
Table 1. The geographical origin of the 12 pecan seedstocks and seed source in the USDA-ARS National Clonal Germplasm Repository (NCGR) for pecans and hickories.
Seedstock Orchard Row Tree Provenance Origin Grafted date 87MX1-2.2 CSP 4 3 Southern Santa Catarina, San Luis Potosi, MX 1990* 87MX5-1.7 CSP 16 9 Southern Jaumave, Tamaulipas, MX 1990* Frutoso BWRom 109 23 Southern Parras, Coahuila, MX 1992 Elliott BWV 5 42 Eastern Milton, Santa Rosa, FL 1993 Moore BWV 4 39 Eastern Waukeenah, Jefferson, FL 1993 Giles BWV 6 11 Northern Chetopa, Cherokee, KS 1991 Major BWV 6 21 Northern Green River, Henderson, KY 1991 Peruque BWV 8 36 Northern Peruque, St. Charles, MO 1991 Posey BWV 7 7 Northern Gibson, IN 1991 Riverside BWV 3 16 Western Big Valley, Mills, TX 2005 San Felipe BWV 6 20 Western Del Rio, Val Verde, TX 2003 VC1-68 CSP 1 15 Western Phoenix, Maricopa, AZ 1995 * One-year-old seedlings from open-pollinated seeds were planted. Open-pollinated seeds were collected as a seedstock from these mature maternal trees in the fall of 2008. Eighty seeds from each seedstock were individually weighed, measured, and divided into 20 nut lots for various treatments. Two lots (2 × 20 seeds) of each seedstock were frozen at −18 °C for 7 d[23,24], while another two lots (2 × 20 seeds) were stored in a refrigerator at 4 °C for 7 d. The first batch (consisting of 20 frozen and 20 non-frozen seeds of each seedstock) was planted in 10.2 cm × 10.2 cm × 60 cm pots, filled with pine bark moss, in the greenhouse in Brownwood, TX, USA on 9 Jan 2009 (first planting). In the second batch, half of the frozen seeds (20) and half of the non-frozen seeds (20) were stratified by placement in 16.5 cm × 14.9 cm Ziploc sandwich bags (20 seeds per bag), layered with moisturized perlite, kept in the refrigerator for 50 d, and planted on 27 Feb 2009 (second planting). Consequently, there were four treatment combinations: Frozen/Non-stratified, Frozen/Stratified, Non-frozen/Stratified, and Non-frozen/Non-stratified (control).
Field data collection
-
Before treatments, seeds were measured for seed length (measured from the base and apex in mm), seed height (measured perpendicular to the plane of the suture at the widest point in mm), seed width (measured in the plane of the suture at the widest point in mm), and seed buoyancy (g). Seed buoyancy is measured as the rise (in mL) in a volume of water at room temperature (20−25 °C) converted to grams[25]. Seed density was calculated using the formula: seed density = seed weight (g)/(seed weight (g) + seed buoyancy (g)). Seed density is an important indicator of seed quality and was used to investigate its effect on seed germination and seedling growth.
The planting and germination dates were recorded on the calendar day and converted to Julian days. The germination days of emergence were calculated by subtracting the Julian planting date from the Julian germination date to determine the total number of days it took for the seeds to emerge. The number of emerged and non-emerged seeds was counted, and the percentage of emerged seeds in each treatment was calculated. Seedling heights in millimeters were measured from the soil line to the top of the seedling, and stem diameters in millimeters were taken approximately 5 mm above the soil line using calipers on 19 Jun 2009 (during the first growing season). Since the differing treatments required separated planting dates, the growth rates were calculated using the following formula: height or diameter growth rate (mm/day) = height (mm) or diameter (mm)/(measuring Julian days − germination Julian days).
Data analysis
-
All analyses were performed with JMP® Pro 17.0.0 (SAS Institute Inc). The following parameters were analyzed: 1) the effect of freezing and stratification treatment on the seed germination rates for the first and second plantings separately, using a Multinominal Logistic Regression; 2) the effects of the freezing and stratified treatments and their interactions using the Likelihood Ratio Tests in the Nominal Logistic Model, and; 3) the effects of the treatments on seedstocks, using the Standard Least Squares model under the hypothesis of no effect of treatments on seed germination. The effects of seedstocks, seed treatments, and treatment × origin interactions were analyzed using a one-way analysis of variance (ANOVA). The means of the days from seed plantings to seedling emergence (days to emergence), seedling height and stem diameter were compared using the Tukey-Kramer HSD test. All variables were compared using a principal component analysis (PCA).
-
Overall, treating seeds by freezing, stratification, or their combination significantly affected seed germination across the 12 seedstocks (p < 0.05) (Table 2, Fig. 1). The freezing treatment significantly impacted seed germination across seedstocks, with many frozen seeds failing to germinate (Fig. 1a). Nearly half of all seedstocks greater than 40% germination failure after freezing (Fig. 1b, Supplementary Table S1). Some seedstocks, such as 'Major', were particularly impacted by freezing compared to others (Fig. 1b). The interaction of the stratified and freezing treatments with seed germination rate was only significant when the seedstock maternal family was included as a factor (likely due to high variation across the different seedstocks) (Table 2). Compared to non-frozen seeds, the germination rate of frozen seeds was reduced from 0% to 45% (average 15.71%) in the first planting (i.e. non-stratified), and 0% to 90% (average 48.5%) in the second planting (i.e. stratified) (Supplementary Table S1). This indicates that the freezing treatment decreased seed germination rates, and frozen/stratified seeds had lower germination rates than non-frozen/stratified seeds (Fig. 1b). Variation in treatment response between seedstocks was observed. Some seedstocks, like 'Moore', 87MX1-2.2, and 87MX5-1.7, maintained high germination rates across all treatments, while others like 'Riverside', 'Peruque', 'San Felipe, and 'Elliott' showed significantly lower germination rates (Fig. 1b, Supplementary Table S1). Overall, the days of emergence were not significantly affected by freezing or stratified treatment or their combination when considering seedstock origins, with 25.4, 27.1, 27.8, and 28.5 d of emergence for southern, western, eastern, and northern respectively. However, stratified seeds germinated faster than non-stratified seeds, regardless of frozen or not (Fig. 2, Table 3).
Table 2. The treatment effect tests on the seed germination rates.
Source Nparm DF L-R ChiSquare Prob > ChiSq Without considering seedstocks Frozen/Non-Frozen 1 1 144.1823 < 0.0001** Stratified/Non-Stratified 1 1 11.5772 0.0007** Frozen/Non-Frozen*Stratified/Non-Stratified 1 1 38.7198 < 0.0001** With considering seedstocks Frozen/Non-Frozen 1 1 91.1617 < 0.0001** Stratified/Non-Stratified 1 1 0.0000 0.9964 Frozen/Non-Frozen*Stratified/Non-Stratified 1 1 0.0000 0.9961 Seedstock 11 11 92.2452 < 0.0001** Seedstock*Frozen/Non-Frozen 11 11 40.7617 < 0.0001** Seedstock*Stratified/Non-Stratified 11 11 30.0626 0.0015** Seedstock*Frozen/Non-Frozen*Stratified/Non-Stratified 11 11 20.1627 0.0432* Nparm: The number of parameters associated with this effect; DF: The degree of freedom for the effect test; L-R ChiSquare is the likelihood ratio chi-square test statistic for the hypothesis that the corresponding regression parameter is zero, given the other terms in the model. Prob > ChiSq: The probability of obtaining a greater chi-square value if the specified model fits no better than the model that includes only an intercept. * Indicates significance at p < 0.05 and ** at p < 0.01. 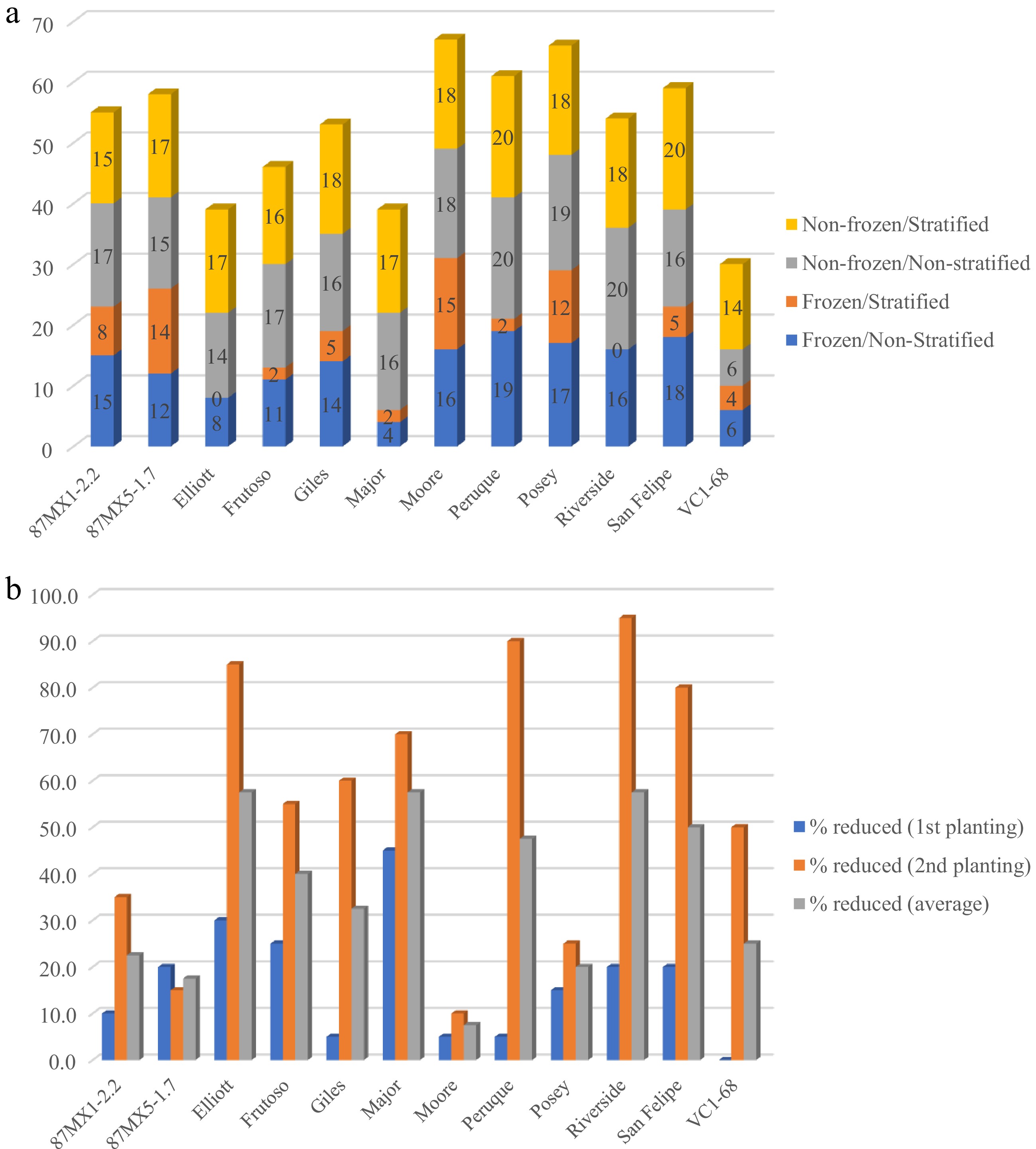
Figure 1.
(a) Numbers of germinated seeds of 12 seedstocks for each treatment of 20 seeds, and (b) percentage of seeds that failed to germinate in the first planting (frozen and non-frozen but non-stratified seeds) and the second planting (frozen and non-frozen but stratified).
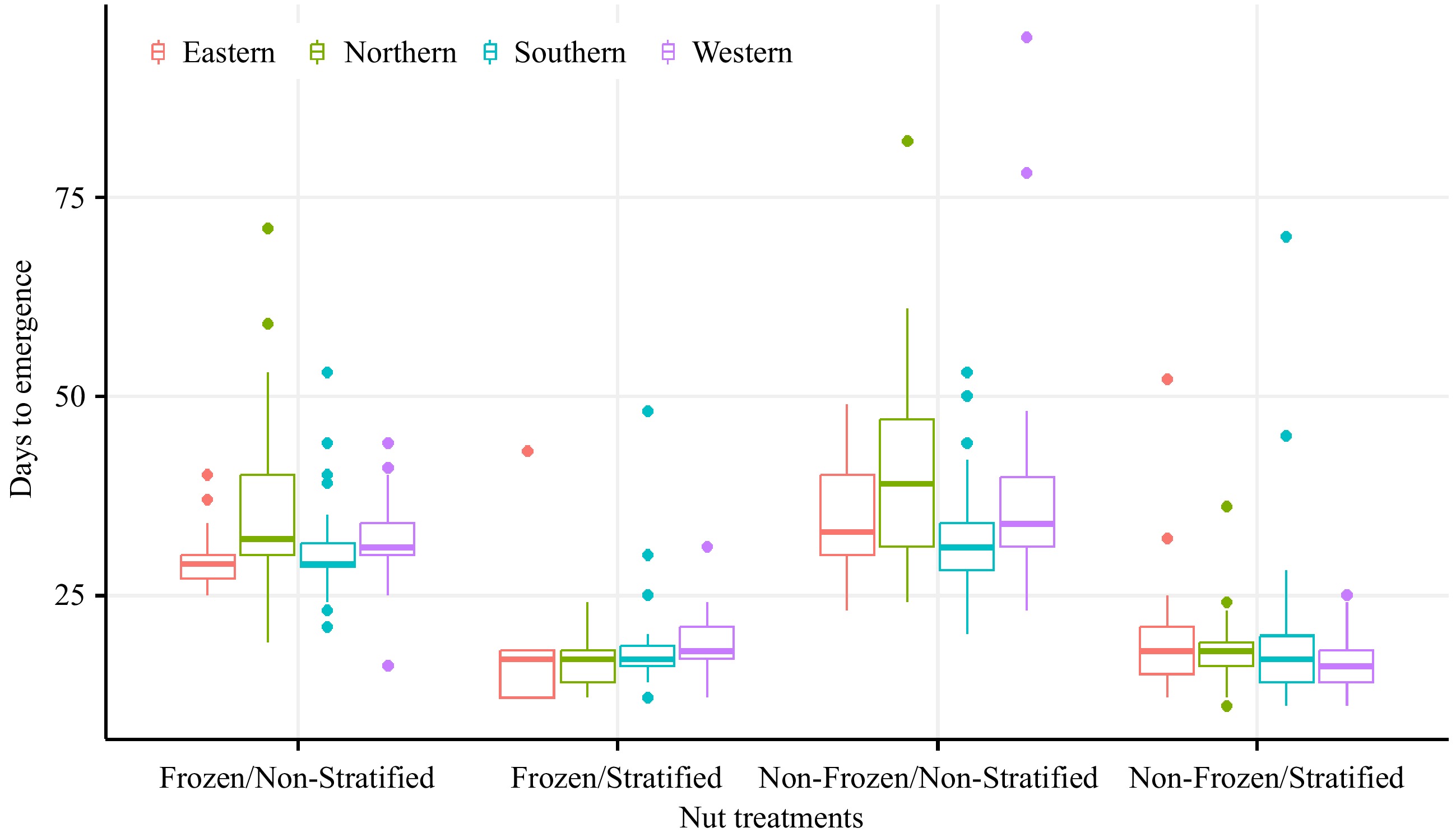
Figure 2.
The visual comparisons of days to emergence of the 12 seedstocks from four regions under different seed treatments.
Table 3. The influence of seed treatments on days to emergence and seedling growth in the first season.
Treatment Days to emergence Height growth rate (mm/day) Diameter growth rate (mm/day) Mean Std dev Std error Mean Std dev Std error Mean Std dev Std error Frozen/Non-stratified 32.455 b 7.321 0.7179 0.396 0.184 b 0.0136 0.035 c 0.008 0.0006 Frozen/Stratified 18.028 c 6.012 1.0641 0.418 0.130 b 0.0205 0.039 b 0.007 0.0009 Non-frozen/Stratified 17.802 c 6.196 0.6232 0.530 0.158 a 0.0118 0.043 a 0.007 0.0005 Non-frozen/Non-stratified 36.911 a 12.827 0.6505 0.438 0.182 b 0.0123 0.037 b 0.007 0.0005 F Ratio 192.691 21.346 39.200 Prob > F < 0.0001** < 0.0001** < 0.0001** Values within the column followed by different letters are significantly different at p < 0.01 using the Tukey-Kramer HSD test. Because the seeds were planted and germinated on different dates, their days to emergence was calculated by subtracting the Julian date of planting from the Julian date the individual seedlings emerged. When comparing the treatment effects across seedstocks, stratification significantly reduced the average days to emergence, with stratified seeds emerging after being planted for approximately 18 d, regardless of freezing treatment (Table 3). The freezing treatment showed a slight, but significant, effect of reducing the days to emergence on non-stratified seeds, compared to the control. For example, frozen but non-stratified seeds emerged over 33 d, which was approximately 4 d faster than control (non-stratified, non-frozen) seeds that took 37 d (Table 3). The results indicate that seed stratification significantly affected the germination date across frozen and non-frozen conditions, compared to the control.
The comprehensive test for the interactions of treatment effects and seedstock origin can be found in Supplementary Table S2. The freezing treatment had no significant interaction with the origin, while the stratification treatment had a significant interaction with the northern and southern seedstocks (Supplementary Table S2). Although seeds from southern seedstock germinated 3 d faster than other seedstocks, the days to emergence of all seeds across the treatments did not have significant differences with origin (Supplementary Table S3).
The germination rate of the non-frozen seed showed no significant difference, whether stratified or not (averaging 86.7% emerged vs 80.8% emerged) (Table 4, Supplementary Table S1). However, stratification significantly decreased the seed germination rate for the frozen seed compared to non-stratified seed (averaging 28.8% emerged to 65% emerged). The results indicate that stratification alone had the highest seed germination rate (86.7% emerged), while combining freezing and stratification significantly reduced seed germination rate (28.8% emerged). The freezing treatment alone decreased seed germination rate, but not significantly (65.0% vs 80.8% emerged) (Table 4, Supplementary Table S1).
Table 4. Seed germination rate (%) of 12 seedstocks under different treatments.
Seedstock Frozen/
Non-StratifiedFrozen/
StratifiedNon-frozen/
Non-stratifiedNon-frozen/
StratifiedAverage 87MX1-2.2 75 40 85 75 68.75 87MX5-1.7 60 70 75 85 72.50 Elliott 40 0 70 85 48.75 Frutoso 55 10 85 80 57.50 Giles 70 25 80 90 66.25 Major 20 10 80 85 48.75 Moore 80 75 90 90 83.75 Peruque 95 10 100 100 76.25 Posey 85 60 95 90 82.50 Riverside 80 0 100 90 67.50 San Felipe 90 25 80 100 73.75 VC1-68 30 20 30 70 37.50 Average 65 28.75 80.83 86.67 Data showed the percentage obtained by dividing the number of germinated seeds by the total number of seeds (20) in each treatment. The eastern seedstock 'Moore' had the highest germination rate (83.8%), followed by the northern seedstock 'Posey' (82.5%). Western seedstock 'VC1-68' had the lowest seed germination rate (37.5%) (Table 4). Interestingly, the northern seedstock 'Peruque' had the highest seed germination rate (95%−100%), except for the frozen, stratified seed (10%). No seed emerged for 'Elliott' (eastern) and 'Riverside' (western) after the Frozen/Stratified combined treatment (Table 4, Fig. 1a). The days to emergence varied among all seedstocks across the treatments, with no significant differences observed except for 87MX5-1.7 and 'Posey'. 87MX5-1.7 exhibited the shortest days to emergence, while 'Posey' took the longest (approximately 8 d difference) (Table 5).
Table 5. Days to emergence of different treated seed and seedling growth of different pecan rootstocks in the first season.
Seedstock Days to emergence Height growth rate (mm/day) Diameter growth rate (mm/day) Mean Std dev Std error Mean Std dev Std error Mean Std dev Std error 87MX1-2.2 24.764 ab 8.658 1.666 0.483 bcd 0.126 0.020 0.037 bc 0.006 0.001 87MX5-1.7 23.526 b 9.659 1.637 0.520 abc 0.116 0.020 0.037 bc 0.005 0.001 Elliott 26.872 ab 9.606 1.979 0.474 bcd 0.145 0.024 0.037 bc 0.006 0.001 Frutoso 28.556 ab 10.400 1.842 0.587 a 0.168 0.022 0.039 bc 0.007 0.001 Giles 28.528 ab 19.122 1.697 0.414 de 0.213 0.020 0.037 bc 0.010 0.001 Major 29.910 ab 14.766 2.004 0.397 de 0.213 0.024 0.041 abc 0.009 0.001 Moore 26.881 ab 11.073 1.510 0.508 abc 0.163 0.018 0.040 bc 0.007 0.001 Peruque 26.629 ab 10.131 1.582 0.225 f 0.100 0.019 0.031 c 0.009 0.001 Posey 31.000 a 15.114 1.510 0.359 e 0.129 0.018 0.040 bc 0.008 0.001 Riverside 27.964 ab 9.303 1.666 0.460 cd 0.149 0.020 0.041 abc 0.006 0.001 San Felipe 27.895 ab 15.239 1.637 0.557 ab 0.108 0.020 0.042 ab 0.005 0.001 VC1-68 24.167 ab 7.905 2.256 0.567 ab 0.110 0.027 0.045 a 0.006 0.001 F Ratio 1.7144 26.042 10.815 Prob > F 0.0665 < 0.0001* < 0.0001* Values within the column followed by different letters are significantly different at p < 0.01 using the Tukey-Kramer HSD test. Seedling growth
-
Because stratified seeds were planted a month later than non-stratified seeds, the number of growing days for the seedlings in the first planting group were not equal to those of the second group. Therefore, directly comparing their heights and diameters is inappropriate. However, the comparisons are possible when data are aggregated across origins based on the stratification treatment (which crosses both planting groups). Stratified, non-frozen seedlings had significantly greater average growth rates for height (0.53 mm height/day vs 0.396 to 0.438 mm height/day) and stem diameter (0.043 mm/day vs 0.035−0.039 mm/day), than all other treatments (Table 3). When seedlings were non-stratified, freezing significantly reduced average stem diameter growth (0.035 mm/day vs 0.037 mm/day), but had no significant effect on the average height growth rate (Table 3). The growth of frozen, stratified seedlings was not significantly different than the non-frozen, non-stratified control (Table 3). These results indicate that stratified or freezing treatments can independently influence seedling growth.
Overall, the seedlings displayed significant variation in vigor, with the greatest plant height observed on 'Frutoso', followed by 'San Felipe', 'VC1-68', and 87MX5-1.7, and the largest diameters observed on 'VC1-68', followed by 'San Felipe', and 'Riverside' (Table 5). In summary, western and southern seedstocks resulted in taller seedlings with larger stem diameters, and northern seedstocks resulted in shorter seedlings and smaller stem diameters (Table 5, Supplementary Table S3, Supplementary Fig. S1). In this test, seed quality (nut density) did not significantly correlate with days to emergence, seedling height, or diameter.
This study contains several variables, such as phenotypic traits, seedstock genotypes, origins, seed quality, and seed treatments. To reduce the complexity and uncover the underlying structure of the data, a principal component analysis (PCA) was conducted. The PCA visualized the data structure and relationship among these variables (Fig. 3). This biplot indicated that the top two principal components captured nearly 90% variances, with PC1 explaining 56.4% of the total variance of phenotypes and PC2 explaining 33.3% of the total variation. The patterns, trends, and relationships among the seed treatments, seed germination, and seedling growth corroborate the main findings of this study. For instance, stratification is negatively correlated with days to emergence, whereas freezing is positively correlated, suggesting that stratification promotes seed germination and freezing inhibits seed germination (though this correlation was not statistically significant).
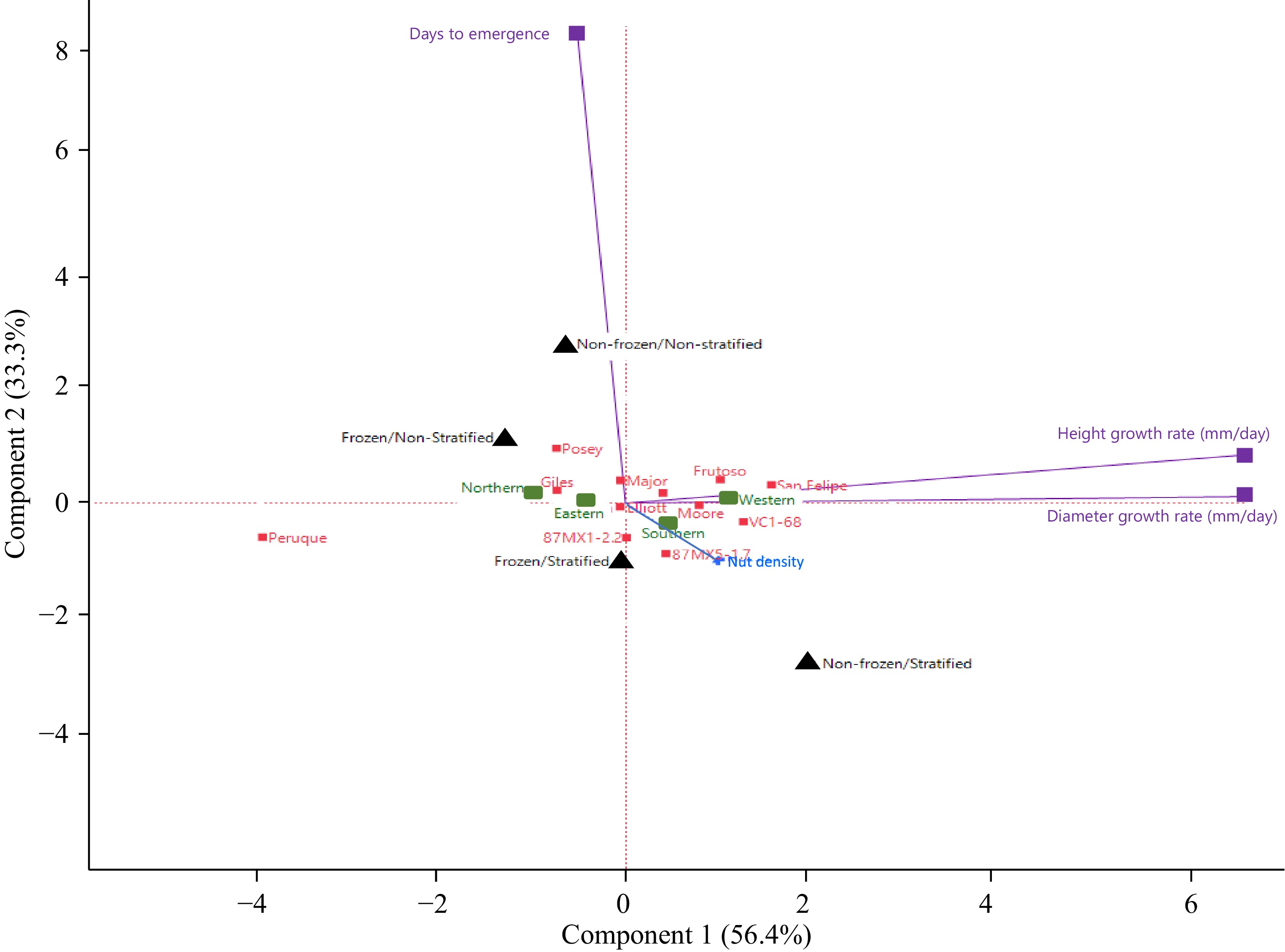
Figure 3.
PCA biplot of nut treatments to 12 seedstocks, origins, and nut density on nut germination, seedling height, and diameter. The explained variance of the axes is given in percentage. The eigenvalues of the first two PCA axes were 1.691 (56.4%) and 0.999 (33.3%), respectively. Treatments are presented in black, seedstocks in red, provenance origins in green, seed quality in blue, and phenotypic traits in purple.
-
Pecan growers face several challenges related to seed germination. Dormancy issues, including thick seed coats and strong seed sutures, often result in slow or uneven germination, impacting the overall efficiency of nursery operations. Additionally, pecan seeds require specific environmental conditions for successful germination and deviations from these conditions can lead to poor seedling establishment. These challenges necessitate precise management of pre-planting treatments to ensure optimal germination rates and seedling vigor. This study provides a detailed examination of how freezing and stratification treatments influence pecan seed germination and subsequent seedling growth across 12 seedstocks. The present findings indicate that these treatments significantly influence seed performance, which is essential for effective rootstock management and its horticultural practices, offering insights into practical nursery management and broader implications for nut crop cultivation.
Influence of treatments on seed germination rates
-
The data revealed a notable impact of freezing treatment on seed germination, with a significant proportion of frozen seeds failing to germinate. Specifically, nearly half of the seedstocks exhibited over 40% germination failure post-freezing, underscoring the potential detrimental effects of this treatment. Seedstock 'Major' displayed significant sensitivity to freezing, further illustrating the variability among different seedstocks. This response highlights the importance of selecting appropriate seed treatment protocols based on specific seedstock characteristics[26−30]. Interestingly, the interaction between stratification and freezing treatments was significant only when considering the maternal family of the seedstocks, suggesting that inherent genetic variability plays a crucial role in how these treatments affect germination rates[31,32]. The overall reduction in germination rates for frozen seeds—averaging only 15.71% in the first planting and 48.5% in the second—indicates a clear trend: freezing negatively impacts seed viability. In contrast, stratified seeds generally demonstrated higher germination rates, reinforcing the idea that stratification serves as a beneficial treatment to enhance seed performance[17,30,32,33].
The present findings indicate that stratification significantly decreases the time required for germination after planting, whereas freezing alone has a significant but decreased influence on days to emergence. Stratification, a process that involves exposing seeds to cold temperatures to break dormancy[6,21,27−29,33], proved beneficial across all seedstocks, leading to quicker and more uniform germination. The freezing treatment had a marginal impact on seed days to emergence but was still 4 d quicker than the control (non-frozen/non-stratified seed) (Table 3). However, the freezing treatment significantly decreased germination rates. This is consistent with findings in other nut crops where excessive cold exposure can negatively impact seed viability[27,28,33,34]. Therefore, freezing seeds without stratification would not be recommended as a meaningful practice for accelerating seedling germination, but is an essential step used by nurseries for killing pecan weevil infestations[23,24]. The present study did not evaluate the effectiveness of freezing for pecan weevil elimination; however, it is important to carefully balance the effects of a freezing treatment with stratification to prevent negative impacts on seed germination.
Days to emergence
-
While freezing treatment was shown to delay germination rates, it was somewhat surprising that it did not significantly alter the days to emergence across different seedstock origins (Supplementary Table S3). Stratification, however, consistently resulted in faster emergence, reducing the average days to emergence to approximately 18 d across treatments. This finding aligns with previous research indicating that stratification can facilitate quicker germination and emergence by breaking seed dormancy[27−29]. The slight but significant reduction in days to emergence for frozen, non-stratified seeds—emerging 4 d earlier than the control—suggests that while freezing is generally detrimental, it may still have minor effects that need further investigation. The overall lack of significant differences in days to emergence across seedstock origins, aside from the notable trend in southern seedstocks emerging faster emphasizes the need for broader evaluations of how genetic factors influence germination dynamics[31].
Germination rates across seedstocks
-
The results showed substantial variation in germination rates among the seedstocks, with 'Moore' achieving a high germination rate of 83.8% compared to the significantly lower rate of 37.5% observed in 'VC1-68'. This is a surprising result because 'VC1-68' has been using rootstock by the US pecan growers, especially in California and southern Texas. This variability is crucial for understanding which seedstocks may be better suited for specific environmental conditions or treatment protocols. Notably, the northern seedstock 'Peruque' exhibited exceptional germination rates under non-frozen conditions but dramatically declined when subjected to the combined freezing and stratification treatment.
These findings suggest that certain seedstocks have innate characteristics that make them more resilient to specific treatments, further highlighting the importance of tailoring seed management strategies to individual seedstock needs. Additionally, the absence of germination in certain seedstocks after combined treatments raise questions about potential thresholds of stress that seeds can tolerate, which could guide future breeding and selection efforts.
Seed quality is another factor affecting germination[31]. This study showed that well-filled seeds (higher density) germinated faster and exhibited quicker seedling growth (Fig. 3), a finding that is consistent with previous research[6], even though this interaction was not statistically significant in the present study.
Seedling growth analysis
-
While seedling growth comparisons between stratified and non-stratified treatments were complicated by differences in planting dates, aggregated data across origins allowed for meaningful insights. Stratified, non-frozen seedlings exhibited significantly higher growth rates in both height and stem diameter compared to other treatments. This aligns with existing research on nut crops, such as almonds and walnuts, where cold stratification has similarly improved germination rates by overcoming physiological dormancy barriers[17,31,33]. This suggests that stratification not only improves germination but also promotes better seedling development. Conversely, freezing led to a significant reduction in the stem diameter growth rate compared to the control. When freezing was combined with stratification, no significant differences in growth from the control were observed. Similar trends have been observed in pistachios, in which cold stratification improved seedling growth, whereas excessive freezing hindered it[34]. Nevertheless, this finding reinforces the hypothesis that stratification enhances not only germination but also early seedling vigor, which is crucial for establishing strong plants in competitive environments.
The observation that freezing negatively impacted stem diameter growth in non-stratified seedlings, while height growth remained unaffected, suggests differential responses of growth parameters to stress. This differential impact emphasizes the complexity of seedling development, where some traits may be more sensitive to environmental stressors than others.
Variability among seedstocks
-
The analysis revealed significant variation in seedling vigor across different seedstocks, with 'Frutoso' displaying the tallest seedlings and 'VC1-68' exhibiting the largest diameters. Notably, the western and southern seedstocks produced taller seedlings with larger diameters compared to northern seedstocks. This pattern raises important considerations for seedstock selection based on intended agricultural outcomes, such as yield potential or adaptability to specific environments. Interestingly, seed quality as measured by nutrient density did not correlate significantly with days to emergence or seedling growth metrics. This lack of correlation suggests that factors influencing seed performance may extend beyond intrinsic seed quality, warranting further exploration into the complex interactions among genetics, environmental conditions, and seed treatment protocols.
Principal component analysis (PCA)
-
In this study, principal component analysis (PCA) was employed to simplify the complexity of the dataset, which included variables such as phenotypic traits, genotypes, origins, seed quality, and seed treatments. By conducting PCA, the aim was to uncover the underlying relationships among these variables and visualize the data structure. The resulting biplot revealed that the first two principal components (PC1 and PC2) together accounted for nearly 90% of the total variance—specifically, 56.4% for PC1 and 33.3% for PC2. This substantial explanation of variance indicates that these components effectively capture the critical patterns within the data.
The PCA also highlighted notable trends, particularly concerning seed treatments and their impact on germination and seedling growth. For example, the analysis suggested that stratification may enhance seed germination by being negatively correlated with the number of days to emergence. Conversely, freezing treatments appeared to delay germination, as indicated by a positive correlation with days to emergence. While these correlations were observable trends, it is important to note that they were not statistically validated. Overall, the PCA results provide valuable insights into how different factors influence seed performance, laying the groundwork for further investigation into optimal seed treatment strategies.
Implications for seedstock management
-
The findings from this study have significant implications for seed management practices. Understanding the differential responses of various seedstocks to freezing and stratification can guide farmers and seed producers in selecting appropriate treatments tailored to specific seed types. For example, seedstocks that are particularly sensitive to freezing may benefit from exclusive stratification treatments to enhance germination rates without the added stress of freezing. Moreover, the observed variability in seedling vigor and growth emphasizes the necessity of evaluating seedstocks based on their specific growth characteristics, which can influence overall crop performance. By integrating knowledge of seed treatment impacts and genetic variability, stakeholders can develop more effective seed management strategies that maximize germination success and seedling establishment.
Future directions
-
The study underscores the need for a nuanced approach to seed treatment. While stratification is crucial for enhancing germination and seedling growth, freezing treatments should be optimized to balance pest control with seed viability. Future research should focus on refining these treatments to minimize their negative impacts. For instance, exploring different freezing durations and temperatures could help mitigate adverse effects on seed germination. Additionally, investigating other pre-planting treatments, such as chemical scarification[32,35], varying stratification periods[26,36], or temperature ranges[30] could provide further insights into improving seedling production efficiency. While this study provides valuable insights into the effects of freezing and stratification on seed germination and growth, several avenues for future research remain. First, a deeper exploration of the genetic mechanisms underlying the observed variability among seedstocks would provide a clearer understanding of how certain traits can be enhanced through breeding programs[6,37−40]. Additionally, longitudinal studies tracking the long-term performance of seedlings originating from different treatments could offer insights into how early germination and growth impacts overall plant health and productivity. Finally, investigating the interactions between environmental conditions, such as temperature and moisture levels[27,28,41], with seed treatments could further refine our understanding of optimal seed management practices[12,17,37].
-
In conclusion, the results of this study demonstrate the significant effects of various seed treatments on germination rates and seedling growth across 12 pecan seedstocks. The freezing treatment led to notably high germination failure, with nearly 50% of the seedstocks exhibiting over 40% failure rates. In contrast, stratification consistently improved germination, with stratified seeds emerging approximately 18 d after planting, compared to longer durations for non-stratified seeds. Specifically, non-stratified, frozen seeds took an average of 33 d to emerge, while control seeds averaged 37 d. Moreover, the analysis revealed distinct variations among seedstocks, with 'Moore' achieving the highest germination rate at 83.8%, while 'VC1-68' struggled with a low rate of 37.5%. The interaction between freezing and stratification treatments significantly affected the germination rates, with frozen, stratified seeds showing only 10% germination for the northern seedstock 'Peruque'. Seedling growth metrics further emphasized the benefits of stratification, as stratified, non-frozen seedlings exhibited a growth rate of 0.53 mm/day in height, outperforming all other treatments. These findings indicate that effective management of seed treatments is crucial for optimizing germination and seedling vigor, which can ultimately enhance commercial pecan production. Future research should continue to explore these interactions to refine cultivation strategies and improve yield outcomes.
This research was supported by the US Department of Agriculture – Agriculture Research Service National Programs through CRIS project 3091-21000-046-000-D (Crop Germplasm Research Unit, TX, USA). The authors highly appreciate the efforts of Dr. LJ Grauke, a retired Horticulturist in the USDA-ARS Pecan Breeding and Genetics Program, for establishing this project, and retired senior technician Lynn Johnson for his efforts in seed measurement, germination, seedling management, and data collection. This article reports the results of research only. Mention of a trademark or proprietary product is solely for the purpose of providing specific information and does not constitute a guarantee or warranty of the product by the US Department of Agriculture and does not imply its approval to the exclusion of other products that may also be suitable.
-
The authors confirm contribution to the paper as follows: study conception and design: Cox T, Kubenka K; analysis and interpretation of results: Wang X, Hilton A, Chatwin W; draft manuscript preparation: Wang X; review of the manuscript: Chatwin, W, Hilton A, Kubenka K, Tondre B. All authors have read and agreed to the final version submitted for publication.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
- Supplementary Table S1 Numberes of seeds emerged and germiantion rates of freezing treatment in the different planting time.
- Supplementary Table S2 Scaled Estimates using the nominal factors expanded to all levels.
- Supplementary Table S3 The influence of pecan rootstock origin on days to emergence and seedling growth in the first season.
- Supplementary Fig. S1 The visual comparisons of seedling height (A), and stem diameter (B) of the seedstocks from four regions under different seed treatments.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang X, Kubenka K, Hilton A, Chatwin W, Cox T, et al. 2025. The effects of freezing and stratification on pecan (Carya illinoinensis) seed germination and seedling growth. Technology in Horticulture 5: e002 doi: 10.48130/tihort-0024-0030
The effects of freezing and stratification on pecan (Carya illinoinensis) seed germination and seedling growth
- Received: 09 September 2024
- Revised: 24 October 2024
- Accepted: 29 October 2024
- Published online: 09 January 2025
Abstract: Pecan (Carya illinoinensis) cultivation is crucial for commercial production and relies on selecting rootstocks adapted to local environments. Historically, pecan breeding has prioritized scion improvement over rootstock selection due to propagation challenges. However, rootstocks significantly impact scion growth, phenology, and productivity. Pecan nurseries use open-pollinated seeds from regionally favored cultivars (seedstocks) as rootstocks, and seedstock influences germination. Challenges arise from seed dormancy, with some varieties stratification or having thick shells, which affect germination and seedling growth. Pecan nurseries use freezing to eliminate pecan weevil infestations and stratification to synchronize seed germination, but their effects are not well quantified. This study investigates how freezing and stratification impact seed germination and seedling growth across 12 pecan seedstocks from diverse origins. Results indicate that both freezing and stratification, or their combination significantly affect seed germination. Stratification improved germination rates, with non-stratified frozen seeds averaging only 15.7% compared to 48.5% for stratified seeds. Stratified seeds also emerged faster, averaging 18 d, whereas non-stratified seeds took 37 d. The effect of stratification on germination was not influenced by freezing. Although freezing reduced germination rates, especially when combined with stratification, seedstock origins did not significantly affect germination. Stratification interacted significantly with northern and southern origins. The study underscores the need for a nuanced approach to seed treatment. While stratification is crucial for enhancing germination and seedling growth, freezing treatments should be optimized to balance pest control with seed viability. Future research should focus on refining these treatments to minimize their negative impacts.
-
Key words:
- Pecan rootstock /
- Provenance /
- Geographical origins /
- Seed treatment /
- Seed emergence /
- Germination rate /
- Growth rate













