-
Currently, due to world climate change, there is an increase in temperature and precipitation negatively impacting ecosystems[1]. These climate changes contribute to waste in the food supply chain[2].
Climate change threatens food security by drastically reducing agricultural production due to abiotic stresses and stimulating the spread of new and old pathogens[3,4]. Recently, the emergence of diseases related to the fungus Nigrospora sp. has been discovered in various plants and fruits such as olive trees[5], palms[6], blueberries[7], kiwis[8], passion fruits[9], and even in banana trees where this fungus acted as an endophyte[10].
Endophytic microorganisms are found in almost all plant species in the world and reside within plant tissues[4]. These microorganisms have a mutualistic relationship with plants by inhibiting pathogens such as bacteria E. coli, B. subtilis, and P. oryzae, and fungi such as C. albicans through their bioactive secondary metabolites[11]. However, mutualistic functionality can transform into pathogenicity depending on the host, fungal genotype, and abiotic conditions[12].
The spores of Nigrospora sp. are dispersed by wind, rain, and insect vectors[13]. Furthermore, higher temperatures in winter can reduce the mortality of insect vectors promoting infections by Nigrospora sp. in different weakened or injured plants throughout the year and threaten crops with economic importance[14]. Warm and humid conditions of tropical and subtropical climates are the most ideal for this fungus[5]. Therefore, alternatives to combat new post-harvest diseases are necessary to minimize the environmental and economic impacts associated with food waste.
Papaya is a tropical fruit with a sweet flavor and numerous health benefits[15]. However, this fruit has a relatively short shelf life, especially when compared to some other fruits[16]. Papaya is a climacteric fruit; therefore, it continues to ripen after harvest[17]. Fruit maturation is related to an increase in the hormone ethylene and, as they ripen, changes occur in softness, sweetness, and aroma[18]. Specifically, the increase in ethylene levels accelerates respiration rates and enzymatic activities that modify the fruit's texture and taste. Consequently, while ripening enhances palatability, it also shortens the shelf life of papayas.
In addition to ripening, post-harvest diseases are also mainly responsible for the short shelf life of papayas[19]. There is a growing interest in exploring natural alternatives, such as the application of essential oils due to their antimicrobial properties[18], aiming to extend the shelf life of fruits and ensure consumer safety[20], in addition to minimizing post-harvest diseases.
Essential oils derived from plants, such as oregano and clove have antimicrobial properties against bacteria and fungi[21]. Therefore, they are a natural and sustainable alternative for preserving fruits[22]. These bioactive compounds damage cell membranes, interfere with metabolic processes, and inhibit fungal growth[23]. The main compound of clove essential oil is eugenol, capable of inhibiting enzymatic activity, and inducing oxidative stress in fungi with consequent cell death[24].
The use of essential oils as antimicrobials in fruits is a field of continuous research and development because, despite their proven effectiveness, their application and dosage must be optimized to guarantee safety and benefit. Furthermore, to our knowledge, this is the first study of a potentially sustainable method to control post-harvest disease caused by Nigrospora sp. in papayas and shows the importance of evaluating preventively potential harmful fungus reported on other tropical and temperate zones crops[25]. The main objective of this research was to evaluate the antimicrobial action of essential oils (EOs) as alternatives for the control of emerging fungal diseases, such as those caused by the fungus Nigrospora sp. in papayas. To achieve this, in vitro and in vivo studies were carried out using clove and oregano essential oils as strategies to combat post-harvest infections.
-
The evaluation of the antifungal activity of the essential oils of clove and oregano was made in two stages: first, the tests were in vitro by the direct contact method and then in vivo with the inoculation of the fungus Nigrospora sp. in papayas to analyze the inhibition of this disease in the fruit.
Materials
-
The essential oils were purchased from Harmonie aromaterapia (Florianópolis, SC, Brazil) and fungal strains are from the Embrapa Instrumentation collection. All reagents used for analysis were of analytical grade. Papayas from the Solo group, cultivar Golden, were carefully transported in refrigerated trucks at 10 °C from the city of Araraquara to the post-harvest laboratory, Embrapa Instrumentação (São Carlos, SP, Brazil) (a distance of 30 km and time of 45 min) and sanitized with a 0.02% (v/v) NaClO solution for 15 min. Immediately afterwards, papaya fruits at stage 2 of maturation, with up to 25% of the skin surface covered by a yellow color, were homogenized without defect, pattern, and size[26].
Gas Chromatography coupled to Mass Spectrometry (GC-MS)
-
Clove and oregano essential oils were initially diluted in dichloromethane in a ratio of 1:10 and stored in 1.5 mL bottles at −28 °C. For analysis, 1 μL of the samples were diluted in dichloromethane (10% v/v) and injected into a Shimadzu GC-MS model GCMS-QP2010 Plus, on an HP-5MS fused silica capillary column (30 m × 0.25 mm i.d. × 0.25 μm). The chromatographic conditions were: injector at 250 °C in split mode 1:20 for 1.0 min; helium carrier gas at 1.0 mL·min−1; oven temperature ramp of 60 °C (1 min), with an increase of 3 °C·min−1 up to 240 °C; interface temperature: 240 °C, electron ionization source +70 eV, scanning mode between 35 and 350 m·z−1. To obtain the temperature programmed retention index (LTPRI) of volatile compounds, a solution of n-alkanes (C8−C20) was injected into the GC-MS under the same conditions as the sample. The analytes were identified by comparing the LTPRI and mass spectra of the sample with literature data[27].
In vitro analysis − evaluation of the antifungal activity of EOs
-
This analysis was performed according to the methodology of Plaza et al.[28]. Inhibition of fungal growth by EO was obtained by measuring fungal growth in potato dextrose-agar (BDA) culture medium with EO concentrations of 62.5, 125, 250, 500, 750, and 1,000 μL·L−1. The emulsifier Tween 80 (0.05% v/v) was used to homogenize the EOs in the PDA medium. A control treatment containing only the emulsifier, and the culture medium was also used. A disk (5 mm in diameter) with the mycelium (inoculum) of the fungus was transferred to the Petri dishes with the PDA medium and then kept at 25 °C. Fungal growth measurements were taken every eight hours, in two perpendicular directions (diameter expressed in centimeters) and measured by Eqn (1):
$ \mathrm{P}\mathrm{I}\; \left(\mathrm{\text{%}}\right)=\dfrac{\left(\mathrm{G}\mathrm{r}\mathrm{o}\mathrm{w}\mathrm{t}\mathrm{h}\; \mathrm{ }\mathrm{c}\mathrm{o}\mathrm{n}\mathrm{t}\mathrm{r}\mathrm{o}\mathrm{l}\mathrm{ }-\mathrm{T}\mathrm{e}\mathrm{a}\mathrm{t}\mathrm{m}\mathrm{e}\mathrm{n}\mathrm{t}\; \mathrm{ }\mathrm{g}\mathrm{r}\mathrm{o}\mathrm{w}\mathrm{t}\mathrm{h}\right)}{\rm{Control\; growth}}\times100 $ (1) where PI represents the percentage of inhibition. Among the concentrations evaluated, the lowest concentration that completely inhibited fungal growth was considered the Minimum Inhibitory Concentration (MIC).
Scanning electron microscopy
-
The changes in the morphology of fungi caused by the antimicrobial action of essential oils were analyzed using a Scanning Electron Microscope (SEM-SEM JEOL JSM-6701F, Tokyo, Japan). The sample was prepared according to Yu et al.[29] with some modifications. The fungus Nigrospora sp. was cultivated for seven days at 25 °C. Then, 1 mm discs of mycelium containing the fungus were removed and deposited in petri dishes and kept for three days at 25 °C. After this period, clove and oregano essential oil were added to different plates and incubated for 24 h. The tests were carried out in triplicate and with control plates (without essential oil). A disc with a diameter of 1 mm was removed with the fungus and placed in tubes with glutaraldehyde (3%, v/v) overnight and then immersed in phosphate buffer (0.05 M, pH 6.8). The samples were dehydrated in acetone solutions (30%, 50%, 70%, and 90%, v/v) and dried in liquid carbon dioxide at the critical point. Subsequently, the samples were deposited on stubs and coated with gold.
In vivo analysis − application of essential oils on papayas
-
Nigrospora sp. was cultivated in PDA culture medium at 25 °C for seven days[30]. The papayas were previously sanitized in 0.02% (v/v) NaClO solution for 10 min after arriving at the laboratory. Then, the papayas were inoculated at five points in the equatorial region, making micro-wounds (1−2 mm deep) with the aid of a flamed needle number 5, on which 1 mm disks of mycelia from the petri dish with the fungus were deposited. Then, 1 mL of the oils were manually applied to the fruits. After covering the papayas with different treatments, they were stored at 25 °C and 70% relative humidity for eight days. For the incidence analysis, all papayas were first sanitized in a 0.02% (v/v) NaClO solution for 10 min, before applying the oils and inoculating the fungi. Then the oils were applied in a curative way (24 h after microorganism inoculation) and in preventive mode (24 h before microorganism inoculation). Then, the papayas were separated into eight treatments: Inoculated control (papayas without oils only inoculated); Control without inoculation (papayas without oil and without being inoculated); Control-CEO (papayas coated with clove oil without inoculation); CEO-P (papayas coated with clove oil in preventive mode and inoculated); CEO-C (papayas coated with clove oil cloves in curative mode and inoculated); Control-OEO (papayas coated with oregano oil without inoculation); OEO-P (papayas coated with oregano oil in preventive mode and inoculated); OEO-C (papayas coated with oregano oil oregano in curative mode and inoculated). The positive and negative controls were papayas in the inoculated control group and papayas in the control without inoculation group respectively. The papayas were separated into three groups for non-destructive analyses (colorimetry, mass loss, and size measurement): control (papayas without oil coating); CEO (papayas coated with oil clove essential); and OEO (papayas coated with oregano essential oil). Papaya quality attributes were evaluated on days 0, 2, 5, and 8 of storage. For non-destructive analyses, ten papayas were used per treatment and for incidence analyses five papayas were used.
Post-harvest non-destructive analysis on papayas
-
Fruit weight loss was evaluated on days 0, 2, 5, and 8 of storage, calculated in relation to the initial weight and presented as percentage. The diameter and length of each fruit were measured with a measuring tape. The color measurements were performed on the external surface of the fruit (on the peel) with a Minolta® CR-400 Chroma Meter colorimeter (Minolta Camera Co., Osaka, Japan). Chroma (C*) was calculated using Eqn (2) and Hue angle (h°) using Eqn (3). Three measurements were taken on each fruit in the equatorial region and at equidistant points.
$ {C}^{*}=\left(\right({a}^{*}{)}^{2}+({b}^{*}{)}^{2}{)}^{1/2} $ (2) $ {h}^{\circ}={tan}^{-1}\left(\dfrac{{b}^{*}}{{a}^{*}}\right) $ (3) Severity disease analysis
-
The diameter of the lesions on the fruits was performed on days 2, 5, and 8 of storage with a digital caliper and calculated using the area under the disease progress curve (AUDPC)[31].
$ AUDPC=\sum \left[\left(\dfrac{{Y}_{i}+1 +{Y}_{i}}{2}\right)\right]\left[{T}_{i}+1 -{T}_{i}\right] $ (4) where Yi + 1 = lesion diameter at time Ti + 1 and Yi = diameter of the lesion in time Ti.
Statistical analysis
-
To evaluate the differences between means, analysis of variance (ANOVA) and Tukey's test (p < 0.05) were used in the Sisvar software[32].
-
In Table 1, the main compounds in clove essential oil are observed to be eugenol and β-caryophyllene, with percentages of 90.75% and 7.81%, respectively. The main compounds of oregano essential oil are carvacrol (67.89%) and p-cymene (8.69%). The remaining significantly identified compounds are listed in the table, but compounds corresponding to peaks with a relative area of less than 1% were not listed.
Table 1. Major compounds of Syzygium aromaticum and Origanum vulgare essential oils.
Compound Syzigium aromaticum
(% area)Origanum vulgare
(% area)Eugenol 90.75 − β-caryophyllene 7.81 − β-myrcene − 1.45 α-terpinene − 1.22 p-cymene − 8.69 γ-terpinene − 6.15 Thymol − 8.13 Carvacrol − 67.89 Caryophyllene − 1.86 Total 98.56 95.39 These data corroborate the studies by Oliveira et al.[18] and Ferreira et al.[33], which found values of 89.73% and 96.33% for the eugenol compounds present in S. aromaticum oil. Tsoumani et al.[34] obtained percentages of 68.79% and 8.32% for the compounds carvacrol and p-cymene from the essential oil of O. vulgare. The composition of essential oils can vary depending on geographic origin, where they are extracted from plants (roots, leaves, and stems), and genetic factors[35]. Kaur et al.[36] proved the antifungal activity of eugenol against Fusarium moniliforme and Helminthosporium oryzae in their study. In another study comparing the action of eugenol with the synthetic antifungal clotrimazole, the natural compound showed greater efficacy against C. glabrata, C. albicans, and C. tropicalis[37]. The antifungal activity of carvacrol was proven against 27 clinical isolates of Malassezia furfur, a fungus associated with human mycoses[38]. Carvacrol was also effective against Botrytis cinerea, another fungus that causes post-harvest fruit rot[39].
In vitro analysis − evaluation of the antifungal activity of EOs
-
Table 2 presents the Minimum Inhibitory Concentration values of clove and oregano essential oil against the fungus Nigrospora sp. Both oils inhibited fungal growth at the same MIC value, at an EO concentration of 125−250 μL·L−1.
Table 2. Percentage inhibition of mycelial growth of Nigrospora sp. and Minimum Inhibitory Concentration (MIC) of Syzygium aromaticum and Origanum vulgare essential oils at different concentrations (μL·L−1).
Concentration (μL·L−1) Syzygium aromaticum Origanum vulgare 0 0.00 ± 0.00 0.00 ± 0.00 62.5 59.48 ± 2.83 35.11 ± 4.82 125 66.69 ± 0.28 82.83 ± 2.37 250 100.00 ± 0.00 100.00 ± 0.00 500 100.00 ± 0.00 100.00 ± 0.00 750 100.00 ± 0.00 100.00 ± 0.00 1,000 100.00 ± 0.00 100.00 ± 0.00 MIC 125 < MIC ≤ 250 125 < MIC ≤ 250 The main component of clove essential oil is eugenol, and oregano is carvacrol[40]. Both compounds are phenols and have antifungal properties[41]. The antifungal mechanisms of these compounds are through the rupture of the cell membrane, damage to the structure, and cell lysis[42]. These compounds also induce the production of reactive oxygen species (ROS) that damage fungal cellular components[43,44].
Although the antifungal mechanisms of these oils are similar, they differ in their active compounds and therefore, the potency of antifungal activity may vary depending on the type of fungus targeted and the specific concentrations of active compounds in the oil[45]. The other minor compounds in essential oils also contribute to their antifungal effects[46].
Scanning electron microscopy
-
Figure 1a & d show that the fungi in the control group presented normal and tubular morphology. Fungi treated with essential oils of clove (Fig. 1b, e) and oregano (Fig. 1c, f) exhibited crushed, distorted, and shrunken hyphae. Fungi under the influence of oregano essential oil still has ruptured mycelia. These images show that the main compounds in these oils, eugenol (present in CEO) and carvacrol (present in OEO) can cause a lot of damage to the plasma membranes of fungi, such as damaging the cellular structure and causing the lysis of these structures.
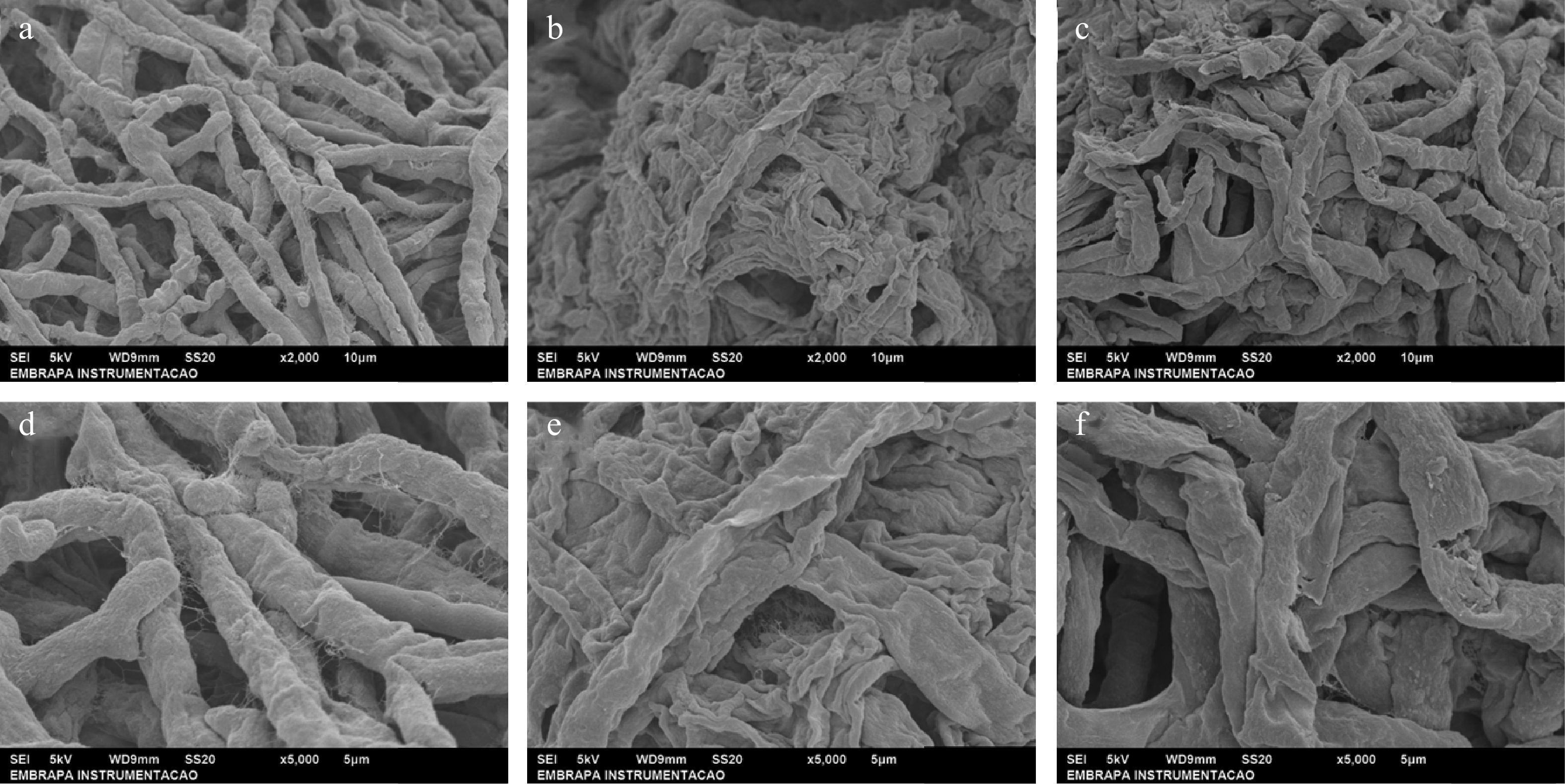
Figure 1.
Images obtained by scanning electron microscopy (SEM) of the morphology of the fungus Nigrospora sp. under the action of the essential oils Syzygium aromaticum and Origanum vulgare at (a)−(c) magnification 2,000× and scale bar 10 μm, and (d)−(f) magnification 5,000× and scale bar 5 μm. (a), (d) Control, (b), (e) CEO, (c), (f) OEO.
Zhou et al.[47] verified with SEM images that F. oxysporum mycelia became wrinkled and rough under the action of eugenol. Zhang et al.[39] also observed in their work that B. cinerea mycelia was crushed and ruptured by the carvacrol. They also found a marked decline in the total lipid content of the fungal cells, suggesting that the cell membrane structures were destroyed. In the study by dos Santos et al.[48], R. stolonifer hyphae became wrinkled and lost cytoplasmic material when subjected to the action of O. vulgare essential oil. With the loss of cytoplasmic components, there is a loss of rigidity and integrity of the fungal cell wall, resulting in the death of the microorganism[49].
In vivo analysis - application of essential oils on papayas
Physicochemical parameters of papaya
-
Figure 2 shows that over time, fruits coated with essential oils lost less weight compared to control fruits. This effect must be related to the hydrophobic nature of essential oils[50]; thus EO forms a thin barrier on the surface of the fruit[51]. This barrier helps prevent fruit water loss through transpiration, so papaya fruit retain more moisture. Fruit respiration can also be inhibited, limiting the diffusion of gases such as oxygen and carbon dioxide into and out of the fruit[52].
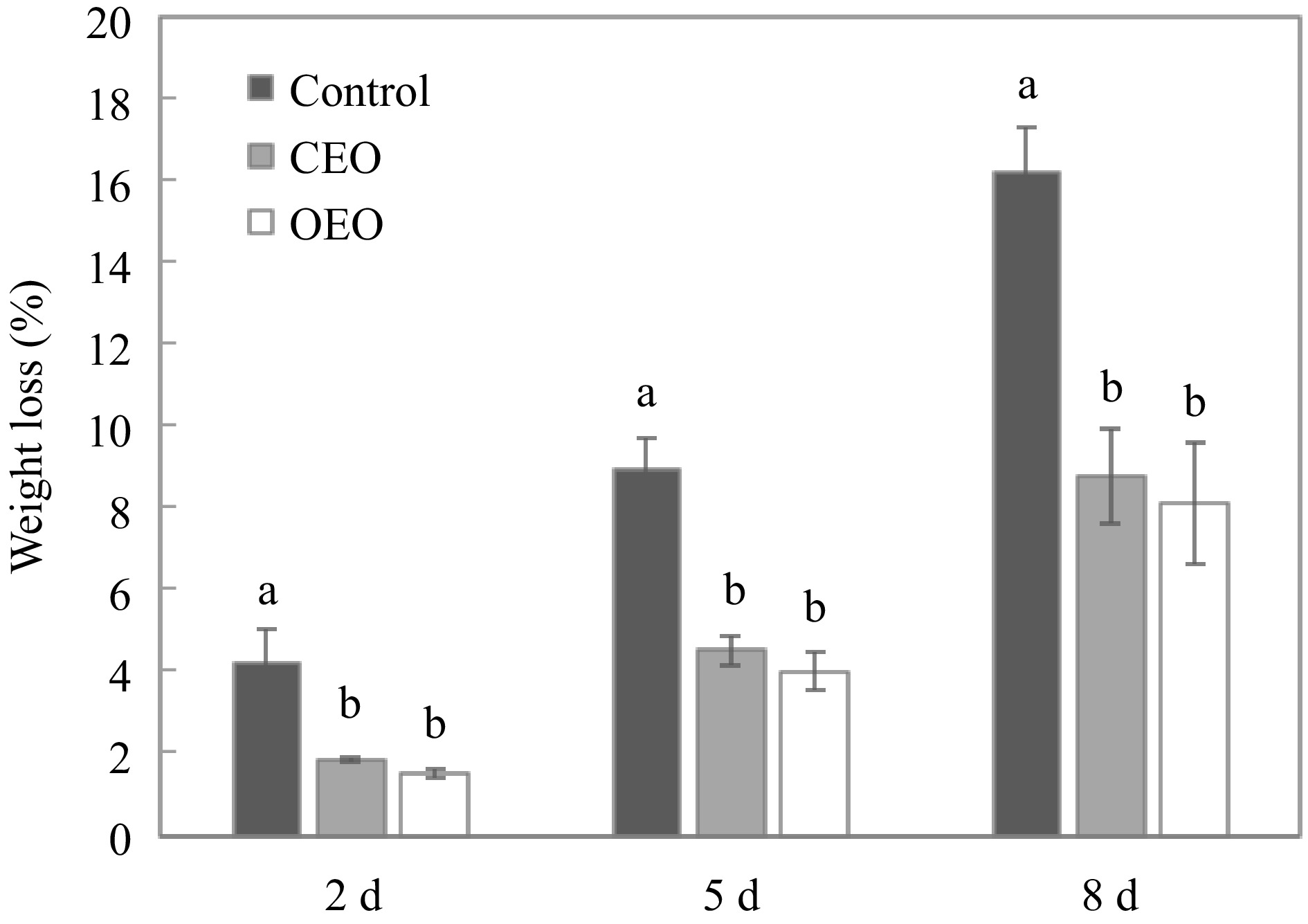
Figure 2.
Weight loss of papaya during storage at 25 °C and 70% RH treated without essential oil (control), with Syzygium aromaticum essential oil (CEO) and with Origanum vulgare essential oil (OEO). For each storage period, different letters indicate significant differences among treatments (p < 0.05).
Another factor that may have influenced fruits with essential oils to lose less weight is the bioactivity of essential oils as antimicrobial and antioxidant[53], resulting in inhibiting the growth of microorganisms and reducing the oxidation rate in fruit tissues, and delaying ripening and senescence[54]. Thus, the fruit firmness rate might be reduced due to slower cell degradation. Furthermore, for this research, the thin layer of essential oil on the surface of the fruit acted as a physical barrier, protecting the fruit from superficial injuries that can lead to increased water loss and microbial entry, thus accelerating weight loss and spoilage[55].
Table 3 shows that control fruits presented a shorter length and smaller diameter over storage time of 8 days compared to the fruits treated with the essential oils.
Table 3. Length and diameter of papaya fruits over storage time (8 d) treated without essential oil (control), with Syzygium aromaticum essential oil (CEO), and with Origanum vulgare essential oil (OEO).
Storage time (d) Length (cm) Diameter (cm) 0 2 5 8 0 2 5 8 Control 17.26 ± 0.56a 16.54 ± 0.95a 15.75 ± 0.63b 15.09 ± 0.83b 25.25 ± 0.44a 24.82 ± 0.68a 23.97 ± 0.57b 23.05 ± 0.58b CEO 17.28 ± 0.98a 17.20 ± 0.82a 17.15 ± 0.62a 17.11 ± 0.88a 25.19 ± 0.52a 25.15 ± 0.78a 25.11 ± 0.65a 25.09 ± 0.80a OEO 17.25 ± 0.71a 17.21 ± 0.97a 17.18 ± 0.88a 17.13 ± 0.63a 25.22 ± 0.65a 25.16 ± 0.54a 25.10 ± 0.71a 25.07 ± 0.75a Means followed by different letters on the same column indicate significant differences among treatments (p < 0.05). Weight loss in papaya fruit can notably impact its size, length, and diameter due to the high content of water and changes that occur during dehydration[56]. As the fruit cells lose water, they become dehydrated and shrink. Thus, a reduction in the total volume of the papaya occurs[57]. Water loss also causes a loss in fruit turgidity. The fruit pulp becomes softer and less rigid, contributing to a change in the total weight and mass, affecting the fruit size[58]. Therefore, volume reduction, dehydration, and softening contribute to a decrease in the papaya length and diameter. As shown in the mass loss results, the essential oils in papayas prevented the fruits from having considerable changes in their length and diameter (Table 3).
Table 4 shows the color parameters L*, C*, and hº. The values of L (brightness), chroma (color intensity), and hº (hue) decreased over time in the uncoated and oil-coated papayas during storage. The L* and C* values of the peel of the coated fruits did not change much over time compared to the control fruits, indicating that there may have been less color change and delay in ripening. During papaya maturation, the skin changes from green to yellow. It is due to the degradation of chlorophyll and the synthesis of pigments such as lycopene and carotene[59]. These results are aligned with the work by Culmone et al.[60] where papayas with oregano essential oil showed little change in their L* and C* values compared to control fruits.
Table 4. Color parameters L*, C*, and h° of papayas stored for 8 d at 25 °C and 70% RH treated without essential oil (control), with Syzygium aromaticum essential oil (CEO), and with Origanum vulgare essential oil (OEO).
Time (d) Treatments L* C* (hº) 0 Control 64.10 ± 5.77a 57.56 ± 2.92b 101.05 ± 1.13a CEO 66.01 ± 9.35a 62.90 ± 7.37a 93.22 ± 7.05b OEO 65.50 ± 7.11a 49.67 ± 7.18c 101.67 ± 3.29a 2 Control 63.53 ± 6.16a 54.21 ± 6.93b 87.47 ± 4.91b CEO 65.59 ± 7.38a 60.92 ± 11.79a 84.45 ± 10.67b OEO 64.79 ± 7.99a 47.23 ± 10.65c 94.36 ± 8.36a 5 Control 61.75 ± 6.80a 53.96 ± 6.58b 74.71 ± 5.64a CEO 65.35 ± 5.78a 60.54 ± 8.47a 73.72 ± 8.55a OEO 64.41 ± 10.88a 45.97 ± 9.79c 77.73 ± 8.53a 8 Control 54.98 ± 7.90a 52.81 ± 15.07a 70.54 ± 3.78b CEO 60.14 ± 8.28a 59.29 ± 13.55a 68.81 ± 4.22b OEO 61.70 ± 7.78a 40.94 ± 9.21b 77.27 ± 5.08a Means followed by different letters on the same column indicate significant differences among treatments (p < 0.05). The values presented in this table are the means ± standard deviations. Figure 3 shows the in vivo antifungal activity of essential oils applied to papayas. Fruits coated with the oils had lower rot severity by Nigrospora sp. compared to the control fruits. Clove essential oil acted better curatively than preventively. On the other hand, the oregano essential oil acted better preventively than curatively.
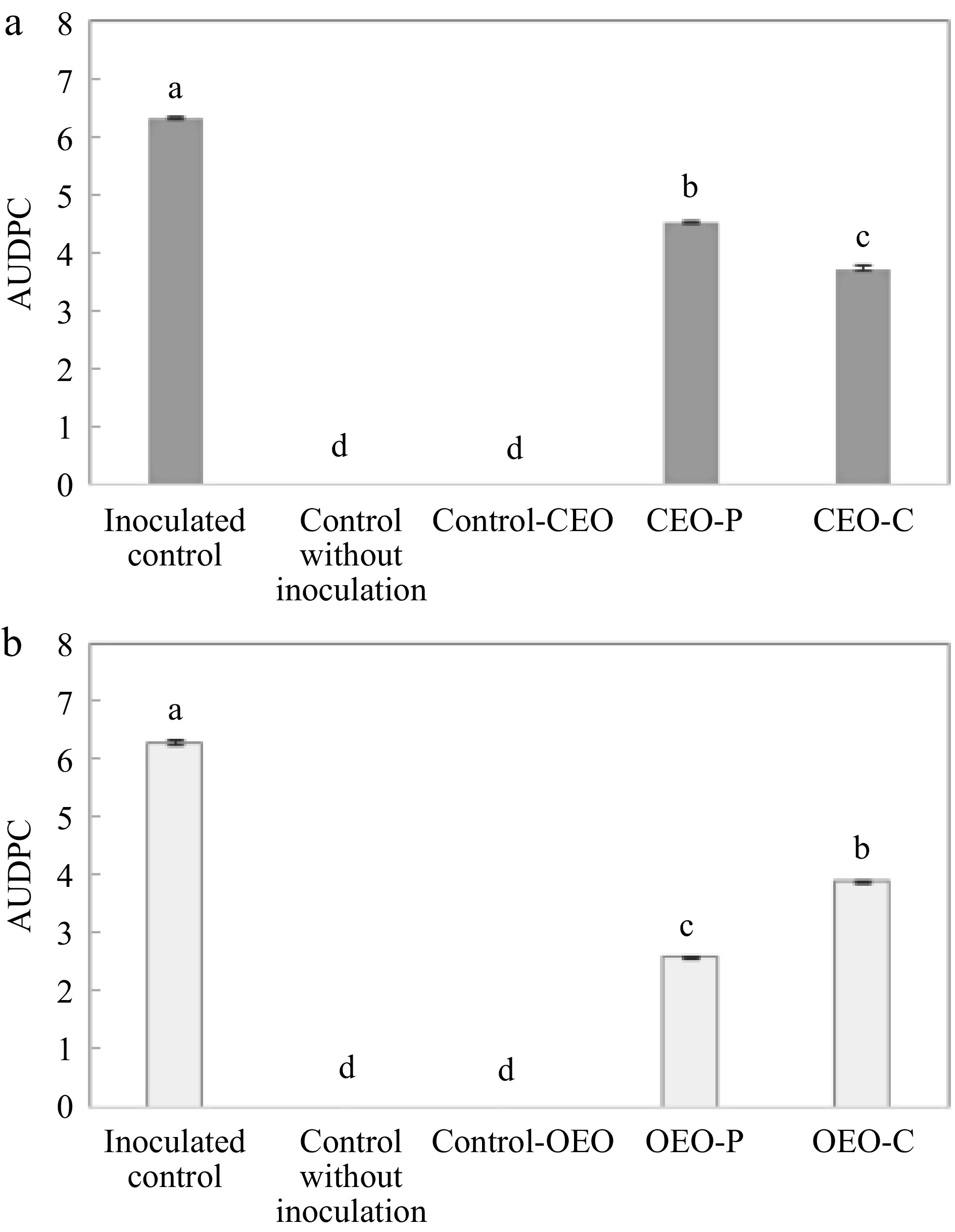
Figure 3.
Area under the disease progress curve (AUDPC) for lesion diameter values (mm) in papaya fruits treated (a) with Syzygium aromaticum essential oil (CEO), and (b) with essential oil of Origanum vulgare (OEO) in a preventive (P) and curative (C) manner. Different letters indicate significant differences among treatments (p < 0.05).
Although the in vitro analysis showed the same MIC value for both oils, we observed that for the in vivo analysis the essential oil of oregano showed lower severity values, indicating that this oil may have a better inhibitory effect. However, it is worth highlighting that the fruits were injured and inoculated, so the source of nutrients and the ideal temperature (28 °C) for this fungus to grow (used in storage) may have favored the small growth of disease in the fruits not obtaining a 100% of inhibitory effect. We also observed that non-inoculated fruits did not present the disease. These fungal growth inhibition results indicate that oregano and clove essential oils can be a great sustainable and safe alternative to increase the post-harvest life of fruits.
-
The essential oils of S. aromaticum and O. vulgare effectively inhibited the growth of the Nigrospora sp. fungus in vitro tests, presenting a MIC of 125−250 μL·L−1. At in vivo analysis, the essential oils of S. aromaticum were more effective in inhibiting the disease in the fruits curatively and O. vulgare essential oil preventatively. Additionally, the oils reduced fruit weight loss, diameter and length losses, and color changes over time. Therefore, the use of essential oils on fruits may serve as an effective alternative for inhibiting the growth of the fungus Nigrospora sp. and for preserving fruits post-harvest. Although they are effective in controlled environments, their large-scale application faces practical challenges, such as costs, technical feasibility, and consumer acceptance. The adoption of essential oils as an alternative to synthetic fungicides can promote more sustainable agricultural practices but require further studies on their interactions in the field and the need for continuous monitoring to ensure post-harvest efficacy and quality.
The authors are grateful for the research funding provided by the São Paulo Research Foundation–FAPESP, Brazil (# 2022/1068-6), National Council for Scientific and Technological Development–CNPq, Brazil (# 383138/2023-0, 138584/2023-0), Coordination for the Improvement of Higher Education Personnel–CAPES, Brazil (# 001), Empresa Brasileira de Pesquisa Agropecuária (# 20.19.03.0124.00.00) – Embrapa, Rede Agronano, CNPq/MCTI Sisnano (# 442575/2019-0) and CNPq Research Productivity fellowship (# 307141/2022-5).
-
The authors confirm contribution to the paper as follows: study conceptualization: Duarte LGR, Ferreira MD; methodology: Duarte LGR, Bogusz S Junior, Ferreira MD; investigation: Duarte LGR, Pedrino IC, Osti YGP, Fukuyama CWT, Nogueira PHB, de A. Astolfo ME; formal analysis: Pedrino IC, Fukuyama CWT, Nogueira PHB, de A. Astolfo ME, da M. Martins ME; data curation, visualization, writing – original draft: Duarte LGR; funding acquisition, resources, writing – review & editing: Ferreira MD; project administration: Duarte LGR, Ferreira MD; supervision: Duarte LGR, Bogusz S Junior, Ferreira MD. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Duarte LGR, Pedrino IC, Osti YGP, Fukuyama CWT, Nogueira PHB, et al. 2025. Nigrospora sp. in post-harvest papayas: efficacy of essential oils in antifungal inhibition. Technology in Horticulture 5: e009 doi: 10.48130/tihort-0025-0002
Nigrospora sp. in post-harvest papayas: efficacy of essential oils in antifungal inhibition
- Received: 22 August 2024
- Revised: 28 December 2024
- Accepted: 08 January 2025
- Published online: 05 March 2025
Abstract: Climate change is negatively impacting ecosystems and encouraging the spread of new post-harvest diseases. This research evaluated two essential oils (EOs) as alternatives for controlling emerging fungal diseases. In vitro and in vivo studies were conducted with clove (Syzygium aromaticum) EO (CEO) and oregano (Origanum vulgare) EO (OEO) against the fungus Nigrospora sp. Both EOs were tested in vitro at concentrations of 62.5−1,000 μL·L−1. In vivo tests on papayas fruits belonging to the Solo group used in curative and preventive applications. Weight loss, fruit length, diameter, and skin color were evaluated over eight days. The Minimum Inhibitory Concentration (MIC) was 125−250 μL·L−1 for both EOs. Clove EO was more effective curatively, while oregano EO was more effective preventively. Papayas fruits treated with EOs lost less weight (9% for CEO and 8% for OEO) compared to controls (16%). Treated fruits maintained the length and diameter. Clove and oregano EOs offer a sustainable alternative to synthetic fungicides for post-harvest fruit preservation.
-
Key words:
- Antifungal /
- Syzygium aromaticum /
- Origanum vulgare /
- In vitro /
- In vivo












