-
Longan (Dimocarpus longan Lour.), belonging to the Sapindaceae family and native to southern and southwestern China, is primarily found in the Hainan and Yunnan provinces. It has 2,000 years of cultivation history and is cultivated in over 20 tropical and subtropical regions, including China, Thailand, Vietnam, America, and Australia. China is the largest producer of longan, with the largest cultivation area, the most abundant cultivars, and the finest fruit quality[1−3], followed by Thailand and Vietnam. The wood is recognized for its strength and durability, making it suitable for use in furniture construction, shipbuilding, and carving. The abundance of flowers makes longan nectar a valuable resource, notable for its high phenolic content and potent antioxidant activity[4]. In traditional Chinese medicine, the tender, translucent flesh of the longan fruit is reputed to stimulate the appetite and spleen, nourish the heart, and calm the spirit. Fresh fruits can be processed into dried longan, canned products, or longan paste for long-term preservation. The fruit core contains substantial starch, and is suitable for brewing alcohol or making activated carbon. Furthermore, flowers, young leaves, aril, seeds, and roots of longan all have medicinal properties. They are primarily recognized for their ability to inhibit tyrosinase, enhance blood circulation, exhibit antioxidant and anti-aging effects, reduce inflammation, regulate the immune system, prevent glycation, combat cancer, enhance memory, improve Alzheimer disease related spleen deficiency, alleviate sleep, and decrease insomnia[5−7]. Consequently, longan is highly favored by the public and esteemed as a treasure among fruits.
As a significant subtropical fruit resource, longan typically has a harvest season concentrated in July and August for most cultivars. Few cultivars mature exceptionally early or late, and the limited storability of longan fruit results in a short market supply period for fresh produce. Currently, only a few fruit trees, such as mango (Mangifera indica) and longan, can achieve off-season flowering by artificial regulation, with mango mainly using paclobutrazol and ethephon[8], while longan primarily relys on KClO3. The application of KClO3 to induce floral transition at the end of the 20th century has allowed fruit farmers to artificially adjust the harvest season and optimize market timing. This advancement has facilitated a year-round fresh fruit supply, aligning with consumer demand and driving the growth of the longan industry. However, this technology cannot induce flowering for other plants in the Sapindaceae family, such as lychee (Litchi chinensis) and rambutan (Nephelium lappaceum), making it a precious model perennial fruit tree for the study of off-season flowering induction.
In the first decade of extensive application of this technology, it significantly impacted the global planting area and yield of longan, comprehensively changing the short market period and concentrated harvest season. Nevertheless, over more than 20 years of successful application of KClO3 to expand longan harvest seasons, the mechanism of KClO3-induced flowering remains a mystery. Extensive research has been conducted from various perspectives, yet the precise mechanisms are still unresolved. Although the effects of KClO3 on inducing flowering in longan have been reviewed by Huang et al.[3], Hau & Hieu[9], these studies did not provide detailed data on flowering rates across different cultivars and application methods, nor did they address the physiological and biochemical reactions involved, recent advances in molecular biology, or potential regulatory models. Therefore, this article aims to review the progress of existing research, thoroughly examine the technical aspects of KClO3 application in longan flowering, and explore recent advances in the effects of KClO3 treatment on longan development, physiology, and molecular biology. Additionally, the potential mechanisms of KClO3-induced longan flowering are discussed and the prospects of nano-materials for flower induction evaluated.
-
The longan exhibits a current-year flower bud differentiation type. Annually, there are one to three cycles of shoot development; following approximately four weeks of low-temperature exposure (ranging from 10 to 14 °C) during winter, the terminal buds of the latest shoots enter a short dormancy. This dormancy phase stops the vegetative growth, allowing for the accumulation of non-structural carbohydrates and a readjustment of hormonal balance, thereby facilitating the transition from vegetative to reproductive growth[1,10,11]. Typically, the normal differentiation of the longan flower bud initiates in mid to late January, and the bud shows morphological differentiation from February to March in subtropical climates[12−17]. A key indicator of inflorescence meristem formation is the emergence of little red inflorescence meristems at the bud apex within the leaf axil, colloquially called the 'small red dot'. Once the inflorescence meristems are formed, the sequential development of primary, secondary, and tertiary lateral branches occurs, followed by the formation of primordia for florets and floral organs. However, in tropical regions like Thailand, insufficient accumulation of chilling requirements may inhibit the natural flowering of certain cultivars and potentially affect their yields[9].
Molecular biological studies on the regulation of natural longan flowering
-
Recently, advances in sequencing and molecular biology techniques have led to the resolution of two versions of the longan genome for cultivar 'Jidanben' and 'Honghezi'[18,19]. Additionally, several genes regulating natural flowering time in longan have been identified (Table 1). These included integrator genes from the six major flowering pathways as well as genes that regulate the development of floral meristem. Some of these genes have been partially validated for their functions, broadly consistent with those found in other plants. However, the most important plant florigen gene, FLOWERING LOCUS T (DlFT) showed functional divergence. Several studies have identified four homologs of the AtFT gene in longan, DlFT1, DlFT2, DlFT3 and DlFT4. Notably, DlFT2 (referred to as DlFT1 in longan[20]) and DlFT3 were shown to promote early flowering when heterologously overexpressed in Arabidopsis (Arabidopsis thaliana). In contract, DlFT1 (referred to as DlFT2 in longan[20]), unlike its counterparts in other plants, was found to inhibite flowering in longan[20,21]. Studies on the cultivar of 'Sijimi', which can be induced to flower in summer through paclobutrazol and ethylene treatments, have revealed a significant increase in DlFT expression level in the leaves[22]. This indicated that DlFTs genes were indeed involved in the floral induction process in longan.
Table 1. Genes regulating the natural flowering of longan.
Other cultivars 'Sijimi' Integrators FT2, FT3: positive*[21,22,32] SVP: positiveRNA-seq[10,29] FT1: negative*[21,22,32] TFL1-1: negative[24] TFL1-2: negative[24] Photoperiod
pathwayGI: positive[27] COL: positiveRNA-seq[10,29] FKF1: positive[27] APRRs: positiveRNA-seq[10,29] ELF4-1/2: negative[28] GI: positiveRNA-seq[10,29] FKF1: positiveRNA-seq[10,29] ELF: positiveRNA-seq[10,28] LFY: negativeRNA-seq[10,29] Flower meristem identity LFY: positive[10] − Others − WRKYs: positiveRNA-seq[30] WRKYs: positiveRNA-seq[30] * 'positive' and 'negative' referred to the results of molecular function verification. 'positive' genes were those that, when overexpressed in other plant species, accelerated flowering time, whereas 'negative' genes delayed flowering. In the absence of experimental verification, classification was based on RNA-seq or RT-qPCR data, with 'positive' genes showing upregulation and 'negative' genes showing downregulation in flower buds compared to leaf buds. Superscript RNA-seq represented the results based on RNA-seq. Conversely, two copies of TERMINAL FLOWER 1 (DlTFL1-1 and DlTFL1-2) have been identified in longan, and overexpression of them resulted in delayed flowering in both Arabidopsis and Nicotiana benthamiana, as well as an increased number of floral branch in Arabidopsis. Additionally, the overexpression led to the downregulation of DlFT and APETALA1 (DlAP1) expression levels[23], similar to the function of TFL1 observed in Arabidopsis and apple[24,25]. Jue et al.[11] cloned the LEAFY (DlLFY), and through transgenic overexpression in Arabidopsis combined with DAP-seq analysis, demonstrated that DlLFY promoted early flowering and inhibited vegetative growth by binding to DlTFL1. Besides, gene function research of genes involved in circadian rhythms showed that Gibberellin Insensitive (DlGI) and DlFKF1 promoted the floral transition[26], but DlELF4-1 and DlELF4-2 delayed the flowering time[27]. The preliminary functions of these genes were mainly consistent with those validated in Arabidopsis, indicating that the functions of flowering regulatory genes are conserved in longan, except for DlFT.
'Sijimi' is a cultivar native to the border between Guangxi province of China and Vietnam. It can flower year-round without the need for cold temperature conditions, making it a crucial material for studying the natural flowering of longan. The rapid development of high-throughput sequencing technology and multi-omics integrated analysis methods has significantly propelled research into the flowering mechanism of 'Sijimi'. Sequencing and analysis of differently expressed genes (DEGs) in flower buds from longan cultivars 'Shixia', 'Lidongben', and 'Sijimi' at various developmental stages revealed that DEGs specific to the photoperiod and circadian rhythm pathways were significant for the flower bud development of 'Sijimi'. For instance, CONSTANS-like (DlCOL), RESPONSE REGULATOR (DlAPRRs), GIGANTEA (DlGI), SHORT VEGETATIVE PHASE (DlSVP), F-BOX 1 (DlFKF1), and EARLY FLOWERING (DlELFs) may positively regulate the formation of the perpetual flowering trait in 'Sijimi', whereas DlLFY may inhibit the formation of this trait[11,28]. In addition to the critical genes involved in the flowering pathways, 18 members of the WRKY family may also play roles in the perennial flowering induction process of 'Sijimi'[29]. Furthermore, high-throughput sequencing of microRNAs (miRNAs) for flower bud in 'Sijimi' and 'Lidongben' identified five differentially expressed miRNAs (Dlnovel-miR137, Dlnovel-miR76, Dlnovel-miR101, Dlnovel-miR37, and Dlcsi-miR3954). These five miRNAs targeted 14 genes, including CONSTITUTIVE PHOTOMORPHOGENIC 1-like (DlCOP1-like), DlCasein kinase II, and DlTCP20. Notably, transgenic Arabidopsis of overexpressing pSAK277-miR137 displayed a delayed flowering phenotype[30].
-
The discovery of KClO3 as a longan flowering inducer has marked a significant milestone in the development of the longan industry. While the cultivar 'Sijimi' can naturally flower throughout the year, the application of KClO3 has provided greater flexibility, controllability, and efficiency in scheduling, cultivar management, and orchard planning. KClO3, a potent oxidizing agent, is extensively utilized in producing herbicides and defoliants. When applied to plants, it can inhibit root growth, induce leaf yellowing and shedding, and potentially lead to plant death[31]. In 1998, Changrui Yan from Pingtung University in Taiwan discovered that KClO3, an oxidizing agent commonly used in fireworks, could induce off-season flowering in longan[32]. Over the past two decades, this technique has been actively tested and applied in major longan-producing regions, including provinces of Taiwan, Hainan, Guangdong, Guangxi, Fujian (Xiamen, Zhangzhou), Yunnan (Lujiang) in China, Chiang Mai in Thailand[33,34], the Mekong River Delta in Vietnam[9,35], and Hawaii in the United States[36]. According to the Chinese 'Lychee and Longan Industry Development Report' of 2023, the global cultivation area of longan was approximately 0.5267 million ha, with an annual output of 4.2 million tons (t). China, Thailand, and Vietnam account for over 90% of the total planting area and production in the world, with China alone cultivating 0.2792 million ha and producing 2.27 million t[37]. Notably, the majority of orchards in Hainan province and western Guangdong province in China primarily use the KClO3-induced flowering technique[38]. Additionally, approximately 50% of fresh fruit production in Vietnam and Thailand is derived from KClO3-induced off-season flowering[2,34,35,39].
Currently, there are two primary industrial applications of the KClO3-induced flowering technique (Table 2). One approach involved cultivating early-maturing longan, which promoted early flowering during the regular growing season. The application of KClO3 advancing the flowering, fruiting, and ripening periods by approximately 2 to 3 weeks compared to trees that flower and fruit naturally, effectively advanced the entire period of longan production. For example, in the approximately 75,300 ha of orchards in western Guangdong province of China, the main cultivars 'Chuliang' and 'Shixia' were treated with KClO3 to promote early maturation of longan[38]. During the maturation period of the second autumn shoots, from late October to mid-November of each year, the cultivar 'Chuliang' was treated with KClO3, resulting in a harvest occurring in mid-June, which was approximately two weeks earlier than with natural flowering induction[38]. Between 2020 and 2021, the total output of longan fruits in Guangdong Province, ranged from 752,000 to 997,600 t[38]. During this period, the production of KClO3-induced early-maturing longan ranged from 458,000 to 663,100 t, which accounted for approximately 64.03% of the total output[38]. The application of this technique held significant importance for regional production period regulation, increasing the supply duration and economic value of longan.
Table 2. Statistics of literature information on the treatment methods and flowering rates of KClO3-induced flowering for longan.
Cultivar Region Treatment method Type and dosage * Cultivation pattern and tree age (year) Processing time Flower bud emergency (d) Flowering
rate of treesFlowering rate
of branchesRef. Shixia Guangxi province, China Dissolved in water and then applied to soil −; 100 g/m2 Plantation; 20 Early Jun. 40 100% 43.50% [67] Shixia Danzhou city, Hainan province, China −; 0.75 kg/tree Plantation; 7 Jul. 9th 45 − − [68] Shixia Lujiang Town, Longyang District, Baoshan City, Yunnan province, China −; 0.5 kg/tree Plantation; 5 Aug. 7th − 100% 35% [69] Shixia −; 0.75 kg/tree Plantation; 5 Aug. 7th − 33% 48% Shixia −; 1.0 kg/tree Plantation; 5 Aug. 7th − 100% 45% Shixia Hainan province, China Ditched and then broadcast over the soil −; 0.5 kg/m Plantation; 8 After three times of branches 28 [70] Shixia Guangdong province, China Injection −; 8 g/tree Plantation; 4 − 30 − − [71] Shixia Ditched and then broadcast over the soil 90% purity; 1 kg/tree Plantation; 7 Oct. 28th − − − [72] Shixia Ditched and then broadcast over the soil −; 1.0~1.5 kg /tree Plantation; − Dec. 3rd − 100% 46.25% [73] Shixia Foliar spraying applied twice AR; 830 mg/L Plantation; − Dec. 10th − 100% 78.38% [73] Shixia Guangxi province, China Dissolved in water and then applied to soil + foliar spraying TG; 0.5 kg/tree Plantation; 30 Early Dec. 47 − 91.78% [45] Shixia − −; 7.5 g/tree Plantation; 12 Oct. 1th − − 52.25% [74] Chuliang Lujiang Town, Longyang District, Baoshan City, Yunnan province, China Dissolved in water and then applied to soil −; 0.75 kg/tree Plantation; 5 Aug. 7th − 100% 35% [69] Chuliang −; 0.5 kg/tree Plantation; 5 Aug. 7th − 66% 32% [69] Chuliang −; 1.0 kg/tree Plantation; 5 Aug. 7th − 33% 33% [69] Chuliang Guangxi province, China Ditched and then broadcast over the soil −; 7.5 g/tree Plantation; 12 Oct. 1th 23 d advance − 29.79% [74] Chuliang Guangdong province, China Ditched and then broadcast over the soil −; 1.0~1.5 kg/tree Plantation; − Dec. 3rd − 50% 3.59% [73] Chuliang Foliar spraying applied twice AR; 830 mg/L −; − Dec. 1st − 100% 4.61% [73] Fenglisui Xiamen city, Fujian province, China Ditched and then broadcast over the soil AR, 99.5% purity; 5 g/tree −; 5 Sep. 21st 40 100% 74.50% [47] Fenglisui AR, 99.5% purity; 5 g/tree + girdling −; 5 Sep. 21st 40 100% 86.8% [47] Fenglisui AR, 99.5% purity; 5 g/tree + branch pulling −; 5 Sep. 21st 40 100% 90.2% [47] Fenglisui Xiamen city, Fujian province, China Dissolved in water and then applied to soil 99% purity; 5 g/tree −; 3 Sep. 21st 38 100% 94.80% [46] Fenglisui 99% purity; 15 g/tree −; 3 Sep. 21st 38 75% 86.20% [46] Fenglisui 99% purity; 25 g/tree −; 3 Sep. 21st 38 50% 75.30% [46] Songfengben 99% purity; 70 g/tree Plantation; 6 Aug. 29th 43 94% 92.80% [48] Songfengben −; 5 g/tree Pot experiment; 3 Nov. 25th 45 100% 60% [48] Songfengben −; 10 g/tree Pot experiment; 3 Nov. 25th − 0 0 [48] Guixiang Guangxi province, China − −; 7.5 g/tree Plantation; 12 Oct. 1st − 32.63% [74] Fuyan Fujian province, China Broadcasting −; 700 g/tree Street tree; − Middle of Jun. − 75.50% − [58] Fenker Taiwan province, China Ditched and then broadcast over the soil −; 80 g/tree Plantation; 14 Oct. 24th 43.8 − 69% [75] Fenker −; 80 g/tree+ girdling Plantation; 14 Oct. 24th 46.4 − 73% [75] E-Daw Ban Pong district, Ratchaburi province, Thailand − −; 300 g/tree Plantation; 8~10 − 27 100% − [76] Daw Thailand − −; 10 g/tree −; 2 − 35 − 91% [77] E-Daw Chiang Mai city, Thailand Broadcasting −; 8 g/tree Plantation; 4−6 21 − 100% [41] E-Daw Chiang Mai city, Thailand Foliar spraying −; 1 g/L Plantation; 4-6 Hot season − − 12.50% [41] E-Daw Rainy season − − 61.70% [41] E-Daw Cool season − − 96.70% [41] E-Daw Chiang Mai city, Thailand Dissolved in water and then applied to soil −; 4 g/m2 Plantation; 4 Aug. − Rainy season 31 − 50.90% [41] E-Daw Sep. − Rainy season 24 − 11.90% [41] E-Daw Oct. − Cool season 20 − 88.60% [41] E-Daw Nov. − Cool season 26.3 − 80.00% [41] E-Daw Dec. − Cool season 39.5 − 88.80% [41] E-Daw Mar. − Rainy season 27 − 77.50% [41] E-Daw Apr. − Rainy season 24.3 − 87.50% [41] E-Daw May − Rainy season 31.5 − 50.00% [41] E-Daw Jun. − Rainy season 21.5 − 36.30% [41] E-Daw Jul. − Rainy season 25 − 51.30% [41] E-Daw Lampang province, Thailand Dissolved in water and then applied to soil −; 5 g/m2 10−12 Nov. 30th 38 − 98.7% [78] Si-Chompoo Chiang Mai, Thailand Broadcasting −; 1 g/tree Plantation; − Apr. 6th 21 − 100.00% [41] Si-Chompoo Chiang Mai, Thailand Injection −; 0.25 mg/cm branch Plantation; − Apr. 6th 49 − 90.00% [41] Si-Chompoo Dissolved in water and then applied to soil −; 1 g/m2 Plantation; 4−6 Nov. 5th 21 − 100.00% [41] Si-Chompoo −; 2 g/m2 Plantation; 4−6 Nov. 5th 21 − 100.00% [41] Si-Chompoo −; 4 g/m2 Plantation; 4−6 Nov. 5th 21 − 100.00% [41] XCV Mekong Delta provinces, Vietnam Ditched and then broadcast over the soil −; 24−36 g/m + girdling (2−3 mm width) Plantation; − − − − − [79] TDB 20−40 g/m + girdling (0.5−1.0 mm width) Plantation; − − − − − [79] E-Daw 40−60 g/m Plantation; - − − − − [79] Biew Kiew State of Hawaii, USA Broadcasting 300 g/tree Plantation; 7 Apr. 5th 49 − [80] Biew Kiew Plantation; 4 Sep. 15th − − 97.80% [81] AR, analytical reagent; TG, technical grade; * m refers to the canopy diameter, and m2 refers to the canopy projection area. Due to the different units in each article and the lack of information on canopy diameter, this statistical table retained the data and units mentioned in the references. The second method involved promoting off-season flowering of longan, particularly in tropical regions. With KClO3 treatment, fruit can be harvested approximately six months later, allowing farmers to schedule the treatment based on local climatic conditions and market demand. This approach helped prevent market supply surpluses and mitigated the impact of adverse weather conditions, such as low temperatures, heavy rain, and typhoons, on yield and market value. The discovery of the KClO3-induced longan flowering technique has significantly expanded the cultivation industry of longan in tropical areas. Significant producers of off-season longan, such as Thailand, Vietnam, and Hainan province in China, have achieved year-round flowering and stable, high yields[2,39]. Apart from the longan harvested in June, July, and August, all other year-round fruits originated from KClO3-induced off-season flowering, mainly targeting the Chinese export market[34]. Half of the cultivated area utilized the KClO3 off-season production method, especially in the warmer eastern region of Thailand, 100% of the cultivated area employed off-season production methods[34]. The earliest fruit harvesting can occur in January, supporting the supply of off-season longan fruits for the traditional Chinese Spring Festival. Additionally, to address issues related to slow sales caused by centralized harvests, each region has adjusted its induction times based on local climatic conditions. In the Hainan province of China, the southern and western regions treated longan trees from August to September, with fruits marketed from March to April. The central and northwestern regions treated the trees from October to November, with fruits available from May to June. In the northeastern and Leizhou areas, treatment occurred from November to December, and fruits were marketed from late June to July[40]. The application of this technology has led to a dramatic increase in longan exports of Thailand, from 8,600 t in 1998 to 170,000 t in 2000, while the cultivation area increased twofold[41]. By 2017, The cultivation area in Thailand had reached approximately 187,564 ha, with a total output of 1,027,500 t and an average yield of 5.4 t/ha. Furthermore, approximately 50% of longan cultivated in the Mekong River Delta provinces of Vietnam relied on KClO3-induced off-season flowering. For these off-season plantations, the yield can reach up to 10 t/ha, which was double the yield of 5.6 t/ha for naturally flowering longan in the northern region[35]. Therefore, the application of the KClO3-induced longan flowering technique has been pivotal in advancing the longan industry. This technology has significantly increased both the cultivation area and production volume of longan, thereby enabling consumers to enjoy fresh longan fruits year-round.
Technical points of KClO3-induced longan flowering
-
Currently, the main cultivars applying this technology include six Chinese cultivars: 'Shixia', 'Chuliang', 'Fenglisui', 'Songfengben', 'Fuyan', and 'Fenke'; three Thai cultivars: 'E-Dew', 'Do', and 'Si-Chompoo'; four Vietnamese cultivars: 'Tieu Da Bo' ('TDB'), 'Xuong Com Vang' (XCV), 'Xuong Com Rao' and 'E-Daw'; and one American cultivar: 'Biew Kiew'[9,35] (Table 2). Following KClO3 treatment, the flowering rate for these cultivars can reach up to 100% of treated trees, with an average flowering rate exceeding 80% for branches. Cultivars such as 'Shixia' (Guangxi)[42], 'Fenglisui' (Xiamen, Fujian)[43,44], and 'Songfengben' (Xiamen, Fujian) showed highly sensitivity to KClO3 treatment and can achieve flowering rates of over 90%[45]. Additionally, the hybrid of 'Dongbao No. 9' and 'Shixia', known as 'Baoshi No.1', also exhibited strong sensitivity to KClO3 and has been cultivated in the Hainan province of China[46]. The time for applying KClO3 was flexible and can induce flowering under various temperature conditions throughout the year in Thailand and the Hainan province of China[39,47,48]. However, the optimal period for treatment was during the dry and cool season, from August to December. In the Panfio district of Sisaket province of Thailand, the longan cultivar 'Phuang Thong' was typically treated in May to minimize the impact of the rainy season[49]. For most cultivars, treatment was typically applied when the leaves were 40 to 45 d old, indicated by a color change from light green to dark green[47]. However, some cultivars required treatment at an earlier stage. For instance, the Vietnamese cultivars 'Long', 'TDB', and 'XCV' needed to be treated when the new leaves were light green and 35 to 40 d old[9].
The methods for applying KClO3 primarily involved soil banding at the drip line of the crown and solution drenching. In some regions, foliar spraying and trunk injections were utilized. However, while soil application and drenching have demonstrated the highest flowering rates, trunk injections offered the best utilization efficiency but resulted in less stable flowering rates. In contrast, foliar spraying tended to be less effective overall[50,51]. Nevertheless, integrating soil application with foliar sprays has been significantly enhanced flowering rates[42]. The quantities of KClO3 application ranged from 4 g to 1.5 kg per tree. Insufficient quantity resulted in poor flowering induction, while excessive quantity could cause severe stress reactions in longan trees[52]. Sensitivity to KClO3 varied among cultivars, gradient treatment experiment on three-year-old 'Fenglisui' and 'Songfengben' revealed applying 5 g of 99.5% pure KClO3 per tree resulted in higher flowering rates compared to higher application rates above 10 g per tree (Table 2)[43,45]. For four to six-year-old longan trees, the 'E-Daw' cultivar achieved flowering rates of 80% and 100% with application rates of 4 and 8 g/m², respectively, while the 'Si-Chompoo' reached 100% flowering at 1 g/m²[39]. In the Mekong Delta region of Vietnam, 'XCV' required 24 to 36 g/m (diameter of the crown), 'TDB' required 20 to 40 g/m, and 'E-Daw' required 40 to 60 g/m[9]. For cultivars such as 'Fenglisui' and 'E-Daw', in addition to KClO3 treatment, supplementary measures were required to enhance the flower induction efficiency. These measures included branch pulling, girdling, appropriate irrigation, and ensuring adequate light. Implementing these techniques can significantly increase the flowering rate in lower-performing cultivars by more than 10.0%[9,44,53,54]. Furthermore, the combined use of KClO3 and growth regulators, such as cytokinin (CK), paclobutrazol and ethephon, has been shown to enhance flowering rates[55−57]. However, it was inevitable that without the application of KClO3, the use of growth regulators alone cannot induce off-season flowering.
Issues of KClO3-induced longan flowering
-
Although the off-season flowering technique using KClO3 for longan was widely used, several issues persist. These issues can lead to low flowering rates, disordered branch and inflorescence development, and adverse impacts on fruit set and quality. Therefore, it is crucial to comprehensively consider factors such as application timing, purity, method, dosage, climatic conditions, cultivar, and tree age (Table 2). These considerations were reflected primarily in the following aspects:
First, in practical production, the application of KClO3 often demonstrated instability in achieving consistent flowering rates and yields. It was highly susceptible to low spring temperatures and uneven water and nutrient supply during fruit development, which could lead to issues such as fruit drop and cracking. Additionally, overripe harvest periods in summer were vulnerable to typhoon disruptions, which affected yield. It is crucial to comprehensively consider factors such as climate, cultivar, dosage, application method, and environmental protection.
Second, in addition to fruit yield, fruit quality is a critical indicator of the economic value of longan. However, there were relatively few studies examining the fruit quality of longan induced by KClO3, and the existing research presented mixed results. Tian et al[58]. suggested that KClO3 treatment could enhance the fruits quality of cultivar 'Chuliang', while polybutylene treatment improved the edible rate and soluble solids content. Conversely, Sritontip et al[59]. found that the application of KClO3 and its related reduction products had no significant effects on longan cultivars. However, KClO3 treatment in Thailand was associated with reduced fruit size and a decrease in fruit base diameter[33]. Pan et al.[50]. conducted experiments on cultivar 'Guiguanzao' longan and discovered that, although KClO3 treatment could reduce the fruit drop rate and increase yield, it negatively impacted fruit quality. Chaikul et al[60]. posited that fruit size may be influenced by the timing of leaf maturation and the continuity of nitrogen supply to trees during flower development. These results indicated that the fruit quality of KClO3-induced longan can also be significantly enhanced through other auxiliary cultivation methods, by applying a balanced range of nitrogen, phosphorus, and potassium compound fertilizers, as well as using growth regulator during the flower and fruit development stages of KClO3-induced longan. However, current research on this topic was also limited, future studies should focus on aligning appropriate technical measures with different seasons, cultivars, and regions of longan to ensure simultaneous improvements in both fruit yield and quality.
Third, the application of KClO3 posed potential risks for both human health and the environment. Regarding human safety, KClO3 possessed potential explosive properties, which presented significant safety hazards during its purchase, transportation, storage, and application. Additionally, although several studies have indicated that the environmental and ecological risks associated with KClO3 treatment for longan were minimal, and that trace amounts of chlorate ions were not considered a significant threat to safety, these safety concerns still warranted careful consideration. Researches have demonstrated that KClO3 can induce the formation of methemoglobin in mice and dogs, leading to red blood cell lysis and kidney failure. Moreover, it may affect thyroid hormone secretion in humans, potentially having a significant impact on individuals with iodine deficiencies[61]. Excessive application of KClO3 to the soil, coupled with the runoff into adjacent farmlands or areas with other crops and fruit trees, can cause chlorate stress in these plants. This stress adversely affected their nitrogen absorption and utilization efficiency, ultimately impacting their yield. Additionally, residual KClO3 in fruits and vegetables posed a risk of human poisoning[62]. Therefore, environmental and pollution issues must be carefully considered when applying KClO3 in longan orchards. Several studies have demonstrated that the environmental impact and residue levels of KClO3 in fresh fruits can be minimal when the compound was applied within appropriate limits and only once per year. Following the routine application of KClO3 to longan trees, the residual amount in the soil typically persisted for only 15 to 30 d. In contrast, KClO3 residues can remain in the leaves can reach up to 5 months; but there was no significant accumulation of KClO3 were detected in mature fruits[39,63]. Moreover, the treatment did not adversely affect the flowering and yield of the subsequent season. However, administering the treatment twice within a three-month period may reduce the flowering rate[39,63]. Excessive use of KClO3 can result in a residual amount in the leaves for over eight months[39,63]. Ongprasert et al.[64] conducted a year-long study on soil chlorate residues in 25 orchards in Thailand. Their findings indicated that the chlorate residue in the surface soil of the application strip reached levels as high as 136 to 340 mg/kg a few days after application. The concentration of KClO3 decreased to 24.2 to 68 mg/kg after 50 to 55 d. The critical concentration affecting earthworm activity is 34 mg/kg. The decomposition speed was faster in high and medium-fertility soils treated with liquid fertilizer compared to those treated with urea. If the concentration of KClO3 was excessively high, the use of diluted honey water could accelerate its decomposition[64]. Additionally, Baubuom et al.[65] demonstrated that KClO3 exhibited a specific dose-effect relationship with the flowering and fruit-setting efficacy of longan. However, the maximum effective concentration of KClO3 should be 43 g/m (where 'm' represented the diameter of the canopy projection), with 25 g/m being sufficient to promote flowering. Excessive application of KClO3 can lead to the presence of chlorate ions, chloride ions, and potassium ions in the fruit peel. Nevertheless, the residue levels in the fruit pulp were minimal and did not pose a significant risk to human health. Therefore, it is crucial to use KClO3 within a reasonable concentration range to effectively promote off-season longan flowering.
-
After treatment with KClO3, the apical meristems of longan trees can transition from a vegetative state to a reproductive state within 20 to 60 d[39,58,66]. The fastest morphological differentiation occurred in an average of 27 d in Chiang Mai city of Thailand, and Hainan province of China[36,67,68]. In contrast, it required approximately 48 d in Guangxi and Fujian provinces of China, as well as Hawaii in the USA[45,66,69] (Table 2). Following the application of KClO3, branches exhibited five distinct phenotypic responses, and the untreated plants exhibited only the latter two phenotypes (Fig. 1d, e)[67,70,71].

Figure 1.
Phenotypic variation of branches induced by KClO3 in off-season flowering of longan. (a) Buds exhibiting shoot-like morphology that undergo direct floral bud differentiation; compound leaves show inhibited growth or abscission. (b) Initial shoot elongation followed by bud transition and morphological differentiation during the red leaf stage. (c) Shoots exhibiting floral transition demonstrate a 'flower with leaf' phenotype. (d) Vegetative shoots develop without entering floral transition. (e) Dormant buds that do not produce flowers or new shoot growth. Scale bars = 2 cm.
(1) The shoot-like bud directly undergones flower bud morphological differentiation, characterized by a slight elongation of compound leaves, which may cease growth or abscise (Fig. 1a).
(2) Initial shoot elongation followed by bud transition and morphological differentiation during the red leaf stage (Fig. 1b).
(3) The bud transitioned from vegetative to reproductive growth during the early stages. However, later the shoot exhibited a 'flower with leaf' state, where some floral apices reverted to leaf buds (Fig. 1c).
(4) Vegetative shoots grow directly without undergoing floral transition (Fig. 1d).
(5) Dormant buds that neither flowered nor produced shoot growth (Fig. 1e).
In Xiamen city of Fujian province, branches treated with KClO3 in late September underwent significant changes in the apical meristem with a conical shape within two weeks post-treatment. Inflorescence primordia developed after 33 d, with the growth point transitioning to a hemispherical shape. By 37 d, both primary and secondary inflorescence primordia were observed[44]. Additionally, KClO3 treatment enhanced the length and girth of new shoots, which was advantageous for cultivating fruiting mother branches for the subsequent year[55]. It also improved the female-to-male flower ratio, fruit set rate, and fruit quality[42,58].
Impact of KClO3 treatment on the physiology and biochemistry of longan
-
KClO3 significantly influenced the physiological and biochemical profiles of the longan tree. Throughout its absorption, transportation, and utilization, it markedly affected various aspects including abiotic stress responses, photosynthesis, non-structural carbohydrate content, and the levels and balance of plant hormones.
Firstly, KClO3 treatment induced abiotic stress responses in longan trees, leading to significant alterations in various physiological indices. Notably, photosynthetic processes were markedly inhibited, as evidenced by reductions in chlorophyll a, chlorophyll b, and xanthophyll content, as well as the decline in the photosynthetic rate, stomatal conductance, CO2 exchange rate, transpiration rate, and leaf water potential[70,72]. Additionally, the structural integrity of thylakoid membranes and grana within the chloroplasts was compromised[61,73]. Within one month of KClO3 application, there was a significant increase in proline and malondialdehyde (MDA), and the activities of superoxide dismutase (SOD), and peroxidase (POD) in both leaves and apical buds. Concurrently, there was a notable accumulation of reactive oxygen species (ROS) throughout the plant[67]. Furthermore, root vitality was also significantly diminished[74].
Secondly, following the KClO3 treatment, there was an increase in the non-structural carbohydrate content in leaves, including sucrose, fructose, glucose, and soluble sugars. In contrast, the activity of sucrose-metabolizing enzymes, such as sucrose synthase and sucrose phosphate synthase, decreased. Additionally, protein degradation led to a significant increase in soluble protein and soluble amino acid content, while starch content declined[72]. In the apical buds and branches, similar increases in starch, sucrose, soluble sugars, and soluble amino acids were observed. Unlike the natural flowering response to cool winter temperatures, which typically resulted in a decrease in sugar and starch levels in longan apical buds during late floral differentiation, KClO3 treatment caused the total sugar and starch content to double increase after floral bud differentiation[75,76]. This indicated that the physiological state during KClO3-induced off-season flowering differed from that during the natural flowering season, suggesting the involvement of distinct floral induction pathways.
Plant hormones are integral to the regulation of flowering in plants. Among the six primary flowering pathways, gibberellin (GA), CK, and auxins (IAA) played crucial roles by modulating the GA pathway and influencing the expression of genes responsible for floral meristem identity[77−79]. During the KClO3-induced flowering process in longan, significant alterations in plant hormone levels were observed across various tissue types before and after induction. The most pronounced changes were in CK and GA levels: there was a notable increase in CK content across all tissues and a significant decrease in GA content[45,69]. Additionally, ethylene (ETH) and abscisic acid (ABA) were also implicated in the floral induction process, but the specific hormones directly involved in the induction of longan flowering remain unclear. Initially, IAA, CK, and ABA levels increased in the roots that first encountered KClO3, while GA levels decreased[45,69]. Following the transduction of the KClO3 signal to the branches, there was a significant increase in zeatin (ZT) levels in the branches, accompanied by a marked production of N6-isopentenyl adenine/isopentenyl adenosine (iP/iPA) type CKs from the xylem and phloem at the shoot apex. During the morphological differentiation phase, characterized by the appearance of the 'small red dot', there was an observed increase in ABA levels[10,80]. Concurrently, the levels of IAA, CK, and ETH in the leaves also showed a significant increase. In contrast, GA3 content decreased during the physiological differentiation phase but increased during the morphological differentiation phase, although it remained significantly lower compared to the levels in the apical buds, thereby establishing a distinct concentration gradient[44,53,81,82]. In the apical buds, CK levels were markedly elevated, and there was a concurrent increase in salicylic acid (SA) and ETH levels; however, the content of GAs was notably reduced[10,44,53,81,82]. Tiyayon et al. suggested that mature leaves may played a role in the conversion of iP/iPA to ZT/ZR[83], and the increase of CKs in the apical buds was mainly associated with elevated levels of iP/iPA in the branches[84]. However, under shaded conditions, an increase in CKs was also observed in KClO3-induced longan buds, but the flower bud failed to achieve floral transition[53]. Furthermore, simultaneous treatment with CKs, paclobutrazol, and KClO3 increased the flowering rate, which correlated with an increase in CK content and a decrease in GAs[55−57]. These observations suggested that a high CK/GA ratio may be critical for the KClO3-induced off-season flowering process in longan.
Advances in the molecular mechanism of KClO3-induced longan flowering
-
Research on the mechanism of off-season flowering induction in longan has primarily focused on physiological and biochemical aspects, with molecular mechanism studies being limited. However, in the past three years, there have been significant advances in the molecular research on KClO3-induced off-season flowering in longan, largely due to the rapid development of high-throughput sequencing technologies, particularly utilizing the Illumina platform for next-generation sequencing. Huang et al.[85] conducted transcriptome sequencing analysis on the apical buds of the cultivar 'Shixia' for KClO3 and control treatment at 2, 6, and 30 d post-treatment. They identified 24 differentially expressed genes (DEGs) involved in flowering pathways. Notably, at 2- and 6-days post-treatment, the circadian clock-associated gene LATE ELONGATED HYPOCOTYL (DlLHY) was significantly upregulated, while DlGI and CONSTANS (DlCO) were significantly downregulated (Table 3). Genes involved in the gibberellin pathway, such as GA REQUIRING 4 (DlGA4), DlGA5, REPRESSOR OF GA1-3-LIKE 1 (DlRGL1), SCFSL Y1 (DlSLY1), and GIBBERELLIC ACID INSENSITIVE (DlGAI), were upregulated on the 2nd day. Additionally, DlGA5 and DlRGL1 were also upregulated on the 6th day, while DlGA4, DlRGL1, GA INSENSITIVE DWARF1 (DlGID1), and SLEEPY1 (DlSLY1) upregulated after 30 d of treatment. Concurrently, the vernalization pathway gene VERNALIZATION INSENSITIVE 3 (DlVIN3) was significantly upregulated in the first two periods. Moreover, the expression of FLOWERING LOCUS T (DlFT), APETALA 1 (DlAP1), and LEAFY (DlLFY) increased rapidly during floral morphological differentiation at 30 d post-treatment[85].
Table 3. Genes regulating the KClO3 induced flowering of longan.
Pathway Genes Positive* Negative* Integrators FT1*[21]; FT[71] FT2*[21]; FT3[21]; SVP[20]; SOC1[20,71]; FLC[20,93] Photoperiod pathway LHY[71]; CO[93] LHY[93]; CO[71]; GI[71]; FKF1[71]; CCA1[71]; ZTL[71] Vernalization VIN3[71] Hormone GID1, SLY1[71]; ARF3[32] ABI5[71]; GA2ox[93]; CBF1[93] Aging pathway AP2[32]; SPL8[32]; SPL5[93] Autonomous pathway GLK1, UGT87A2-5, VIM1[93] CML24[93] Flower meristem identity AP1[20,71,93,95]; LFY[71,93]; MADS98, AG[71,93], SEP1/2/3/4, AP3-1/2, PI, AG, AGL6[20,93] AG[32]; AGL42[32]; MADS111, MADS82[20,93] Others MYB23/33/9/116[94];
Early stage: 3 C3Hs; 2 HMGs; 3 MYBs; 1 bHLH; 1 CPP[71];
Middle stage: 3 AP2-EREBPs; 2 C2-like; 1 CCAAT-like; 1 MYB; 1CPP[71];
Last stage: 10 MADSs; 4 HBs; 1 LEAFY; 3 AP2-EREBP-like[71]Early stage: 1 C3Hs; 1 CCAAT-like[71];
Middle stage: 1 EIL; 4 b-ZIPs; 2 TRAFs; 1 C2-like; 1 bHLH[71];
Last stage: 2 C3Hs; 1 GRAS; 1 C2-like; 1 bHLH; 2 MADSs;
1 MYB[71]* This table was organized based on the results of RNA-seq from reference, with 'positive' genes showing upregulation and 'negative' genes showing downregulation in flower buds compared to leaf buds. Li et al. conducted transcriptome sequencing analysis on apical buds four weeks after KClO3 treatment, during the morphological differentiation phase, and found 2,663 DEGs significantly upregulated after KClO3 treatment, including APETALA 2 (DlAP2), AUXIN RESPONSE TRANSCRIPTION FACTOR 3 (DlARF3), and SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 8 (DlSPL8), while AGAMOUS (DlAG) and agamous-like 42 (DlAGL42) were downregulated[86]. Wang et al. reported differing results, noting that DlLHY was downregulated and DlCO was upregulated in KClO3 treated bud. Additionally, 56 genes involved in regulating flower time were implicated in the flower induction process. MADS-box family members such as DlAP1, DlMADS98, SEPALLATA1/2/3/4 (DlSEP1/2/3/4), DlAP3-1, DlAP3-2, PISTILLATA (DlPI), DlAG, and DlAGL6 responded to KClO3 regulation and were upregulated at 41 and 54 days post-treatment, during inflorescence differentiation[19,87]. Chen et al. analyzed the expression patterns of MYB family members and found that four MYB family members, DlMYB23, DlMYB33, DlMYB 9, and DlMYB 116, exhibited significantly increased expression levels in the apical buds after KClO3 treatment[88]. Results of KClO3-induced longan flowering in Thailand showed that DlFT1 decreased slowly from 11 to 18 d post-treatment but rapidly increased fourfold in the week preceding floral morphological differentiation. In contrast, the expression of DlFT2 and DlFT3 in mature leaves significantly decreased at various time points post-treatment[21]. Furthermore, the expression of DlAP1-1 and DlAP1-2 was markedly upregulated[89].
Integrating these research findings indicated that genes involved in the photoperiod pathway, GA pathway, vernalization pathway, age pathway, autonomous pathway, flowering integrator genes, and floral meristem regulatory genes were all involved in the KClO3-induced off-season flowering process in longan. This suggested that within the regulatory network of KClO3-induced flowering, the transition of leaf buds to floral buds following KClO3 stimulation may primarily occur during the interaction of hormone and floral pathway genes. However, these studies were still insufficient to elucidate the specific mechanisms involved.
-
The absorption and translocation processes of KClO3 in plants were believed to involve pathways similar to those of potassium nitrate (KNO3), primarily mediated by nitrate reductase (NR) and nitrite reductase (NiR). These enzymes converted KClO3 into potassium chlorite (KClO2), potassium hypochlorite (KClO), potassium chloride (KCl), and molecular oxygen[90,91]. The phytotoxic effects of KClO3 were attributed to its metabolic process intermediates, such as chlorite and hypochlorite, which can cause yellowing, abscission, and plant leaves in severe cases. In longan, KClO3 was rapidly translocated from the roots to the leaves within five days of treatment, with peak accumulation in the leaves observed around 15 d. It was then metabolized into KCl, with the highest KCl content recorded at 28 d and remaining elevated for up to 150 d[61,63,73].
Although KClO3 was well-known for inducing off-season flowering in longan, various other chlorates, chlorites, bleaching agents (which released chlorates under high-temperature conditions), sodium hypochlorite (NaClO), and calcium hypochlorite (Ca(ClO)2) have also been shown to promote off-season flowering in longan to varying degrees. However, these alternatives did not match the effectiveness of KClO3 in terms of flowering rate and inflorescence length[59,92]. This suggested that KClO3 primarily enhanced longan flowering through the products of its redox process. Additionally, enzymes involved in KClO3 metabolism have significant effects on longan flowering. The sole application of KClO3 significantly reduced the activity of NR, while the co-application with potassium nitrate (KNO3) increased both NR activity and the accumulation of KClO3[61,73]. Inhibiting NR activity has been shown to slow the rate of KClO3-induced flowering in longan[84], indicating that the absorption, transport, and reduction of KClO3 intermediates played a critical regulatory role in longan flowering. However, the mechanisms of KClO3 absorption, transport, and utilization within longan trees remained unclear. Furthermore, there was a lack of in-depth research on the regulatory mechanism of NR during the flowering process in longan, and more detailed studies are needed to understand how KClO3 treatment activates the flowering induction and transition systems in longan.
Although there was no specific molecular regulatory mechanism identified for longan, considerable researches have investigated the absorption and utilization pathways of chlorates in rice, wheat, and Arabidopsis. In plants, the nitrate absorption system comprised two main components: a high-affinity transport system (HATS) that operated under low nitrate concentrations and a low-affinity transport system (LATS) that functioned at high nitrate concentration levels[93]. In contrast, chlorate absorption primarily occurred through an ATP-dependent, pH-sensitive HATS[94,95]. The nitrate transporter families NRT1/PTR (nitrate transporter 1, NPF) and NRT2 were crucial for the translocation of both nitrate and chlorate in plants. Key transport proteins identified included NRT1/PTR/NPF, NRT2, chloride channels (CLCs), and slow anion channel-associated one homolog (SLAC1/SLAH)[96−98]. In Arabidopsis, the structural genes of AtNRT1 included AtNRT1.1, AtNRT1.2, AtNRT1.5, and AtNRT1.1 and AtNRT1.2 were involved in the nitrate absorption process. The AtNRT1.1 knockout mutant, Atchl1-5 exhibited chlorate tolerance but showed reduced nitrate uptake, decreased leaf stomatal conductance, and diminished AtNRT2.1 signaling[99,100]. AtNRT1.5 facilitated the transport of K+ from roots to the stems. The uptake of K+ by root cortical cells was accompanied by a proton (H+) extrusion process mediated by H+-ATPase, and the presence of K+ enhanced the absorption and assimilation efficiency of NO3−[101,102]. PICLORAM RESISTANT 30 (AtPIC30) encoded a major facilitator superfamily (MFS) transporter in Arabidopsis, responsible for the absorption and transport of the systemic herbicide picloram. Mutations in AtPIC30 led to defects in chlorate transport, indicating its involvement in the chlorate translocation process[103].
Chlorates and nitrates exhibited competitive interactions during the plant absorption and assimilation processes, which were commonly utilized to assess the nitrogen uptake, assimilation, and signal transduction in crops with various genetic backgrounds. These interactions were also used to screen for nitrate reductase (NR) mutant materials[104−106]. The interaction between NR and nitrite reductase (NiR) influenced plant resistance to KClO3. High-resistance genotypes tended to have lower nitrogen use efficiency, while sensitive genotypes exhibited higher efficiency. In wheat (Triticum aestivum), nitrate treatments inhibited chlorate uptake, and the plant response to chlorate was associated with NR activity[107]. The presence of nitrates reduced the conversion efficiency of chlorates in Arabidopsis, but it displayed greater sensitivity to chlorates in the absence of nitrates, where chlorate treatment induced the expression of AtNR genes at the mRNA level[31]. In rice (Oryza sativa), treatment with KClO3 led to downregulation of OsNR2 in the roots of both NR and NRT introgression lines, through expression in leaves and stems varies. Specifically, OsNR2 was upregulated in the NR introgression lines and downregulated in the NRT introgression lines. OsNIA2 was expressed at higher levels than OsNIA1 in roots, stems and leaves, and the transcription of OsAMT1.3/2.3 (responsible for ammonium root ion transport) and OsGLU1/2 was affected in both NR and NRT introgression lines[108]. Furthermore, in Arabidopsis, the ethylene signal deficiency mutant ctr1-1 showed resistance to KClO3 and reduced NR activity. Mutation of the downstream AtEIN2 component (ein2 mutant) suppressed the function of Atctr1-1, restoring sensitivity to KClO3 and NR activity, indicating a regulatory role of ethylene signal in KClO3 metabolism[109]. Based on studies in rice, wheat, and Arabidopsis, it was likely that KClO3 in longan also played functions through reduction and transport mechanisms involving NR, NiR, and NRT, while specific research in longan was currently limited.
-
Most current research indicated that oxidative stress induced by KClO3 played a key role in the flowering of longan[42]. KClO3 can induce abiotic stress in plants, leading to the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS). The accumulation of these ROS and RNS can result in oxidative damage and lipid peroxidation[108]. ROS primarily included the superoxide anion (O2−·), hydrogen peroxide (H2O2), hydroxyl radical (·OH), and nitric oxide (NO). Excessive accumulation of intracellular ROS can result in oxidative DNA damage and alterations in protein function, leading to cellular pathology and cell death. Consequently, ROS have long been regarded as harmful signals. However, numerous studies have that ROS was also involved in the plant flowering process (Fig. 2). For instance, research on tomato (Solanum lycopersicum) has shown that, under normal conditions, the apical meristem accumulated H2O2. This hydrogen peroxide formed intramolecular and intermolecular disulfide bonds with the transcription factor TERMINATING FLOWER (SlTMF), which regulated the maturation of tomato apical meristem[3]. These disulfide bonds facilitated the aggregation of intrinsically disordered regions (IDRs), enhanced covalent interactions, and triggered protein phase separation. This process enabled the binding of proteins to the promoter of the F-box family floral meristem differentiation gene ANANTHA (SlAN), forming a reversible transcription condensate. This condensate precisely regulates the expression of flowering genes[3], while, an artificial increase in H2O2 content can delay flowering in tomatoes. Similarly, high H2O2 concentrations under stress conditions, such as high temperatures and chemical treatments, can inhibit flowering in rice and mango (Mangifera indica)[110,111]. In Arabidopsis, nitric oxide (NO) can suppress flowering by inhibiting the expression of photoperiod pathway and autonomous pathway genes, such as AtCO and AtGI, while enhancing the expression of FLOWERING LOCUS C (AtFLC). Excessive NO treatment or mutations leading to excess NO production can result in delayed flowering[112]. Conversely, overexpression lines of THYLAKOID ASCORBATE PEROXIDASE (AtAPX), as well as wild-type and mutant lines of Arabidopsis, exhibited H2O2 levels ranging from low to high. Their phenotypes showed an increase in days to flowering; specifically, low to moderate H2O2 levels transitioned flowering from late to early. This suggested that H2O2 also played a role in initiating flowering in Arabidopsis[113].
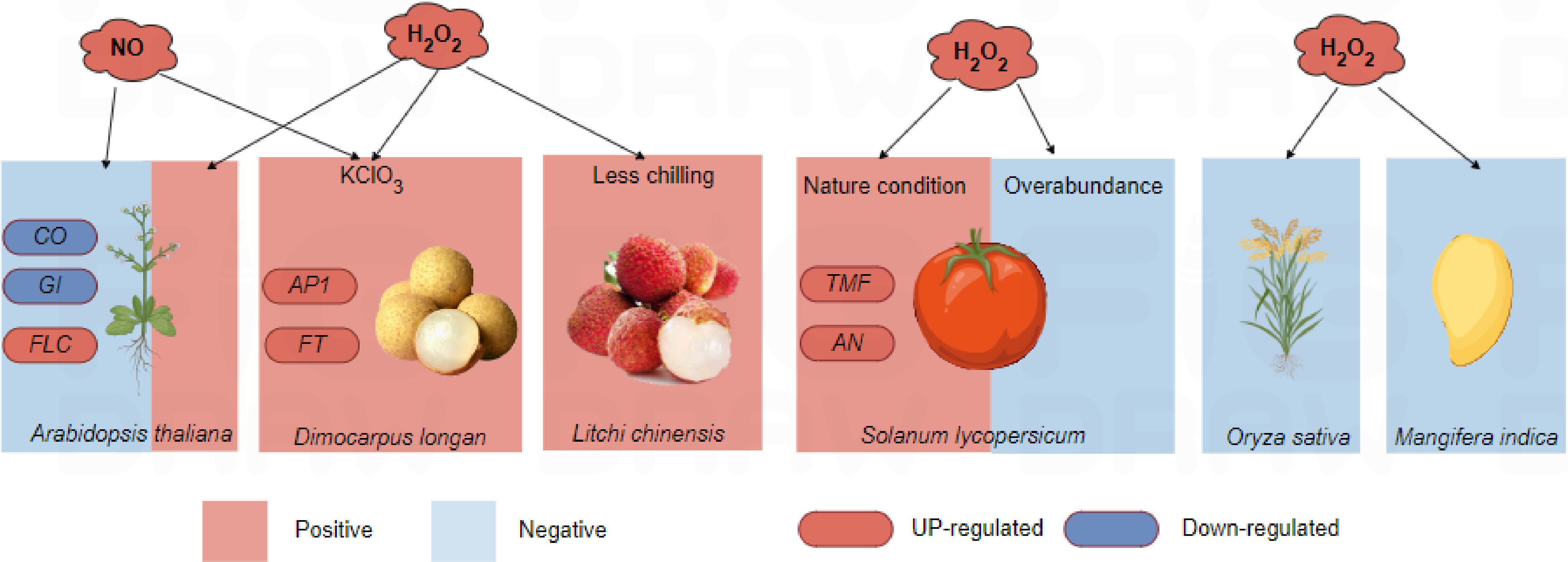
Figure 2.
The influence of ROS on plant flowering. NO, nitric oxide; H2O2, hydrogen peroxide; CO, CONSTANS; GI, GIGANTEA; FLC, FLOWERING LOCUS C; AP1, APETALA1; FT, FLOWERING LOCUS T; TMF, TERMINATING FLOWER; AN, ANANTHA. Red marked NO, H2O2 and genes meant their increasement in content and gene expression level, and the red box indicated that they promoted flowering of the corresponding plants, while the blue box indicated that the gene expression level was down-regulated, and the blue box indicated that plants were inhibited flowering.
Longan and lychee exhibited flowering responses to H2O2 levels similar patterns to the tAPX mutant. In lychee, the flowering induction rate was typically up to 100% under low-temperature conditions (8~15 °C). However, when the temperature was adjusted to 13~18 °C, the flowering rate dropped to 20%. Treating lychee branches with the H2O2 generator methyl viologen (MV) can increase the flowering rate to 80%, indicating that the increased H2O2 from MV compensates for the lack of chilling required for flowering induction[114,115]. In longan treated with KClO3, the application of MV and the NO generator sodium nitroprusside (SNP) promoted flowering. This treatment also significantly upregulated the expression of DlFT and DlAP1 in the leaves. Notably, MV treatment significantly increased DlFT and DlAP1 in floral buds. Conversely, treatment with the H2O2 scavenger dimethylthiourea (DMTU) and NO synthase inhibitor (L-NNA) significantly inhibited longan flowering and suppressed DlFT expression in the leaves[46,116]. Moreover, oxidative stress-related genes in the leaves were significantly downregulated from 2 to 8 d after KClO3 treatment, while oxidative defense-related genes were significantly upregulated from 0.5 to 8 d after treatment[42]. These findings suggested that the H2O2 production in longan after KClO3 treatment may play a crucial role in the induction of flowering. However, the mechanisms underlying ROS and H2O2 generation and their roles in the flowering network needed to be fully elucidated. Additionally, KClO3 treatment did not promote flowering in lychee, suggesting that longan may have specific factors responsive to KClO3. Further investigations are required to understand the specific mechanism of KClO3-induced flowering in longan.
-
Floral transition is a complex physiological process resulting from the interplay between external environmental cues and internal regulatory mechanisms. Most subtropical fruit trees require a period of low temperatures and the accumulation of sufficient chilling requirements to induce the floral transition. The application of potassium chlorate (KClO3) to longan facilitated flowering under a range of temperature conditions, significantly accelerating the process compared to traditional low-temperature induction. Current research on the mechanism of KClO3- induced flowering in longan has explored various dimensions, from morphological and physiological biochemistry to molecular biology. Emerging evidence suggested a potential connection between hormonal balance, ROS generation induced by KClO3, and the initiation of the flowering network. This review synthesized previous research findings to propose a hypothetical model for KClO3-induced flowering in longan (Fig. 3). Upon application, KClO3 was absorbed into the root system through the HATS and NRTs. ClO3− was then converted into chlorite ClO2−, ClO−, and Cl− through the action of NR and NiR. These ions were transported to the leaves and buds, where they induced upregulation of CKs and downregulation of GAs, leading to ROS production. It was hypothesized that these factors may activate the expression of the floral meristem identity genes through pathways related to photoperiod, hormones, aging pathways and integrators, ultimately leading to floral transition.
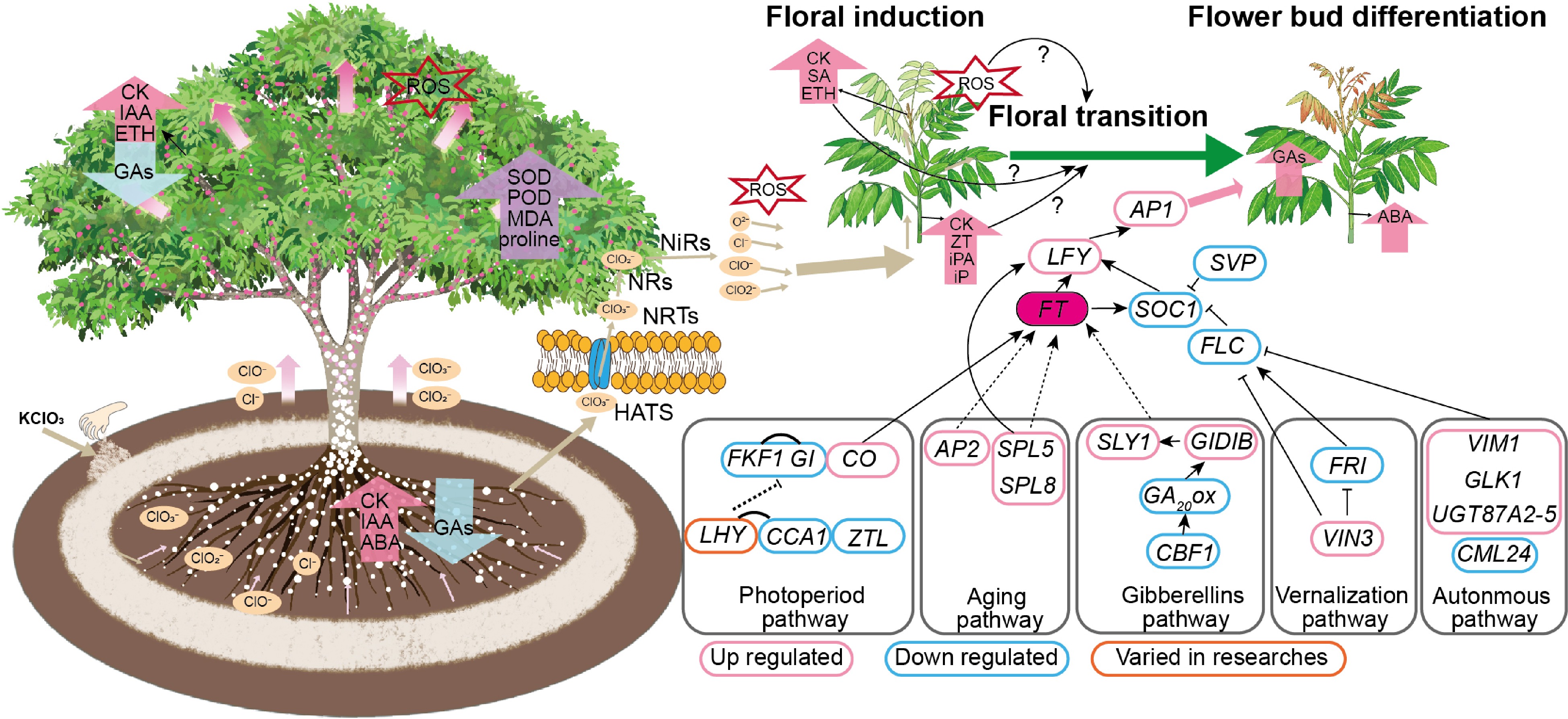
Figure 3.
Hypothetical model for KClO3-induced flowering in longan. Note: Cl−, chloride ion; ClO−, hypochlorite ion; ClO2−, chlorite ion; ClO3−, chlorate ion; CKs, Cytokinin; GAs, Gibberellins; ROS, Reactive Oxygen Species; H2O2, hydrogen peroxide; NR, Nitrate Reductase; NiR, Nitrite Reductase; NRT1, Nitrate Transporter 1; HATS, High-Affinity Transport System; FKF1, Flavin-Binding, Kelch Repeat, F Box 1; CO, CONSTANS; GI, GIGANTEA; CCA1, CIRCADIAN CLOCK ASSOCIATED 1; LHY, LATE ELONGATED HYPOCOTYL; ZTL, ZEITLUPE; SPL, SQUAMOSA PROMOTER BINDING PROTEIN-LIKE; AP2, APETALA 2; GID1B, GA INSENSITIVE DWARF1B; GA1, GA REQUIRING 1; FLC, FLOWERING LOCUS C; VIN3, VERNALIZATION INSENSITIVE 3; FRI, FRIGIDA; VIM, VARIANT IN METHYLATION; FY, WDR33; MYB30, MYB domain protein 30; AP1, APETALA1; FT, FLOWERING LOCUS T; AGL24, AGAMOUS-like 24; LFY, LEAFY; FUL, FRUITFULL; SOC1, SUPPRESSOR OF OVEREXPRESSION OF CO 1. The expression of genes was based on the comprehensive analysis of the results of references, down and up-regulated meant that these genes were commonly down or up-regulated in several references.
However, whether the hormone change induced by KClO3 or the ROS burst primarily initiated the flowering pathway remained unclear. Other potential mechanisms may be involved in activating the flowering regulatory network. Litter research on the mechanism of action of the whole system has limited a comprehensive understanding of the mechanism underlying off-season flowering in longan. Therefore, a systematic study of the key mechanisms through which KClO3 triggered flowering signals in longan is needed, encompassing various tissues including roots, trunks, branches, leaves, and buds. Future research should employ a multi-omics approach, integrating genomics, genome-wide association studies (GWAS), transcriptomics, proteomics, epigenomics, and metabolomics, alongside gene silencing, and genetic transformation in longan and molecular interaction experiments. We need to elucidate the molecular regulatory network and identify the key genes involved in the molecular regulatory network related to the absorption, transportation, transformation, and flowering induction of KClO3 within longan trees. Such comprehensive studies are essential to fully elucidate the molecular mechanisms underlying KClO3-induced flowering in longan.
The application of nanotechnology in agriculture has expanded rapidly, with nano-fertilizers providing advantages such as high efficiency, target delivery, and reduced environmental impact compared to traditional fertilizers[117]. For instance, low-concentration iron-based nanomaterials and nano-nitrogen fixation materials[118,119] have demonstrated the ability to precisely target specific organelles across different plant species, thereby offering enhanced control over plant growth and development. Given these advancements, investigating the mechanism of KClO3 induced flowering in longan could pave the way for developing low-dose, high-efficiency functional nanomaterials specifically for artificial flowering induction. Such innovations would not only refine the effectiveness of KClO3-induced flowering, but also increase the possibility of inducing off-season flowering across species, such as closely related lychee and rambutan.
We acknowledge funding support from the National Key Research and Development Program (2018YFD1000104).
-
The authors confirm contribution to the paper as follows: study conception and design: Zhang J; data collection: Mei J, Zhao G; analysis and interpretation of results: Mei J, Zhao G; draft manuscript preparation: Mei J, Hua X. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated and analyzed during the current study are available from the corresponding author by request.
-
The authors declare that they have no conflict of interest.
-
Received 23 July 2024; Accepted 22 October 2024; Published online 21 January 2025
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press on behalf of Hainan University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Mei J, Zhao G, Hua X, Zhang J. 2025. Advances in research of potassium chlorate-induced flowering in longan. Tropical Plants 4: e003 doi: 10.48130/tp-0024-0043
Advances in research of potassium chlorate-induced flowering in longan
- Received: 23 July 2024
- Revised: 19 September 2024
- Accepted: 22 October 2024
- Published online: 21 January 2025
Abstract: Longan (Dimocarpus longan Lour.) is a fruit tree of significant economic value in tropical and subtropical regions. The introduction of potassium chlorate (KClO3) as a flowering inducer has facilitated remarkable advancements in longan cultivation, enabling the year-round supply of fresh fruits. This innovation has substantially increased both the cultivation area and the economic value of the longan, marking a milestone in the industry development. This paper outlines the characteristics and current research advancement regarding both natural and KClO3-induced flowering in longan, while addressing the challenges associated with KClO3-induced flowering in this species. Recent findings are encompassed at the morphological, physiological, biochemical, and molecular biological levels. Additionally, the absorption and transport pathways of chlorate and nitrate in longan and other plants are summarized, as well as the effects of oxidative stress on floral transition. Furthermore, potential future research directions are explored, including elucidating the mechanism of KClO3-induced flowering in longan through multi-omics analysis and investigating the application of nanomaterials to enhance artificial flowering induction in longan and other species. This provides insights supporting further in-depth research into the mechanism of KClO3-induced flowering in longan, thereby contributes to the framework of knowledge and potential applications in horticulture.
-
Key words:
- Floral transition /
- Off-season flowering /
- Longan /
- Potassium chlorate












