-
Lotus, Nelumbo nucifera Gaertn. (commonly known as Asian lotus), is one of the most commercially significant aquatic plants in the world. It belongs to the Nelumbonaceae family, which contains a single genus Nelumbo, with the American lotus (Nelumbo lutea Pear) as the only other extant species. The Asian lotus is geographically widely distributed in most Asian countries, as well as in Australia and Russia (Fig. 1). The American lotus, also known as yellow lotus, occurs primarily in eastern regions of North American and the Caribbean[1]. Due to geographical isolation by the Pacific Ocean, the two lotus species exhibit distinct morphologies in plant size, leaf, and petal shape, particularly in their petal colors. The Asian lotus displays diverse flower colors, including white, pink, red, pale yellow, as well as a variety of color patterns, while the American lotus only exhibit yellow flowers (Fig. 1). The two species, however, are genetically crossable and share rather similar aquatic habits and life cycles. Both of the two species have eight pairs of chromosomes (2n = 16), with approximately 800 Mb genome sizes, highly conserved gene contents, and little chromosomal rearrangements[2,3].
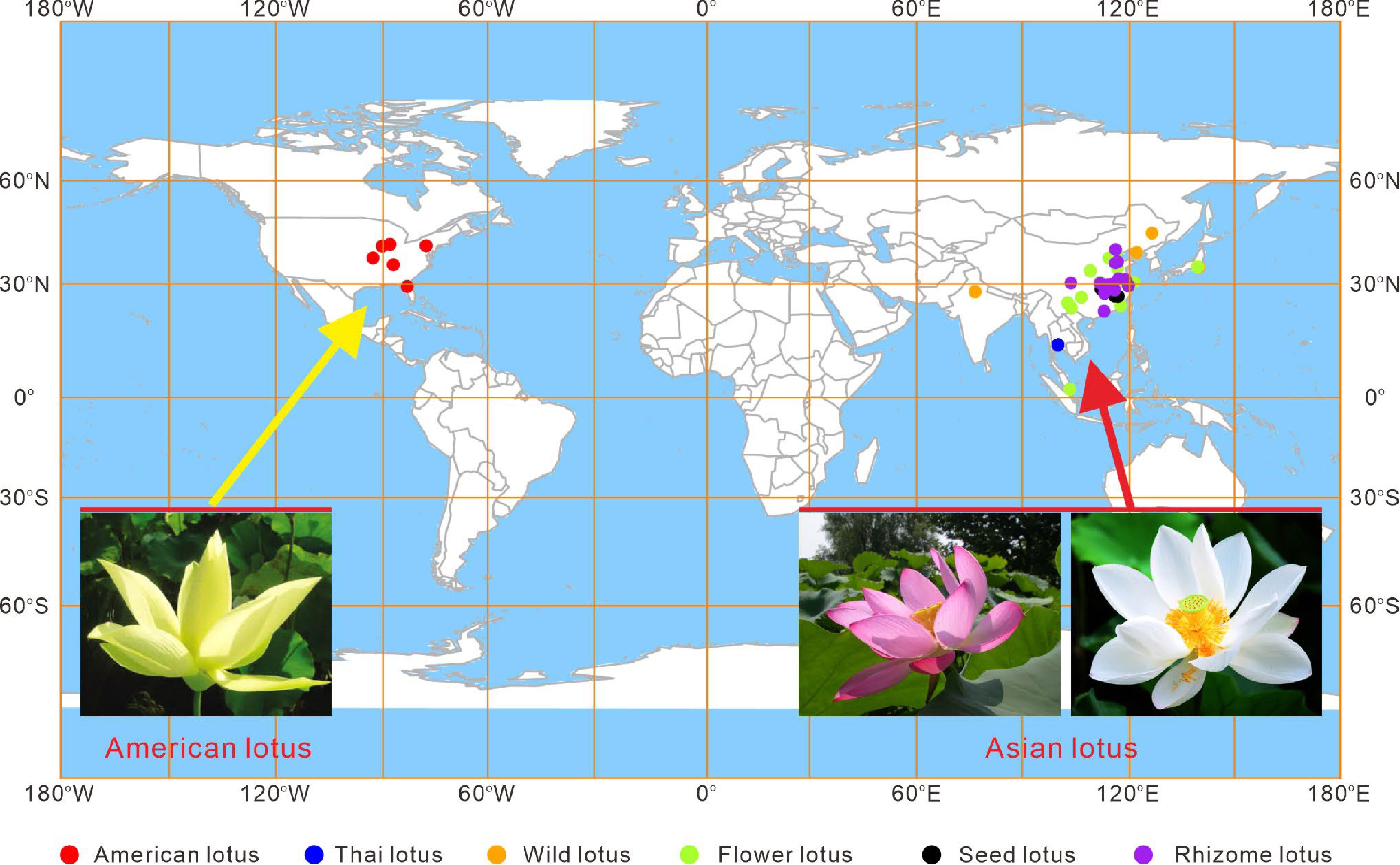
Figure 1.
Geographical distribution of lotus accessions collected in the Wuhan National Lotus Germplasm Bank at the Wuhan Botanical Garden of the Chinese Academy of Sciences. The different colored dots indicate cultivars belonging to different categories.
While the American lotus predominantly grows naturally in the wild, the Asian lotus has been domesticated and cultivated over 3,000 years, for its nutritional, ornamental, and medicinal attributes[4]. The total lotus cultivation area in China is estimated to cover over 530,000 ha, and so far at least 4,500 lotus cultivar germplasm resources have been conserved[5]. Most of these cultivars are hybrids developed by traditional crossing, while approximately 300 are local genotypes collected from wild lakes all over the world (Fig. 1)[2]. These lotus cultivars are grouped into four categories based on their agricultural uses, including the flower lotus for ornamental purpose; the rhizome lotus for edible rhizome production; the seed lotus for harvesting fresh or mature seeds; and the medicinal lotus for pharmaceutical uses[6].
As an ancient traditional herb, all lotus plant tissues have been used in the treatment of various diseases, such as fever, sunstroke, sweating, obesity, ischemia, cancer, diabetes, hepatopathy, and hypertension[7−10]. Its medicinal properties are largely ascribed to the abundantly accumulated active components in different lotus organs, including alkaloids, flavonoids, terpenoids, polysaccharides, saponins, and minerals. Of these compounds, benzylisoquinoline alkaloids (BIAs) are regarded as the most important pharmacological ingredients[11,12]. Here, we summarize the recent advances in the chemical profiles, pharmacological activities, extraction methods, and elucidations of its biosynthesis pathway.
-
BIAs are a class of tyrosine-derived nitrogenous secondary metabolites with over 2,500 known structures[13]. The core chemical structure of BIAs consist of a benzene ring, a pyridine ring, and a second aromatic ring (Fig. 2)[14]. Like other plant-derived alkaloids, most BIAs are significantly bioactive[15]. Typical pharmacologically significant BIAs include morphine and codeine with analgesic traits, sanguinarine and berberine with antimicrobial properties, as well as noscapine, papaverine, and tubocurarine with antitussive, vasodilative, and muscle relaxant properties, respectively. The occurrence of BIAs in plants is restricted primarily in the order Ranunculales and eumagnoliids. However, their accumulation has been detected in other families, including Rutaceae, Lauraceae, Cornaceae, and Nelumbonaceae.
The lotus plant richly accumulate BIAs in almost all its organs[11]. To date, at least 61 alkaloids (Table 1) have been identified in lotus since the first isolation of nuciferine, roemerine, and O-nornuciferine alkaloids from the plant in the 1960s[16,17]. Notably, all reported lotus alkaloids are BIAs except Oleracein E and Methylcorypalline, which contain only basic isoquinoline structures. Based on their structures, these BIAs can further be divided into three subclasses, including 1-benzylisoquinolines, aporphines, and bisbenzylisoquinolines (Figs 2−4). 1-Benzylisoquinoline alkaloids are basically intermediates in the biosynthesis of aporphines and bisbenzylisoquinolines, and are mostly accumulated in trace amounts in lotus tissues[18]. In contrast, aporphines and bisbenzylisoquinolines are the end BIA products in lotus, with their accumulation levels accounting for over 95% of the total BIA content[11].
Table 1. BIAs detected in the lotus (Nelumbo nucifera) tissues. Alkaloids are assigned with numbers as shown in Figures 2–5.
No. Alkaloid Formula Enantiomer Organ Reference 1-BENZYLISOQUINOLINE 1 Lotusine C19H24NO3+ E, S, F [25] 2 Methyl lotusine L 3 Armepavine C19H23NO3 (−)-R and (+)-S S, F, L [21] 4 Coclaurine C17H19NO3 (+)-R S, L [26] 5 N-norarmepavine C18H21NO3 (+)-R L [27] 6 N-methylisococlaurine C18H21NO3 L [16,28] 7 N-methylcoclaurine C18H21NO3 (−)-R L, S [16] 8 Isococlaurine C19H24NO3+ L 9 Methylhigenamine S [17] 10 Norcoclaurine-6-O-glucoside S 11 Norcoclaurine C16H17NO3 (+)-R and (+)-S S, L [26,29] 12 Argemexirine S, L 13 6-demethy-4-methyl-N-methylcoclaurine C18H21NO3 S [30] 14 Nor-O-methylarmepavine C20H25NO3 S [30] 15 4’-N-methylcoclaurine C19H23NO3 L, S [17] 16 4’-methyl coclaurine L, S [17] 17 Bromo methyl armepavine L, S [17] 18 Methoxymethy lisoquinoline L, S [17] 19 Higenamine P [31,32] 20 Higenamine glucoside P [33] APORPHINE 21 Nuciferine C19H21NO2 (−)-R S, F, L [34,35] 22 N-nornuciferine C18H19NO2 (−)-R S, L [26] 23 Roemerine C18H17NO2 (−)-R S, F, L [16,26,32] 24 O-nornuciferine C18H19NO2 (−)-R S, F, L [32,36] 25 Anonaine C17H15NO2 (−)-R S, L [16,26] 26 Lirinidine C18H19NO2 (−)-R S, L [37] 27 Nuciferine-N-Methanol F 28 Nuciferine-N-Acetyl F 29 Anonaine-N-Acetyl F 30 Caaverine C17H17NO2 (−)-R S, L [20,38] 31 Oxidation-nuciferine S, L, F [19,30] 32 Asimilobine C17H17NO2 (−)-R S, F, L [17,20] 33 Methyl asimilobine S, L [17] 34 N-methyl asimilobine S, L [16,39] 35 Roemerine-N-oxide S, L [16,40] 36 N-methyl asimilobine-N-oxide S, L, F [16,19] 37 Nuciferine-N-oxide S, L, F [16,19] 38 Dehydroanonaine C17H13NO2 L [16] 39 Dehydronuciferine C19H19NO2 L [16] 40 Dehydroaporphine C18H15NO2 L [41] 41 Nelumnucine S, L 42 Dehydroroemerine L [16] 43 Liriodenine C17H9NO3 L [16,40] 44 7-hydroxydehy dronuciferine C19H19NO3 L [16] 45 Pronuciferine C19H21NO3 (+)-R and (−)-S S, F, L [16,26,40] 46 Glaziovine S 47 Lysicamine C18H13NO3 L [38,42] 48 Cepharadione L [38] BISBENZYLISOQUINOLINE 49 Neferine C38H44N2O6 1R, 1'S S, F, E [17,31] 50 Liensinine C37H42N2O6 1R, 1'R S, F, E [35] 51 Isoliensinine 1R, 1'S S, F, E [25] 52 N-norisoliensinine C36H40N2O6 S, F, E [25] 53 6-hydroxynorisoliensinine C36H40N2O6 S, F, E 54 Methyl neferine S, E [10,17] 55 Nelumboferine C36H40N2O6 S, E [10,17] 56 Negferine C38H44N2O6 L [17,26] 57 Nelumborine F [17] 58 Dauricine S, F [43] TRIBENZYLISOQUINOLINE 59 Neoliensinine C63H70N3O10 1R, 1'S, 1''R E [44] L, Leaf; E, embryo; F, flowers; S, seeds; R, rhizome; LS: leaf sap; NS, not specified. Unlike BIAs in the Ranunculales, lotus BIAs exhibit several unique features. Firstly, lotus BIAs show strict tissue specific accumulation patterns. Although BIAs are found in all the plant tissues, highest levels of approximately 3,000 μg/g fresh weight (FW) are accumulated in the laminae and plumules, followed by petals and petioles with about 500 and 100–200 μg/g FW, respectively. Conversely, lowest BIAs levels (less than 20 μg/g FW) occur in the rhizomes and stamens. Interestingly, the lotus bleeding sap contains extremely high BIAs level of up to 10,000 μg/g FW[11]. In addition, lotus leaves and petals predominantly accumulate aporphine BIAs, including nuciferine, O-nornuciferine, N-nornuciferine, roemerine, and anonaine, while the plumules mostly contain bis-BIAs of liensinine, isoliensinine, and neferine[11]. Secondly, while BIAs prevalently assume the (S)-enantiomer conformation, lotus show enrichment of both (R)- and (S)-conformers. For example, both (R)- and (S)-stereoisomers of armepavine have been isolated from sacred lotus[19−21]. To date, most aporphines isolated from lotus belong to the (R)-configurations (Table 1). Thirdly, all aporphine BIAs isolated from lotus lack the hydroxyl and methyl modifications at the C-3' and C-4' positions, suggesting that the (R)-N-methylcoclaurine might be the key precursor for both aporphine and bis-BIA biosynthesis in lotus[22].
Lotus cultivars exhibit considerably diverse patterns of BIA composition and accumulation. Generally, seed cultivars accumulate the highest concentration of BIAs in leaves, followed by flower cultivars, while lowest levels are observed in the rhizome cultivars[11,23]. Multivariate principal component analysis (PCA) of 92 lotus cultivars showed that the seed cultivars generally accumulate high levels of nuciferine and O-nornuciferine, whereas flower cultivars primarily accumulate high N-nornuciferine, roemerine, and anonaine alkaloids[23]. In contrast, rhizome cultivars contain no dominant BIAs. Cluster analysis of the 92 cultivars also indicated that the American cultivar ‘Meizhouhuanglian’ could group with other Asian lotus cultivars, which suggested the lack of obvious differentiation between the American and the Asian lotus accessions based on BIA content and composition[23]. Developmentally, the lowest BIAs levels are accumulated in the lotus leaves at the bud stages (developmental stage S1). BIAs levels then increase steadily in the leaf growth and expansion stages, peak at S6 stage, then decrease slightly at the S7 senescence stage (Fig. 5a)[11]. Accumulation of BIAs in the lotus plumules is also developmentally regulated[24]. Major BIAs were almost undetectable in the plumules at 12 days post pollination (DAP), which later increased consistently until 21 DAP (Fig. 5b).
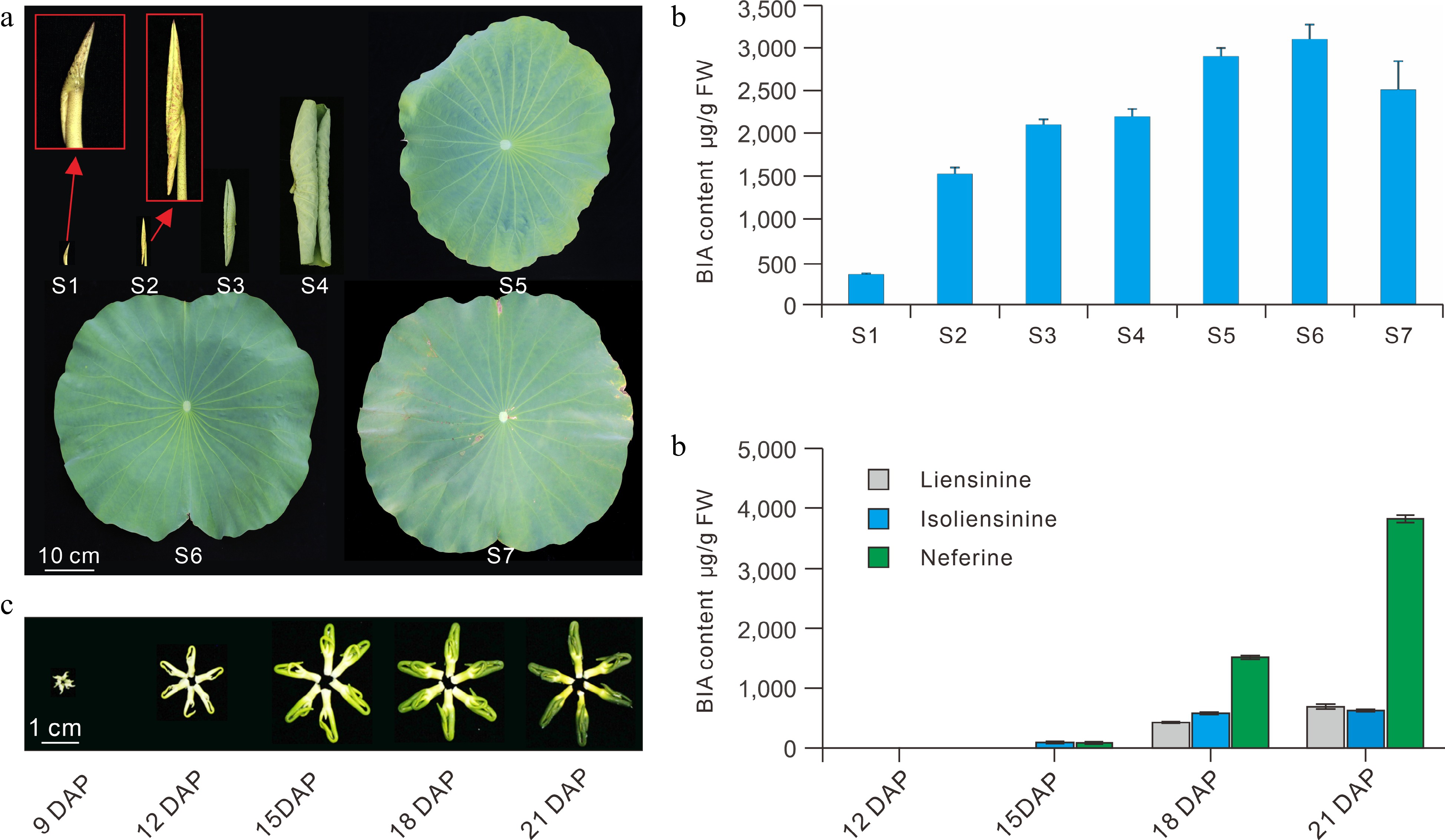
Figure 5.
BIA profiles in the leaf and plumule during development. (a) Lotus leaves showing the seven defined developmental stages. (b) BIA profiles in the seven leaf developmental stages. (c) Lotus plumules showing different developmental stages. (d) BIA content in the plumule at different developmental stages. S, leaf developmental stages; DAP, days post pollination. The figure is modified from previous reports[11,24].
-
Extraction of alkaloids from raw materials is the first analytical step in their identification, quantification, and application. Currently, three main alkaloid extraction methods with varied principles are widely used, including solvent extraction, distillation, expeller pressing and sublimation methods. Solvent extraction is presently the most popularly used method. The plant alkaloids occur mostly as salts of organic and inorganic acids[45,46], thus, acidic and basic solvents are initially required to remove the non-alkaloid compounds. Subsequently, appropriate purification methods, primarily acidic extraction is performed by mixing the ground sample materials with weak acid solutions (e.g., acetic acid in water, methanol, or ethanol), to obtain a desired level of purity. The solvent extraction is carried out in four steps: 1) assimilation of solvents into the target solid matrix; 2) alkaloid dissolution in solvents; 3) solvent diffusion out of a solid matrix; 4) collection of extracted solutes[47]. Theoretically, any factors that enhance the above steps can facilitate alkaloid extraction. Key factors that affect the extraction efficiency, include alkaloids-solvent solubility, sample particle size, solvent to solid ratio, extraction temperature, and extraction duration[48,49].
The lotus BIAs are weak alkalescent compounds, with poor solubility or insolubility in water, but with rapid solubility in organic solvents. Based on the law of similarity and intermiscibility (like dissolves like), crude lotus BIAs extract are usually prepared in alcohol solvents (ethanol or methanol)[10,19]. Other solvents, such as hydrochloric acid, dichloromethane and trichloromethane have also been used for lotus BIA extraction[20]. The solvent extraction efficiency can be enhanced by finely grinding the lotus tissues, solvent refluxing, microwaving, and sonication. A recent comparative study, using three extraction solvent (methanol, 50% methanol, and water) and two different extraction conditions (reflux for 120 min twice or sonication for 30 min twice), suggested that the 'reflux in methanol' method could result in highest BIA recovery from lotus flowers (Table 2)[50]. Moreover, pre-basifying lotus leaves with 10% ammonia water revealed a significant two-fold nuciferine yield relative to that obtained from non-basified leaves[51].
Table 2. Extraction efficiency of alkaloids from lotus flower.
Extraction method Content (mg/g dry weight)a Total 1 2 3 4 5 6 7 8 9 10 Methanol, reflux 1.76 (100) 1.75 (100) 0.07 (100) 0.63 (100) 0.69 (100) 0.83 (100) 1.45 (100) 5.73 (100) 1.30 (100) 0.75 (100) 14.96 (100) 50% Methanol, reflux 1.09 (62) 1.35 (77) 0.05 (71) 0.50 (79) 0.61 (88) 0.78 (94) 1.35 (93) 3.79 (66) 0.94 (73) 0.56 (75) 11.02 (74) H2O, reflux 0.24 (14) 0.35 (20) nd.b 0.21 (33) 0.18 (26) 0.38 (45) 0.78 (54) 2.57 (45) 0.66 (51) 0.29 (38) 5.66 (38) Methanol, sonication 0.88 (50) 1.11 (64) 0.03 (44) 0.39 (62) 0.33 (48) 0.47 (56) 0.97 (67) 2.77 (48) 0.70 (54) 0.42 (56) 8.07 (54) 50% Methanol, sonication 0.98 (56) 1.27 (73) 0.04 (58) 0.49 (78) 0.47 (69) 0.80 (96) 1.38 (95) 3.93 (69) 0.97 (75) 0.59 (79) 10.92 (73) H2O, sonication 0.14 (8) 0.21 (12) nd. 0.12 (20) 0.08 (11) 0.25 (30) 0.53 (37) 1.91 (33) 0.48 (37) 0.19 (26) 3.91 (26) 1. nuciferine; 2. N-nornuciferine; 3. N-methylasimilobine; 4. Asimilobine; 5. Pronuciferine; 6. Armepavine; 7. norarmepavine; 8. N-methylcoclaurine; 9. coclaurine; 10. Norjuziphine. a. relative value (%) against the content obtained by methanol under reflux is given in parentheses. b. less than the quantitation limit. The conventional solvent extraction method is typically time consuming, requires large volume of organic solvents, and high cost waste solvent treatment. Consequently, modern or greener extraction methods, such as supercritical fluid extraction (SFC), high-speed counter-current chromatography (HSCCC), and microwave assisted extraction (MAE) techniques have been developed and applied in BIAs extraction. SFE utilizes supercritical fluid as the extraction solvent, which has high solubility as liquid and high diffusivity as gas. SFE is almost pollution-free, and can efficiently extract alkaloids with no residual organic solvents[52]. The solvating properties of supercritical fluid can dramatically rise when the pressure and temperature are near their critical points. The supercritical carbon dioxide (S-CO2) is one of the most widely used supercritical fluids due to its low critical temperature (31 °C), low cost, non-toxicity, chemical inertness, and non-flammability properties[46,53−54]. However, due to the relatively low polarity of S-CO2, polar agents, such as methanol and water are used as modifiers to raise its polarity and enhance its efficiency for BIAs extraction from lotus tissues. A previous study showed that the highest nuciferine yield of 325.54 μg/g could be achieved with extraction conditions set at 70 °C, 30 MPa, flow rate 0.2 ml/min, 2 h extraction time, and with 10% (v/v) diethylamine and 1% (v/v) water in methanol as the polarity modifier[51].
The HSCCC is a liquid-liquid partition chromatography technique that uses no solid support matrix. This method permits the introduction of crude samples directly into the hollow column, and eliminates the adsorbing effects on stationary phase material as well as artifact formation, thus offering maximum sample recovery capacity and a wide range of solvent system selection[55]. Ma et al. used a simple two-phase solvent system comprising of n-hexane-ethyl acetate-methanol-acetonitrile-water (5:3:3:2.5:5, v/v/v/v/v) to successfully purify four main aporphine alkaloids, including 2-hydroxy-1-methoxyaporphine, pronuciferine, nuciferine, and roemerine from a crude extract of lotus leaves[56].
In addition to the above-mentioned methods, several other extraction methods, such as maceration, percolation, soxhlet, and pressurized liquid extraction are available for selection. Modern and green methods are preferred mostly due to their energy-efficiency, speed, and less solvent requirement, and high extraction yield. However, traditional techniques are still widely used at the industrial level due to their low investment cost. Overall, the selection of suitable extraction techniques depends not only on the physical, chemical, and stability of the target alkaloids, but also on time and cost of the extraction methods.
Separation, identification, and quantification
-
Following extraction, the resulting crude BIA extracts are mixtures of a variety of natural products, such as phenolics, carotenes, glycosides, and terpenes, which require further separation and purification. The separation of BIAs depends largely on their physical and chemical characteristics, such as adsorption properties, partition coefficients, molecular sizes, solubility, and ionic strengths[47]. BIAs are mostly water-insoluble but soluble in organic solvents, while their acid salts are water-soluble or soluble in dilute acids, thus, these compounds can be precipitated by adding solvents (e.g., sodium bicarbonate, ammonia, or tartaric acid) that alter the reaction pH levels. Similar theory has been used to develop pH-zone-refining counter current chromatography techniques for lotus BIAs isolation[35,57].
Isolation of pure lotus BIAs using conventional methods, such as column chromatography and thin-layer chromatography (TLC) is extremely difficult due to their high structural similarity. The structurally similar BIAs can instead be rapidly separated by counter-current chromatography (CCC) according to their different partition coefficients in two immiscible solvents. The CCC is a liquid-liquid partition chromatography technique with no solid support matrixes, and has been widely used for the preparative isolation of lotus BIAs. Accurate selection of the solvent system is highly crucial when using the CCC method. Generally, the two-phase solvent system should have: no decomposition or denaturation effects on the target compounds, sufficient sample solubility and suitable partition coefficient values, and satisfactory retention capacity of the stationary phase[58]. For efficient separation, the partition coefficient (K) value of the target alkaloids and the separation factor between two BIAs (K2/K1) should be close to 1 and greater than 1.5, respectively[55]. Other factors, such as the rotary speed, the mobile phase flow rate, and the column temperature also affect the target alkaloid separation.
Preparative separation of liensinine and its analogues from lotus plumules using CCC was first reported by Wu et al.[59]. The authors used two-phase solvent systems of light petroleum (b.p. 60–90 °C)–ethyl acetate–tetrachloromethane–chloroform–methanol–water (1:1:4:4:6:2, v/v) and ethyl acetate–tetrachloromethane–methanol–water (1:6:4:1, v/v) (Table 3) to successfully isolate bis-BIAs from lotus plumules in small- and large-scale, respectively. To limit the application of deleterious tetrachloromethane and chloroform solvents, another simplified HSCCC solvent system of n-hexane–ethyl acetate–methanol–water (5:8:4:5, v/v, containing 0.5% NH4OH) was developed and successfully used to separate liensinine, isoliensinine, and neferine from crude lotus extract[60]. Later, several pH-zone-refining CCC solvent systems, which generally use 10 mM triethylamine in the upper organic phase and 5 mM HCl in the aqueous phase for adjustment of pH and K values of target compounds were developed[35,57,61,62]. These two phase solvent systems were used to successfully isolate over 98% pure BIA singletons from both plumules and leaves. Other than the CCC technique, a preparative liquid chromatography has also been developed for high purity laboratory-scale isolation of liensinine, isoliensinine, and neferine[63].
Table 3. Major two-phase solvent systems developed for preparative separation of lotus BIAs through counter-current chromatography (CCC) techniques.
Solvent systems Mix ratios (v/v) Target BIAs Reference Light petroleum (60–90 °C)–ethyl acetate–tetrachloromethane–chloroform–methanol–water 1:1:4:4:6:2 Small scale
Plumule BIAs[59] Ethyl acetate–tetrachloromethane–chloroform–methanol–water 1:6:4:1 Small scale
Plumule BIAs[59] n-hexane–ethyl acetate–methanol–water 5:8:4:5
0.5% NH4OHPlumule BIAs [60] n-hexane–ethyl acetate–methanol–water 5:5:2:8
10 mM triethylamine
5 mM HClPlumule BIAs [57] Diethyl ether – Na2HPO4/NaH2PO4 (pH = 7.2 – 7.5) 1:1 Plumule BIAs [62] Petroleum ether (60–90 °C)–ethyl acetate–methanol–water 5:5:2:8
10 mM triethylamine
5 mM HClLeaf BIAs [61] n-hexane-ethyl acetate-methanol-water-[C4mim][PF6] 5:2:2:8:0.1
10 mM triethylamine
3 mM HClWhole plant BIAs [35] After extraction and purification, lotus BIAs are subjected to further identification and quantification processes. High-performance liquid chromatography (HPLC) techniques, particularly the reversed-phase LC methods, were predominantly employed[45]. Identification of known BIA compounds is normally accomplished by HPLC separation and UV detection, followed by electrospray ionization (ESI), and tandem mass spectrometry (MS/MS). The reversed-phase C18 columns are most ideal, and the application of aqueous acetonitrile containing 0.1% triethylamine as mobile phase has been shown to effectively separate both lotus leaf and plumule BIAs with strong peak signals and fine peak shapes[23,63,64]. Other optimized HPLC parameters for lotus BIAs characterization include: column temperature 30◦C; 270–280 nm UV/photodiode array (DAD) detection wavelength; the gradient elution mode, 40%–80% acetonitrile at 0–15 min, 80% acetonitrile at 15–18 min, 80%–40% acetonitrile at 18–19 min, 40% acetonitrile at 19–25 min; and a flow rate 0.8 ml/min. The MS detection of lotus BIAs are preferably conducted in the positive mode, with cone voltage of 20 V and a nebulizer gas temperature/pressure of 150 °C/21 psi. The needle, shield, and capillary voltage parameters are normally used with default settings. Such HPLC-DAD-ESI-MS/MS methods have been used to identify the major leaf BIAs, including nuciferine, N-nornuciferine, O-nornuciferine, anonaine, and roemerine, as well as plumule BIAs, such as liensine, isoliensinine, and neferine (Table 4; Fig. 6; Fig. 7).
Table 4. Identification of major lotus leaf and plumule alkaloids and their HPLC-MS/MS ion characteristics.
Peaks TRa (min) Molecular weight m/z
[M + H]+Major fragment ions Alkaloids 1* 7.56 281 282 251/219 N-nornuciferine 2* 9.03 281 282 265/250 O-nornuciferine 3* 10.17 265 266 249/219 Anonaine 4* 12.43 295 296 265/250 Nuciferine 5* 13.39 279 280 249 Roemerine 6∆ 6.61 610 611 503/283/206 Liensinine 7∆ 9.47 610 611 489/297/192 Isoliensinine 8∆ 17.72 624 625 503/297/206 Neferine * The retention time (TRa (min) of peaks 1–5 as obtained by Chen et al.[23].
∆ The retention time of peaks 6–9 as reported by Chen et al.[63].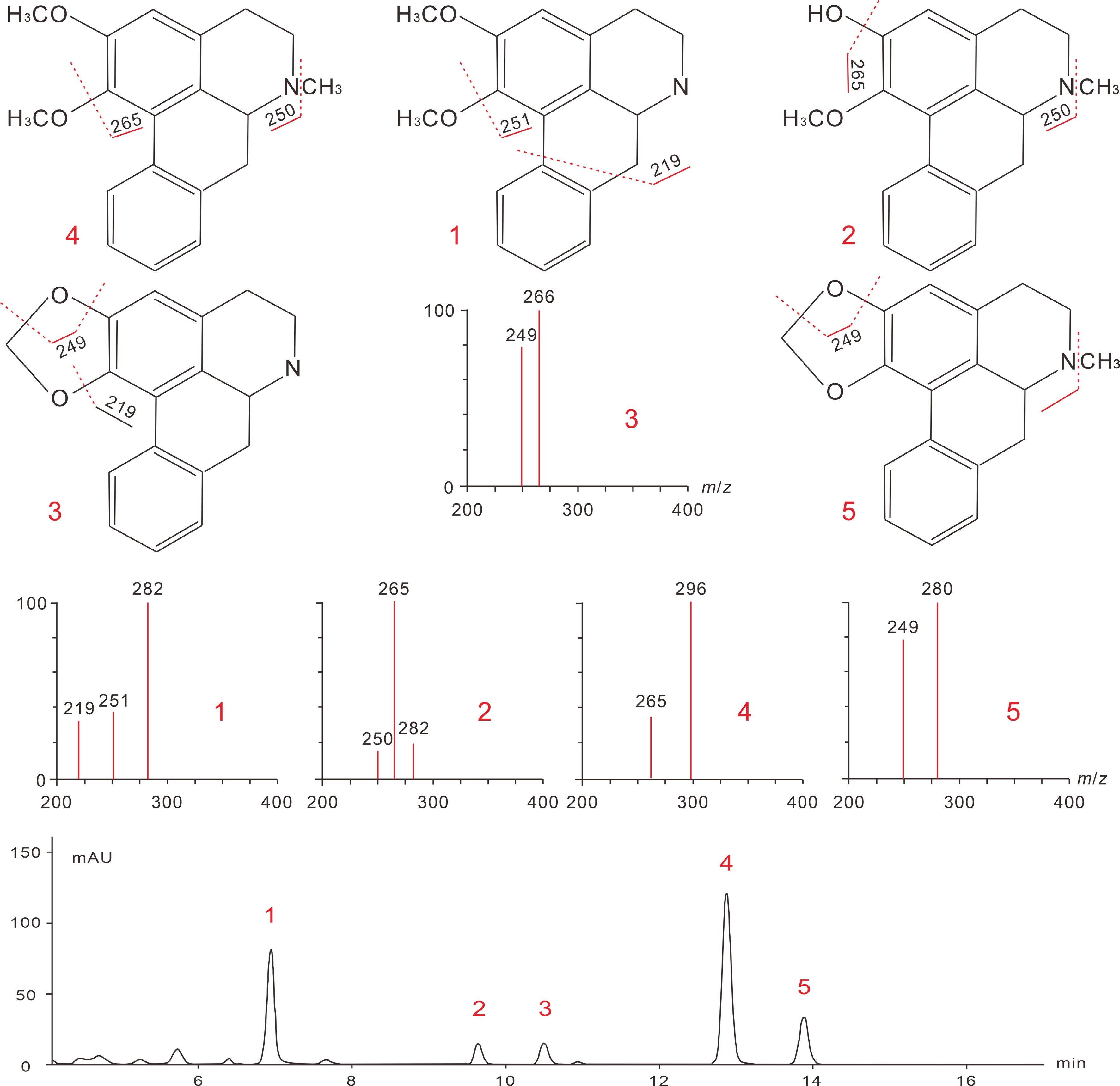
Figure 6.
Analytical mass spectra of lotus leaf BIAs and their fragmentation pathways. The chemical structures and the corresponding peak numbers 1 to 5 represent signals for N-nornuciferine, O-nornuciferine, anonaine, nuciferine, and Roemerine, respectively. Figures are modified from Luo et al. [65] and Chen et al. [23].

Figure 7.
Analytical mass spectra of lotus leaf BIAs and their fragmentation pathways. The chemical structures and the corresponding peak numbers 6 to 8 represent signals for liensinine, isoliensinine, and neferine, respectively. Figures are modified from Chen et al. [64], Deng et al. [11], and Lai et al. [66].
Currently, lotus BIAs are predominantly identified using the ultra-performance liquid chromatography technique equipped with quadrupole time-of-flight mass spectrometry (UPLC-QTof-MS). In contrast to traditional HPLC-MS methods, the UPLC-Q-Tof-MS technique exhibits higher sensitivity and resolution, and mass measurement accuracy, making it a powerful and reliable analytical technique for plant metabolite identification[65,66]. Under optimized UPLC-Q-Tof-MS conditions, over 20 BIAs were identified from lotus leaves and plumules, based on their chromatographic characteristics, UV spectra, exact mass, and MS fragments[31,65,67]. Moreover, these studies further verified previous observations that liensinine, isoliensinine, neferine, and lotusine are the major BIAs in lotus plumules, while those of aporphines, including nuciferine, roemerine, Anonaine, N- and O-nornuciferine are the major BIAs in lotus leaves.
-
In addition to their nutritional roles, lotus is also a key source of herbal traditional Chinese medicine. Lotus was originally used to treat various diseases, such as pharyngitis, chest pain, cough, vomiting blood, fever, and heat stroke[68,69]. To date, extensive studies have identified a wide range of lotus bioactive ingredients, their pharmacological efficiencies, and health benefits. As key bioactive lotus constituents, BIA alkaloids have nowadays received significant attention. In the following sections, we present an overview of pharmacological activities of lotus BIAs from various tissues.
Lotus leaves
-
As introduced above, lotus leaves accumulate primarily aporphine-type BIAs, with Nuciferine, N- and O-nornuciferine as the richest components. Diversified pharmacological activities of lotus leaves therefore attribute predominantly to these three BIAs. The nuciferine, for example, has shown anti-obesity, anti-virus, antioxidant, anti-cardiovascular, antimicrobial, and anti-cancer activities.
Lipid-regulating activity
-
Lipids are maintained through dynamic balances within the cell as Triglyceride (TG) by a series of synthesis and catabolism related transcription factors (TFs) and enzymes. Lotus alkaloids can exert lipid-regulating effects in several ways, including mainly inhibition of lipid synthesis and absorption, inhibition of cell proliferation and differentiation, and interaction with proteins. A study on the effects of nuciferine on blood lipids in male golden hamsters, feeding with normal diet, high-fat diet (HFD), or HFD supplemented with nuciferine (10 and 15 mg/kg·BW/day), revealed a reduction in total cholesterol (TC), TG, low-density lipoprotein and free fatty acids in hamsters treated with different doses of nuciferine after eight weeks[70]. In addition, nuciferine could alleviate dyslipidemia, and liver steatosis by inhibiting the expression of hepatic genes related to lipid metabolism in hamsters fed with a high-fat diet[70,71]. Similarly, a recent study demonstrated that lotus leaf extracts could inhibit adipogenesis in 3T3-L1 preadipocytes and suppress obesity in high-fat diet-induced obese rats[72]. Overall, these pharmacological effects demonstrate the potential of lotus leaf BIAs as natural lipid-lowering agents.
Hypoglycemic activity
-
Currently, approximately 90% of global type 2 diabetic patients are characterized by hyperinsulinemia and insulin resistance. Therefore, controlling the blood glucose levels of patients is crucial for diabetes treatment[73]. Huperzine has been reported to lower blood sugar via various mechanism in the body. Conversely, nuciferine can increase the glucose uptake by fat and muscle cells, thereby promoting insulin secretion by pancreatic β-cells[56]. Nuciferine is also directly involved in the process of insulin secretion and has been shown to augment insulin secretion in the pancreatic β-cells by shutting down or stimulating the amplification of adenosine triphosphate pathway[74].
Antioxidant activity
-
Free radicals or oxidants that break down cells and tissues can affect metabolic function and cause different health challenges. Antioxidants are substances that effectively inhibit oxidation reaction of free radicals at low concentrations[75]. The antioxidant capacity of aporphine alkaloids isolated from the lotus leaves, including (R)-N-methylasimilobine, lysicamine, and (R)-nuciferine have been screened using antiradical scavenging, metal chelating, and ferric reducing power assays[38]. In addition, a previous study demonstrated that lotus leaf-fermented broth could exhibit 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, ferric ion reducing power, superoxide dismutase-like activity, tyrosinase inhibition, and nitrite scavenging activity, which provided scientific basis for the application of sugar-fermented lotus leaves as an antioxidant condiment[76]. A recent study reported that, to enhance, achieve, and maintain higher scavenging activity of DPPH radicals and hydroxyl radicals for lotus leaves, the optimal treatment should include oven drying the leaves at ~55–65 °C to maintain alkaloids and amino acids content[77].
Antimicrobial activity
-
Bacteria is known to be one of the leading causes of numerous diseases. Over exposure or misuse of antibiotics can result in antibacterial resistance. Chinese herbal medicine has been demonstrated to be an excellent alternative for the treatment of such bacterial infections to avoid antibiotic resistance[78]. The n-butanol compound lotus leaves extracts can effectively inhibit peridontitis-causing bacteria, such as Actinobacillus actinomycetemcomitans Y4, Actinomyces viscosus 19246, Porphyromonas gingivalis 33277, Fusobacterium nucleatum 25586, and Actinomyces naeslundii wvl 45[79]. Moreover, the lotus leaf alkaloids not only have anti-mitogenic effects, but also exhibit a stronger antibacterial effect on Escherichia coli. The lotus leaf alkaloids and flavonoids are considered as key bioactive components responsible for the antibacterial activity.
Anti-cancer activity
-
A key characteristic of cancer is the indefinite cell proliferation, and lotus leaf alkaloids have been shown to exhibit antiproliferative properties against several cancer cells. For example, in the presence of nicotine, nuciferine could significantly inhibit the proliferation of non-small cell lung cancer cells, suppress the activity of Wnt/β-catenin signaling, enhance Axin stability, and induce apoptosis. In addition, nuciferine could also down-regulate the expression levels of β-catenin and its downstream targets, including c-myc, cyclin D, and VEGF-A[80]. Methanolic lotus leaves extract, containing nuciferine, N-methylasimilobine, (-)-lirinidine, 2-hydroxy-1-methoxy-6a, and 7-dehydroaporphine, significantly inhibited melanogenesis in B16 melanoma cells[19]. Furthermore, the inhibitory potential of nuciferine against the growth of human breast cancer cells has been reported[81]. The inhibitory effect of nuciferine is likely due to its ability to block cancer cell division cycle and enhance cancer cell apoptosis.
Anti-cardiovascular activity
-
A recent study reported that 40 mg/kg nuciferine could not only significantly reduce aortic lesion and vascular plaque in ApoE(-/-) mice fed with a high-fat diet, but also attenuate migration and proliferation of vascular smooth muscle cells in vivo and in vitro through the Calm4/MMP12/AKT signaling pathway[82]. Nuciferine has also been associated with reduced vascular wall inflammation and significant down-regulation of vascular inflammatory factors, such as IL-1β, TNF-α, MCP-1, and NF-κB, with the latter being a major regulator during all phases of the inflammatory response[83]. Additionally, lotus leaf alkaloids are linked with vascular remodeling by down-regulating MMP-2 and 9 expression and inducing TIMP-2 expression. MMP is an important protease that degrades the extracellular matrix (ECM), while TIMP is a matrix metalloproteinase inhibitor, both of which contribute to atherosclerosis development[84].
Lotus plumules
-
In contrast to lotus leaves, lotus plumules accumulate mainly bis-BIAs, with liensinine, isoliensinine, and neferine the most abundant components. The bioactivities of these bis-BIAs contribute largely to the pharmacokinetic properties of lotus plumules.
Antioxidant activity
-
Antioxidant compounds, including alkaloids, phenolics, and saponins could be obtained from lotus plumules using 50% ethanol solvent extraction, and DPPH as well as nitric oxide in-vitro assays to test the hydro alcoholic extract of lotus plumules (HANN) revealed excellent free radical scavenging activity[85]. Neferine not only exhibited scavenging activity against ABTS, DPPH, NO, ONOO− and
${\text{O}^-_2} $ Anti-inflammatory activity
-
Neferine is a crucial skin anti-inflammatory and anti-aging agent. For example, a previous study demonstrated that neferine could reduce the phosphorylation level of the MAPK/NF-κB pathway, and inhibit mast cell degranulation and cytokine expression by suppressing elevated intracellular calcium in mast RBL-2H3 cells stimulated with A23187/phorbol ester (PMA)[89]. In lipopolysaccharide-induced human endothelial cells, neferine could significantly inhibit the formation of inflammatory mediators, such as NO, tumor necrosis factor-α, cyclooxygenase-2, inducible nitric oxide synthase, and interleukin 1β due to its ability to regulate mitogen-activated protein kinase and nuclear factor-κβ pathways[90]. In addition, neferine could inhibit lipopolysaccharide and dextran sulfate sodium-induced inflammation both in vitro and in vivo, alter protein expression of iNOS, COX-2, receptor-interacting protein 1 (RIP1), RIP3, mixed lineage kinase domain-like protein (MLKL), and increase the protein expression of caspase-8 in colon tissues, all of which affected the incidence and prevalence of ulcerative colitis (UC)[91]. As the combined effects of oxidative stress and inflammation leads to pathogenesis of numerous diseases, the antioxidant and anti-inflammatory mechanisms of lotus seed alkaloids need to be further explored.
Anti-proliferative activity
-
Liensinine and isoliensinine are two main bis-BIAs found in the lotus seed plumules, and both have been reported to have inhibitory effects against cell proliferation. For example, liensinine could functionally regulate the transforming growth factor β1-induced proliferation, migration, and signaling pathways of human tenon fibroblast cells via the mitogen-activated protein kinase 7 gene to enable rapid patients recovery after glaucoma surgery[92]. Liensinine could also not only significantly inhibit the proliferation of GBC cells in vitro by suppressing their G2/M phase growth in a dose- and time-dependent manner, but also induce apoptosis in gallbladder cancer cells by inhibiting the Zinc finger X-chromosomal protein (ZFX)-induced PI3K/AKT pathway[93]. In addition, previous reports have shown that liensinine could inhibit over-proliferation of gastric cancer cells and osteosarcoma cells[94,95]. Isoliensinine is a liensinine isoform, which has also been shown to exhibit inhibitory effects against angiotensin II (Ang II)-induced proliferation of porcine coronary artery smooth muscle cells (CASMCs)[96], as well as HepG2, Huh-7, and H22 hepatocellular carcinoma (HCC) cells[97]. Moreover, BIA alkaloids mixtures, including neferine, liensinine, and isoliensinine extracted from lotus plumules could delay or inhibit the abnormal proliferation and migration of pulmonary artery smooth muscle cell (PASMCs) by regulating the expression of p-SRC and PIM1[98].
Anti-cardiovascular activity
-
Extensive anti-cardiovascular activities of Neferine, liensinine, and isoliensinine, such as anti-arrhythmic, anti-thrombic, and anti-hypertensive have been documented. Wicha et al.[99] demonstrated that neferine not only had hypotensive effects on NG-nitro-L-arginine methyl ester(L-NAME)-induced rats, but also induced vascular relaxation via the endothelial nitric oxide synthase (ENOS)/nitric oxide (NO)/soluble guanylate cyclase (SGC) pathway. Neferine has shown potential effectiveness in preventing episodes of reentrant ventricular tachycardia and sudden cardiac death after myocardial ischemic injury, and was effective against ischemic arrhythmias. In addition, neferine was shown to significantly inhibit adenosine diphosphate (ADP), collagen, arachidonic acid (AA) and platelet-activating factor (PAF)-induced platelet aggregation in rabbits. A more recent study showed that, unlike neferine, isoliensinine could effectively scavenge early afterdepolarizations (EADs) and delayed afterdepolarizations (DADs) by inhibiting INaL and ICaL in ventricular myocytes, thus, demonstrating its potential anti-arrhythmic effects[100].
Anti-cancer activity
-
To date, numerous studies have demonstrated the roles of alkaloids from lotus plumules, especially neferine in the treatment of different types of cancer. Neferine is a common chemosensitizer of vincristine that enhances the anti-tumor effect of vincristine by inhibiting gastric cancer cell proliferation (SGC7901) leading to their apoptosis. Neferine has also been shown to inhibit the growth of lung adenocarcinoma A549 cells, arrest their cell cycle in G1 phase, and further induce apoptosis through lipid peroxidation, depletion of the mitochondrial membrane potential, MAPKs activation, DNA degredation, and intracellular calcium accumulation[101]. In addition, neferine could cure liver cancer cells (HepG2) and breast cancer cells (MCF-7) by inhibiting their cell proliferation and growth through different mechanisms[102]. Chang et al.[103] reported that in addition to neferine, liensinine could also act as an anti-tumor agent by inhibiting normal mitochondrial energy supply, impairing lysosomal function, and inhibiting the growth of non-small cell lung cancer both in vitro and in vivo. In addition, liensinine could inhibit the growth of gastric cancer cells by increasing ROS levels and inhibiting the PI3K/AKT pathway[94]. Similarly, isoliensinine could cause apoptosis in HepG2, Huh-7, and H22 hepatocellular carcinoma (HCC) cells by decreasing NF-κB activity and constitutively phosphorylating NF-κB p65 subunit at Ser536 in HCC cells[97]. Overall, lotus seeds are now widely used in various cancer treatments due to their broad antagonistic properties.
In addition to the pharmacological effects mentioned above, lotus BIAs possess substantial functions in protection against photoaging related skin problems[104], reduced clinical manifestation of Alzheimer disorders[105], suppression of CCl4-induced liver damage, and bleomycin-induced pulmonary fibrosis[106,107]. Other minor BIAs isolated from lotus, such as members of the 1-benzylisoquinolins type BIAs have also shown notable anti-HIV, anti-inflammatory, anti-arrhythmic, and anti-thrombotic properties[17]. Thus, lotus BIAs have significant potential for the development of novel drugs in treating various life-threatening human diseases, such as microbial infection, inflammation, atherosclerosis, cancer, obesity, neurological disorders, and diabetes.
-
As mentioned above, lotus BIAs are predominantly aporphines and bis-BIAs, which accumulate mainly in lotus leaves and plumules[11,18]. Notable, 1-benzylisoquinolines, which are mostly biosynthetic intermediates and derivatives of the aporphines and bis-BIAs, have also been detected in lotus tissues in trace amounts. The biosynthetic pathways of lotus BIAs have been proposed based on their identified structures[12,22,29,108]. Noteworthily, lotus BIAs primarily occur as R-enantiomers, which is contrary to the predominant S-enantiomers occurrence in the Ranunculales.
Similar to other plant BIAs, the biosynthesis of lotus BIAs starts also from the L-amino acid tyrosine. Tyrosine is first converted into L-dopamine (L-DOPA) and 4-hydroxyphenylacetaldehyde (4-HPAA), respectively by the catalyzation of tyrosine decarboxylase (TYDC)[109]. L-DOPA and 4-HPAA are subsequently condensed by norcoclaurine synthase (NCS) into (R)-norcoclaurine, the first BIA scaffold in plants[110] (Fig. 8). Two additional enzymatic steps, catalyzed by norcoclaurine 6-O-methyltransferase (6OMT) and (R)-coclaurine N-methyltransferase (CNMT) respectively, yield the core intermediate (R)-N-methylcoclaurine of almost all BIAs[15]. In other BIA accumulating species, N-methylcoclaurine normally undergoes two further 3'-hydoxylation and 4'-Omethylation reactions, catalyzed by (S)-N-methylcoclaurine 3'-hydroxylase (CYP80B) and 3'-hydroxy-N-methylcoclaurine 4'-O-methyltransferase (4'OMT), respectively, to yield (S)-reticuline, which is another central precursor and a major branch point of numerous BIA structures, including morphine and berberine. Interestingly, neither reticuline nor corytuberine have been isolated from lotus tissues to date. In addition, all identified aporphine-type BIAs in lotus lack the hydroxyl and methyl modifications at the C-4' and C-3' positions, suggesting that the two enzymatic steps catalyzed by CYP80B and 4’OMT are not necessary for aporphine biosynthesis in lotus[22,110]. Consistently, a recent study showed that pronuciferine is the lotus aporphine biosynthesis precursor, and it is synthesized from the core intermediate (R)-N-methylcoclaurine by the enzymatic actions of NnCYP80G and 4-O-methyltransferase (7OMT)[12] (Fig. 8). According to the lotus BIA structures, production of nuciferine from the pronuciferine precursor may arise from an unknown dehydration reaction[18]. Additional modifications, such as N-demethylation, O-demethylation, and methylenedioxy-bridge formation, catalyzed by the N-demethlase (NDM), O-demethlase (NDM), and CYP719A, respectively[22,111], transforms nuciferine into other diverse aporphines (Fig. 8).
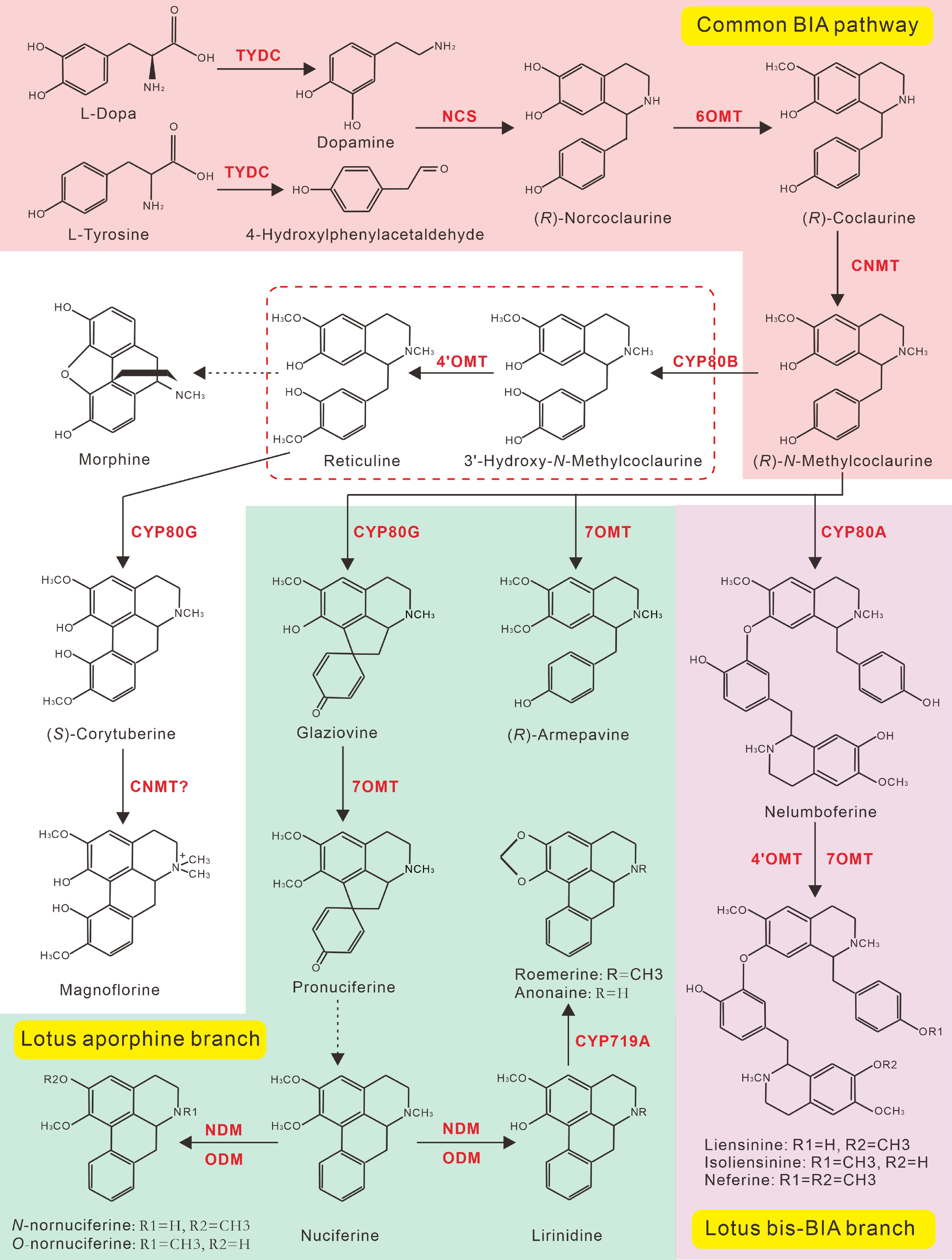
Figure 8.
Proposed BIAs biosynthetic pathway in lotus. Steps marked with red, green, and purple background represent the common reactions for the biosynthesis of (R)-N-Methylcoclaurine, the lotus aporphine branch, and the bis-BIA biosynthesis branch, respectively. All biosynthetic enzymes are shown in red. The (R)-N-Methylcoclaurine is the branch point for aporphine and bis-BIA biosynthesis in lotus. Dotted arrows indicate multiple enzymatic or unknown steps.
The lotus bis-BIAs can also directly be synthesized from the (R)-N-methylcoclaurine precursor (Fig. 8). The 7-O3' coupling reaction, catalyzed by CYP80A enzyme, transfers two molecules of (R)-N-methylcoclaurine into Nelumboferine, which is the first bis-BIAs in the bis-BIA biosynthesis pathway[12,108]. Two further enzymatic steps, catalyzed by the 4'OMT and 7OMT enzymes respectively, yield the three major bis-BIAs, including liensinine, isoliensinine, and neferine. Other enzymatic modifications, including N-demethylation, double 4'-Omethylation, 8-O3' and 3'-3' intermolecular couplings, result in the production of other diverse bis-BIA structures (Fig. 4).
BIA biosynthetic and regulatory genes
-
The currently advanced next generation sequencing techniques have facilitated the availability of at least seven high quality lotus genome versions as references for isolation of BIAs biosynthetic genes[2,112−115]. The previously functionally characterized BIAs pathway genes in the Ranunculales[116] have been retrieved and used to query and predict most of their corresponding homologs from the lotus genomic and transcriptomic data based on sequence similarities. As a result, at least 5, 4, and 1 TYDC, NCS, and CNMT candidate gene copies, respectively have been predicted in the early lotus BIA biosynthesis pathway[22]. Similarly, seven OMT genes have been identified, with four and three of which forming clusters with the 6OMT/4’OMT and 7OMT clades, respectively. In addition, downstream lotus BIA biosynthetic gene sequences, including CYP80A, CYP80G, CYP719A, NDM, and ODM have been isolated[22,29].
The lack of stable lotus transformation system has hindered functional characterization of most its BIA pathway enzymes. Consequently, functional studies have mostly involved correlation analysis between gene expression levels and spatial temporal BIA accumulation patterns in different lotus tissues or developmental stages[22,29,117,118]. In vitro enzymatic assays were recently used to functionally characterize four lotus OMTs, with NnOMT1 exhibiting 6OMT activity and accepting both S- and R-substrates, while NnOMT5 mainly showing 7-O-methyltransferase activity with strong S-enantiomer stereospecific preference[119]. In contrast, Nn6OMT and Nn7OMT have both been shown to display 6-O and 7-O methyltransferase activities[108,120]. Notably, none of these NnOMTs accepted aporphine substrates, indicating that O-methylation reactions proceed primarily from 1-benzylisoquinoline intermediates.
The core intermediate (R)-N-Methylcoclaurine is the lotus BIA biosynthesis branch point, from which intermolecular C-O phenol coupling reaction catalyzed by NnCYP80A produces diverse bis-BIAs, while the intramolecular C-C phenol coupling reaction catalyzed by NnCYP80G yields aporphines (Fig. 8). Two recent studies[12,108] have functionally characterized the enzymes responsible for the lotus CYP80A and CYP80G members. The expression of NnCYP80G in yeast could efficiently convert the (R)-N-Methylcoclaurine substrate into a pronuciferine glaziovine in lotus, while in the presence of NnOMT5, glaziovine could efficiently be transferred into pronuciferine[12]. Similarly, the expression of NnCYP80A augmented with CPR and CYB5 in yeast could catalyze the production of nelumboferine from (R)-N-Methylcoclaurine substrate[108]. In addition, NnCYP719A, which is the third P450 in lotus could efficiently convert caaverine and lirinidine substrates into anonaine and roemerine, respectively.
Although the sequences of other lotus BIA biosynthetic genes, such as NnTYDCs, NnNCSs, NnCNMT, NnODMs, and NnNDMs have already been isolated based on similarities with homologs from other BIA producing species, they are still yet to be functionally validated. Since none of the isolated lotus NCSs exhibit NCS activity, a non-enzymatic Pictet-Spengler condensation of dopamine and 4-HPAA might yield norcoclaurine in lotus[108]. However, since both R and S enantiomers of norcoclaurine have been detected in lotus, it is unlikely to entirely rule out the possibility of additional enzyme requirement for the Pictet-Spengler condensation and the formation of (S)-norcoclaurine in lotus, as well as a second enzyme for stereochemical inversion of (S)-norcoclaurine to (R)-norcoclaurine.
Unlike their characterization, little knowledge is still available on the regulation of lotus BIA genes. A previous comparative transcriptomic analysis of high and low BIA accumulating lotus cultivars showed that the expression levels of most BIA biosynthetic genes were significantly higher in the former than the latter cultivar[22], suggesting transcriptional regulation of lotus BIA biosynthesis. Correlation analysis between gene expression profiles and BIA contents revealed 16 candidate TFs, such as WRKYs, MYBs, ERFs, and bHLHs that potentially regulate lotus BIAs biosynthesis. As an important group of secondary metabolites, lotus BIA biosynthesis is regulated by mechanical wounding and jasmonate (JA) treatment[11,121]. To date, the only two functionally characterized BIA regulator TFs are NnWRKY70a and NnWRKY70b, which both belong to the group III WRKY TFs and are JA responsive. Overexpression of NnWRKY70a and NnWRKY70b in the lotus petals significantly enhanced BIA biosynthesis. Functional validation assays showed that NnWRKY70a and NnWRKY70b could directly bind and activate one or more BIA biosynthetic gene promoters, while NnWRKY70b could physically interact with NnJAZ1 and two other WRKY TFs (NnWRKY53b and NnWRKY70a), thus suggesting its potential interaction with other WRKY TFs to regulate lotus BIA biosynthesis via the JA signaling pathway[122].
-
Lotus contains a vast number of BIAs, most of which are R-conformers. Aporphines and bisbenzylisoquinoline alkaloids are the two major lotus BIAs types, and are predominantly accumulated in the leaves and plumules, respectively. The lotus BIAs are generally weak alkalescent compounds with poor solubility or insoluble properties in water, but soluble in organic solvents. The crude lotus BIAs are commonly extracted with ethanol or methanol solvents, assisted with reflux, sonication, and microwave techniques. Critical fluid extraction and high-speed counter-current chromatography are relatively greener, energy-efficient, time- and solvent-saving, as well as high yielding BIA extraction techniques. The UPLC-Tof-MS is currently the most popular method for BIA isolation, characterization, and quantification. Lotus BIAs harbors significant medicinal properties with potential application in the treatment of various life-threatening diseases, such as obesity, inflammation, cancer, HIV, and aniocardiopathy.
The availability of high-quality lotus genome sequences have facilitated the isolation of most genes or enzymes involved in lotus BIA biosynthesis and regulation. However, functional characterization specifically through protein expression in engineered yeast strain and downstream enzyme activity assays have only been successfully conducted for NnOMTs, NnCYP80A, and NnCYP80G. Future intensive work on functional characterization of putative genes and enzymes is needed to fully elucidate the lotus BIA biosynthetic pathways. In addition, although the tissue specific accumulation of lotus BIAs has been clarified, the exact cell types involved in lotus biosynthesis and storage remain largely unknown. Combining cutting edge spatial metabolomics with single-cell RNA sequencing would not only give valuable clues on the localization of BIAs and their biosynthetic enzymes, but also facilitate the isolation of BIA biosynthesis regulators as well as possible intermediate transporters. The unique stereochemistry of BIAs in this basal eudicot species is equally worthy of research both at the molecular and biochemical levels.
The ultimate aim studying lotus BIAs is of course to develop clinical drugs based on these molecules. There is no doubt that BIAs in lotus bear significant pharmacological activities. It should be noticed, however, unmodified lotus BIAs possess suboptimal efficiency in absorption, metabolism, excretion and toxicity properties. Research should be directed on total chemical synthesis, structural modifications, creation of BIA hybrids or new BIA analogs, with an aim to develop lotus BIA-based novel drugs for treatment of degenerative diseases, cancer, and the continuous threat of novel infections like COVID-19.
-
The authors confirm contribution to the paper as follows: study design: Li J, Deng X; wrote the manuscript: Wei X, Deng X; revised the manuscript: Zhang M, Ogutu C, Yang M. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This project was supported by funds received from the National Natural Science Foundation of China (Grant no. 32070336 and 32370428) and the Natural Science Foundation of Shandong Province (Grant no. ZR2021MC163).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wei X, Zhang M, Yang M, Ogutu C, Li J, et al. 2024. Lotus (Nelumbo nucifera) benzylisoquinoline alkaloids: advances in chemical profiling, extraction methods, pharmacological activities, and biosynthetic elucidation. Vegetable Research 4: e005 doi: 10.48130/vegres-0024-0004
Lotus (Nelumbo nucifera) benzylisoquinoline alkaloids: advances in chemical profiling, extraction methods, pharmacological activities, and biosynthetic elucidation
- Received: 23 November 2023
- Revised: 31 December 0023
- Accepted: 09 January 2024
- Published online: 05 February 2024
Abstract: As a member of the only two species in the Nelumbonaceae family, lotus (Nelumbo nucifera) accumulates abundant benzylisoquinoline alkaloids (BIAs) in almost all of its tissues. Evidence from both traditional and modern medicine suggest great potential of the lotus BIAs in developing novel drugs for the prevention and treatment of diverse life-threatening diseases. This review provides a comprehensive summary on the up-to-date advances in the chemical profiling, extraction methods, pharmacological activities, and biosynthesis of lotus BIAs. Currently, a total of 59 BIAs structurally belonging to the 1-benzylisoquinoline, aporphine, and bis-BIA categories have been identified in various lotus tissues, with their predominant accumulation in the leaf and plumule. In contrast to the common S-conformers in Ranunculales, most lotus BIAs are R-conformers. Solvent extraction is still the most widely used BIA extraction method in lotus at the industrial level, however, numerous greener and highly advanced extraction techniques have also been developed. High-performance liquid chromatography (HPLC) followed by electrospray ionization (ESI) and tandem mass spectrometry (MS/MS) techniques are currently the most commonly used methods for separation, quantification, and characterization of lotus BIAs. Moreover, the pharmacological activities of major BIAs isolated from lotus leaves and plumules are discussed, and their biosynthetic pathways proposed based on recent functional characterization studies of lotus BIA biosynthetic genes. Finally, a summary discussion is provided on the future research trajectories in elucidating lotus BIA biosynthesis, storage, and transportation, as well as their potential application in clinical drug development.
-
Key words:
- Lotus /
- Benzylisoquinoline alkaloid /
- Extraction /
- Pharmacological activity /
- Biosynthesis















