-
In nature, plants are faced with many challenges posed by adverse environments, such as drought, extreme temperature and salinity. To cope with these disadvantages, plants adapt to abiotic stress by accumulating compatible solutes, such as soluble sugars and some free amino acids, which is often considered to be the basic strategy for their protection and survival under stress[1]. Among these compatible substances, most sugars not only play roles in osmotic regulation but also play a signaling role, such as glucose[2−4], sucrose[4−6] and trehalose-6-phosphate[7−9]. Sugars are the basis of energy storage and material transport in plants. Different types of sugars are formed by metabolism after photosynthesis and play key roles in many metabolic processes throughout the whole life cycle of plants. In the process of plant growth and development and environmental response, sugars mostly act as signal molecules to regulate a variety of physiological and biochemical processes[10]. Trehalose is a kind of nonreducing disaccharide with special physical and chemical properties that has strong hydration ability under drying and freezing conditions and can replace the bound water on the surface of biomolecules to improve the stability of proteins and biofilms[11,12]. Trehalose is widely found in a variety of organisms, including bacteria, yeasts, fungi and algae, as well as some insects, invertebrates and plants[13]. Trehalose is easily induced by stress, stimulates plant resistance mechanisms[14], and plays an important role in dealing with a variety of abiotic stresses, such as drought stress[15,16], salt stress[15,17] and extreme temperature stress[18,19]. This review discusses the advances of trehalose in regulating plant growth and development and response to abiotic stress.
-
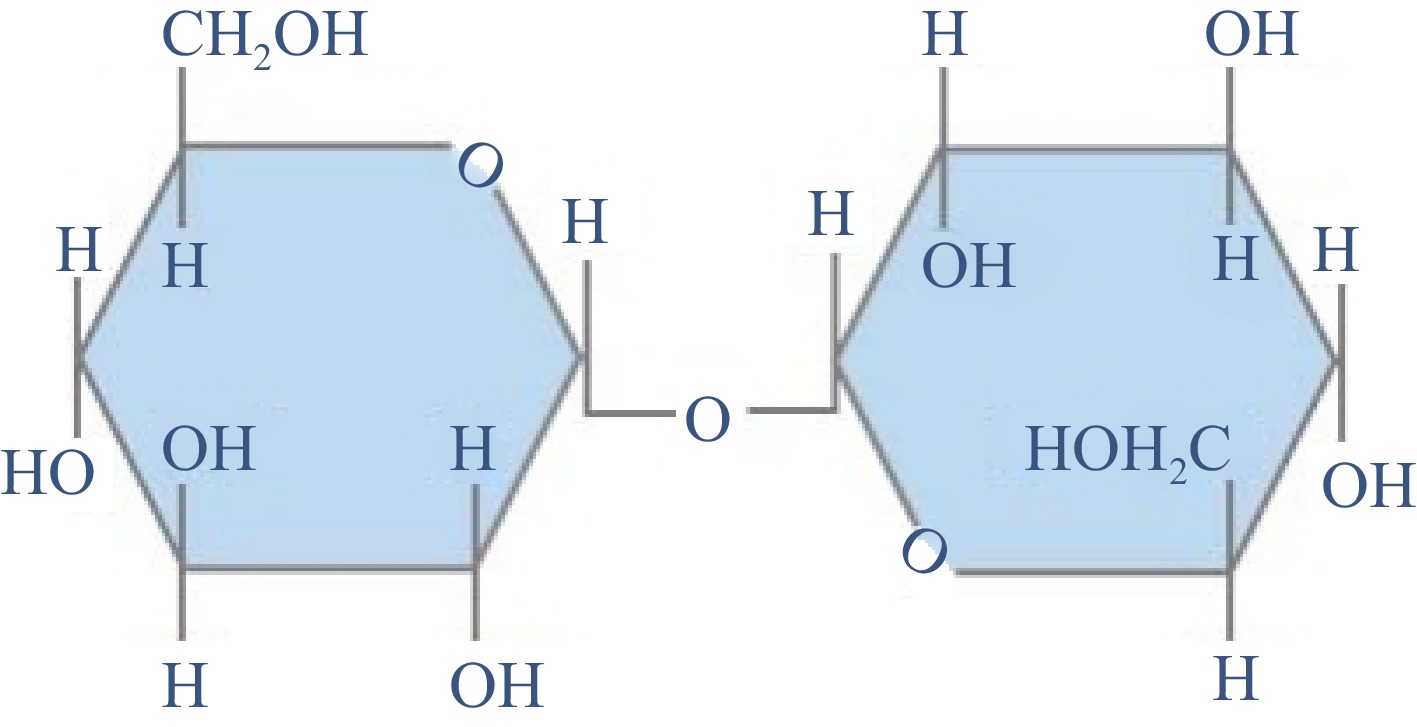
Trehalose is composed of two glucose subunits linked by a 1,1-glycosidic bond[20]. Since both reducing subunits are involved in the formation of glycosidic bonds (Fig. 1), trehalose has certain molecular stability and unique properties compared with other disaccharides[21]. Trehalose can resist acid hydrolysis and maintain its stability under acidic pH and high temperature conditions[22]. Trehalose cannot form hydrogen bonds inside it, which creates its high degree of hydrophilicity[23]. When the organism is dehydrated or frozen, trehalose can replace water molecules to form hydrogen bonds with surrounding macromolecules and membranes, thus playing a protective role. In the case of extreme dehydration, trehalose can be crystallized into glass, avoiding the denaturation of biomolecules and restoring its functional activity during rehydration[24].
-
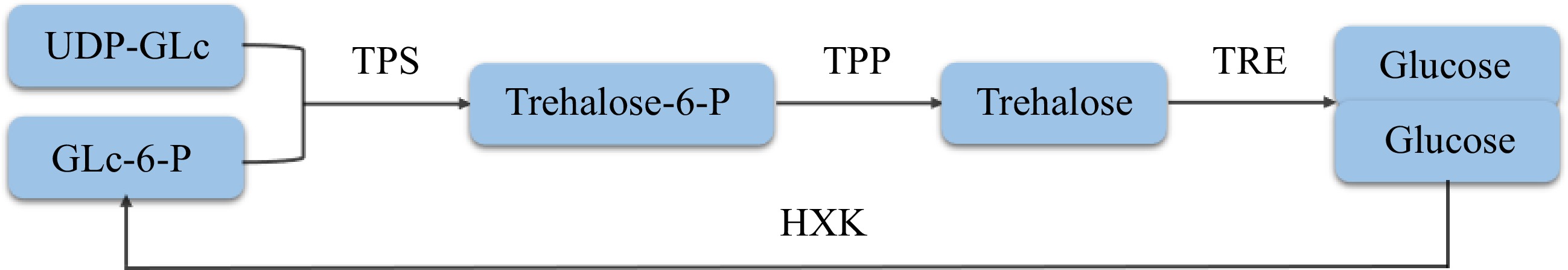
Trehalose in plants was first found in Selaginella tamariscina and then identified in algae, liverworts and other lower plants, while trehalose in angiosperms was found later[25]. The biosynthesis of trehalose in plants mainly consists of the monosaccharides uridine-glucose diphosphate (UDP-GLc) and glucose-6-phosphate (GLc-6-P) as precursors (Fig. 2), catalyzed by trehalose-6-phosphate synthase (TPS) to form trehalose-6-phosphate (Trehalose-6-P, T6P). Then, trehalose is formed under the catalysis of trehalose-6-phosphate phosphatase (TPP)[22,25]. Approximately 20 years ago, two enzymes of the trehalose biosynthesis pathway, trehalose-6-phosphate synthase (TPS) and trehalose-6-phosphate phosphatase (TPP), were identified in Arabidopsis thaliana[26,27]. In Arabidopsis thaliana, there are 11 genes encoding TPS or TPS-like protein (AtTPS1-AtTPS11) and 10 genes encoding TPP protein (AtTPPA-AtTPPJ)[28,29]. A total of 14 TPS and 13 TPP genes were identified in rice, of which OsTPP1 is regulated by abiotic stress[30]. In cassava, there are 12 TPS and 10 TPP genes that encode proteins that play a key role in trehalose synthesis[31]. Compared with the synthetic pathway, the decomposition of trehalose is simpler. Trehalose is directly hydrolyzed into two molecules of glucose under the action of trehalase (TRE), while glucose can be formed into GLc-6-P under the action of hexokinase (HXK)[10]. Trehalose in plants has only one biosynthesis and decomposition pathway, which plays an extensive role in plant growth and development and stress response[29].
-
Many studies have shown that trehalose and the key intermediate substances in its metabolism play crucial roles in regulating plant growth and development (Fig. 3). In plants, trehalose participates in various life and cell metabolism processes. For example, trehalose induced the expression of NADPH-dependent thioredoxin reductase C (NTRC) during the day and inhibited it at night, which affected the activity of ADP-glucose pyrophosphorylase (AGPase) and regulated the daily starch accumulation and metabolism of Arabidopsis thaliana[32]. Trehalose and its biosynthetic gene can also participate in abscisic acid (ABA)-mediated root elongation, stomatal movement and seed germination. In rice, appropriate concentrations of trehalose and sucrose inhibited the synthesis of soluble sugars and seed germination by promoting ABA synthesis[33]. In another study with Arabidopsis thaliana, in the presence of ABA, ABF2 enhanced the expression of AtTPPE by directly binding to its promoter, increased trehalose content and triggered the accumulation of reactive oxygen species (ROS) in roots and stomata, thus enhancing the effect of ABA on inhibiting root growth and promoting stomatal closure[34]. In addition, in legumes, PvTPS9 can regulate the metabolism of trehalose in symbiotic root nodules and the whole plant, indicating the role of trehalose in affecting the growth of root nodules and plants[35].

Figure 3.
Function of trehalose in plant growth and development.




In recent years, key genes of trehalose biosynthesis have been reported to play key roles in plant flower development. Kataya et al. found that AtTPPI homozygous T-DNA insertion lines showed smaller leaves, shorter roots, delayed flowering and salt sensitivity[36]. Meanwhile, deletion of AtTPS1 led to late flowering, indicating that AtTPS1 is necessary to regulate timely flowering[37]. Zhao et al. transferred JcTPPJ into Arabidopsis thaliana and found that 35S:JcTPPJ transgenic plants had higher sucrose content in inflorescences, showing late flowering and style atypia, indicating that JcTPPJ may regulate floral organ development by regulating sucrose status in plants[38]. These three studies suggest the potential role of trehalose synthesis genes in regulating plant development. In the process of plant flowering, trehalose synthesis genes may also regulate the development of floral organs by affecting the content of other soluble sugars (such as sucrose). Additionally, in lotus, low light stress blocked photosynthesis, decreased the content of soluble sugar in the rhizome, decreased the expression of NnTPS1, activated the activity of NnSnRK1, induced programmed cell death and finally led to flower bud abortion[39]. In Solanaceous crops, trehalose application affected style length via roots interaction with rhizosphere and promoted pistil to stamen ratio[40]. There is little evidence that trehalose is directly involved in the regulation of plant flowering, but some reports have shown that trehalose synthesis genes are involved in flower organ development, which still needs further study.
Trehalose was also reported to play important roles in improving crop yield and quality. Islam & Mohammad reported that foliar spraying of 10 mM Tre significantly enhanced plant photosynthesis, mineral acquisition and root cell activity, ultimately improving the biomass, yield and quality of Indian mustard[41]. In apple, potassium treatment significantly reduced the activity of trehalase (TRE) and increased trehalose content in fruit, which improved fruit firmness and quality[42].
As the synthetic precursor of trehalose, the function of T6P in plant growth and development has been widely studied. T6P, as a signaling molecule, is related to plant growth and development and sucrose metabolism[43]. In Arabidopsis thaliana, the change in sucrose directly affected the levels of T6P in plants, regulated the levels of PIF4 protein by regulating GRIK1-mediated KIN10 activation and finally affected auxin signaling to regulate hypocotyl elongation[44]. Meanwhile, during the growth of Arabidopsis thaliana, sucrose activated the T6P pathway, which suppressed the level of mature miR156, leading to the upregulation of the SPL gene and regulating the plant development[9]. In recent years, it has been reported that T6P plays a key role in the regulation of embryonic maturation[7], axillary bud growth[45,46] and leaf starch degradation[47]. Whether the actions of T6P are related to trehalose, and whether endogenous trehalose may act as a signal molecule related to T6P signal in regulating plant growth, development and stress response. These need to be further studied.
-
Trehalose protects biological cells and bioactive substances from destruction under adverse environmental conditions such as dehydration, drought, high temperature, freezing, high osmotic pressure and toxic reagents and plays an important role in plant resistance to abiotic stress[48]. Overexpressing OsTPS1 showed elevated trehalose and proline concentrations and upregulation of stress response-related genes, such as WSI18, RAB16C, HSP70 and ELIP, resulting in enhanced resistance of rice seedlings to abiotic stresses[15]. In sweet potato, IbTPS1 is induced by drought, salt, heat and other environmental stresses. The tolerance of yeast to dehydration, salinity and oxidation was improved by expressing IbTPS1 in yeast, indicating that IbTPS1 is a candidate gene for improving the plant stress resistance[49], which may be related to the increase of endogenous trehalose levels. Similarly, application of exogenous trehalose can also improve plant stress resistance[50−53]. However, whether exogenous trehalose improves plant resistance by inducing endogenous trehalose or stimulating other signaling molecules in plants, thereby mobilizing the resistance system and improving resistance, still need further exploration.
Drought stress
-
Drought stress can destroy cellular ROS homeostasis, increase the accumulation of oxygen free radicals, inhibit plant growth, and damage the plant osmotic regulation system, biofilm system, respiration and photosynthesis metabolism[54]. Han et al. found that cassava, a drought-tolerant crop, had a high expression of MeTPS1 in tissues before and after stress, and the content of trehalose increased, which improved the tolerance to drought stress[31], suggesting that trehalose plays a key role in plant resistance to drought stress.
Trehalose application was widely reported to improve the plants drought stress tolerance via reducing oxidative damage and restoring photosynthetic capacity (Table 1). For example, spraying exogenous trehalose increased the contents of antioxidants such as ascorbic acid (AsA) and reduced glutathione (GSH) in maize roots and leaves, increased the activities of antioxidant enzymes such as superoxide dismutase (SOD) and ascorbate peroxidase (APX), decreased the production rate of superoxide anion (
${\text{O}^-_2} $ Table 1. Roles of trehalose in regulating plant stress resistance.
Stress types Regulatory mechanisms Species Treatments References Drought stress Enhance antioxidant capacity Zea mays 0 and 30 mM Tre spraying plants [16] Helianthus annuus 0, 10, 20 and 30 mM Tre spraying plants [101] Triticum aestivum Medium + 50 mM Tre [50] Raphanus sativus 0, 25 and 50 mM Tre soaking seeds and spraying plants [56] Ocimum basilicum 30 mM Tre and 1 mM SA alone or in combination with irrigating [102] Chenopodium quinoa 0, 5, 10, 15, 20 mmol·L−1 Tre spraying plants [54] Zea mays 10 mmol·L−1 Tre spraying plants [55] Protect photosynthetic mechanism Zea mays 0 and 30 mM Tre spraying plants [57] Zea mays 1% Tre and different forms of zinc spraying plants [58] Cause anatomical changes of leaves Raphanus sativus 25 mM Tre soaking seeds and spraying plants [59] Regulate endogenous ABA level and signal transduction Solanum lycopersicum 1.5, 15 and 45 mM Tre spraying plants [63] Salt stress Enhance antioxidant capacity Oryza sativa 25 mM Tre soaking seeds [67] Oryza sativa Nutrient solution + 10 mM Tre hydroponics [68] Zea mays Nutrient solution + 10 mM Tre hydroponics [52] Arabidopsis thaliana Nutrient solution + 0.5, 1 and 5 mM Tre hydroponics [51] Cucumis melo 2%, 3%, 4%, 5% Tre spraying plants [69] Citrullus lanatus Nutrient solution + 0, 5, 10, 20 and 30 mM Tre hydroponics [70] Protect photosynthetic mechanism Fragaria × ananassa Nutrient solution + 10, 30 mM Tre irrigating plants [72] Oryza sativa 0, 10, 20 mM Tre spraying plants [71] Co-regulation of stress response with other substances Oryza sativa Nutrient solution + 10 mmol·L−1 Tre hydroponics [17] Heat stress Protect PSII and regulate plant photosynthesis Triticum aestivum Nutrient solution + 1.5 mM Tre hydroponics [18,78,82] Regulate plant redox dynamic balance and photosynthesis Paeonia lactiflora 30 mmol·L−1 Tre spraying plants [81] Cold stress Enhance antioxidant capacity Capsicum annuum 5%, 10%, 15% Tre soaking fruit [88] Solanum lycopersicum 10 mM Tre spraying plants [86] Cucumis melo 10 mM Tre spraying plants [53,87] Zea mays 3, 6, 9, 12, 15, 18 mmol·L−1 Tre irrigating plants [85] Osmotic adjustment Oryza sativa 0, 0.5, 1 and 2 mM Tre/Spermidine soaking seeds [19] Oryza sativa Nutrient solution + 5 mM Tre irrigating plants [90] Triticum aestivum 0, 5, 10, 20, 40, 50 mmol·L−1 Tre soaking seeds and hydroponics [89] Regulate nitrogen assimilation and polyamine synthesis Triticum aestivum 1, 10, 50 mmol·L−1 Tre spraying plants [91] Heavy metal stress Enhance antioxidant capacity Oryza sativa Nutrient solution + 10 mM Tre hydroponics [94] Oryza sativa 0, 10, 20, 40, 60 mmol·L−1 Tre hydroponics [95] Triticum aestivum 0, 25, 50 mM Tre spraying plants [96] Nitrogen deficiency Activate nitrate and ammonia assimilation Nicotiana tabacum 8 mM Tre spraying plants [100] Acid rain stress Enhance antioxidant capacity, maintain the stability of plasma membrane Hordeum vulgare 0, 5, 10, 15 mM Tre soaking seeds [98] Alkali stress Enhance antioxidant capacity, osmotic adjustment Oryza sativa 0, 5, 10, 15, 20 mmol·L−1 Tre spraying plants [99] In addition, exogenous trehalose could increase plant drought stress by inducing leaf anatomical changes, such as increasing leaf epidermis thickness, vascular bundle area, midvein thickness and number of vascular bundles[59]. Also, trehalose can participate in seed germination under stress. Under drought conditions, 0.5 mmol·L−1 trehalose increased starch degradation by upregulating the expression of the calcium-dependent CBL1-OsSnRK3.1/3.23 gene and activating the OsK1a-OsMYBS1/2-OsAmy3/8 pathway, and induced trehalose synthesis, thereby enhancing sugar metabolism, maintaining seed germination, significantly increasing drought tolerance during germination[60].
ABA is an important plant hormone that responds to various abiotic stresses, and its level increases rapidly under drought conditions[61]. In recent years, the relationship between trehalose and ABA in the process of drought has been gradually clarified. In Arabidopsis thaliana, drought stress caused ABA signal response factors ABF1 and ABF4 to activate AtTPPI expression, changed the trehalose metabolism pathway, led to stomatal closure, improved water use efficiency, and made plants adapt to stress[62]. Trehalose also can upregulate the expression of ABA signal-related genes SlPYL1/3/4/5/6/7/9, SlSnRK2.3/4, SlAREB1/2 and SlDREB1, activate the ABA signal pathway and regulate stomatal closure and cell water loss under drought stress[63]. Besides that, overexpression of trehalose synthetic gene OsTPP3 also increased the ABA content and drought resistance of plants by increasing the expression of genes related to ABA biosynthesis[64]. These suggest that trehalose could increase plant drought resistance by inducing ABA production.
Salt stress
-
Salt stress mainly includes osmotic stress and ion toxicity, redox disorder, which leads to nutrition deficits and disrupts the energy balance of plants[65] and also affects water absorption and utilization, the anatomical structure of leaves and photosynthesis, eventually limited plant growth[65,66]. Therefore, how to enhance plants salt stress tolerance is particularly important for plant normal growth and development.
Studies showed that feed with trehalose could trigger the expression of salt tolerance-related transcription factors genes, such as bHLH, NAC, WRKY, etc, and increase the level of endogenous trehalose in rice, thus enhancing the activity of antioxidant enzymes (Table 1)[67−69], increasing K+ level and the ratio of K+/Na+ in leaves and stems, maintain ion dynamic balance and redox state[51], regulating antioxidants and the glyoxylase system[52], which synergistically improve the salt tolerance of plants[70]. Foliar spraying trehalose can alleviate the adverse effects of salt stress on rice by improving growth traits, chlorophyll content, gas exchange characteristics, chlorophyll fluorescence and other parameters[71]. In strawberries, external application of 30 mM trehalose significantly alleviated the inhibition of salt stress on strawberry growth. It could alleviate the inhibition of PSII function by increasing carotenoid content, thus reducing the injury caused by salt stress[72]. Additionally, overexpressing of trehalose synthesis genes AtTPPD could improve plant salt stress resistance via regulating sugar metabolism[73]. These suggest that trehalose could regulate sugar metabolism, which need further investigation. Over all, trehalose may improve plant salt stress tolerance and promote plant growth and development by regulating the balance of antioxidant system, ion exchange and other metabolism (such as sugar metabolism).
Additionally, trehalose can coordinate with other substances to regulate plant salt tolerance, such as osmoregulatory substances and growth-promoting bacteria. Nounjan & Theerakulpisut showed that external application of proline and trehalose could increase the activity of antioxidant enzymes in rice under salt stress or in the recovery stage, among which exogenous trehalose had the most obvious promoting effect on the activity of antioxidant enzymes in rice[17]. In another study with tomato, 1-aminocyclopropane-1-carboxylate (ACC) deaminase and trehalose had synergistic protective effects on tomato plants under salt stress during interaction with the plant growth-promoting strain Pseudomonas sp. UW4[74]. Strigolactones can upregulate the expression of the Tre biosynthetic genes TPS1, TPS2, TPP1 and TPP2, enhance the activity of TPS and TPP, accelerate the conversion of glutamic acid to Tre, and inhibit the degradation of Tre by weakening the activity of trehalase, thereby improving tomato plant salt stress resistance[75]. Under salt stress, the upregulation of OsNCED3 leads to ABA accumulation, thereby activating the expression of OsTPP3, increasing the Tre content of rice seedlings, and enhancing their salt tolerance[76].
Heat stress
-
In plants, high temperature often leads to the outbreak of ROS, which destroys proteins, DNA and lipids and leads to adverse changes in plant growth, development and physiological status[77,78]. Osmotic protective agents, such as proline[79,80] and trehalose (Table 1)[81,82], play a positive role in alleviating heat stress. Trehalose can significantly promote the PSII complex to maintain a stable oxygen evolution rate and cell redox homeostasis, which eventually alleviated the damage caused by heat stress[78,81,83]. Meanwhile, trehalose effectively regulated the level of photosynthesis-related proteins, alleviated the chloroplasts structure damage and increased the proton gradient (ΔpH) and ATP synthase activity by promoting cyclic electron flow (CEF) to alleviate PSII photoinhibition caused by heat stress in wheat[78,82]. Thus, trehalose can alleviate heat caused damage mainly by maintaining normal photosynthesis in plants. However, most studies are limited to physiological mechanisms, and the related molecular regulatory mechanisms need to be further studied.
Cold stress
-
Cold stress is one of the main abiotic stresses in a plants life, including chilling/cold injury (> 0 °C) and freezing injury (< 0 °C). Cold stress is the main environmental factor affecting plant growth and development, limiting geographical distribution and reducing crop yield. Trehalose can be widely detected in cold-tolerant crops, indicating that trehalose may be involved in the regulation of plant tolerance to cold stress (Table 1)[84].
Cold stress can cause an imbalance of the antioxidant system and serious damage to the cell membrane structure of plants. Liu et al. reported that irrigation trehalose could increase the activities of antioxidant enzymes in maize roots, enhance the ability of cells to scavenge ROS, and maintain the stability of the cell membrane structure and function of maize seedling roots, thus alleviating the damage caused by cold stress[85]. And exogenous trehalose may play roles in H2O2→NO→antioxidation→cold tolerance pathway[86,87] and maintain the integrity of cell structure[53]. In addition, trehalose could alleviate the chilling injury of pepper fruit at low temperature and maintain quality by reducing the damage to the cell membrane structure caused by ROS[88].
Cold stress also leads to osmotic stress, and trehalose can improve plants resistance to stress by regulating some osmotic regulatory substances. For example, trehalose could increase the content of osmotic regulating substances (such as proline and soluble sugar) which may result in increasing plant cold tolerance[19,89]. In addition, application of trehalose regulated the water absorption of arbuscular mycorrhizal fungi (AMF) and mycorrhizal symbiotic rice under cold stress by inducing the expression of GintAQPF and OsPIPs, thus creating more suitable growth conditions for rice[90]. These results indicate that trehalose plays a regulatory role in osmotic stress induced by low temperatures.
Studies have also shown that trehalose can regulate plant resistance to cold stress in other ways. For example, trehalose could promote floret fertility to alleviate the decrease in the number of grains per spike caused by cold stress at the booting stage, mainly because trehalose regulates nitrogen assimilation, the GSH-AsA cycle and spermidine synthesis[91]. Liu et al. transferred the TaTPS11 gene of cold-tolerant wheat varieties into Arabidopsis thaliana and found that the overexpression line had higher carbohydrates, such as sucrose, fructose and starch, which improved the cold tolerance of Arabidopsis thaliana[92]. In rice, 'SAPK10-ABF1-TPS2' participates in plant cold tolerance by regulating the homeostasis of trehalose[93].
Other stresses
-
In addition to drought, salt and extreme temperature, plants often suffer from other stresses during their growth and development, such as heavy metals, acid rain, and nutrient deficiency. Studies have shown that trehalose can play a role when plants are poisoned by heavy metals (Table 1). When exposed to Cu stress, trehalose application can increase the level of endogenous trehalose and regulate antioxidant and glyoxylase systems, thus improving rice tolerance to Cu stress[94]. Additionally, exogenous trehalose could effectively alleviate the decrease in chlorophyll a and b contents in rice caused by Cd stress, reduce the excessive accumulation of ROS, and form Cd-Tre chelates to reduce Cd toxicity[95], improve the growth, physiology, and defense systems, thereby increase the crop quality and yield[96]. Elevating endogenous trehalose by transferring AtTPS1 gene into tobacco showed the same roles in improving plant Cd and Cu stress tolerance[97]. In barley, exogenous trehalose increased the activity of antioxidant enzymes and plasma membrane H+-ATPase and the content of chlorophyll to maintain the stable pH of the plasma membrane in roots and leaves, thus resisting acid rain stress[98]. In rice, spraying an appropriate concentration of trehalose could effectively improve alkali tolerance and alleviate the damage of alkali stress to rice seedlings, which is mainly achieved by improving the ability of ROS scavenging and osmotic regulation[99]. Foliar spraying trehalose could partly alleviate the symptoms of nitrogen deficiency in tobacco by upregulating nitrate and ammonia assimilation, increasing the activities of enzymes such as nitrate reductase (NR) and glycolate oxidase (GO), and changing the contents of
${\text{NH}^+_4} $ ${\text{NO}^-_3} $ In general, when plants encounter abiotic stress, trehalose application can enhance the antioxidative ability to maintain ROS homeostasis, protect photosynthetic institutions, and regulate osmotic regulators in plants, such as endogenous trehalose[67] and proline[19]. Moreover, the overexpression of two key enzyme (TPP and TPS)-related genes in the trehalose biosynthesis pathway can also alleviate the damage caused by abiotic stress by increasing the level of endogenous trehalose and regulating the changes in downstream stress-related genes[15], plant hormones[64] and sugar[73].
-
Sugar plays a key role in many metabolic processes throughout plant life. Trehalose, as a multifunctional biomolecule, plays an important role in seed germination[33], plant development and reproduction[37] and yield and quality formation[42]. Trehalose shows great potential in regulating plant growth and development and improving plant stress tolerance (Fig. 4). Although the preliminary physiological effects of trehalose on key plant physiological processes and stress have been elucidated, most of them are at the physiological level, and the related molecular mechanisms need to be further studied. For example, how trehalose is involved in the regulation of redox balance, photosynthesis and glucose metabolism and how to regulate downstream stress-related genes to improve plant resistance remain to be studied. Furthermore, the current reports on the interaction between trehalose and plant hormones are mainly focused on ABA. Whether there is a certain relationship between trehalose and other plant hormones (such as ethylene, auxin and gibberellin) in the regulation of plant growth, development and stress resistance. And whether endogenous trehalose also acts as a signal molecule and is related to the mechanism of action of exogenous trehalose needs further exploration.
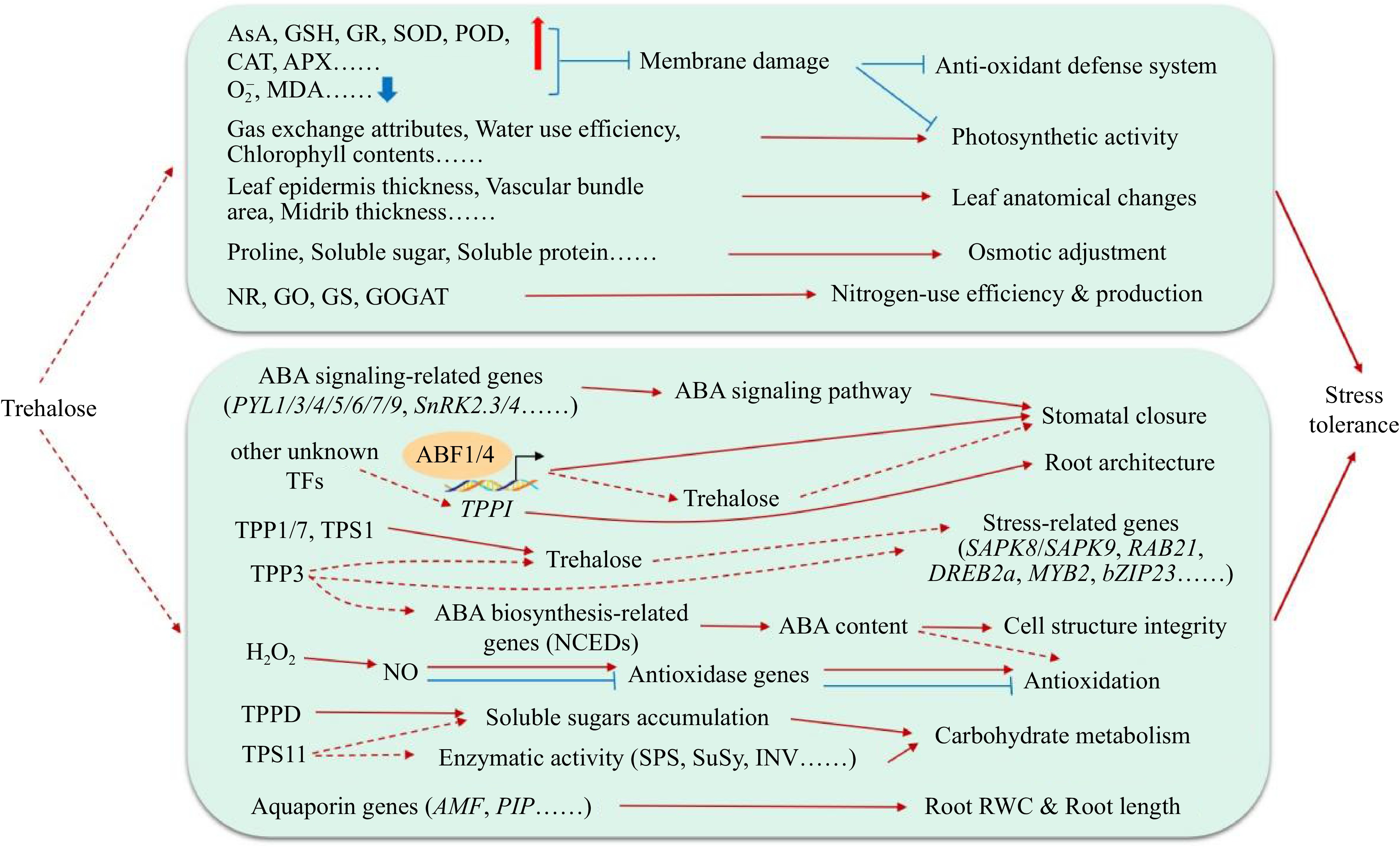
Figure 4.
Function of trehalose in the abiotic stress response of plants.




-
The authors confirm contribution to the paper as follows: literature collection: Zhang Y, Xu D; tables and models design: Liang A; manuscript layout and format: Li M, Shi J; draft manuscript preparation: Han Y, Liu T; manuscript modification and review: Qi H. All authors reviewed the results and approved the final version of the manuscript.
-
The data generated during and analyzed during the current study are available from the corresponding author upon reasonable request.
This work was supported by grants from the Basic Research Project of Liaoning Provincial Department of Education (JYTZD2023117), the China Agriculture Research System of MOF and MARA (CARS-25), the China Postdoctoral Science Foundation (2020M670793) and the and the Research Start Funding of Shenyang Agricultural University (880419015).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Han Y, Liang A, Xu D, Zhang Y, Shi J, et al. 2024. Versatile roles of trehalose in plant growth and development and responses to abiotic stress. Vegetable Research 4: e007 doi: 10.48130/vegres-0024-0007
Versatile roles of trehalose in plant growth and development and responses to abiotic stress
- Received: 03 October 2023
- Revised: 30 November 2023
- Accepted: 23 January 2024
- Published online: 27 February 2024
Abstract: Trehalose is a natural nonreducing disaccharide that is found in most organisms, such as yeasts, bacteria, invertebrates and plants. Trehalose plays an important role in regulating plant growth and development and stress response. Thus, in this review, we discuss the physical and chemical properties of trehalose, its function in plant growth and development, and the regulatory mechanism of its response to abiotic stresses such as drought, salt, and extreme temperature. The purpose of this review is to provide a reference for further analysis on the mechanism of trehalose in regulating plant growth and development and stress resistance.
-
Key words:
- Trehalose /
- Plant growth and development /
- Abiotic stress /
- Antioxidant /
- Signaling