-
Facility vegetable production in China is primarily limited to a few economically beneficial crops such as tomatoes, cucumbers, eggplants, and peppers. However, the single repetitive planting system, high multiple cropping index, blind use of pesticides and fertilizers[1−3], combined with the semi-closed environment of the facilities have led to secondary salinization of the soil, and decrease in organic matter content as well as enzyme activity[4−8]. Research has shown that continuous cropping in a closed facility can hinder crop growth and development[9] and quality of produce[10,11]. Continuous cropping has become a bottleneck, restricting the sustainable development of the facility vegetable industry in China[12]. Thus, solving the continuous cropping obstacles of facility vegetables has become a critical issue[13].
Currently, there are many in-depth studies on the causes and mechanisms of cropping constraints[14−17]. Some measures have been proposed to prevent and resolve these constraints. In recent years, the use of summer cover crops to restore the soil for continuous cropping has received attention[18]. Fallow is essentially a short-term form of crop rotation[19], compared to traditional crop rotation. It does not change the original planting habits of only planting some short-lived crops during the fallow period[20]. Planting catch crops can not only effectively increase surface coverage and suppress weeds[21,22], but reduce water evaporation and salt accumulation, block soil N loss[23,24], promote increased yield of subsequent crops[25−27] and reduces mineral nutrient leaching[28,29]. Isık et al. found that cover crops such as sorghum, and Sudan grass significantly suppressed the growth of early organic lettuce weeds[30]. Xiao et al. observed that reduced nitrogen and phosphorus loss, increased soil organic matter, and also considerable economic benefits weere obtained during the summer fallow period in plastic greenhouses by planting catch vegetables such as leafy turnip, pepper leaf and edible amaranth[31]. Qi et al. studied continuous cropping corn and wheat as green manure in plastic greenhouses for two years during the summer fallow season. Compared to the control, with corn and wheat as catch crops, the EC of soil decreased, and soil organic matter content increased by 21.32% and 51.61%. The of soil urease, catalase, and sucrase activities also all significantly increased.
In China, there is typically a 1–2-month summer break period (July–September), which is the high-temperature season and also the off-season for leafy vegetables in northern China. There have been numerous reports of planting cover crops during summer breaks, such as legumes, green manures (hairy vetch, soybeans), grains (such as wheat, rice, sweet corn), as well as vegetables (like pak choi and amaranth)[22,26,32−34]. These crops however had relatively low economic value and limited benefits for farmers in terms of increasing their income. For the first time, we utilized the previous intercrops of New Zealand spinach (I-NZS), Malabar spinach (I-MS), and Chinese mallow (I-CM), as well as newly planted New Zealand spinach (NZS), which is of great economic value, for summer filling in cucumber continuous cropping greenhouses. In addition, it also included summer-filled edible amaranth (EA) and water spinach (WA).
In this study, we used no catch crop as the control (CK) , and after planting the six catch crop treatments mentioned above, we investigate: (1) whether the current status of secondary salinization in continuous cropping soils has been mitigated? (2) does the soil organic matter content of continuous cropping soils increase? (3) how does soil enzyme activity change in continuous cropping soils?
-
This experiment was conducted at the Horticulture Field of Northwest A&F University in Yangling, China (34°17' N, 108°4' E). A steel frame structure plastic greenhouse with a width of 8 m and a length of 40 m was used in this study. The greenhouse had been used to grow cucumbers for eight consecutive years, leading to the secondary salinization of greenhouse soil and damage to the cucumber plant system. The parameters analyzed for control soil yielded the following data: the EC value was 379 μS·cm−1, the Na+ content 1.09 g·kg−1, the TN content 1.35 g·kg−1, the TP content 0.965 g·kg−1, and the TK content was recorded as 8.36 g·kg−1.
Experimental materials
-
This study selected five types of vegetables as catch crops for summer fallow cultivation, including water spinach (Ipomoea aquatica F.), Chinese mallow (Malva verticillata L.), Malabar spinach (Basella alba L.), edible amaranth (Amaranthus mangostanus L.), and New Zealand spinach (Tetragonia tetragonioides L.). These five vegetables were chosen from the leafy vegetable family, which have a tendency to remove salt. Therefore, we selected them for further summer filling experiments within a cucumber greenhouse facility.
Experimental design and crop management
-
This experiment was conducted based on the spring intercropping of cucumber with leafy vegetables in 2020. For the experiment, a trench of 50 cm deep was dug in the intercropping area and a plastic cloth was used to vertically divide the area. There were seven treatments, including: (1) using no catch crop as the control (CK); (2) broadcasting water spinach 20 g per area (WS); (3) broadcasting edible amaranth 1 g per area (EA); (4) planting New Zealand spinach seedlings with 3–4 leaves stage, averaging 48 plants per area (NZS); (5) planting cucumber about 10 d after spring, broadcasting Chinese mallow in the intercropping area (5 g per area) during the cucumber intercropping period, and leaving Chinese mallow in the intercropping area after the cucumber is removed (I-CM); (6) planting cucumber about 10 d after spring, broadcasting Malabar spinach in the intercropping area (40 g per area) during the cucumber intercropping period, and leaving Malabar spinach in the intercropping area after the cucumber is removed (I-MS); (7) planting New Zealand spinach (1 row × 12 plants·row−1) in the intercropping area 28 cm apart from each other in two rows of cucumber per plot during the cucumber intercropping period, and leaving New Zealand spinach in the intercropping area after the cucumber is removed (I-NZS). Each treatment was repeated three times, for a total of 21 filling areas, each with an area of 4.2 m2 (3.5 m × 1.2 m). The experiment lasted 33 d, from June 29, 2020, to August 1, 2020. All treatments were not fertilized and were watered normally during the growing period (Supplemental Fig. S1). Special attention needs to be paid to the fact that the last three treatments (I-CM, I-MS, I-NZS) utilize the previous (spring 2020) residual crop.
Soil sample collection
-
Soil sampling was carried out at the beginning and end of the experiment. A 5 cm diameter soil auger was used to collect the top 0~10 cm of soil. 'S' mixed sampling method was used to collect soil samples in each plot. After collection, the soil samples were crushed and mixed in an iron pan, and then placed in a cool place to air dry. The soil samples were used for analysis of soil chemical characteristics and determination of soil enzyme activity.
Determination of soil chemical properties
-
Soil samples with a diameter of 1 mm are used to determine the content of soil pH, EC, and total nitrogen (TN), total phosphorus (TP), and total potassium (TK). Soil samples with a diameter of 0.150 mm are used to analyze the content of soil organic matter (SOM), calcium (Ca), magnesium (Mg), sodium (Na+), and chloride (Cl−). The EC value of the soil leachate is determined by electrical conductivity, following the method of Paladino et al.[35]. The SOM content was determined by the ASI method, following the method of Mehlich[36]. The TN content was quantified by the Kjeldahl method, whereas the TP contents were measured by the molybdenum blue-ascorbic acid method. Additionally, the method of Fan et al. used to examine the TK content[37]. The content of Ca, Mg, and Na+ is determined by atomic absorption spectrophotometry, following the methods of Han et al.[38]. The Cl− content is determined by a mercury thiocyanate spectrophotometer, following the methods of Cherif et al.[39]. Except for Cl−, the contents of other mineral elements in the table are the sum of soluble and insoluble elements.
Determination of soil enzyme activities
-
The crushed soil sample, after being screened through a 0.150 mm sieve, is used for determining soil enzyme activity. Soil urease activity (URE) is determined using the phenol-sodium salicylate colorimetric method while soil neutral phosphatase activity (NP) was quantified using the sodium phosphate-phenol colorimetric method. The soil sucrase activity (SUC) was inspected using the 3,5-dinitrosalicylic acid colorimetric method. We also investigated the catalase activity (CAT) using ultraviolet spectrophotometry. The soil dehydrogenase activity (DHA) was tested using the chloro-triphenyl-tetrazolium method. The above methods of determination are referenced by Guan[40]. The incubation time of soil catalase was 20 min, the other four kinds of soil enzymes were cultured for 24 h.
Data analysis
-
The data was analyzed using Microsoft Excel 2010 for statistical analysis and IBM SPSS Statistics 23 (Duncan's new multiple comparison method) for significance analysis (p < 0.05). Graphs were generated using GraphPad.Prism.9.5.0.730 and R language (the 'Hmisc' and 'corrplot' packages).
-
At the end of the summer season, the soil EC, Cl−, TN, TP, and TK content under leafy vegetable crops were significantly reduced compared to the CK. On the contrary, the soil SOM content increased significantly compared to the CK. Under the I-MS, I-NZS, and EA treatments, the Ca contents were significantly reduced compared to the CK. The Mg content in the soil used for EA, I-CM, and I-NZS was considerably reduced compared to that of CK. Except for the I-CM treatment, the Na+ content of other filling treatments was significantly reduced. New Zealand spinach, due to its larger biomass, absorbs large amounts of soluble salts, Ca2+, and Na+, and has a stronger ability to utilize N, P, and K (p < 0.05). There are also differences among different plants in terms of nutrient utilization, with I-NZS and I-MS showing a stronger ability to absorb nitrogen (Table 1).
Table 1. Effects of summer catch leafy vegetables on soil chemical properties of continuous cucumber cropping.
Treatments EC
(μS·cm−1)SOM
(g·kg−1)TN
(g·kg−1)TP
(g·kg−1)TK
(g·kg−1)Ca
(g·kg−1)Mg
(g/kg−1)Na+
(g·kg−1)Cl−
(g·kg−1)CK −21 ± 6.18d 0.432 ± 0.00a 0.273 ± 0.07e 0.010 ± 0.01d −0.05 ± 0.07d −0.02 ± 0.32c −0.04 ± 0.24b 0.017 ± 0.04d −0.002 ± 0.02d WS 40 ± 1.41b −0.615 ± 0.11c 0.487 ± 0.12d 0.170 ± 0.02b 0.42 ± 0.41c 0.22 ± 0.15c 0.39 ± 0.12ab 0.162 ± 0.04b 0.036 ± 0.0abc EA 81 ± 13.06a −0.649 ± 0.20c 0.676 ± 0.05c 0.207 ± 0.06b 1.05 ± 0.15b 1.54 ± 0.50b 0.99 ± 0.66a 0.336 ± 0.01a 0.051 ± 0.02a NZS 18 ± 6.65c −0.623 ± 0.08c 0.730 ± 0.10bc 0.075 ± 0.03c 0.58 ± 0.15c 0.43 ± 0.20c 0.46 ± 0.39ab 0.069 ± 0.02c 0.022 ± 0.00c I-CM 27 ± 9.43c −0.777 ± 0.24c 0.549 ± 0.06d 0.415 ± 0.04a 0.95 ± 0.13b 0.77 ± 0.02c 0.91 ± 0.39a 0.032 ± 0.02d 0.043 ± 0.01ab I-MS 40 ± 3.68b −0.592 ± 0.08c 0.819 ± 0.09ab 0.379 ± 0.07a 1.37 ± 0.20a 2.62 ± 0.57a 0.38 ± 0.33ab 0.134 ± 0.04b 0.021 ± 0.01c I-NZS 87 ± 6.50a −0.458 ± 0.04b 0.900 ± 0.05a 0.223 ± 0.04b 1.61 ± 0.14a 1.91 ± 0.58ab 0.80 ± 0.00a 0.135 ± 0.01b 0.029 ± 0.00bc CK: bare cultivated land as a control, WS: water spinach, EA: edible amaranth, NZS: newly planted New Zealand spinach, I-CM: Chinese mallow, I-MS: Malabar spinach, I-NZS: the previous New Zealand spinach. EC: electrical conductivity, SOM: organic matter, TN: total nitrogen, TP: total phosphorus, TK: total potassium. The soil chemical property data are the values corresponding to the initial stage of the summer catch period minus the values corresponding to the end of the summer catch period, the negative sign '−' indicates an increase in the value of a soil chemical property and vice versa. The significant difference in confidence level p < 0.05. Effects of summer catch leafy vegetables on soil enzyme activities of continuous cucumbers cropping
-
The majority of the soil enzymes are released by microorganisms. Exploring the changes in soil enzyme activity in high-intensity cucumber cropping facilities can reflect the activity of microorganisms to some extent. At the end of the summer catch crop, except for the NZS treatment, the decrease in urease activity was significant for all other summer catch crop treatments when compared to the CK. Excluding I-NZS treatment, all catch crop treatments showed a significant increase in neutral phosphatase activity. The sucrase activity for all catch crop treatments was significantly lower than CK. The changes in catalase activity were significantly different for all catch crops except EA. The dehydrogenase activity for all catch crop treatments was significantly higher than CK (Table 2).
Table 2. Effects of summer catch leafy vegetables on soil enzyme activities of continuous cucumber cropping.
Treatments URE
(mg NH3-N/g soil)NP
(mg Phenol/g soil)SUC
(mg Glu/g soil)CAT
(mmol H2O2/g soil)DHA
(μg TTC/g soil)CK 0.929 ± 0.21a −0.023 ± 0.01b 0.64 ± 0.13d 0.74 ± 0.11a 10.53 ± 0.20a WS 0.386 ± 0.00b −0.086 ± 0.00c 5.05 ± 0.53bc −0.28 ± 0.02d −1.27 ± 0.14b EA −0.230 ± 0.00c −0.168 ± 0.02ef 6.33 ± 0.66a 0.69 ± 0.21a −3.29 ± 0.31d NZS 0.802 ± 0.25a −0.137 ± 0.02de 4.27 ± 0.19c 0.07 ± 0.00c −2.68 ± 0.25c I-CM 0.360 ± 0.03b −0.133 ± 0.02d 4.97 ± 0.33bc −1.31 ± 0.23e −5.48 ± 0.40e I-MS 0.319 ± 0.02b −0.185 ± 0.02f 5.21 ± 0.74b −1.63 ± 0.00f −3.52 ± 0.42d I-NZS 0.164 ± 0.12b 0.072 ± 0.01a 4.49 ± 0.08bc 0.32 ± 0.07b −11.18 ± 0.05f CK: bare cultivated land as a control, WS: water spinach, EA: edible amaranth, NZS: newly planted New Zealand spinach, I-CM: Chinese mallow, I-MS: Malabar spinach, I-NZS: the previous New Zealand spinach. URE: urease activity, NP: neutral phosphatase activity, SUC: sucrase activity, CAT: catalase activity, DHA: dehydrogenase activity. The enzyme activity data are the values corresponding to the initial stage of summer catch period minus the values corresponding to the end of summer catch period, the negative sign '−' indicates an increase in soil enzyme activity and vice versa for a decrease in enzyme activity. The significant difference confidence level p < 0.05. Analysis of yield and net income of summer catch leafy vegetables
-
The economic and biological yields of different summer catch crops are significantly different, with I-NZS and I-MS having the highest yields (Figs 1 & 2). For WS, EA, NZS, I-CM, I-MS, and I-NZS treatments: (1) the average economic yields of leafy vegetables were 4,071, 7,691, 7,439, 6,877, 30,395, and 28,262 kg·ha−1, respectively (Fig. 1); (2) the actual selling prices of leafy vegetables were CNY¥ 3, 2, 10, 7, 4, and 8 kg−1, respectively (Supplemental Table S1); (3) leafy vegetables profit margins were 45%, 50%, 20%, 55%, 55%, and 55%, respectively (Supplemental Table S1); (4) net profits were USD
${\$} $ 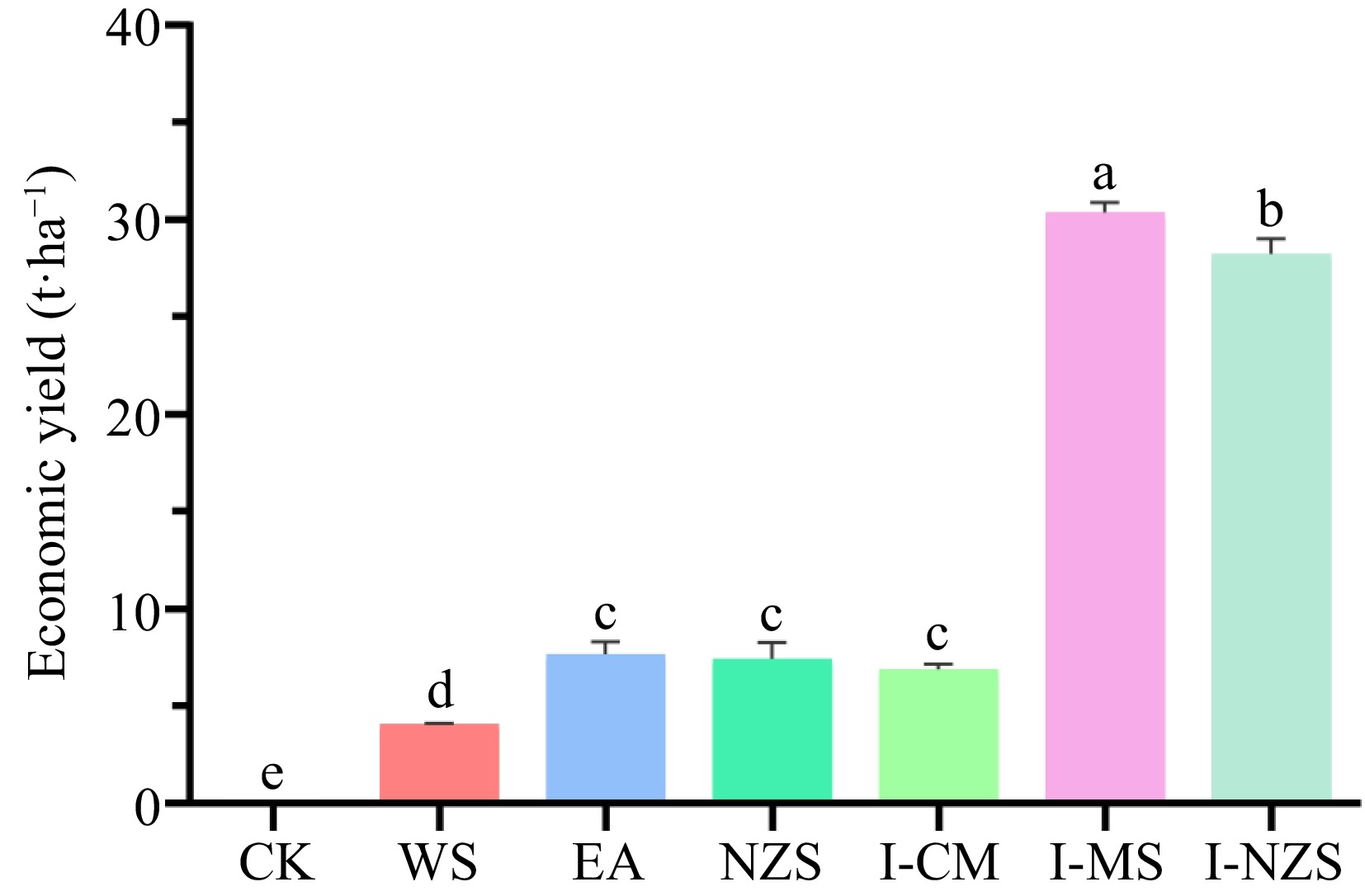
Figure 1.
Economic yield of summer catch leafy vegetables. CK, bare cultivated land as a control; WS, water spinach; EA, edible amaranth; NZS, newly planted New Zealand spinach; I-CM, previously intercropped chinese mallow; I-MS, previously intercropped malabar spinach; I-NZS, previously intercropped New Zealand spinach. The significant difference confidence level is p < 0.05.
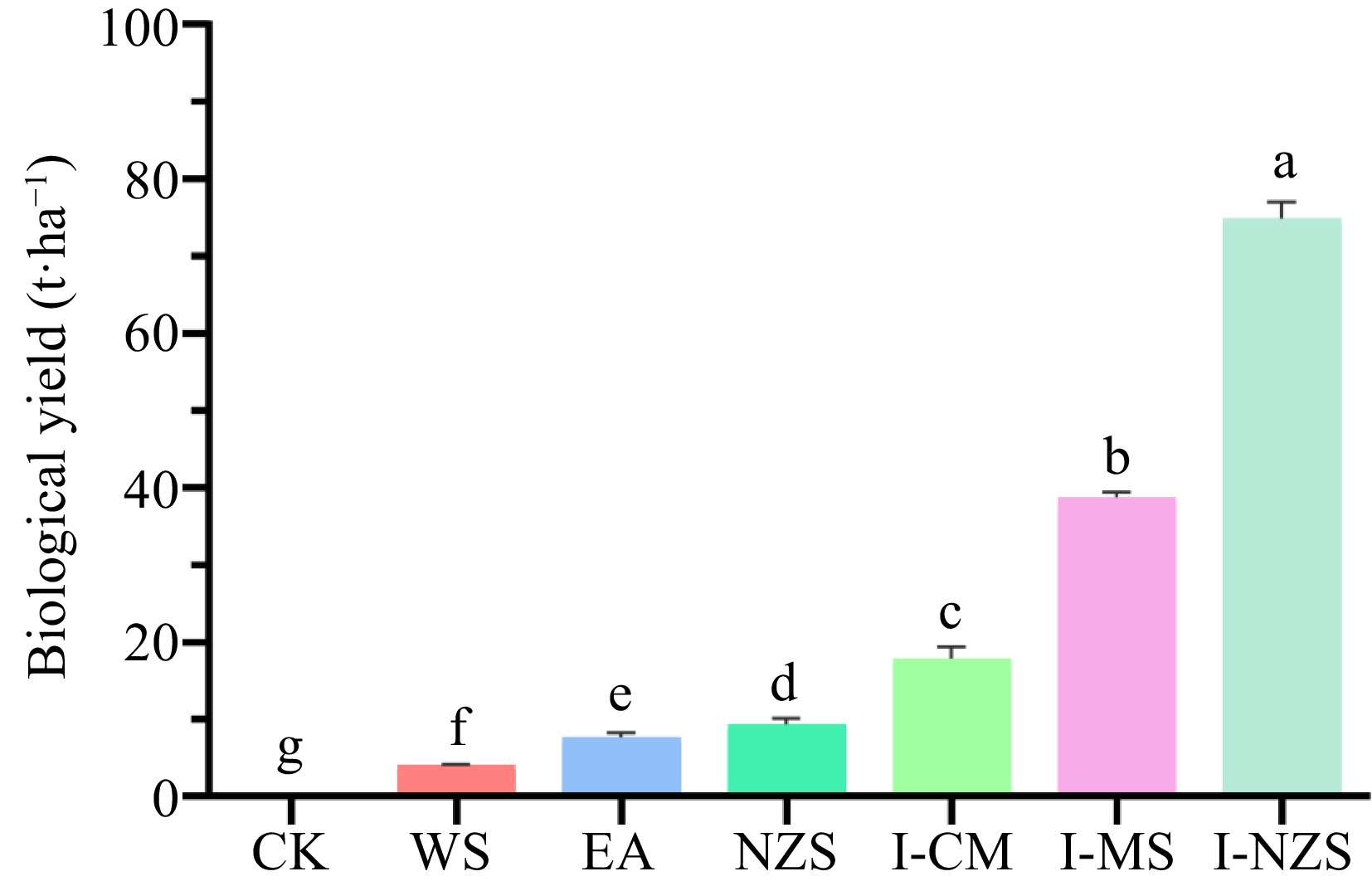
Figure 2.
Biological yield of summer catch leafy vegetables. CK, bare cultivated land as a control; WS, water spinach; EA, edible amaranth; NZS, newly planted New Zealand spinach; I-CM, previously intercropped chinese mallow; I-MS, previously intercropped malabar spinach; I-NZS, previously intercropped New Zealand spinach. The significant difference confidence level is p < 0.05.
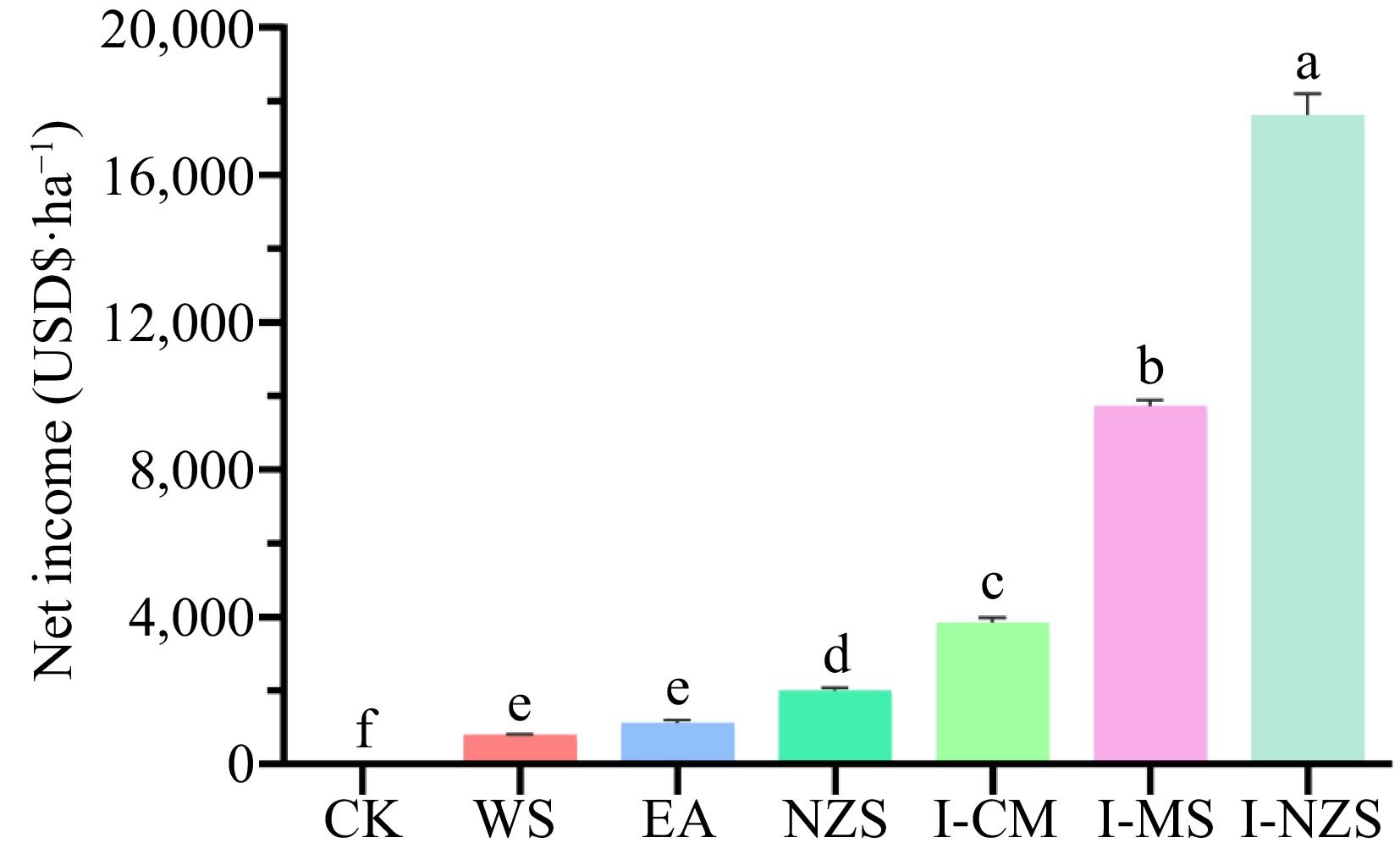
Figure 3.
Net income of summer catch leafy vegetables. CK, bare cultivated land as a control; WS, water spinach; EA, edible amaranth; NZS, newly planted New Zealand spinach; I-CM, previously intercropped chinese mallow; I-MS, previously intercropped malabar spinach; I-NZS, previously intercropped New Zealand spinach. The significant difference confidence level is p < 0.05.
Relationship between the yield of summer catch crops and soil
-
The economic yield of leafy vegetables is positively correlated with the biological yield of leafy vegetables. The decrease of TN, TK, Ca, EC, and TP are negatively correlated with the dehydrogenase, urease, and catalase activity. The relationship between soil EC and soil properties such as TN, TK, Ca, Na+, TP, Mg, Cl−, and the decrease in sucrase activity is positive. Meanwhile, a negative relationship between urease and dehydrogenase activity was also observed (Fig. 4).
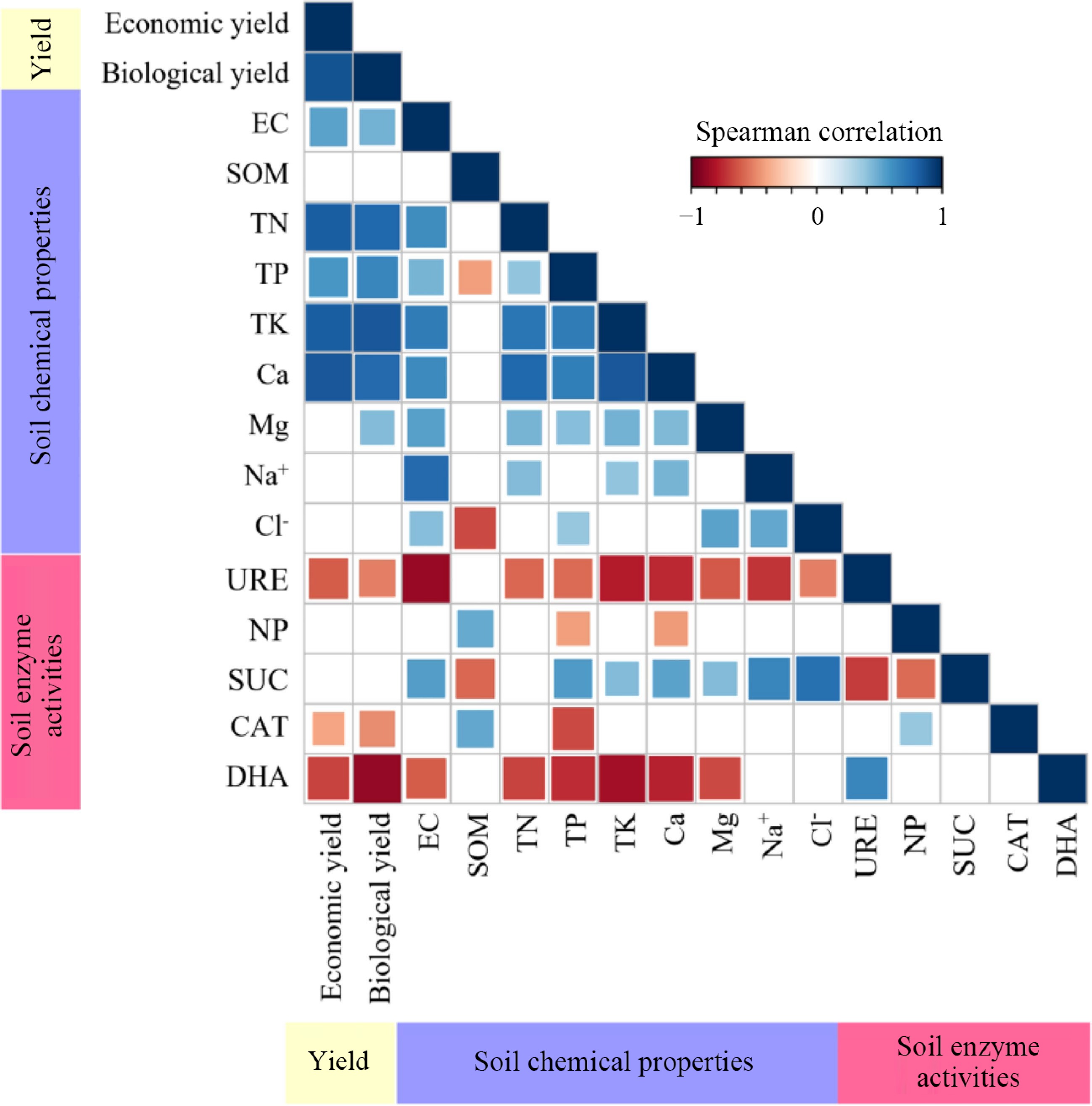
Figure 4.
The relationship between the yield of summer catch leafy vegetables and soil. EC, electrical conductivity; SOM, organic matter; TN, total nitrogen; TP, total phosphorus; TK, total potassium; URE, urease activity; NP, neutral phosphatase activity; SUC, sucrase activity; CAT, catalase activity; DHA, dehydrogenase activity.
-
High concentrations of salt can reduce the quality of agricultural soil[41], and eventually bring harm to the production[42−45]. Secondary salinization of soil has become the main constraint in protected vegetable production, and it occurs mainly in the top 0~10 cm[46]. Soil EC is an indicator for evaluating soil soluble salt content[47]. This paper studied the reducing effect of water spinach, Chinese mallow, Malabar spinach, edible amaranth, and New Zealand spinach on the secondary salinization of soil in cucumber greenhouse. The results not only showed a significant positive correlation between the reduction of soil EC and the decrease of Ca, Mg, Na+, and Cl− (Fig. 4), but also indicated a significant positive correlation with the biological yield of leafy vegetables (Fig. 4). All five cover crops can significantly reduce the soluble salt content in the soil (Table 1), indicating that summer cover cropping can effectively reduce secondary salinization. Among all treatments, EA and I-NZS treatments showed the greatest decrease in soluble salt content in the soil (Table 1), highlighting the importance of selecting appropriate vegetable catch crops. Considering the comprehensive salt removal effect, economic benefits, and labor savings, the best summer cover crops were I-MS and I-NZS (Table 1, Fig. 3). This occurred because these two crops were interplanted with spring cucumbers and were left for summer cover cropping post-cucumber harvest. During this period, the growth time of Malabar spinach and New Zealand spinach became longer, resulting in greater biomass. These two crops were not only efficient for salt removal but also proved to be lucrative during the off-season when the supply was relatively short.
Soil SOM is a key factor in soil fertility[48] and can directly or indirectly impact crop growth by providing nutrients or improving soil physical and chemical properties[45,49]. A soil SOM content below the critical threshold can result in a serious decline in soil quality[50]. Cover crops can increase soil SOM content[51]. It has been reported that multiple cropping broccoli can significantly increase soil organic carbon post-bean harvest period, possibly due to root deposition[52]. There are also opinions suggesting that the residues of cover crops (such as fallen leaves) are also beneficial in increasing soil SOM content[53]. This experiment found that at the end of the summer filling period, the soil SOM content of all the filled leafy vegetables increased significantly (Table 1), with EA's soil SOM increasing by 19.46% (Table 1). Our study was in line with the study of Xiao et al. who reported that the soil SOM of summer filled amaranth increased by 16.47%[31]. The soil SOM content of the CK decreased (Table 1), which may be due to degradation and loss of soil SOM in the summer break[54]. The changes in soil SOM of different treatments indicated that summer-filled leafy vegetables were beneficial in improving soil SOM values.
Most soil enzymes come from soil microorganisms[55−57] and play a key role in the decomposition of soil organic matter, nutrient cycling, and humus synthesis [51,58]. Soil enzyme activity is sensitive to changes[59], and directly alters the soil microbiome[60]. Generally, soil with higher enzymatic activity is highly fertile[61]. Previous reports have indicated that soil urease, phosphatase, sucrase, catalase, and dehydrogenase can serve as sensitive enzyme indicators for soil in facilities[62]. Soil urease is used by microorganisms as both an intracellular and extracellular enzyme and mainly functions outside of cells[63,64]. Urease is used by the microorganism to catalyze the hydrolysis of urea in soil into ammonium carbonate, which further decomposed to release
${\text{NH}^+_4} $ Previous studies have shown that continuous cultivation can reduce soil enzyme activity [5,76], while fallow is beneficial for improving soil quality[26]. Karasawa & Takahashi found that covering crops appropriately in summer or winter can improve soil phosphatase activity[77]. This study showed that compared with the CK, the soil-neutral phosphatase activity (except for I-NZS) and dehydrogenase activity of summer-cover leafy vegetables increased significantly (Table 2). The soil urease activity of EA was high (Table 2), which was consistent with the report of Tian et al.[22]. The soil sucrase activity of summer cover leafy vegetables was significantly lower than that of CK (Table 2), indicating that summer break can restore soil fertility to some extent, which was contrary to the view of Zhang et al.[78]. This difference may be attributed to the different types of summer-cover leafy vegetables. Some studies have shown that soil enzyme activity is influenced by factors such as season[79] and cover crops[80]. In this experiment, all the neutral phosphatase and dehydrogenase activities of summer cover leafy vegetables increased significantly (Table 2), while the urease and sucrase activities of the treatments decreased (Table 2). The catalase activity of different treatments showed both increases and decreases (Table 2), which could be facilitated by the combined effects of season and treatment[22].
-
Summer filling of leafy vegetables is an effective strategy to alleviate soil succession disorder in the cucumber greenhouse. Compared to CK, all summer-filling treatments significantly improved soil secondary salinization, increased the soil organic matter content, and enhanced the soil dehydrogenase activity. In addition, the economic yield, biological yield, and net income of leafy vegetables were significantly higher than CK, especially in the I-MS and I-NZS treatments. Considering the net benefits of summer filling and the effects of mitigating the facility's successive soils, we recommend using the I-MS and I-NZS treatments during the summer break period, i.e., to fully utilize the previous crop, Malabar spinach and New Zealand spinach.
-
The authors confirm contribution to the paper as follows: performed the research: Wang D, Yao J; data analysis and provided technical help: Sharif R, Chen K, Lv J; draft of the manuscript: Wang D; designed the experiments, supervised this study and revised the manuscript: Li Y. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Work in YL's lab was supported by the Shaanxi Province's Major Research and Development Projects (2022ZDLNY03-12) and Xi'an Science and technology planning projects.
-
The authors declare that they have no conflict of interest.
- Supplemental Table S1 Yield and income of summer catch leafy vegetables.
- Supplemental Fig. S1 Field photos of summer catch leafy vegetables in 2020. Note: CK bare cultivated land as a control, WS water spinach, EA edible amaranth, NZS newly planted New Zealand spinach, I-CM Chinese mallow, I-MS Malabar spinach, I-NZS the previous New Zealand spinach.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang D, Yao J, Sharif R, Chen K, Lv J, et al. 2024. Evaluation of the impact of different summer catch crops on continuous cropping soil properties under plastic tunnel cultivation. Vegetable Research 4: e009 doi: 10.48130/vegres-0024-0008
Evaluation of the impact of different summer catch crops on continuous cropping soil properties under plastic tunnel cultivation
- Received: 26 October 2023
- Revised: 17 January 2024
- Accepted: 26 January 2024
- Published online: 28 March 2024
Abstract: In China, the issue of facility succession disorder is widespread. Summer catch crop is an effective method for overcoming the continuous cropping barrier in facility vegetables. In this study, we designed six summer filling treatments, reporting for the first time on these treatments, which included newly planted New Zealand spinach (NZS) as well as spring-planted New Zealand spinach (I-NZS), Chinese mallow (I-CM), and Malabar spinach (I-MS) for cucumber intercropping and subsequent summer fallow. Compared to CK, all six fallow treatments significantly reduced soil electrical conductivity (EC), increased soil organic matter (SOM) contents, and soil dehydrogenase activity (DHA) (p < 0.05). Among the six summer-filling treatments, I-MS and I-NZS treatments had significantly higher leafy vegetable economic yields (30.4, 28.3 t·ha−1, respectively) and net income (USD${\$} $ 9,730, 17,622 ha−1, respectively) than the other treatments (p < 0.05). The research results provide a practical reference for alleviating the soil secondary salinization of cucumber greenhouse. They also provide a technical basis and support for improving the effectiveness of facility-based agriculture and enriching cropping systems.













