-
Powdery mildew is a devastating disease and can severely affect fruit yield and quality of melon plants worldwide[1]. It is caused by Podosphaera xanthii (P. xanthii) and Golovinomyces cichoracearum (G. cichoracearum), two biotrophic pathogens that can exclusively parasitize melon plants and produce white powdery substances on the surface of infected plant tissues such as leaves[2]. Previous studies have well characterized the lifecycle of mildew fungi, which generally initiates with primary spore germination followed by appressorium generation and terminates with the production of secondary spores[3]. Appressorium, a kind of highly specialized infection structure, can facilitate the penetration of mildew pathogens through the melon plant surface to generate haustoria, which are responsible for the exchange of materials such as water, nutrients, nucleotides and proteins between hosts and mildew pathogens[4]. In China, P. xanthii races 1 and 2F are considered the major causal pathogens for powdery mildew that occurs in melon production[5].
Two inoculation strategies are commonly used to evaluate the pathogenicity of different crop germplasms and/or breeding lines upon powdery mildew[6−15]. The first strategy is a liquid-mediated (LM) one, wherein the collected fungal pathogens from source plants are well suspended in inoculation buffers such as ddH2O containing variable concentration levels of Tween-80 and then landed on target plants by spraying of the conidial suspension[6,7,12−15]. The second strategy is a medium-free (MF) one, wherein target plants are placed in an inoculation box with a 50-µm nylon mesh cover and the collected mildew spores are gently blown from source leaves to the plants through the cover mesh[8−11]. The development of powdery mildew is subsequently monitored and compared among inoculated plants. LM inoculation is now predominantly applied in Cucurbit species such as melon[6,7,12−15], while the utilization of MF strategy is restricted to Arabidopsis and cereal crops such as wheat[8−11]. It remains largely unknown how melon plants respond to powdery mildew when P. xanthii pathogens are inoculated on target plants via the MF strategy. The inoculation strategy can influence the ability to successfully cause infection as well as accurately and reproducibly detect presence of the pathogen[16−18], both of which play crucial roles in efficient identification of plant germplasm/breeding lines with high disease resistance. Despite its wide application in Cucurbit crops, several notable drawbacks, such as the uneven distribution of inoculated pathogens and longer time course of disease development on host plants have been demonstrated for LM inoculation[19,20]. It is thus an urgent need to establish an alternative strategy for efficiently evaluating the interplay between host Cucurbit plants and mildew fungi.
In this study, an MF strategy suitable for melon plants was first developed, and the pathogenic course was then compared between LM- and MF-inoculated melon seedlings. All evidence from phenotypic, qPCR, and microscopic investigations pointed to the better performance of MF inoculation relative to LM inoculation, revealing its great potential in high-efficiency detection of melon-powdery mildew interactions.
-
Melon (Cucumis melo L.) inbred line 'YJM' was used as plant materials. After surface sterilization, 'YJM' seeds were placed on wetted filter paper and kept in an incubator with an air temperature of 28 °C and relative humidity (RH) of 60% ± 10% in darkness. The germinated seeds were then sown in 50-hole trays that were filled with nutrient soil (charcoal:vermiculite:perlite = 1:1:1, v/v/v), and placed in a growth chamber with photoperiod of 16 h (day)/8 h (night), air temperature of 26 °C (day)/26 °C (night), and RH of 60% ± 10%. Melon seedlings were challenged by P. xanthii at the three-leaf stage. A total of 50 seedlings were treated with LM and MF strategies, respectively, and three independent experiments were performed.
Spore preparation and inoculation
-
To prepare mildew pathogens, P. xanthii spores were first collected from diseased melon plants in the greenhouse of Shandong Agricultural University, Taian, Shandong, China, and then propagated on the leaves of melon seedlings followed by several rounds of subcultures every 20 d according to a previous study[7]. Finally, 100 well-diseased leaves were obtained, of which 50 were randomly selected as mildew source for LM inoculation by following the protocol introduced by Wang et al.[7]. The remaining leaves were used for MF inoculation according to the protocol described by Song et al.[21] with some modifications: melon seedlings were placed at the bottom of a cardboard settling tower (56 cm in length, 18 cm wide and 25 cm heigh), and mildew pathogens from source leaves were blown to target seedlings through a 50-μm nylon mesh that was covered on the top of the tower using a hairdryer. Thereafter, the inoculated seedlings were transferred to the growth chamber with the same environmental settings as the abovementioned.
Determination of P. xanthii biomass
-
To estimate pathogen biomass, leaf samples were collected from inoculated seedlings at 0, 12, 24, 48, 72 and 96 h post inoculation (HPI) and frozen in liquid nitrogen. Genomic DNAs were extracted using the CTAB method, and then qualified and quantified with a Nanodrop microvolume spectrophotometer (THERMO, USA). Using the extracted genomic DNAs as templates, quantitative PCR (qPCR) analysis was carried out for PxTUB2 and CmACT7, respectively, and mildew fungus biomass was then calculated according to the method introduced previously[22]. All primers used in the qPCR investigation are provided in Table 1.
Table 1. Primers for qPCR assay of powdery mildew biomass.
Gene ID Forward (5'→3') Reverse (5'→3') PxTUB2g TTGTAGGAATCACATCCC
TTTCTCTTCTTCCGGTTGCATGGGT
GGTTCCmACT7 GGCTGGATTTGCCGGTGA
TGATGCGGAAGGAGGAAATCAGTGT
GAACCMicroscopic investigation
-
To monitor P. xanthii development, leaf samples were collected from inoculated melon seedlings at 8, 12, 24, and 48 HPI and fixed in 2.5% (w/v) glutaraldehyde (pH = 7.2) for 3 h. The fixed leaves were stained with trypan blue according to the method of Frye & Innes[23] and then imaged under a light microscope. For scanning electron microscopy (SEM) investigation, the fixed samples were prepared following the method of Xu et al.[24], and imaged under a TESCAN5136 platform with the cold field emission mode (TESCAN, Czech Republic).
Data analysis
-
All data were processed with Microsoft Excel 2013 software, and displayed as the mean of biological repeats ± standard deviations (SD). Statistical analysis was carried out with SPSS software version 13.0 (SPSS, USA) by following the rules of one-way ANOVA at a 0.05 significance level.
-
Due to no referred information about MF inoculation of fungal pathogens for Cucurbit species, an MF inoculation strategy with 'YJM' seedlings was first elaborated, wherein mildew spores were directly blown to target seedlings through a nylon mesh cover using a hairdryer (Fig. 1 right panel). 'YJM' seedlings at the three-leaf stage were then challenged by P. xanthii via either LM inoculation (Fig.1 left panel), a commonly used one for Cucurbit plants, or MF inoculation. To ensure the comparable distribution of mildew pathogens, the second expanded leaves, which were counted from the top buds, were randomly collected from the LM and MF groups for spore counting under light microscope with 20-fold magnification, respectively. A total of 50 microscopic views from five samples were investigated for each treatment, and no significant differences in spore density were observed between LM and MF groups (Fig. 2a & b), demonstrating that P. xanthii pathogens were indeed evenly landed on melon seedlings via both inoculations under experimental conditions in this study. Disease development was then monitored for both treatment groups over a 5-d investigation course, and white spots were detected at 5 d post inoculation (DPI) for the LM group while at 3 DPI for the MF group, leading to much more severe powdery mildew symptoms on MF leaves than LM ones (Fig. 2c). The disease index was further calculated for both groups at the end of the investigation course, and this parameter from LM-inoculated samples was 78.46% lower than that of MF-inoculated ones (Fig. 2d). All evidence suggested that, given the comparable pathogen strength, powdery mildew was apparently promoted when melon seedlings were challenged by P. xanhii via MF inoculation relative to LM inoculation.
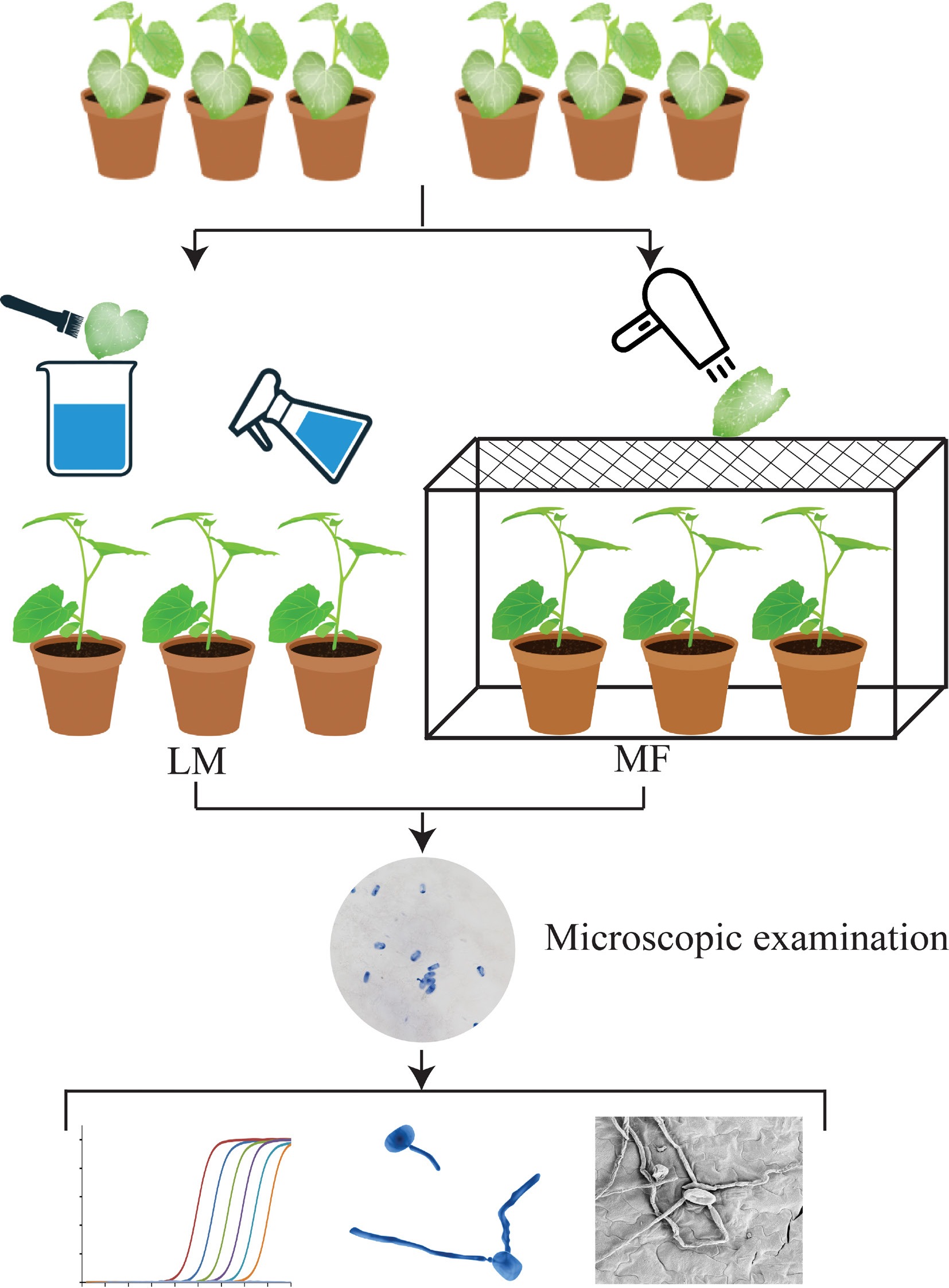
Figure 1.
Schematic illustration for liquid-mediated (LM) and medium-free (MF) inoculations that are used to investigate melon-powdery mildew interactions in this study.
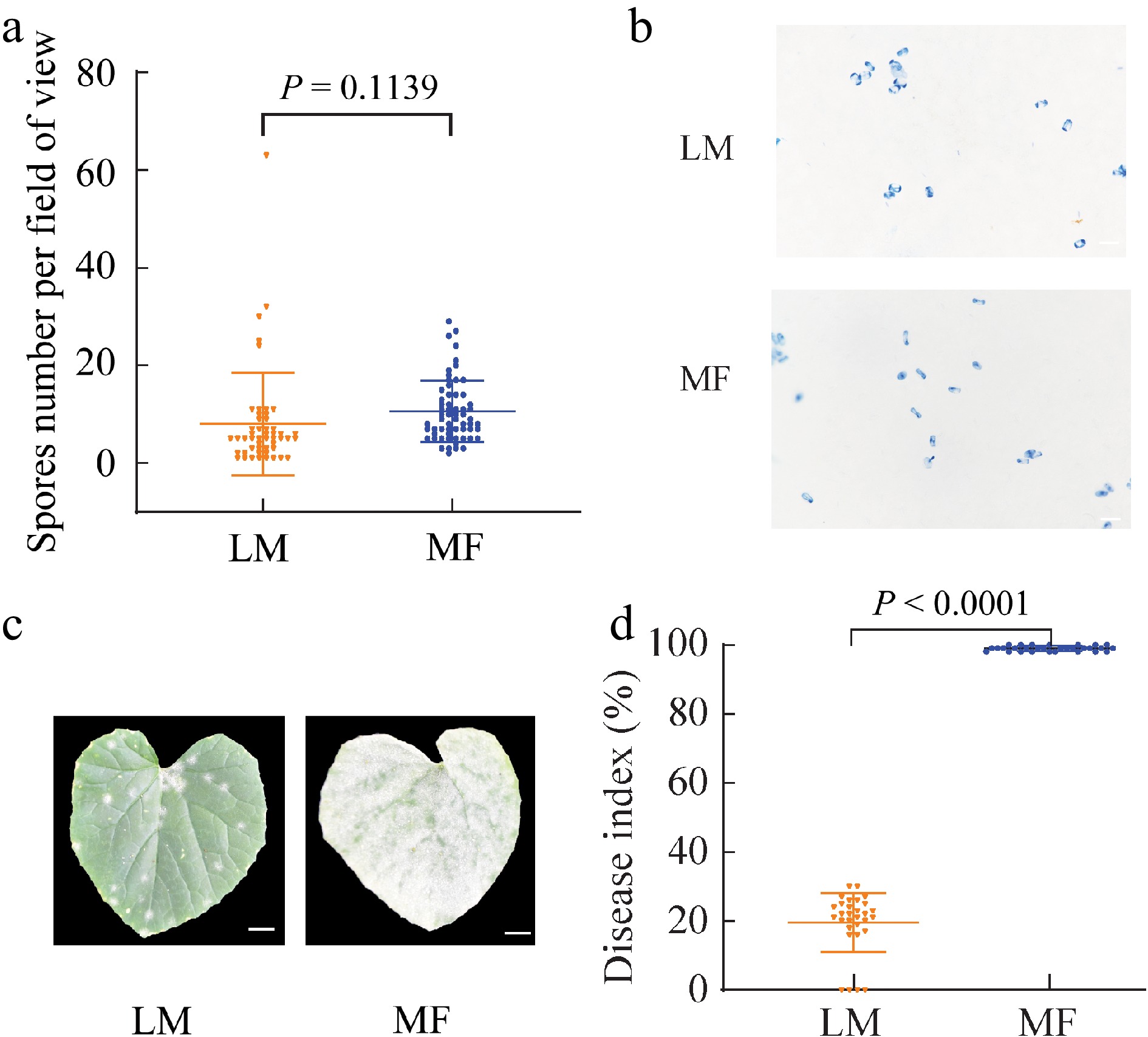
Figure 2.
Spore density, phenotypes and disease index on the leaves of melon seedlings infected by different methods. (a) Spore number on the leaves of melon seedlings immediately after being treated by liquid-mediated (LM) and medium-free (MF) inoculations. (b) Trypan blue staining for P. xanthii on the leaves of melon seedlings immediately after being treated by LM and MF inoculations. (c) Disease symptoms on the leaves of melon seedlings treated by LM and MF inoculations at 5 d post inoculation (DPI). (d) Disease index of melon seedlings treated by LM and MF inoculations at 5 DPI. The value of each group represents mean of (a) 50 or (d) 30 biological repeats ± standard deviations (SD). Scale bar is equal to (b) 200 μm or (c) 1 cm.
qPCR verification of distinct pathogen development between LM- and MF-inoculated melon seedlings
-
To compare the development of powdery mildew at molecular level, a qPCR-based assay, wherein PxTUB2g and CmACT7 were used as fungal growth marker and host internal control genes respectively[22], was adopted to evaluate the dynamic variations in P. xanthii biomass of both LM- and MF-inoculated seedlings over a 4-d investigation course. Apparent divergences were found in not only the starting time point of pathogenic genomic DNA amplification, which could be considered an indicator of P. xanthii growth capacity, but also the abundance of pathogenic genome DNAs, which could be considered an indicator of P. xanthii biomass. For LM-inoculated seedlings, the apparent amplification of pathogenic genome DNAs was observed at 96 HPI, while this time point was shortened to 12 HPI for MF-inoculated seedlings, 84 h earlier than that of LM samples (Fig. 3). Moreover, the DNA abundance of P. xanthii was at a comparable level for both groups at 0 HPI, while separated tremendously at subsequent investigation time points (1.42 of LM vs 20.64 of MF at 12 HPI, 2.43 vs 40.11 at 24 HPI, 2.73 vs 89.02 at 48 HPI, 2.74 vs 104.03 at 72 HPI, and 20.18 vs 349.36 at 96 HPI) (Fig. 3). These observations further supported the conclusion that powdery mildew was stimulated in melon seedlings upon MF inoculation relative to LM inoculation.
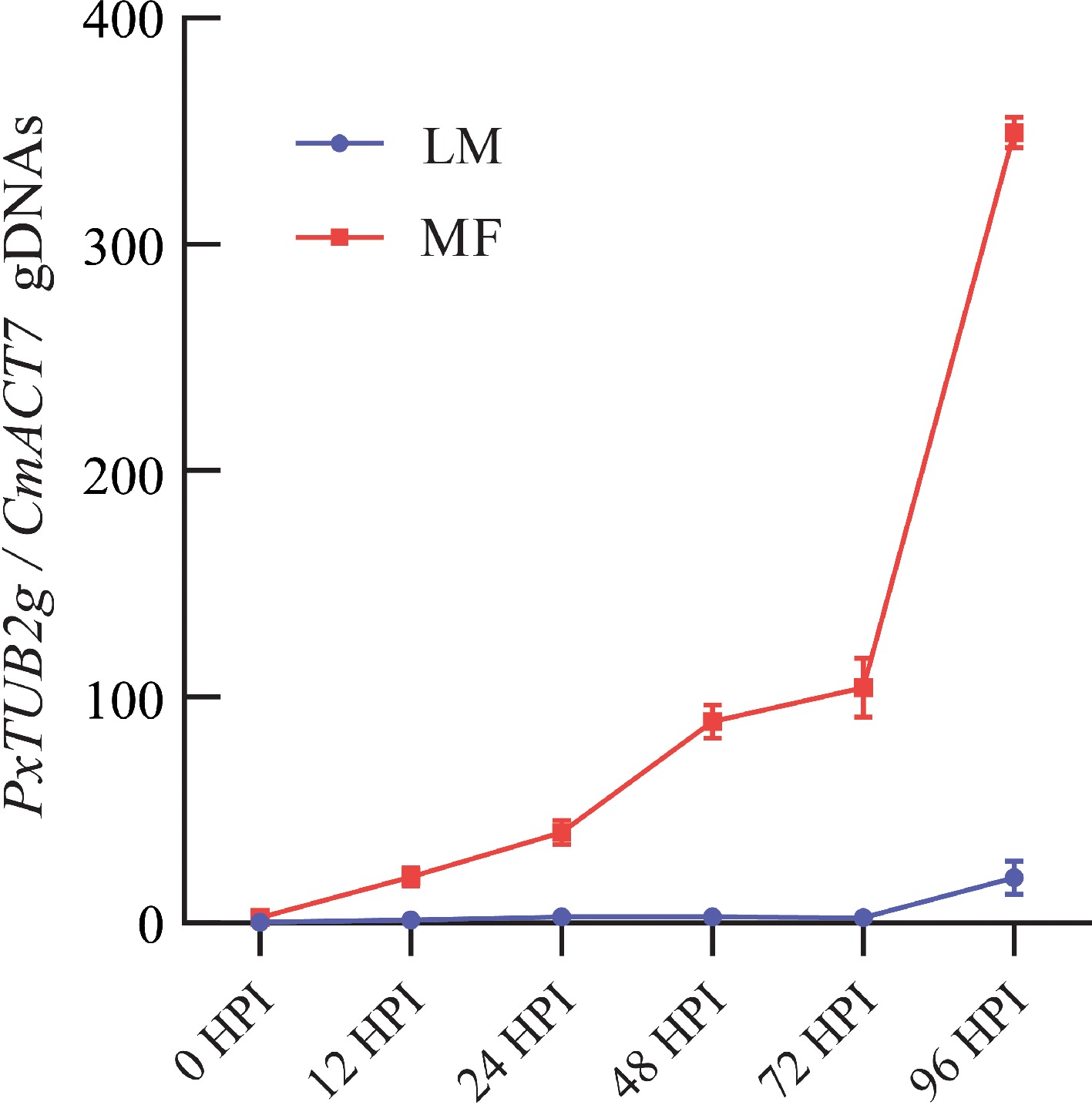
Figure 3.
Quantification of powdery mildew pathogens on the leaves of melon seedlings infected by different methods. The development of P. xanthii was quantitatively assayed by qPCR on the leaves of melon seedlings treated by liquid-mediated (LM) and medium-free (MF) inoculations at 0, 12, 24, 48, 72, and 96 h post inoculation (HPI), respectively. The value of each group represents mean of three biological repeats ± standard derivations (SD).
Microscopic verification of distinct pathogen development between LM- and MF-inoculated melon seedlings
-
Whether the distinct disease development could be attributed to the differences in mildew pathogen survival and growth between LM- and MF-inoculated groups was questioned. To this end, morphological features of P. xanthii were explored by both trypan blue staining and SEM assays. We focused on the inoculated samples at three time points of 12 HPI, 24 HPI, and 48 HPI, because the abovementioned results, particularly those from the molecular investigation, pointed to the fact that the divergence in disease development occurred between LM and MF samples at a very early stage after inoculation. Indeed, spore germination and hyphae growth of P. xanthii were only observed until 48 HPI for LM-inoculated seedlings while uncovered at 12 HPI for MF-inoculated samples, and further stimulated at the subsequent investigation time points (Fig. 4a & b). Being consistent with microscopic observations, statistical significance was also detected in the germination rate of P. xanthii spores between LM- (1.75%) and MF-inoculated samples (40.93%) at 48 HPI (Fig. 4c). A cellular explanation was thus provided for the distinct development of powdery mildew in melon seedlings challenged by P. xanthii via LM and MF inoculations.
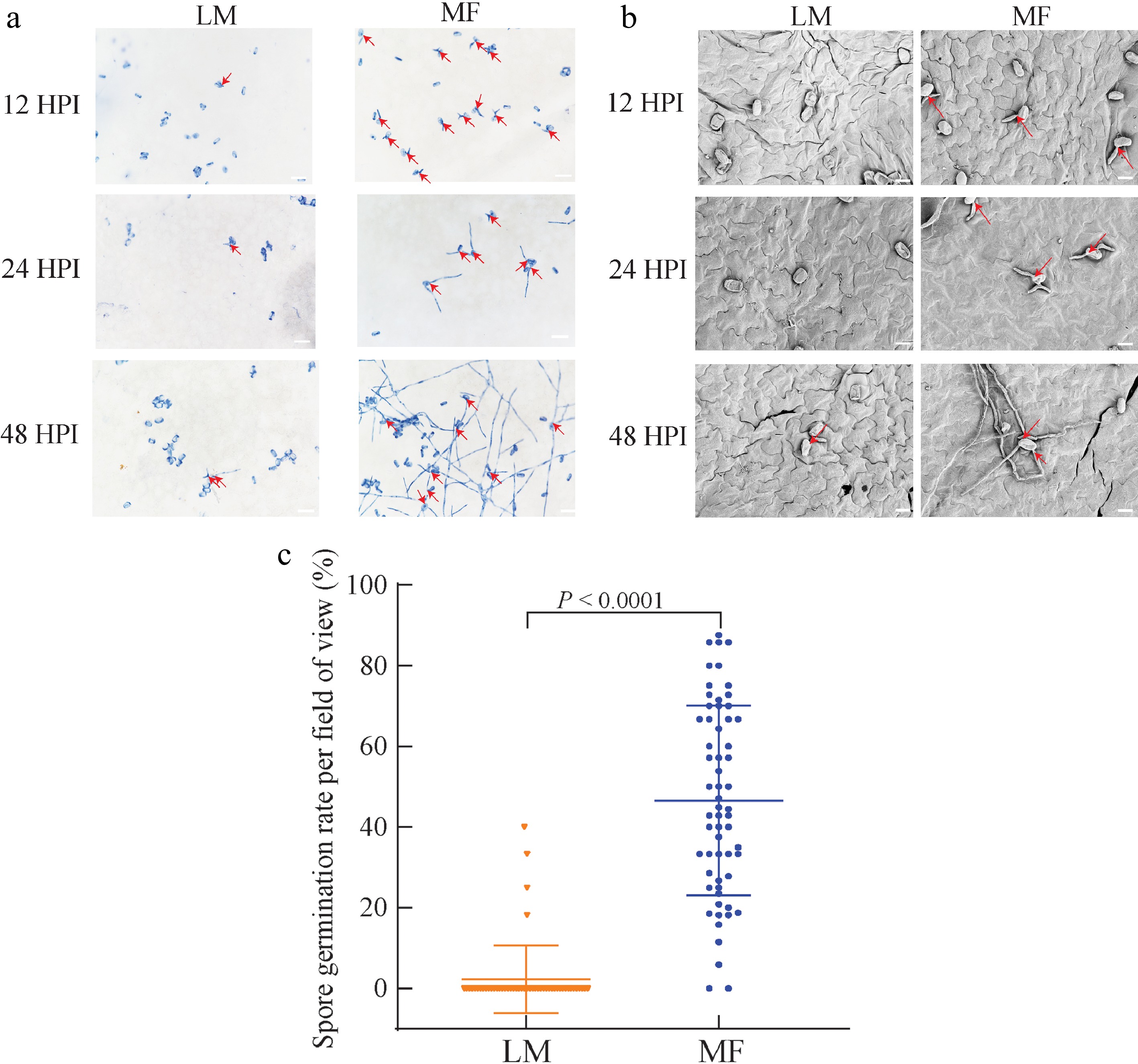
Figure 4.
Mildew pathogen development and spore germination on the leaves of melon seedlings infected by different methods. (a) Trypan blue staining for P. xanthii on the leaves of melon seedlings treated by liquid-mediated (LM) and medium-free (MF) inoculations at 12, 24, and 48 h post inoculation (HPI). (b) Scanning electron microscope (SEM) investigation for P. xanthii on the leaves of melon seedlings treated by LM and MF inoculations at 12, 24, and 48 HPI. (c) Spore germination on the leaves of melon seedlings treated by LM and MF inoculations at 96 HPI. The value of each group represents mean of 60 biological repeats ± standard deviations (SD). Scale bar is equal to (a) 200 μm or (b) 20 μm.
-
The invasion by powdery mildew pathogens induces architectural and physiological re-organization in host plants, thus resulting in a series of cellular dysfunctional events such as cell wall integrity alterations and metabolic disturbances[25,26]. Illumination of plant-P. xanthii interactions via proper experimental methods could benefit our mechanistic understanding about both the pathogenic features of powdery mildew and the adaptive responses of host plants upon mildew stress. Although LM and MF inoculations are two common strategies to explore the interplay between host plants and disease pathogens[8−15], the predominant application of LM inoculation has been well documented in previous studies regarding cucurbit-powdery mildew interactions rather than MF inoculation[27,28].
In this study, a comparative investigation of infection effectiveness was carried out between LM and MF inoculations, via which melon seedlings were challenged by P. xanthii respectively (Fig. 1). The results from phenotypic, qPCR, and microscopic assays showed faster initiation and massive spread of powdery mildew on MF-inoculated seedlings relative to LM-inoculated ones (Figs 2−4), suggesting that MF inoculation would be a promising strategy to efficiently characterize the interplay between melon-mildew pathogens and identify germplasms/breeding lines with high disease resistance.
Several possible reasons could be proposed to explain this divergence in growth responses of P. xanthii upon LM and MF inoculations. Firstly, the more suitable microenvironment, particularly the parameter of RH that is considered a determinant environmental factor in powdery mildew development[29−31], might be maintained on the leaf surface of melon seedlings inoculated by the MF method. In contrast, suspension buffers used in LM inoculation might impose negative influences on the microenvironment, such as the increased RH, on the leaf surface of infected seedlings, thus restricting the development of P. xanthii. Also, some unexpected consequences, such as spore rupture resulting from excessive water absorption[32,33], might occur when mildew pathogens are prepared in suspension buffers, while these damages are largely circumvented in MF inoculation. Additionally, the inoculation homogeneity, of which the MF method commonly displays better performance than that of the LM method[6], might account for this divergence in pathogen growth on P. xanthii-infected melon seedlings via both strategies to some extent.
-
In this study, it is presented that powdery mildew, one of the most devastating fungal diseases was substantially promoted in P. xnathii-infected melon seedlings via MF inoculation rather than the commonly used LM inoculation, thus revealing the great usage potential of MF inoculation in high-efficiency detection of melon-powdery mildew interactions. These observations shed new methodological insights into exploring the interplay between melon plants and P. xanthii pathogens, as well as could benefit the future pathogenicity assay in this field.
-
The authors confirm contributions to the paper as follows: study conception and design: Yang X, Shi Q; data collection: Wang J, Wang S; analysis and interpretation of results: Wang J, Wang S, Yang X; technical assistance: Guo Y, Hu Z, Yin M; draft manuscript preparation: Wang J, Wang S; manuscript revision: Yang X. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated and/or analyzed during the current study are available from the correspondence author on reasonable request.
This work was supported by National Natural Science Foundation of China (32272795), the Taishan Scholar Youth Expert Program of Shandong Province (tsqnz20230605), Natural Science Foundation of Shandong Province (ZR2022MC029), and the Agricultural Variety Improvement Project of Shandong Province (2022LZGCQY006).
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Jianquan Wang, Shuoshuo Wang
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Wang J, Wang S, Guo Y, Hu Z, Yin M, et al. 2024. Efficient detection of melon-powdery mildew interactions by a medium-free inoculation. Vegetable Research 4: e024 doi: 10.48130/vegres-0024-0022
Efficient detection of melon-powdery mildew interactions by a medium-free inoculation
- Received: 23 February 2024
- Revised: 05 June 2024
- Accepted: 11 June 2024
- Published online: 05 August 2024
Abstract: To investigate the interplay between host plants and fungal pathogens, two inoculation strategies, liquid-mediated (LM) and medium-free (MF) ones are commonly used. For the LM strategy, host plants are infected by spraying of target pathogens that are suspended in proper solutions, while the collected pathogens are directly blown onto host plants for MF inoculation. In contrast to the widespread application of LM strategy, MF inoculation has never been adopted in previous studies about melon-powdery mildew interactions. In this study, an MF strategy suitable for Cucumis melo L. was developed, and its effectiveness was evaluated with the seedlings of inbred line 'YJM'. qPCR results showed that in comparison to the LM group, the germination, and growth of Podosphaera xanthii (P. xanthii) pathogens were apparently promoted, and therefore, the disease symptoms were able to be detected more efficiently on the leaves of melon seedlings that were treated via MF inoculation. This stimulated pathogen development on MF-treated leaves was further supported by microscopic observations relative to LM-treated ones, revealing the great potential of MF inoculation in high-efficiency detection of the interplay between melon and P. xanthii. Altogether, the present results shed new methodological insights into melon-powdery mildew interactions and could contribute to pathogenicity assays in this field.












