-
Non-destructive and rapid meat analytical techniques have grown in popularity. Nuclear magnetic resonance spectroscopy (NMR) is a versatile and powerful non-destructive technique that can be used to determine the properties of solid/semi-solid foods. Due to its versatility, an NMR system is often utilized to analyze various food components. Its quantitative nature allows the food industry to formulate effective solutions. Not only does it provide useful information on the structure of food, but also its quantitative nature allows the food industry to develop effective solutions. It can analyze a food sample in less than two minutes. Despite the increasing popularity of NMR among food scientists, it is still considered an under utilized method due to its high cost and low sensitivity.
An NMR system is a type of laboratory device that uses a magnetic field to generate electromagnetic signals. Signal generation begins with the perturbation of sample nuclei due to oscillations caused by a magnetic field. An NMR system can assess the chemical shifts in nuclei when a magnetic field is applied[1]. The area of the NMR signal is directly linked with the number of nuclei that aid with thorough and reliable analysis. Thus, NMR spectroscopy provides insight into the regio/stereo chemistry of a substance as it serves as one of the rarest techniques applicable for crystallized compounds. The sensitivity of this technique is associated with the presence of magnetic atoms in the samples. Since meat products have high contents of 1H, an NMR system can be utilized to evaluate fresh meat by exciting 1H spin.
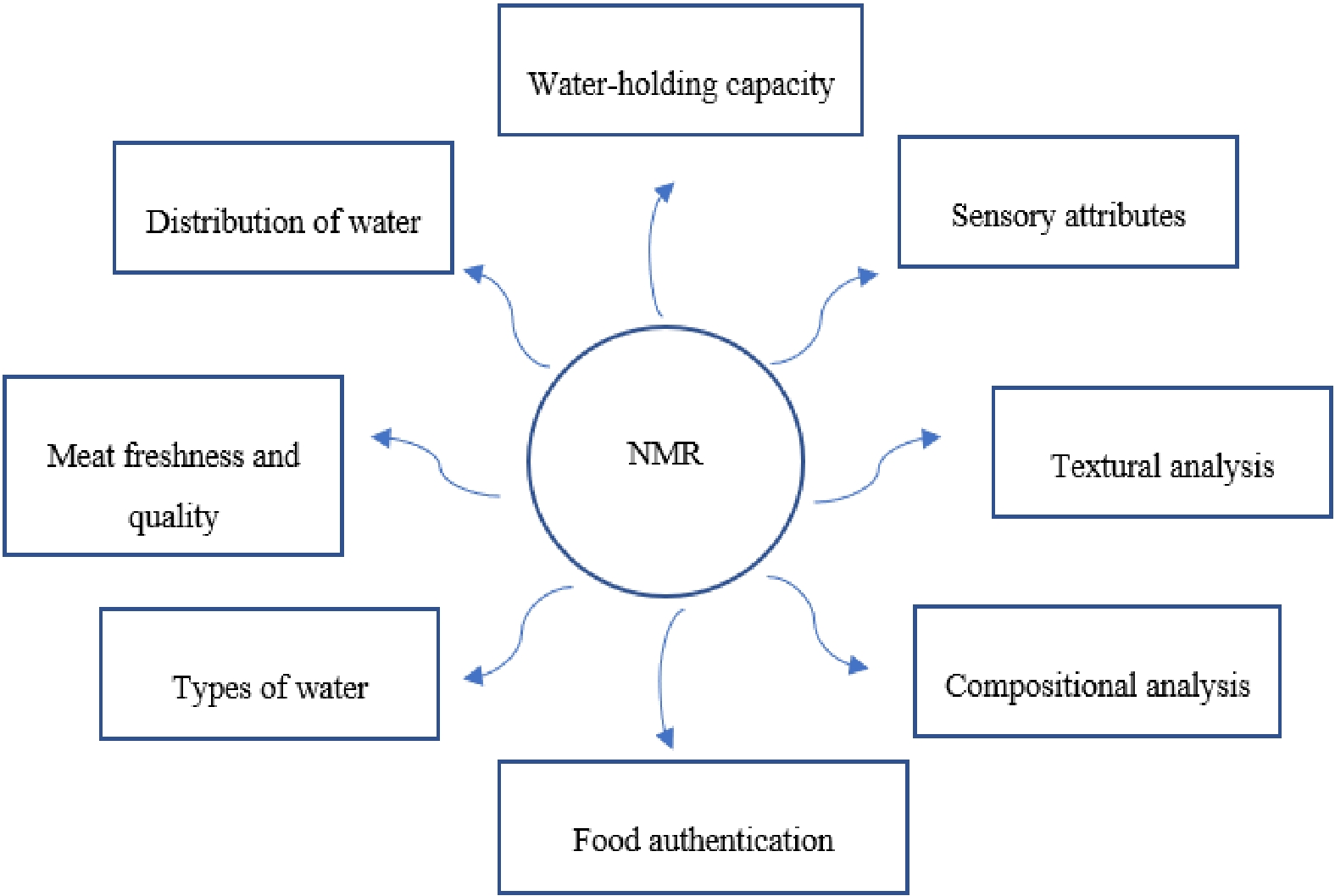
By the 20th century, NMR was used for the study of solid-state matrices. Initially, it was only used for the analysis of moisture in food samples. However, subsequently, NMR systems have been widely used to assess food quality, authentication and classification, evaluation of sensory characteristics, and assessment of molecular mechanisms involved in food production[2]. Various studies related to the analysis of compounds in meat have been successfully carried out using NMR[3]. NMR systems have been utilized to assess the changes in the overall quality of meat, poultry and fish products due to post-mortem, as well as due to thermal or non-thermal processing. Besides the rapid determination of water and fat percentages in the muscle systems, this analytical method can also assess the fat and water distribution dynamics[4]. In addition, 1H-NMR technology can also assess their subsequent interactions with the adjacent macromolecules while ensuring maximum accuracy[5]. The correlation between water distribution and meat quality has also been established in previous studies[6]. Furthermore, NMR has allowed detailed analysis of the intrinsic and extrinsic properties. The intrinsic properties that can be assessed using NMR systems include inquiries related to the animal development stage and muscle type. The extrinsic factors are related to processing and storage conditions including time and temperature combinations that influence the physicochemical behavior of meat. Unlike other spectroscopy techniques, NMR can be performed in optimum physiological conditions, such as salt concentration, pH or temperature. Moreover, it requires low room-temperatures to ensure accuracy. Thus, this technique has huge potential in the meat industry (Fig. 1).
-
Convenient hardware, easy-to-use software, cryoprobes and other technological advances in NMR such as multinuclear/multidimensional and solvent suppression have widely promoted its applications in food. The advantages of NMR spectroscopy over other technologies provide remarkable measures that assure the high quality of food products. Moreover, this technique also helps the food industry improve food safety and nutrition. The non-destructive character and high accuracy of NMR are helpful to thoroughly examine various food components, thereby contributing to tremendous advances in the food industry. Therefore, NMR can be used to evaluate meat quality parameters due to its reliability and rapidity[7] as illustrated in Table 1.
Table 1. Use of NMR in meat quality assessment.
NMR technique Food examination Reference Low field nuclear magnetic resonance Ice crystal sizes and water and oil migration during frozen storage and cooking of stuffed fish balls Fan et al.[66] 1H NMR Metabolic profile analysis of ground beef at different irradiation doses Zanardi et al.[67] High resolution NMR Lipid oxidation in Italian dry-cured α-tocopherol enriched pork sausages Siciliano[68] 23Na nuclear magnetic resonance spectroscopy Assessment of the sodium binding state in salted pasta, chicken, carrot and trout El Sabbagh et al.[69] 60 MHz 1H NMR spectroscopy Authentication of beef versus horse meat Jakes et al.[23] 1H NMR spectroscopy Lipid profile analysis of pig-bred and traditionally reared categories of Iberian dry-cured hams Pajuelo et al.[ 70] 1H NMR based metabolomics Identification of geographical origin of beef Jung et al.[44] 1H NMR spectroscopy Evaluation of changes in polar metabolite concentrations in beef during storage Graham et al.[ 46] NMR Quantitation of moisture and structural alterations in chicken meat cooked by convection Shaarani et al.[27] NMR spectroscopy Evaluation of the effects of irradiation dose (0, 1, 2 and 6 kGy) and storage time (1, 6 and 12 days) on the metabolic changes in pork tenderloin García-García et al.[ 71] 1H NMR Determination of cyclopropane fatty acids in meat and fish Lolli et al.[60] NMR Sensory evaluation of chicken and fish protein hydrolysates Steinsholm et al.[72] LF NMR Water component analysis of meat during cooking Micklander et al.[73] LF NMR Effect of thawing on the quality of frozen rabbit meat Jia et al.[74] LF NMR Moisture content determination of beef Jakes et al.[23] LF NMR Determination of water activity in chicken Venturi et al.[75] High-resolution magic angle spinning NMR (HR-MAS) is an excellent technique for the analysis of solid food samples. It allows the simultaneous analysis of both semi-solid and solid food samples without solvent extractions. It is advantageous for the analysis of samples in their natural state[8]. In terms of quality control, NMR spectroscopy has been utilized for various applications such as monitoring the stability and shelf life of food as well as studying various process effects. In 2018, a study was carried out to determine the quality of salmon after it was stored under varying conditions for 2 weeks. The researchers were able to monitor the changes during storage at 0 and 4 °C through NMR[9]. Moreover, due to the wide spectral overlaps that occur in 1H NMR spectra, 13C NMR is becoming an attractive technique for the analysis of food components. Preliminary studies demonstrate its effectiveness in the evaluation of the chemical composition of meat and meat products[10].
-
Various studies have been carried out on fresh meat using the use of nuclear magnetic resonance systems with CPMG as pulse sequence and T2 as relaxation time. NMR relaxation data can be used to study the changes in muscle fibers associated with thermal treatment. The reduction of water content and alteration of molecular interactions during cooking can be indicated as variations in T2 values[11]. The results of these studies show that this method can identify the main physiological parameters of fresh meat and its effects on cooking and freezing. The relaxation times of meats obtained from four different animals during several developmental stages were quantified. The scientists successfully identified three distinct water populations that were associated with different spin-spin relaxation times (T2). The study discovered that muscle type and developmental stage profoundly dictate the T2 data. Moreover, variation in relaxation times at different growth stages was associated with protein content. The results of the study revealed a linkage between NMR relaxation data and the muscle microstructure[12]. Scientists evaluated the effects of low-field NMR on the quality of hake during a freezing storage period of 6 months. They discovered that the three different water populations in the compartments exhibited varying levels of bound and free water. The researchers attributed the increase in free water to the release of water from the cellular compartments. It also affected the fish's holding capacity. A linear model based on the relaxation times T21 and T22 revealed that the water release was associated with the storage time (R2 = 0.98). Sánchez-Alonso et al. explored the use of NMR to analyze the effects of varying freezing conditions (walk-in freezing, liquid nitrogen flushing, or air blast freezing at −10 and −20 °C) on the quality attributes of fish[13,14]. Although the relaxation times of unfrozen samples were the same as those of frozen ones, the T21 protein increased at −10 °C. The increase was associated with the free water content of the samples. The freezing conditions did not change the quality of the fish, however, water movement during freezing was considered a benchmark to evaluate the quality deterioration of the fish.
The relaxation times of a sample can be used to measure the longitudinal (T1) and transverse (T2) relaxation times of the exciting signal emitted by a particular component of electromagnetic radiation. Due to the varying components that contribute to the relaxation signal, it is possible to determine the proton diffusion using time-domain NMR[15]. Ingestion of carrageenan to shrimps can reduce the spin-spin relaxation rates. LF-NMR determines the quality control parameters including WHC and its effect on the recovery of chicken meat with T1 and T2 relaxation time[16]; texture and quality of frozen fish using T2 relaxation time analysis[13]. In a study by Greiff et al.[17], 0−3.0% salt hake basing was identified on PCA using low-field NMR transverse relaxation time (T2). The researchers concluded that low-salt meat products can be investigated for their nutritive and sensory properties through 1H LF-NMR.
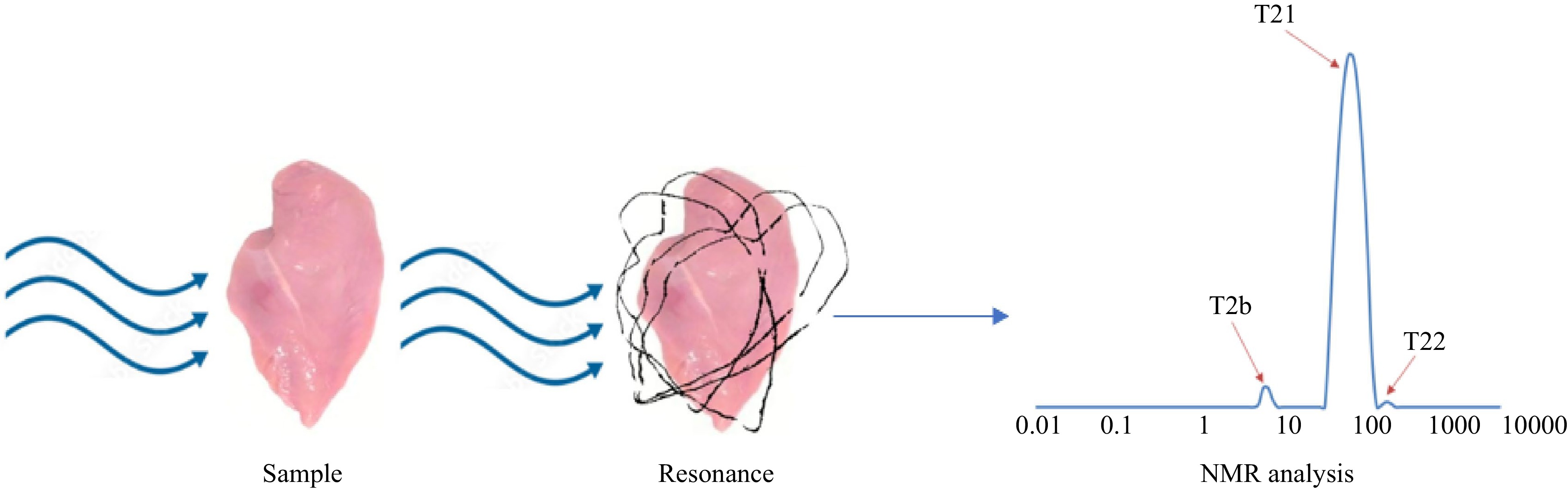
The observed water mobility of the stored salted sardines was computed by using the LF-NMR technique. It was revealed that the three components of T2 exhibited different relaxation times; 11.2−17.5 ms (pool I), 35.3−44.7 ms (pool II) and 161.7−256.5 ms (pool III)[18]. The analysis of protein hydration states corresponded to the evaluation of the motion of water in muscle foods by the addition of fat and water. It was found that the three relaxation components, T2b and T21 (0−10 ms), as well as T22 (40−70 ms), were either protons in macromolecules or water and fat mixtures with meat protein (Fig. 2). Heat-treated meat batters yielded higher concentrations of T23 (200−300 ms) specifically in meat containing higher concentrations of water as compared to fat[19].
Frequencies
-
A radio frequency for high-frequency and low-frequency NMR systems is within the range of 30 MHz and 100 MHz, and > 100 MHz, respectively. They represent acquisition sequence, an antenna, relaxation time and a magnetic field. The most commonly used sequence for fresh meat studies was the Carr-Meiboom-Gill (CPMG) sequence which measures the relaxation time intensity of the nucleus. There are also various acquisition sequences like Total Correlation Spectroscopy Spectrum (TOCSY), Echo Planar (EP), or Fourier Transform (FT) that can be used for estimating the amplitude of a spectrometer[20]. Some NMR systems are commercially available for food analysis, such as The FoodScreenerTM (Bruker, Billerica, Massachusetts, USA), which can perform authentication of honey or compositional analysis of juice, wine and meat. The use of low-frequency NMR for food analysis was initially tested to investigate the relationship between temperature (62–75 °C) and water distribution, as well as their influence on the sensory properties in pork samples[21]. The results indicated that the cooking temperature had a significant effect on the sensory attributes measured by the spectral data with high correlation coefficients i.e. tenderness (R2 = 0.87) and juiciness (R2 = 0.82). Higher values for tenderness and juiciness were obtained from samples that were cooked at 62 °C instead of 75 °C.
Preliminary studies have investigated a strong correlation between the T2 signal intensity and water activity (R > 0.90). Likewise, water content (R = 0.953) and the cooking loss (R = 0.986) of chicken breast were associated with the T2 intensity and the moisture content 12. In addition, beef samples were evaluated for the relationship between the moisture content and the T2 intensity values using NMR. A strong correlation coefficient (> 0.96) denoted that water distribution in the samples could be attributed to the ability of NMR to map the water distribution[22]. The use of the LF-NMR system (60 MHz) was studied to determine the classification of horse and beef mixtures. Results revealed that over 90% of the samples were correctly classified. The high sensitivity of the system presented a strong probability of preventing food fraud[23].
The use of high-frequency molecular recognition (HF-NMR) has been rare in fresh meat. A study published by Liu et al.[24] revealed its capacity to identify 22 metabolites in duck samples. The results of the study demonstrated an accuracy of the classification to be greater than 60%. Moreover, it differentiated among different meat samples by detecting the maximum metabolites of 27-, 50-, 170- and 500-day old duck meat samples. However, fatty acids were not detected in the experiment. Thus, further studies are needed to analyze the composition of the duck meat.
-
Water-holding capacity primarily determines the nutritional quality and sensory attributes of the meat[25]. Thorough identification of the water distribution throughout the skeletal muscle assists food scientists and technologists in better understanding the mechanisms that influence the meat quality and processing yield. Handheld 1H TD NMR sensors measure the water holding capacity of various food items. They can also be used to monitor the changes in water mobility within the food which has been widely studied as a subject of interest to analyze meat quality. NMR is a rapid and noninvasive technique that enables the differentiation of aquatic products through the analysis of water mobility in meat[26]. NMR indicated three different styles of moisture compartmentalization, indicated by three different T2 values i.e. long T21 constituent linked with the presence of moisture between muscle fibers, medium T22 constituent indicating myofibrillar protein densities and short T23 part representing association with other macromolecules. Out of these three components, T21 extracted from the main muscle component demonstrated an increase due to changes linked with protein denaturation. Water loss was revealed as the reduction in M0 and protein density. The decrease in M0 and protein density values was accepted as an indicator of moisture loss[27]. In another study, scientists discovered that the Penicillium roqueforti bacteria enhanced the water holding strength of chicken breast meat due to the increment of the intra-myofibrillar water content, as found by Guo et al.[28]. Similarly, Straadt et al.[29] utilized NMR to explore the relationship between the WHC and the water distribution of cooked loins after aging for different durations. The increasing days of aging were associated with the higher levels of spectral peaks which represented structural changes in myofibrillar proteins. In another study, Herrero et al.[30] used NMR to analyze muscle moisture distribution and the correlation between various physical and chemical parameters of beef using three different models including fibrinogen-thrombin gels (FTG), meat added with fibrinogen-thrombin (ME-FT) and meat emulsions (ME). The data of T2, T1 and apparent diffusion coefficient revealed that the various systems with thrombin and fibrinogen (FTG and ME-FT) exhibited a structure with bulk water, large pores and higher motion of water.
Freshness evaluation
-
NMR has also been used to evaluate the freshness of various meat products. Previously, Tan et al.[31] used MVSA instead of NMR to evaluate fish freshness. In another study targeting the quality evaluation of beef, 1H NMR-based metabolomics was used to evaluate the beef quality. It was found that NMR could be used to improve the management of aging processes to enhance the quality of beef[32].
Assessment of freeze-thaw cycles
-
NMR has been used to study the effects of freezing on the quality-associated changes in meat governed by changes in water state, movement and compartmentalization in muscle fibers. In an experiment by Li et al.[33], the effect of freeze-thaw cycles was examined on the quality and physicochemical behavior of chicken meat using the low-field NMR technique. The samples revealed a rise in purge loss with increasing thawing duration and freeze-thaw cycles, which indicated the presence of loosely bound water in the samples. In addition, T22 revealed a significant association with sensory attributes, freeze-thaw cycles and thawing time with correlation coefficients of 0.731, 0.722 and −0.731, respectively. Li et al.[34] proposed that the freezing-thawing chicken breasts subjected to high-pressure conditions affected the WHC and cooking loss using NMR with an inverse relationship between the two parameters (WHC R = −0.707, cooking loss R = −0.920). The effects of thawing and post-thawing on various quality parameters including color, WHC, texture and lipid oxidation of rabbit meat have been studied using LF-NMR. The results revealed that the water distribution exerted a significant linkage with meat proteins with a strong correlation of 0.95[34]. Moreover, freeze-thaw cycles had a negative effect on the water-holding potential of meat thereby propagating drip loss.
Food authentication
-
Food authentication is also done through the use of NMR sensors. NMR is a fairly new technology that encompasses the majority of its applications related to food authentication. Capturing the true authenticity of food products contributes to a higher market gain and nutritional value, thus it is of utmost importance to the producers and consumers. However, the final food composition is determined by geographical origin, breed, adulteration and harvest year, which make it a challenging process[35]. High-field NMR spectroscopy has been widely used for detecting adulteration due to its ability to provide a fingerprint spectrum[36]. Low-field (LF) and portable TD NMR instruments are becoming more prevalent in detecting adulterations in food. Wang et al.[37] used NMR to detect shrimp samples adulterated with gel injections.
Due to the complexity of the food authentication process, multiple biomarkers are not always adequate to classify a food as authentic or nonauthentic. Usually, discrimination is achieved using a combination of MVSA and NMR as well as HR-MAS, which have wide applications in the analysis of meat and fish-based products. Many studies have verified the use of NMR for meat and fish[38−42]. LF-NMR spectroscopy was used to detect the presence of hydrocolloids in prawns. It was discovered that adulterated prawns can be identified using T2 fitting curves using PCA[26]. Similarly, T2 decay analysis has also been used to identify adulterated pork samples[43].
A wide variety of meat products have been evaluated for their origins using 1H NMR combined with PCA and orthogonal projection to latent structure discriminant analysis (OPLS-DA). Jung et al.[44] conducted a study to identify the origin of beef and discovered that the beef extracts collected from different countries such as Australia, Korea, the United States, and New Zealand were remarkably different in terms of nutrition. Moreover, 1H NMR spectra and OPLS-DA analysis revealed that amino acids i.e. valine, leucine, isoleucine, methionine and tyrosine, and succinate were mainly involved in discriminating the origin of beef. The origin of salmon was then analyzed using support vector machines (SVMs) and 1H NMR. The study results showed that combining 1H NMR and SVMs can help prevent fraud from originating from salmon farms by discriminating against wild and farmed salmon[45]. Due to the increasing awareness of BSE, the geographical origin of beef has become an issue for individuals. Multivariate statistical analyses and 1H NMR spectroscopy to identify the origin of beef was carried out by Jung et al.[44]. Through the use of metabolite profiling, it was possible to identify various markers that indicate the origin of the beef.
Compositional analysis
-
NMR allows the analysis of various physiological and biochemical parameters of meat. It is also used to study the compositional analysis including muscle fiber type, adipose tissue, connective tissue and carcass composition of various tissue components. These parameters have been linked to various meat properties such as pH, moisture, texture, and sensory attributes. It was demonstrated that 1H-NMR could detect the changes in the amino acids and sugars during the postmortem aging of beef[46]. NMR requires minimal sample preparation and can detect the effects of aging on the concentration of various metabolites in meat.
Post-mortem aging and rigor mortis
-
Scientists have examined the effects of antemortem handling and post-mortem aging on the muscles of slaughtered animals by using NMR. The utilization of low-field 1HNMR in the determination of the effect of salting, fillet location and antemortem handling on the fish quality has elaborated its scope in the meat industry. TD NMR investigations have also been conducted to study drip loss in pork loin chops[47], water migration on the postmortem development of yellowfin tuna[48] and evaluation of physicochemical changes in tuna during cold-chain storage[49]. Gudjonsdottir et al.[50] investigated the use of NMR in fish processing methods. One of the most commonly traditionally employed in fish preservation is salting. NMR indicated the irreversible effect of salting and rehydration on the water distribution in cod muscle tissues that resulted in protein denaturation of meat. According to the study by Aursand et al.[4], it was revealed that rested fish demonstrated less severe rigor mortis and hence a better quality as compared to the exhausted fish. The results indicated that the water conditions of fish with exhausted bodies were significantly different from those with salted ones. It was hypothesized that the higher salt concentration in the fish's body was caused by the muscles' contraction during exhaustion. Also, the lower pH level during rigor mortis caused the fish to have higher water content. Various other studies have been conducted using NMR in meat processing methods that influence the quality characteristics of meat during post-mortem aging. These involve assessment of the effects of multiple freeze cycles on beef quality[51], moisture migration within beef during storage, the effect of vacuum-freeze drying on the moisture migration in shrimps[52] and drip loss evaluation of the stored and vacuum-packaged beef[53].
Changes in the metabolome
-
Various studies have been published to identify changes in the metabolome caused by a nutritional approach using NMR. An unsupervised multivariate analysis method using NMR has been utilized to study the effects of varying environmental conditions on the technological traits of meat including water-holding capacity, drip- and cook-losses of meat[54]. Yang et al.[55] researched the effects of high-pressure processing on the sensory properties of marinated meat-based soy sauce. The study focused on the combination of 1D and 2D NMR with MVSA and sensory evaluation to access the effect of two high-pressure methodologies (150 and 300 MPa) on the sensory attributes of marinated meat. It was found that the process led to an increase in taste-related metabolites, though minor dependence on the pressure levels was observed.
Evaluation of fat content and fatty acids
-
1H NMR has the potential to determine the quality of various food products based on their fat composition. It can also detect the presence of primary and secondary lipid oxidation products that can cause oxidative damage to virgin olive oil. Other studies have also highlighted the similar use of NMR in meat fat and different oils[56]. Aside from providing analytical data, NMR can also be used to provide stockbreeding information. For instance, a study conducted by Jaturasitha et al.[57] revealed that a chicken's genetic selection was based on its fat composition. Another study by Nestor et al.[58] demonstrated the presence of the EPA and DHA in Arctic char using this technique. To improve the efficiency of TD NMR measurement times, a handheld sensor has been developed that works as a TD proton MR scanner to accurately quantify the fat of the fresh tuna meat without damaging the product. The relaxation data were obtained using a phase-alternated pair stack (PAPS) CPMG pulse sequence. Eleven sections were scanned in a 12-mm depth probe. The results indicated that the fish's fat by MR sensor yielded a reasonable value[59]. A quantitative analysis of the cyclopropane fatty acids found in various food matrices including meat, fish and dairy products was performed by Lolli et al.[60]. 1H-NMR was used to study the fatty acid profile of chicken meat irradiated with gamma-rays at 0.5−15.0 kGy[56]. Various components of smoked salmon, including the docosahexaenoic acid, macro and micro nutritional components and polyunsaturated fatty acids have been identified through NMR spectroscopy. These methods avoid the need for chemical pre-treatment and extraction and enable the identification of omega-3 fatty acids for processed fish products.
Effect of various processing treatments
-
For the study of non-irradiated beef samples, the data was analyzed using an artificial neural network (ANN) combined with linear discriminant analysis (LDA). Through follow-up analyses, the presence of irradiated beef and non-irradiated beef was identified. The scientists were able to determine the dose of radiation in meat using both techniques[61]. On the contrary, Santos et al.[62] used time-domain 1H Nuclear Magnetic Resonance (TD-NMR) using CPMG and CWFP sequences to discriminate between different sex and race of the bulls. The researchers were able to identify the origin of the beef samples by combining the data from TD-NMR and the PLS-DA. They also made use of the same technology to authenticate aquatic products such as salmon and hake. The researchers concluded that combining the data from these two analytical methods can serve as a reliable tool for determining the origin of beef samples. They also found that NMR can be utilized to discriminate aquatic products such as hake, prawn and salmon. In another study, the effects of ultrasound pretreatment (25, 33 and 45 kHz, 30 min) on the water distribution in beef jerky were studied using the LF-NMR technique. The three peaks revealed bonding with different kinds of water, which included bound water (T2b, 0−10 ms), water in the myofibrillar matrix (T21, 10−100 ms) and free water (T22, > 100 ms) as shown in Fig. 2. The results indicated that the LF-NMR technique can be used to evaluate the water mobility of meat and fish products[63]. In addition, the effects of freeze-thaw cycles and other meat processing treatments have also been investigated using NMR[33].
-
Due to the uncertainties involved in the accuracy and precision of the water content analysis techniques based on NMR, comparative studies have been conducted to examine the various methods used to estimate the moisture content of various meat products. The results of these studies were compared to those obtained using the official methods of the AOAC which are based on oven drying of samples. The NMR results of these studies revealed that the moisture content of various meat types is in accordance with the results obtained using the AOAC Official Methods[64]. However, since no particular method is ideal, the combination of analytical methods with NMR can provide better results. For instance, a combination of NMR and oven-drying can determine the moisture content of various meat products such as turkey, chicken, beef, pork, and ham. The researchers compared the results of the different methods with those obtained by AOAC including the moisture and fat determination methods. NMR and AOAC combination methods have been associated with yielding more consistent results than those achieved by using only one method[65].
-
NMR is a powerful, non-destructive and rapid analytical technique that can accurately analyze a meat sample in less than two minutes for meat quality traits including water holding capacity, water distribution in muscles, meat authentication, determination of fat content, post mortem aging, rigor mortis, and changes in metabolome, among others. It can also be used to assess the effect of various processing methods on meat during thermal, ultrasonic and radiation processing. Generally, T2 relaxation data is exploited against various quality traits, and with the help of algorithms, useful results can be deduced. It has been comprehensively assessed for quality determination of meat and meat products over recent decades, and results suggest that it has great potential for industrial adaptation of real-time quality assessment. NMR relaxometry has gained reasonable popularity among food scientists, yet it has been under utilized in the meat industry and in the labs of food regulatory authorities. Cost, as well as optimization of methods based on various algorithms, particularly, artificial intelligence (AI) methods are the major limiting factors. With the rising concerns of consumers regarding safety, authentication of meat, and nutrition, there is a dire need to use NMR relaxometry for rapid meat assessment by finding new and cost-effective methods, as well as use of AI. Thus, NMR relaxometry has great potential for future use in the meat industry.
The authors are thankful to Pakistan Science Foundation for granting the project PSF/NSLP/P-IU(837) for promoting scientific effort in the department of Food Science & Technology, Faculty of Agriculture & Environment, The Islamia University of Bahawalpur, Pakistan.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Khan MA, Ahmad B, Kamboh AA, Qadeer Z. 2022. Use of NMR relaxometry for determination of meat properties: a brief review. Food Materials Research 2:8 doi: 10.48130/FMR-2022-0008
Use of NMR relaxometry for determination of meat properties: a brief review
- Received: 14 March 2022
- Accepted: 13 May 2022
- Published online: 28 June 2022
Abstract: Nuclear magnetic resonance spectroscopy (NMR) is a non-destructive and rapid meat analytical technique. Since meat has high proton content, the quality of fresh meat can be assessed by exciting proton spin using NMR laboratory apparatus. NMR allows the assessment of intrinsic properties including animal development stages and muscle type, as well as extrinsic factors including processing and storage conditions that affect the technological traits of meat. It can be used to determine the water-holding capacity of meat, water distribution in muscles, meat authentication, fat content, post mortem aging, rigor mortis, and changes in metabolomes, among others. Changes in NMR relaxation times are associated with the changes in muscle components and their distribution as affected by various processing conditions. Since, industrial assessment is now demanding rapidity and real-time assessment, it is therefore essential to evaluate the potential of NMR in contrast to other techniques. Therefore, this review article presents the principle of working of NMR, various methods available, quality traits of meat that can be evaluated using this technique, and a brief history. This review article will help the industry to adopt novel NMR-based meat quality assessment tools for rapid quality determinations.
-
Key words:
- NMR /
- LF-NMR /
- Meat quality /
- Meat authentication /
- 1H NMR