HTML
-
Cultured meat, also known as cultivated meat or lab grown meat, aims to produce meat from in vitro cell culture instead of conventional livestock slaughtering[1,2]. As an emerging cellular agriculture technology, the essence of producing cultured meat is to build muscle tissues based on animal tissue regeneration mechanisms. Thus, various tissue engineering technologies have been applied for cultured meat[3−5]. Although with many developments, it won't be hard to find that they can be classified into two categories, which are two typical difficulties of cultured meat. One is focused on promoting the differentiation of muscle cells, which can be helped by textured/patterned topographies or spatial confinements. The other is dedicated to constructing three-dimensional (3D) tissue structures by top-down or bottom-up approaches. Different from the top-down method that fabricates 3D structures directly, the bottom-up strategy is to first generate building blocks and then assemble them to achieve mass constructions. With these understandings, we will outline the cutting-edge tissue engineering strategies for cultured meat in this perspective from the classifications of textured scaffolds, 3D bioprinting, molding, patterning, and cell sheet engineering. Applied materials will also be introduced when discussing the engineered approaches. Finally, we will discuss the future prospects and challenges in this area.
-
With the advantages of abundant edible resources, 3D porous structure, good biocompatibility, and inexpensive cost, textured scaffolds are widely used for cultured meat. Based on the mechanism of contact guidance, the textured topography is able to promote the differentiation of muscle cells seeded on the structure. The textured scaffolds can be classified into two types, naturally derived plant/animal proteins and decellularized plant tissues.
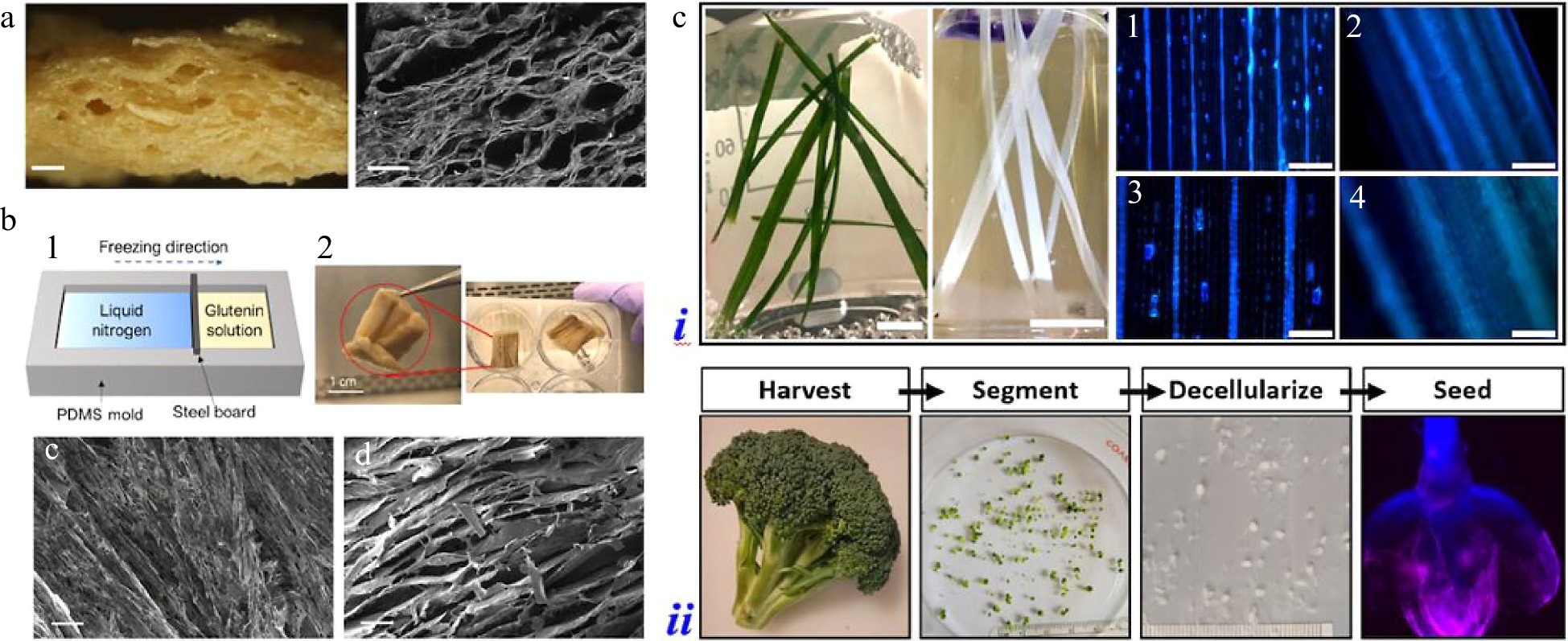
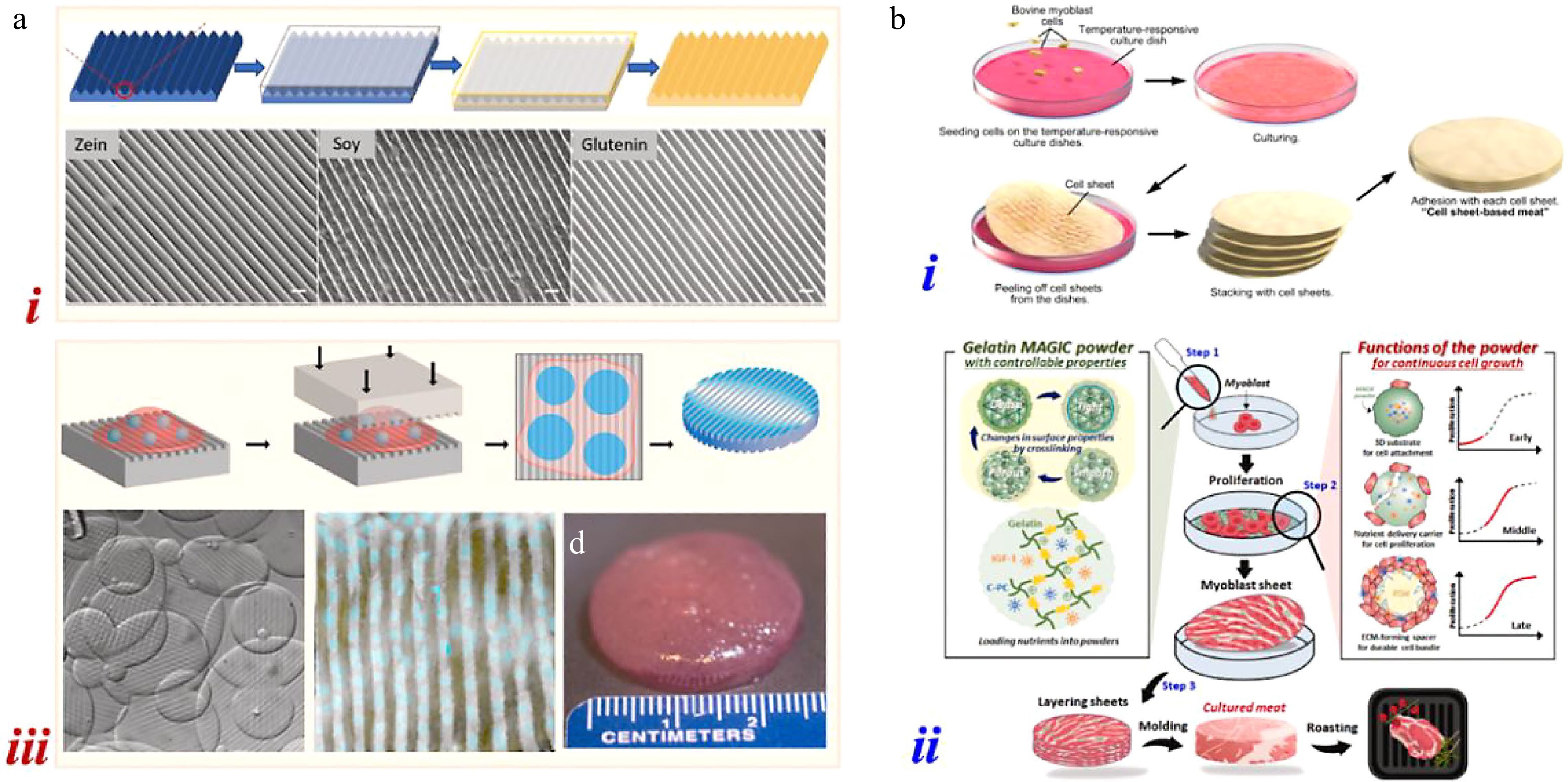
Ben-Arye et al. selected soy protein scaffolds for 3D bovine satellite cell (BSC) culture due to the edibility, suitable texture, and nutritional value, as shown in Fig. 1a[6]. After the adherence of BSCs, cell proliferation and differentiation were found on the scaffolds. Besides, the scaffold co-culture of BSCs, smooth muscle cells, and endothelial cells were investigated, which showed elevated myogenesis and extracellular matrix (ECM) deposition. Similarly, Song et al. (the Zhou group) applied peanut wire-drawing protein scaffolds for the culture of porcine adipose-derived mesenchymal stem cells, while Holmes et al. used homemade 'soda bread' as the scaffolding material to construct cultured meat[7,8]. For better control of the microstructures, Xiang et al. fabricated glutenin scaffolds with fibrous aligned structures with the method of physical cross-linking and directional freeze-drying (Fig. 1b)[9]. The aligned topography was demonstrated to promote the cell alignment and the formation of myotubes via the mechanism of contact guidance. Interestingly, some researchers expressed reservations about the cell adhesive capacity of these plant protein derived scaffolds because of the lack of arginine-glycine-aspartic acid (RGD) sequence. Thus, Lee et al. coated textured vegetable protein with the fish gelatin/agar matrix to introduce a better cell adhesive environment[10]. With this surface modification, they aimed to obtain animal and plant protein-based hybrid cultured meat.
In addition to textured proteins, a ready-formed 3D decellularized scaffold with primarily cellulose backbones could also be used as the culture substrates. With the cost-effective, sustainable, and ethical input advantages, various plant tissues were chosen for decellularization, such as broccoli, sweet pepper, spinach leaves, and green onion. Allan et al. used decellularized amenity grass for in vitro myoblast culture, as shown in Fig. 1c[11]. The striated topography of the grass scaffold supported the alignment and differentiation of myoblasts, which were retained from its natural long narrow structure and parallel vasculature system. Jones et al. chose spinach leaves as the candidate due to the vascular network, which was promising for providing oxygen and nutrient access and maintaining the viability of seeded cells[12]. Broccoli florets were selected as well, which were used as decellularized microcarriers for scaling cell proliferation within reactors (Fig. 1c)[13]. From these examples it could be found that, besides the common reasons like edible, sustainable, and inexpensive merits, decellularized plant tissues were considered as potential scaffolds for cultured meat due to their unique structural characteristics.
-
3D bioprinting, known as an additive manufacturing technique combined with computer modeling and biomaterial engineering, has received increasing interest in the areas of tissue engineering and cultured meat. Based on the stacking layer principle, 3D bioprinting can be used to construct complicated structures for seeding or encapsulating muscle cells.
Su et al. presented a kind of prolamin scaffold with the method of electrohydrodynamic printing[14]. The scaffold was produced by prolamin-based inks which were made up of hordein/secalin and zein. With the highly ordered tessellated structure, porcine skeletal muscle satellite cells adhered, proliferated, and differentiated on the scaffold and further showed the upregulation of myogenic proteins. Ionavici et al. also fabricated plant protein-enriched scaffolds by extrusion-based printing[15]. The mixture of pea and soy protein isolates accompanied with RGD-modified alginate was extruded into agar support bath and printed with well-defined geometries, as shown in Fig. 2ai. In addition to the scaffold that could support the attachment and differentiation of BSCs, they also demonstrated the potential of the pea protein-based material as suitable bioinks for developing cellular constructs. Different from the printed scaffolds which seeded cells on the surfaces, 3D cellular constructs were fabricated by encapsulating cells in bioinks, which required good cell viability over time.
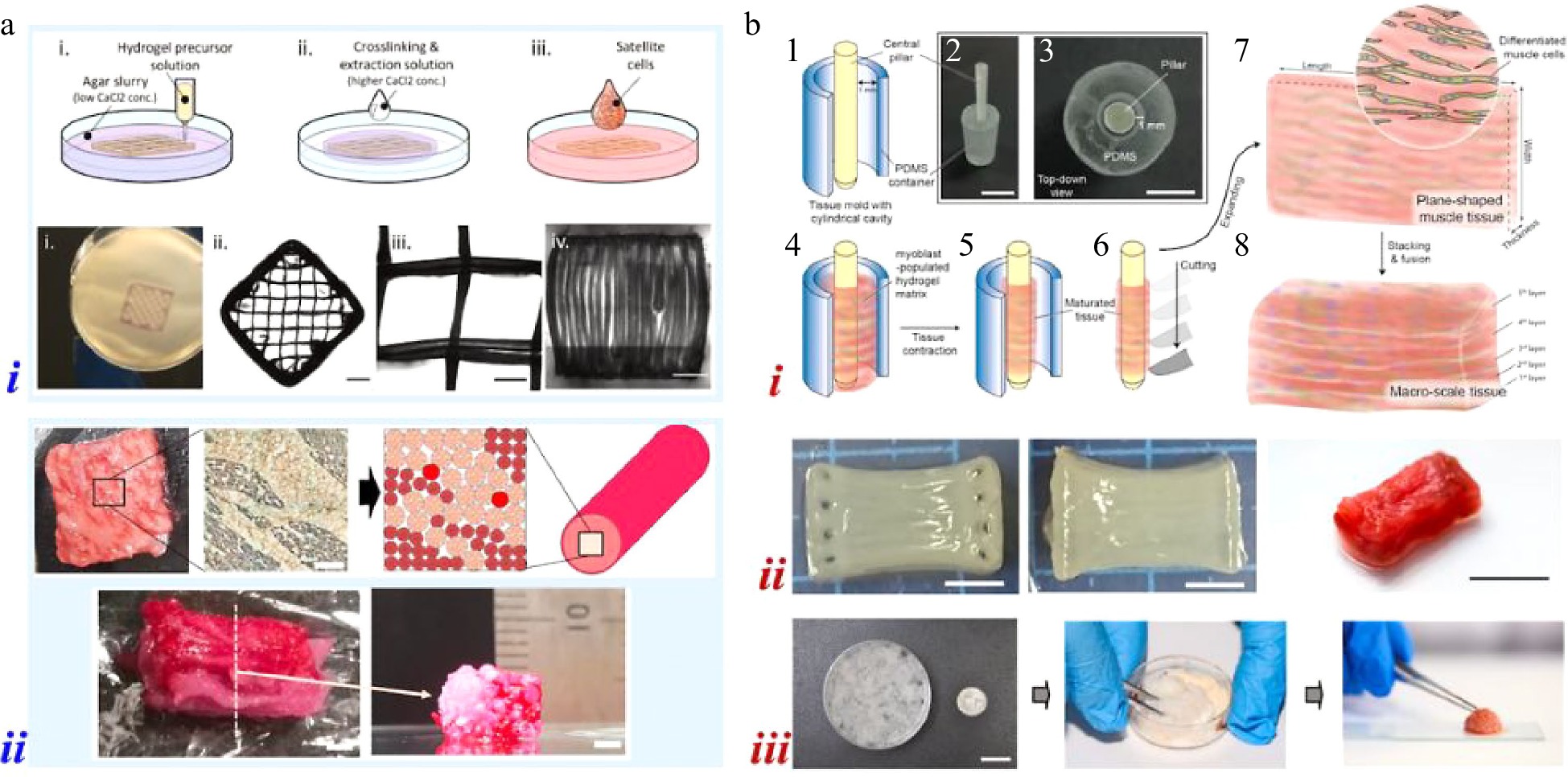
Figure 2.
(a) 3D printing for building muscle tissues: (i) printing of plant protein-enriched scaffolds[15]; (ii) assembly of bovine muscle, fat, and vessel cell fibers[17]. (b) Hydrogel molding for building muscle tissues: (i) mold of a cylindrical container and a central pillar[19]; (ii) pillar immobilized mold[20]; (iii) the first cultured meatball in China[21].
Similarly, Deb Dutta et al. applied the mixture of alginate, gelatin, and plant/insect protein hydrolysates as the bioink for developing 3D bioprinted hydrogel scaffolds encapsulated with BSCs[16]. Furthermore, Kang et al. used tendon-gel integrated bioprinting to build bovine muscle, fat, and vessel cell fibers (Fig. 2aii)[17]. Tendon-gel integrated bioprinting supported anchors for the printed cell fibers, improving the cell alignment and differentiation against contraction. The three different kinds of fibers were cultured separately and further assembled to construct whole cut meat-like tissues by mimicking their histological structures. In construct, Jeong et al. utilized MyoD and PPARγ2 transformed bovine embryonic fibroblast cells to produce steak-type cultured meat with the method of digital light processing-based printing[18]. The two types of transformed cells were mixed in the printing hydrogel and showed myogenesis and adipogenesis respectively by changing the culture medium. In this case, the 3D cell constructs were developed before the differentiation of cells.
-
In addition to 3D bioprinted structures, engineered films with cells either seeded on the surface or embedded inside can be stacked to form tissue constructs for cultured meat. Molding and patterning are two main approaches for engineering films. Molding provides anchors to counter the shrinkage of films which is caused by the cell contraction, while patterning creates a grooved surface to promote cell differentiation.
Nie et al. embedded muscle cells in collagen and Matrigel mixed hydrogels with the mold of a cylindrical polydimethylsiloxane (PDMS) container and a central pillar, as illustrated in Fig. 2bi[19]. The shrinkage of hydrogel induced by myoblast mature and contraction was countered by the pillar. Thus, plane-shaped muscle sheets could be acquired by cutting and expanding the matured tissue hydrogels and further stacking to construct macro-scale skeletal muscle tissues. They also fabricated 3D bovine muscle tissues using cell-laden hydrogels with striped structures, which were generated with the help of pillar immobilized PDMS molds (Fig. 2bii)[20]. The striped structure would promote the alignment of myocytes in hydrogels. Therefore, millimeter-thick muscle tissues containing contractile myotubes could be constructed by stacking the cell-loaded hydrogels. Zhu et al. and co-workers presented a similar work that used PDMS mold with repeating rectangular subunits to generate hydrogel networks[21]. By mixing 50 muscle hydrogel networks together with food additives, the first cultured meatball in China was produced, as shown in Fig. 2biii.
Similar to textured scaffolds, patterned films facilitate the differentiation of muscle cells based on the mechanism of contact guidance. Xiang et al. fabricated grooved films by casting edible hydrogel solutions on PDMS molds, which were replicated from patterned glass substrates (Fig. 3ai)[22]. Naturally derived protein and polysaccharide-based materials such as gelatin, glutenin, zein, alginate, and chitosan were selected for the films. With the patterned surface, BSCs were guided in alignment and showed differentiation on the edible films. Norris et al. also used striped PDMS molds to obtain patterned microcarriers for cultured meat, as shown in Fig. 3aii[23]. Compared to spherical microcarriers, grooved microcarriers promoted the proliferation, myotube alignment, and myogenesis of myoblasts. The obtained cell-microcarrier structures were then centrifugated to produce cultured meat products. Besides the mold assisted patterning of structure topology, films with fibrous surfaces could also be generated by immersion rotary jet spinning. MacQueen et al. spined gelatin fibers and then collected to form films with the help of a rotating reservoir[24]. Myoblasts aligned along the fibers, which contributed to the structural features and protein expression of the obtained meat analogs.
-
Different from the above approaches, cell sheet engineering is a kind of scaffold-free method that can generate cell sheets and then stack them to produce tissue-like structures for cultured meat. Shahin-Shamsabadi & Selvaganapathy fabricated adipocyte and myoblast cell sheets as building blocks for 3D cell mass[25]. With the differentiation of muscle cells, they would fuse together and thus formed a sheet that attached loosely to the culture plate. By changing the medium to a slightly acidic one, the sheet delaminated from the plate and kept the contracted condition. Stable and flat cell sheets were obtained when switched to a slightly basic medium and further assembled layer-by-layer to form meat-like constructs. Besides the medium assisted method, Tanaka et al. also used temperature-responsive culture dishes for the fabrication of cell sheets, as shown in Fig. 3bi[26]. By applying poly(N-isopropylacrylamide), the dish surface was made hydrophilic below 32 °C and hydrophobic at 37 °C. Thus, the cells that attached to each other would detach from the dish when the temperature decreased below 32 °C, resulting in the formation of cell sheets. Park et al. constructed cell sheets with the assistance of gelatin mass growth-inducing culture powder (Fig. 3bii)[27]. The powder provided 3D substrates for cells with nutrient supply and acted as ECM-forming spacers, which contributed to the generation of cell sheets. Cultured meat models were then developed by stacking and molding of obtained cell sheets.
-
Tissue engineering technology plays a critical role in cultured meat due to the essence of regenerating in vitro muscle tissues. However, because of the food characteristics of cultured meat, it is not feasible to copy the technologies without improvement and modification. Thus, some challenges remain to be addressed. Firstly, cheap, food-grade and effective materials are required. Naturally derived materials are either limited to cell adhesion or lack of non-animal sources. Synthetic materials, such as RGD-modified alginate that can provide adherence sites for cells, and gelatin methacrylate that helps with light-based printing, are controversial in the respect of edible properties. Materials that are specially tailored for cultured meat are still desired. The problem of cell viability in 3D tissue constructs also remains unsolved. Cells can only form matured muscle structures on the surfaces, as they will die due to insufficient oxygen and nutrients in the center. The stacking of edible films, microcarriers and cell sheets may overcome this problem, while they have to forgo the structure and mechanical performance. Furthermore, the regeneration of muscle tissue structure and composition has a large room for improvement, which limits the traditional meat characteristic retention of cultured meat. More up-to-date advanced tissue engineering strategies for cultured meat are anticipated.
This work was supported by the National Natural Science Foundation of China (Grant No. 22002061), the Natural Science Foundation of Jiangsu Province (Grant No. BK20200551), the Jiangsu Provincial Double-Innovation Doctor Program (Grant No. JSSCBS20210283), the Postdoctoral Science Foundation of China (Grant Nos. 2021M691615 and 2022T150326), and the Postdoctoral Science Foundation of Jiangsu Province (Grant No. 2021K011A).
-
Guanghong Zhou is the Editorial Board members of Journal Food Materials Research. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of these Editorial Board members and their research groups.
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
| Wang J, Ding X, Zhou G. 2022. Cutting-edge tissue engineering strategies for cultured meat. Food Materials Research 2:20 doi: 10.48130/FMR-2022-0020 |