-
Flavonoids are prominent polyphenols found in many plants, regulating plant growth and protection, and possessing a wide range of pharmacological activities[1]. Flowers of Hibiscus manihot L. (HMLF) are a nutritious and functional food of high economic value, which is beneficial to the prophylaxis and therapy of cardiovascular diseases[2]. As a tea drink in the market, it has also attracted the attention of consumers. After drinking HMLF tea, it can visibly relieve tension, help people relax and adjust their mentality. Moreover, because it is rich in collagen, vitamin E, unsaturated fatty acids and flavonoids, it is a preferred tea with high nutritional value. The concentration of flavonoids in HMLF is tens of times higher than that of other flavonoid-rich plants, making them the primary functional active components[3]. Our previous research has identified rutin, hyperin, isoquercetin, hibifolin, myricetin, quercetin-3′-O-glucoside and quercetin as the primary bioactive flavonoids in HMLF, exhibiting antioxidant, anti-inflammatory, antibacterial and cardioprotective properties[4,5]. Therefore, using eco-friendly and sustainable extraction media for rapid and effective incremental extraction of HMLF flavonoids holds tremendous research value.
Ionic liquids (ILs) have served as eco-friendly media for extracting natural products from plant materials because of their merits of low melting point, strong solubility, low vapor pressure and ease of recovery[6−8]. They are safer and more ecologically favorable than conventional toxic organic solvents. However, their high viscosity hinders the raw materials' dispersion, affecting the extraction efficacy of target constituents[9]. Integration of ILs with microwave techniques has been shown to successfully destroy plant tissue cell walls, release the bioactive components in the cells, and speed up the mass transfer to stimulate the extraction of different bioactive ingredients[10,11]. Other studies have also demonstrated the potential of ILs in combination with ultrasound and mechanochemical-assisted extraction methods for extracting various compounds from plant materials[12,13]. Therefore, ILs possess the potential in extracting natural flavonoids from HMLF and are worth investigating.
High-speed homogenization (HSH) is an ordinary technique with wide application in the preparation and processing of plant extracts for diet or healthy manufacturing[14,15]. The HSH works primarily by using a high-speed rotating head to disperse the particle material in the extraction solvent by generating an intense frequency mechanical effect of fluid shear and cavitation force. Simultaneously, because of its low energy consumption, minimal environmental problems, convenient manipulation and benign dispersancy effectiveness, HSH is widely applied in biopharmaceutical, pathological analysis and other fields[16,17]. Microwave-assisted extraction (MAE) is an environmentally friendly, low-energy and widely used advanced technology that accelerates the mass transfer of target compounds with microwave energy[18]. In the extraction process, the sample is heated thoroughly by convection, which leads to a shorter extraction time and higher extraction efficiency in comparison with conventional extraction techniques[19,20]. The established technique HSH-MAE, which integrates HSH and MAE, benefits from the high efficacy and thermal radiation of both HSH and microwave, which are advantageous for extracting natural bioactive substances.
In this research, IL-HSH-MAE was designed to extract seven target flavonoids from the flowers of H. manihot L., and single-factor experiments were used to maximize the extraction parameters, including the type and concentration of IL, homogenate speed and time. Also, Box-Behnken design (BBD)-response surface methodology (RSM) was utilized to demonstrate how three pivotal working parameters affected extraction efficiency. The dominance of the established IL-HSH-MAE method was proved by comparing it with other extraction methods (IL-HSH, IL-MAE, 60% ethanol-HSH-MAE and 60% ethanol-MAE). Therefore, IL-HSH-MAE possesses the potential for incremental and rapid extraction of target flavonoids and can be exploited as an efficient and green substitution for extracting natural products from plant matrices.
-
HMLF were gathered in Harbin (China), shade dried, comminuted (0.36 mm) and reposited at room temperature. Rutin (≥ 98%), hyperin (≥ 98%), isoquercetin (≥ 98%), hibifolin (≥ 98%), myricetin (≥ 98%), quercetin-3'-O-glucoside (≥ 98%), quercetin (≥ 98%), and HPLC-grade acetonitrile and
${\text {H}}_{\text{3}}{\text{PO}}_{\text{4}} $ Table 1. Ionic liquids used in this study.
Ionic liquid Anion Cation [C2mim]Br 1-ethyl-3-methylimidazolium Br− [C4mim]Br 1-butyl-3-methylimidazolium Br− [C6mim]Br 1-hexyl-3-methylimidazolium Br− [C8mim]Br 1-octyl-3-methylimidazolium Br− [C4mim]Cl 1-butyl-3-methylimidazolium Cl− [C4mim]BF4 1-butyl-3-methylimidazolium ${{\text{BF}^-_{ 4}}} $ [C4mim]H2PO4 1-butyl-3-methylimidazolium ${\text {H}_2{\text{PO}}_4^- }$ [C4mim]OH 1-butyl-3-methylimidazolium OH− Extraction device
-
A high-speed homogenization (HSH) device (IKA-T18, Germany) and microwave-assisted extraction (MAE) system were coupled for extracting target constituents from HMLF. The homogenate speed and time of the dispersion instrument were adjustable by rotating the knob on the dashboard to satisfy the trial criteria as represented by our preliminary study[17]. MAE was fulfilled on the same device according to our previous research[2].
Extraction procedure
-
HMLF (5.0 g) and a respective volume of ionic liquids aqueous solution or 60% ethanol were added into an extraction vessel for pretreatment with HSH under dark conditions. Then, the flask was placed in the MAE system for the extraction with microwave power fixed at 500 W. After extraction, the extraction solution was filtered by 0.45 μm membrane and analyzed by HPLC. Three duplicates of each trial were carried out.
HPLC analysis
-
Rutin, hyperin, isoquercetin, hibifolin, myricetin, quercetin-3′-O-glucoside and quercetin were contemporaneously analyzed by the same apparatus as outlined in our previous work[21]. The standard solutions of seven target flavonoids were prepared as follows: The target flavonoid standard was accurately weighed, dissolved in a 10 mL volumetric bottle with HPLC-grade methanol, and shaken well to obtain the standard storage solution with a concentration of 1.0 mg/mL, and diluted into a series of required standard solutions with different concentrations with HPLC-grade methanol. The linearity range of rutin, hyperin, isoquercetin, hibifolin and quercetin-3'-O-glucoside was 5−1,000 μg/mL, while that of myricetin and quercetin was 1.25−500 μg/mL. HPLC analysis was accomplished on a C18 column (250 mm × 4.6 mm, 5 μm) with a flow rate of 1 mL/min at 30 °C and 254 nm (Fig. 1). 0.5% H3PO4 solution (A) and acetonitrile (B) were used as mobile phases and eluted in the following procedure: 0−40 min, 10%−17% B; 40−59 min, 17%−33% B; 59−62 min, 33% B; 62−65 min, 33%−10% B.
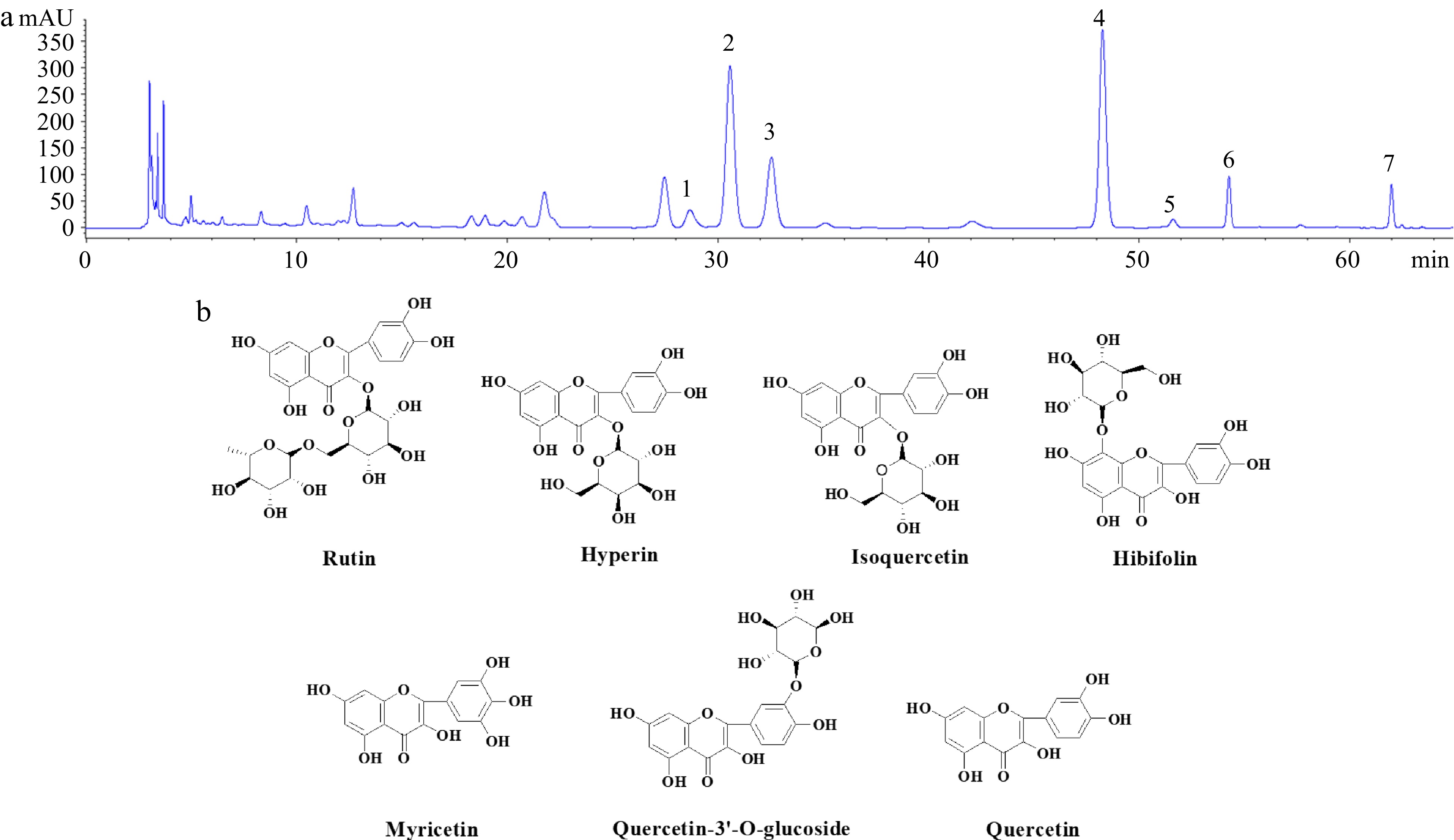
Figure 1.
(a) HPLC chromatograms of the samples from the flowers of Hibiscus manihot L. (b) Chemical structures of seven target flavonoids. 1, rutin; 2, hyperin; 3, isoquercetin; 4, hibifolin; 5, myricetin; 6, quercetin-3′-O-glucoside; 7, quercetin.
Experimental design
-
The IL-HSH-MAE conditions were rationally confirmed by single-factor experiments (type and concentration of IL, homogenate speed and time) and BBD. Three key factors, liquid/solid ratio (X1), extraction temperature (X2) and extraction time (X3) at three levels, were considered as independent variables affecting the extraction yields of seven target flavonoids (Y1−Y7). In the BBD experiment (Table 1), 17 runs were executed in random order and were statistically evaluated using Design-Expert 8.0 software.
-
ILs possess characteristic properties that make them extremely beneficial for a broad scope of applications. The physicochemical property is described by the structure of ionic liquids and the different combinations of anion and cation influence the extraction efficiency of target analytes[9]. In this study, eight ILs with various anions (Br−, Cl−,
${\text {BF}_4^-} $ $\text{H}_2{\text {PO}}_4^- $ ${\text {BF}_4^-} $ $\text{H}_2{\text {PO}}_4^- $ 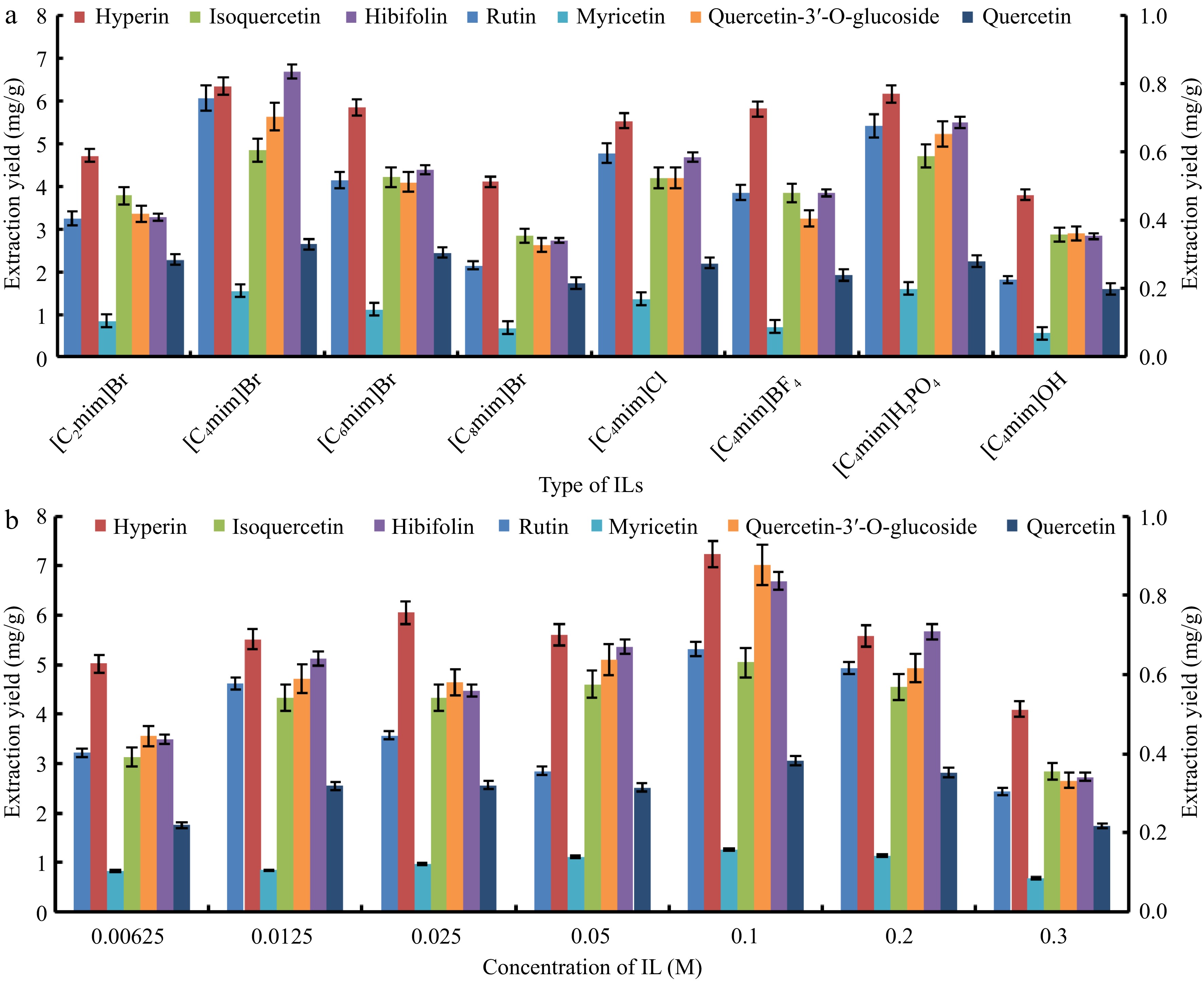
Figure 2.
The effect of (a) type and (b) concentration of ionic liquids on the extraction of seven target flavonoids from the flowers of Hibiscus manihot L.
The concentration of IL played a vital role in the extraction efficiency of target components from plant matrices[22]. The effect of IL concentrations varying from 0.00625 M to 0.3 M was examined to screen the optimal IL concentration for instantaneously extracting seven flavonoids. As exhibited in Fig. 2b, the yields of seven target flavonoids cumulatively enhanced with the concentration of [C4mim]Br increased from 0.00625 to 0.1 M, with peak yields observed at 0.1 M. This is mainly because the solubility and extraction capacity of the solvent system was improved with the addition of [C4mim]Br. Whereas, when the concentration was more than 0.1 M, the extraction yields decreased apparently, which might be due to the high concentration of IL leading to the increase in viscosity of the solution and descend in the mass transfer efficiency that hindered the extraction process resulting in lower extraction yields[23]. Thus, [C4mim]Br of 0.1 M was selected for further experiment.
Effect of homogenate speed and time on extraction efficiency
-
The experimental results of extracting seven target flavonoids from HMLF with different homogenate speeds ranging from 6,000 rpm to 10,000 rpm are shown in Fig. 3a. It can be found that the extraction efficiency enhanced significantly as the homogenate speed increased, while decreasing distinctly when the homogenate speed exceeded 7,000 rpm. Homogenate time was also found to be important in the process of homogenizing the pretreatment of HMLF. As can be seen from Fig. 3b, when the homogenate time reached 120 s, the extraction yields of seven target flavonoids reached the highest with a total extraction yield of 21.06 mg/g, which was significantly higher than other times. This is mainly because homogenization will promptly crush solid materials with a solvent system. Longer homogenate time will reduce the particle size of solid materials. Appropriate reduction of particle size is conducive to mass transfer because smaller particle size provides a larger specific surface area[24,25]. However, the extraction yields decreased gradually as the homogenate time increased to 150 s, possibly because the excessively small particle size would enable the plant material to float on the surface of the solvent system, thus limiting the pretreatment efficiency. Therefore, the homogenate speed and time were 7000 rpm and 120 s, respectively, as the HSH pretreatment conditions of HMLF.
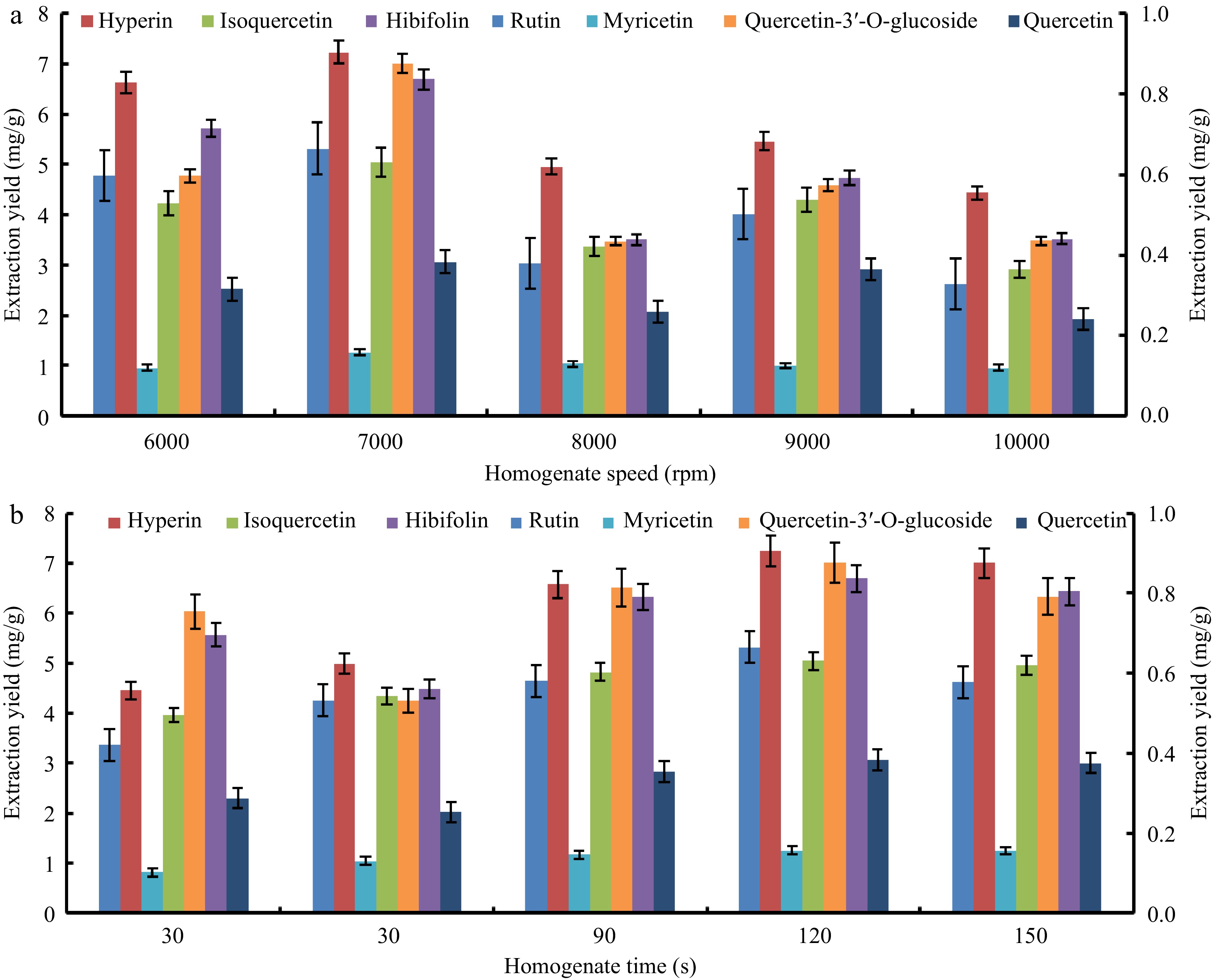
Figure 3.
The effect of (a) homogenate speed and (b) time on the extraction of seven target flavonoids from the flowers of Hibiscus manihot L.
Optimization of the target flavonoids extraction process parameters
-
The effects of the type and concentration of IL, homogenate speed and time of IL on the extraction efficiency of HMLFF were screened through single-factor experiments, followed by the optimization of three key variables (Table 2). The total extraction yield ranged from 11.15 mg to 22.18 mg/g, suggesting that the parameters studied possessed a significant effect on the extraction yields of target compounds. The quadratic regression models for HMLFF were competent with satisfactory R2 values (> 0.94), and the analysis of variance (ANOVA) results in Table 3 showed the validity and suitability of the established models for optimizing the extraction process, with F-values > 14.56 and p-values << 0.01. For hibifolin, X2 and X2X3 significantly affected the extraction yield (p < 0.05), whereas X1,
$ X_1^2 $ $ X_2^2 $ $ X_3^2 $ $\begin{aligned}Y_1=& 0.90-0.079X_1+0.064X_2+0.011X_3-0.017X_1X_2-\\&0.061 X_1X_3- 0.052 X_2X_3-0.17X^2_1-0.19X_2^2-0.10X_3^2\end{aligned} $ (1) $\begin{aligned}Y_2=&7.40-0.60X_1+0.40X_2-0.16X_3+0.004767X_1X_2-\\&0.37 X_1X_3-0.46 X_2X_3-1.34X^2_1-1.36X_2^2-1.19X_3^2\end{aligned} $ (2) $\begin{aligned}Y_3=&4.80-0.38X_1+0.31X_2+0.064X_3-0.004051X_1X_2-\\&0.12 X_1X_3-0.36 X_2X_3-0.60X^2_1-0.51X_2^2-0.56X_3^2\end{aligned} $ (3) $\begin{aligned}Y_4=&7.22-0.63X_1+0.46X_2+0.15X_3-0.091X_1X_2+\\&0.19 X_1X_3-0.62 X_2X_3-1.70X^2_1-1.09X_2^2-1.61X_3^2\end{aligned} $ (4) $\begin{aligned}Y_5=&0.17+0.022X_1+0.016X_2-0.001053X_3-0.002083X_1X_2+\\&0.00205 X_1X_3-0.0018 X_2X_3-0.034X^2_1-0.019X_2^2-0.018X_3^2\\[-19pt]\end{aligned} $ (5) $\begin{aligned}Y_6=&0.85-0.056X_1+0.021X_2+0.036X_3+0.004823X_1X_2-\\&0.022 X_1X_3-0.029 X_2X_3-0.19X^2_1-0.17X_2^2-0.20X_3^2\end{aligned} $ (6) $\begin{aligned}Y_7=&0.41-0.036X_1+0.018X_2-0.014X_3-0.027X_1X_2+\\&0.018 X_1X_3-0.024 X_2X_3-0.067X^2_1-0.080X_2^2-0.087X_3^2\end{aligned} $ (7) Table 2. Results of the Box-Behnken design (BBD) for the extraction yields of seven target compounds from the flowers of Hibiscus manihot L.
Runs Factors Extraction yield (mg/g) X1a X2b X3c Y1 Y2 Y3 Y4 Y5 Y6 Y7 1 −1(20) −1(50) 0(15) 0.48 4.94 3.72 4.88 0.11 0.55 0.28 2 1(30) −1(50) 0(15) 0.44 3.78 3.06 3.18 0.07 0.40 0.23 3 −1(20) 1(70) 0(15) 0.67 5.63 4.32 5.87 0.16 0.58 0.35 4 1(30) 1(70) 0(15) 0.56 4.48 3.64 3.80 0.11 0.45 0.20 5 −1(20) 0(60) −1(10) 0.66 5.15 3.76 4.42 0.15 0.45 0.31 6 1(30) 0(60) −1(10) 0.54 4.65 3.17 3.40 0.10 0.40 0.23 7 −1(20) 0(60) 1(20) 0.84 5.84 4.33 4.04 0.13 0.56 0.25 8 1(30) 0(60) 1(20) 0.48 3.85 3.25 3.78 0.09 0.42 0.24 9 0(25) −1(50) −1(10) 0.51 4.24 3.07 3.07 0.10 0.39 0.21 10 0(25) 1(70) −1(10) 0.71 6.07 4.45 5.36 0.15 0.49 0.31 11 0(25) −1(50) 1(20) 0.60 4.56 3.72 4.92 0.14 0.53 0.23 12 0(25) 1(70) 1(20) 0.60 4.54 3.66 4.73 0.12 0.51 0.23 13 0(25) 0(60) 0(15) 0.90 6.80 4.42 7.42 0.17 0.79 0.40 14 0(25) 0(60) 0(15) 0.90 7.97 4.74 6.84 0.17 0.87 0.41 15 0(25) 0(60) 0(15) 0.94 7.32 5.04 7.46 0.16 0.88 0.38 16 0(25) 0(60) 0(15) 0.93 7.56 4.96 6.99 0.17 0.83 0.45 17 0(25) 0(60) 0(15) 0.83 7.38 4.82 7.38 0.16 0.88 0.43 a Liquid/solid ratio (mL/g); b Extraction temperature (°C); c Extraction time (min). Table 3. ANOVA statistics of the quadratic model for the extraction yields of seven target compounds from the flowers of Hibiscus manihot L.
Variables Y1 Y2 Y3 Y4 Y5 Y6 Y7 F-value p-value F-value p-value F-value p-value F-value p-value F-value p-value F-value p-value F-value p-value Model 14.57 0.0009 25.78 0.0001 16.36 0.0007 22.40 0.0002 21.01 0.0003 57.28 <0.0001 17.53 0.0005 X1 14.15 0.0071 22.63 0.0021 23.95 0.0018 16.96 0.0045 46.74 0.0002 24.45 0.0017 15.40 0.0057 X2 9.47 0.0179 10.07 0.0156 16.74 0.0046 9.08 0.0196 23.05 0.0020 3.33 0.1109 3.91 0.0884 X3 0.27 0.6207 1.70 0.2340 0.69 0.4334 0.98 0.3543 0.11 0.7542 10.13 0.0154 2.26 0.1767 ${\text X_1^2 }$ 33.36 0.0007 59.09 0.0001 32.58 0.0007 64.49 <0.0001 59.48 0.0001 141.92 <0.0001 27.99 0.0011 ${\text X_2^2 } $ 44.16 0.0003 61.05 0.0001 23.03 0.0020 26.41 0.0013 18.15 0.0037 112.47 <0.0001 39.32 0.0004 ${\text X_3^2 } $ 12.34 0.0098 46.84 0.0002 28.20 0.0011 57.83 0.0001 15.52 0.0056 168.60 <0.0001 46.19 0.0003 X1X2 0.33 0.5839 0.0007125 0.9794 0.00139 0.9713 0.17 0.6886 0.21 0.6624 0.090 0.7732 4.34 0.0757 X1X3 4.22 0.0791 4.34 0.0756 1.23 0.3048 0.74 0.4187 0.20 0.6674 1.95 0.2055 1.97 0.2037 X2X3 3.11 0.1212 6.73 0.0357 11.06 0.0127 8.24 0.0240 16.13 0.0051 3.28 0.1129 3.23 0.1155 Lack of fit 2.89 0.1660 0.33 0.8050 0.55 0.6754 4.10 0.1032 5.07 0.0755 0.29 0.8316 1.13 0.4360 R2 0.9493 0.9707 0.9546 0.9664 0.9643 0.9866 0.9575 The 3D-RSM was built to illustrate the interactions of three independent variables on seven target flavonoid yields in Fig. 4. It can be found that the liquid/solid ratio and extraction temperature possessed more significant effects on the extraction yields of target flavonoids. Fig 4a, g & h depict the interaction of liquid/solid ratio and extraction temperature on rutin, myricetin and quercetin yields, respectively. It was noted that the extraction yields rose promptly as the liquid/solid ratio raised from 20 to 24 mL/g and the extraction temperature raised from 50 to 62 °C. When the liquid/solid ratio constantly increased and the extraction temperature rose, the extraction yields showed no notable change. The liquid/solid ratio was stimulative for extracting target flavonoids, the increase in liquid/solid ratio may elevate contact surfaces and endocellular constituent dissolution, whereas an overabundance of fluid may restrict extraction efficacy[26]. The interaction effect of liquid/solid ratio and extraction time on rutin, hyperin and hibifolin are presented in Fig 4b, c & e. It was concluded that the appropriate prolongation of extraction time possessed a positive impact on the extraction yields of target flavonoids. The yields of rutin, hyperin and hibifolin markedly increased to the maximum values as the liquid/solid ratio and extraction time raised, while started to decline once those parameters exceeded 15 min and 24 mL/g. Initially, a prolonged extraction period exposed the sample to microwaves completely, leading to cell wall breakage and greater liberation of endocellular constituents[27]. Figure 4d, f & i demonstrated that increasing the extraction temperature from 50 °C to 62 °C and extraction time from 10 min to 15 min boosted the extraction yields of isoquercetin, hibifolin and quercetin considerably. Usually, higher extraction temperature and longer extraction time facilitated the extraction of target flavonoids from HMLF. This is mainly because elevated temperature facilitates molecular interactions, promotes the dissolving of target analytes in solutions, and modifies the wetness of the sample as well as the penetrability and diffusibility of the matrices[28]. In addition, the viscosity of ILs decreased with the increase of extraction temperature, thus affecting the permeability of ILs solution and improving the extraction efficiency, while too high temperature may cause the degradation of bioactive components[29]. Based on the aforementioned findings, the optimum operating parameters for the simultaneous extraction of seven target flavonoids with the extraction yields of 0.91, 7.50, 4.89, 7.32, 0.17, 0.85, 0.42 mg/g were as follows: liquid/solid ratio of 23.85 mL/g, extraction temperature of 61.7 °C and extraction time of 14.98 min. In the actual experimental operation process, 24 mL/g, 62 °C and 15 min were chosen to extract HMLFF using 0.1 M [C4mim]Br, and the experimental data complied with the predicted values with low RSD, indicating that the models were suitable and reliable to optimize the extraction parameters for extracting HMLFF.
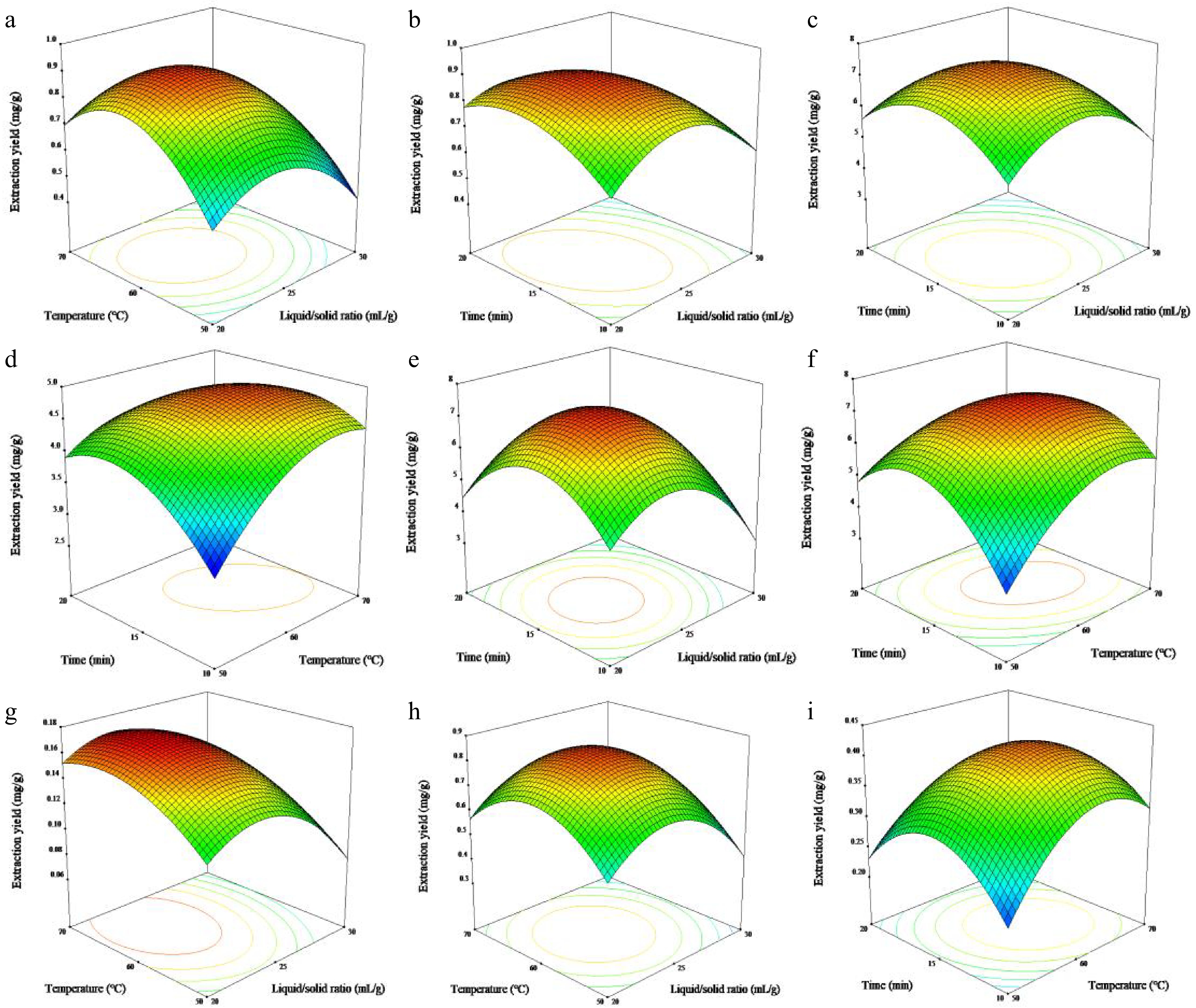
Figure 4.
Response surfaces representations for (a) & (b) rutin, (c) hyperin, (d) isoquercetin, (e) & (f) hibifolin, (g) myricetin, (h) quercetin-3′-O-glucoside and (i) quercetin in the flowers of Hibiscus manihot L. (a), (g) & (h) varying liquid/solid ratio and extraction temperature; (b), (c) & (e) varying liquid/solid ratio and extraction time; (d), (f) & (i) varying extraction temperature and extraction time.
Comparative analysis of different extraction methods
-
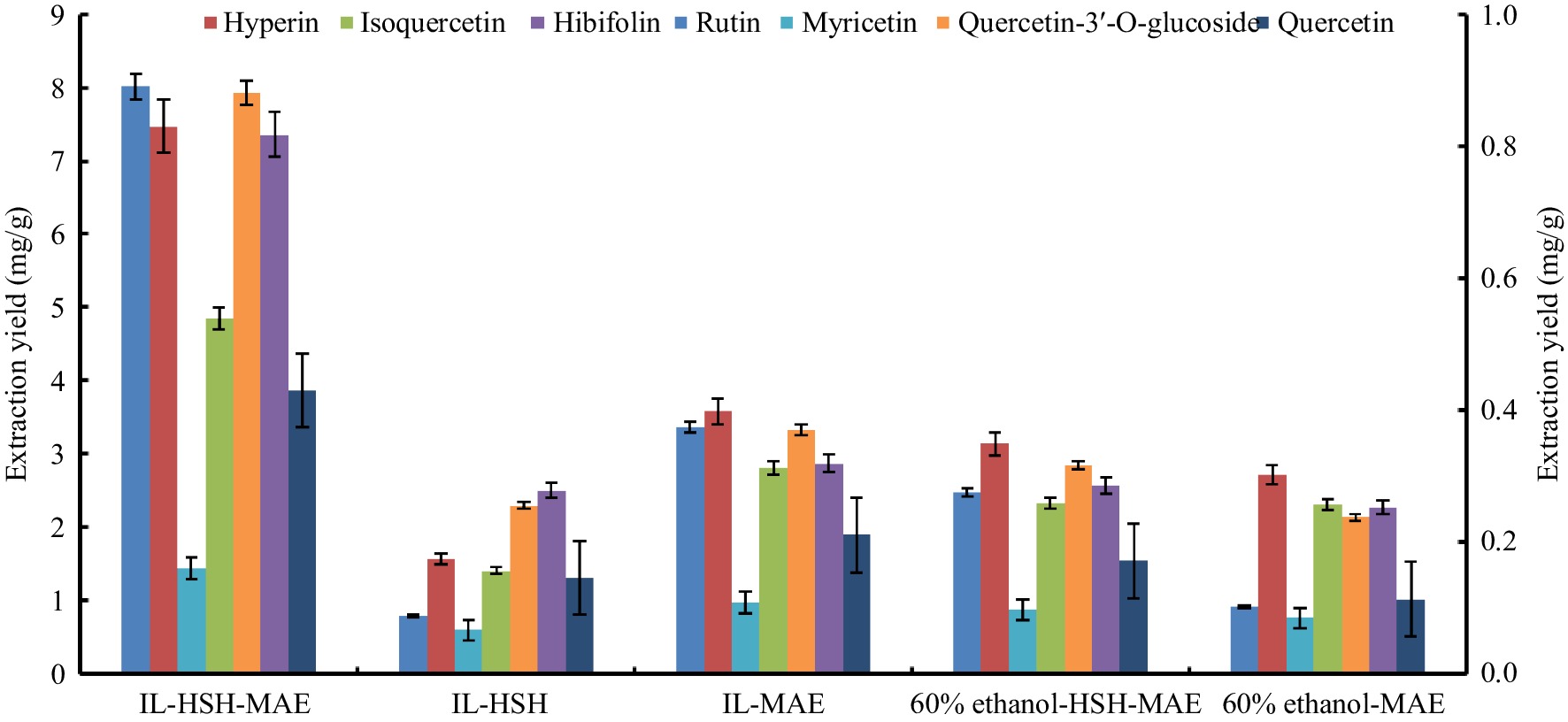
The extraction capabilities of extraction techniques comprising IL-HSH-MAE, IL-HSH, IL-MAE, 60% ethanol-HSH-MAE and 60% ethanol-MAE on the extraction yields of HMLFF were performed and contrasted (Fig. 5). As exhibited in Fig. 5, the total extraction yield by IL-HSH-MAE reached 22.04 mg/g, which was 2.13−3.65 folds greater than IL-HSH, IL-MAE, 60% ethanol-HSH-MAE and 60% ethanol-MAE. The remarkable extraction performance of IL-HSH-MAE was credited to the broad solubility range and exceptional potential of IL in extracting non-polar and polar compounds, in contrast to conventional toxic organic solvents. Additionally, the thermal and mechanical effects of IL aided in expediting the penetrability of the extraction media to plant tissues, enhancing cell wall destruction and mass transfer efficiency, shortening extraction time, and facilitating the target components quick release into the solvent system, thus improving the extraction efficiency[30]. All the data showed the superiority of the newly established IL-HSH-MAE in incrementally and rapidly extracting seven target flavonoids from HMLF. Therefore, the IL-HSH-MAE method has been identified as a proficient and eco-friendly way for extracting natural bioactive ingredients from plant matrices.
-
In the present study, ionic liquid as a promising alternative to the traditional toxic organic solvent that was successfully applied for extracting HMLFF combined with HSH-MAE. The extraction yields of seven target flavonoids reached 0.89, 7.47, 4.85, 7.36, 0.16, 0.88 and 0.43 mg/g, respectively, under the optimum extraction conditions as follows: 0.1 M of [C4mim]Br, homogenate speed of 7000 rpm, homogenate time of 120 s, liquid/solid ratio of 24 mL/g, extraction temperature of 62 °C and extraction time of 15 min. Besides, the developed method IL-HSH-MAE exhibited higher extraction yields compared with other extraction methods. Therefore, the IL-HSH-MAE technique was a prospective strategy with the preponderance of high efficiency and fast extraction of functional active ingredients from plant materials.
The authors gratefully acknowledge the financial support from China Postdoctoral Science Foundation (2021M692893, 2021M702927), National Natural Science Fund of China (82204552), Natural Science Foundation of Zhejiang Province (LQ22H280007), Research Project of Zhejiang Chinese Medical University (2022JKZKTS10), Zhejiang Province Traditional Chinese Medicine Science and Technology (2023ZR079, 2023ZR087). We appreciate the experimental support from the Shanghai Qixia Technology Co., Ltd. and Public Platform of Medical Research Center, Academy of Chinese Medical Science, Zhejiang Chinese Medical University.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Liu J, Fu Y, Cui Q. 2023. Efficient, rapid and incremental extraction of bioactive compounds from the flowers of Hibiscus manihot L.. Beverage Plant Research 3:11 doi: 10.48130/BPR-2023-0011
Efficient, rapid and incremental extraction of bioactive compounds from the flowers of Hibiscus manihot L.
- Received: 17 March 2023
- Revised: 11 April 2023
- Accepted: 17 April 2023
- Published online: 08 May 2023
Abstract: Flavonoids are the primary functional components in the flowers of Hibiscus manihot L. (HMLF). In this study, an efficient and green ionic liquid-high-speed homogenization coupled with microwave-assisted extraction (IL-HSH-MAE) technique was firstly established and implemented to extract seven target flavonoids from HMLF. Single-factor experiments and Box-Behnken design (BBD) were utilized to maximize the extraction conditions of IL-HSH-MAE, which were as follows: 0.1 M of [C4mim]Br, homogenate speed of 7,000 rpm, homogenate time of 120 s, liquid/solid ratio of 24 mL/g, extraction temperature of 62 °C and extraction time of 15 min. The maximal total extraction yield of seven target flavonoids attained 22.04 mg/g, which was considerably greater than the yields obtained by IL-HSH, IL-MAE, 60% ethanol-HSH-MAE and 60% ethanol-MAE. These findings suggested that the IL-HSH-MAE method can be exploited as a rapid and efficient approach for extracting natural products from plants. The process also possesses outstanding superiority in being environmentally friendly and having a high extraction efficiency and is expected to be a luciferous prospect extraction technology.