-
Tropical biomes are the most diverse plant communities on the earth, and it is vital to quantify this diversity on large spatial scales for many purposes[1]. Tropical marine plants mainly include mangroves, seaweeds and microalgae[2]. Microalgae refers to a group of microscopic photosynthetic organisms with extensive and heterogeneous phylogeny, including autotrophs, heterotrophs (e.g., apicomplexans and dinoflagellates) and mixotrophs (e.g., Chromeridophyta and Chromera velia). They are usually divided into at least two major phylogenetic lineages: Stramenopiles (diatoms, eustigmatophytes and brown algae) and Archaeplastida (or Plantae, including red algae, blue-green algae and green algae, which have a common ancestor with modern land plants)[3, 4]. In microbial eukaryotes, the SAR clade includes Stramenopila (e.g., diatoms), Alveolata (e.g., dinoflagellates) and Rhizaria (Chlorarachniophyceae), containing an immense diversity of lineages[5]. The ocean is a rich source of food, minerals, and medicinal commodities. These life forms support the diversity of the ocean and provide abundant compounds as source of food, minerals and medicinal commodities[6].
In addition to tropical marine microalgae, mangroves and seaweeds receive increasing attention as 'blue carbon'[7]. Seagrass beds and mangroves can provide natural and functional products, either individually or through ecological networks[8]. On one hand, mangroves can produce a large number of antimicrobial secondary metabolites, namely antimicrobials, among which phytoanticipins are inactive direct precursors of antimicrobials or phytoalexins that exist in plant tissues prior to pathogen attack and have important medicinal value[9]. On the other hand, seaweed extract can significantly reduce plasma glucose level, inhibit α-amylase, α-glucosidase, protein tyrosine phosphatase 1B (PTP1B), etc.[10−12].
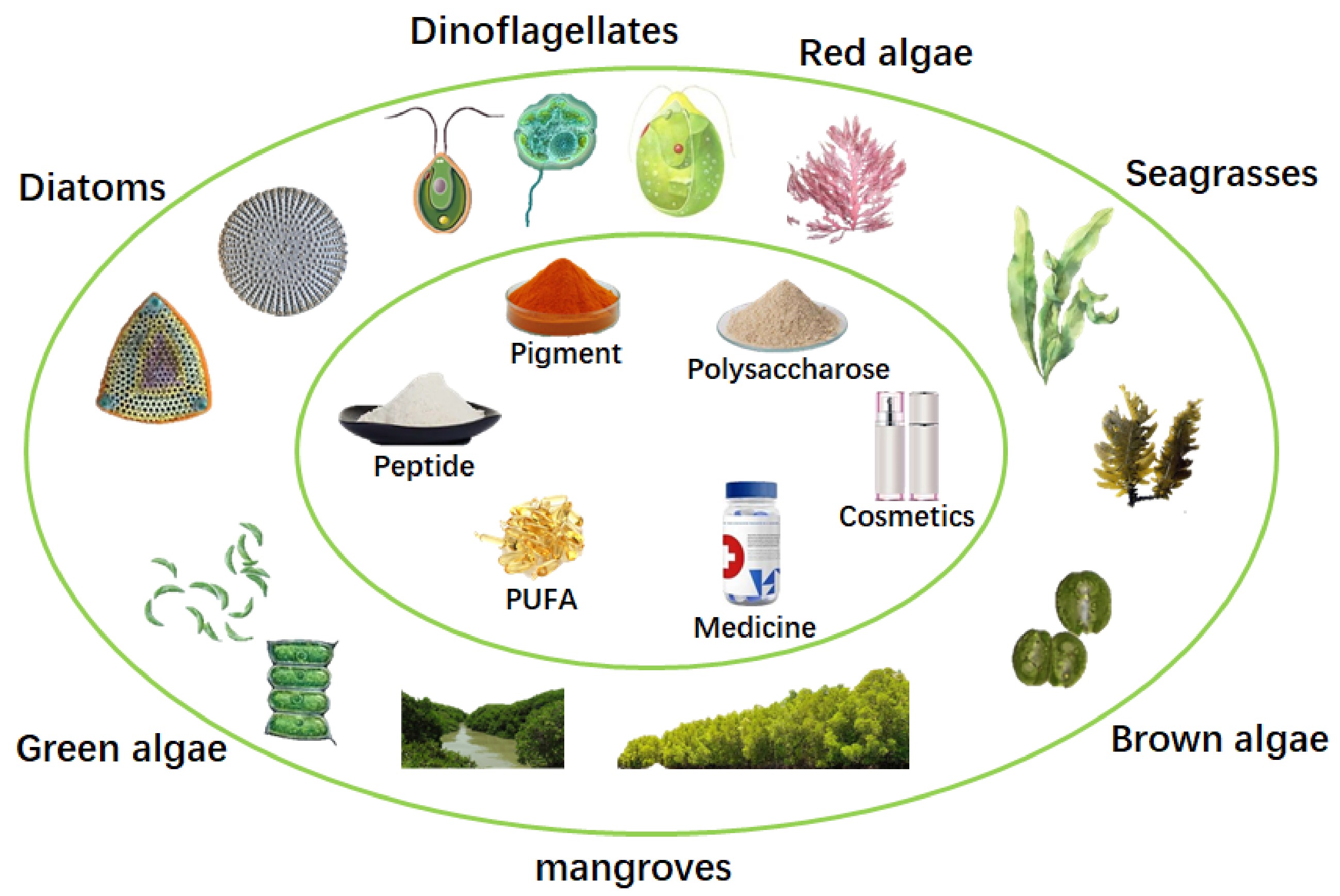
Tropical marine plant metabolites account for 13% of natural products, including many value-added compounds, such as sterols, essential fatty acids, polysaccharides, terpenes, lactones, carotenoids, proteins, peptides, vitamins and mineral oxides (Table 1[13−77]). Moreover, a series of bioactive substances in tropical marine plants had been discovered for the therapy of the globally threatening diseases, such as acquired immune deficiency syndrome[78, 79], Dengue fever[80, 81] and cancer[82−86]. Therefore, tropical marine plants are of great significance for the development of biochemistry, biomass energy resources, and pharmacology[87]. Among marine plants, microalgae, there are an estimated 72,500 species, which are of great ecological importance, as they contribute nearly half of the global organic carbon fixation[88]. However, researches on the isolation and identification of high-value compounds from diatoms, dinoflagellates, red algae, brown algae, green algae, seagrasses and mangroves are still rough now (Fig. 1). This mini-review examines several aspects of value-added compounds from tropical marine algae, including current knowledge on their isolation, measurement and identification. Biotechnological and medical applications involving these value-added compounds are also discussed.
Table 1. Natural products from tropical marine plant metabolites.
Class Species Metabolites Reference Tropical microalgae Chlorophyceae Haematococcus pluvialis Astaxanthin [18] Dunaliella salina β-carotene [19] Chlorella zofingiensis Astaxanthin [20] Scenedesmus almeriensis Lutein [21] Muriellopsis sp. Lutein [22] Chlorella sorokiniana Lutein [23] Parietochloris incisa Arachidonic acid [24] Chlorella pyrenoidosa Peptides [25] Tetraselmis suecica Peptides [26] Rhodophceae Porphyridium purpureum Arachidonic acid [27] Porphyridium cruentum Eicosapentaenoic acid [28] Porphyridium sp. Sulfated polysaccharides [29] Stramenopiles Nannochloropsis sp. Eicosapentaenoic acid [30] Nannochloropsis oculata Peptides [31] Nannochloropsis sp. Phenolics [32] Bacillariophyceae Phaeodactylum tricornutum Eicosapentaenoic acid [33] Phaeodactylum tricornutum Sulfated polysaccharides [34] Dinophyceae Crypthecodinium cohnii Docosahexaenoic acid [35] Labyrinthulomycota Schizochytrium spp. Docosahexaenoic acid [36] Ulkenia spp. Docosahexaenoic acid [37] Oscillatoriaceae Arthrospira platensis Phycocyanin [38] Tropical seaweeds Rhodophceae Porphyra sp. Desmosterol [39] Platysiphonia miniata Icosatetraenoic acid [40] Eucheuma cottonii Polyphenol [41] Chlorophyceae Udotea flabellum Sulfated polysaccharide [42] Codium fragile Sulfated polysaccharide [43] Ulva lactuca Protein [44] Phaeophyceae Ecklonia cava Phlorotannins [45] Laminaria ochroleuca Fucosterol [39] Padina antillarum Epoxyeicosatrienoic acids [46] Ecklonia cava 6,6'-bieckol [47] Ecklonia radiata Polysaccharide [48] Sargassum swartzii Fucoidan [49] Turbinaria turbinata Fucoidan [50] Dictyota dichotoma Fucoidan [51] Ascophyllum nodosum Phlorotannin [52] Spatoglossum macrodontum Polyunsaturated fatty acid [53] Fucus vesiculosus Laminarin [54] Laminaria digitata Laminarin and fucoidan [55] Undaria pinnatifida Fucoxanthin [56] Hijikia fusiforme Carotenoids and curcumins [57] Tropical mangroves Acanthaceae Acanthus ebracteatus Megastigmane [58] Acanthus ebracteatus Bioactive polysaccharides [59] Acanthus ilicifolius Phenylethanoids [60] Acanthus illicifolius 2-benzoxazolinone [61] Acanthus ilicifolius Lignan glucosides [62] Acanthus ilicifolius Phenylethanoid glycosides [63] Acanthus ilicifolius Benzoxazolinone glucoside [64] Acanthus mollis Hydroxamic acids [65] Euphorbiaceae Excoecaria agallocha Diterpenes [66] Excoecaria agallocha Diterpenoids [67] Excoecaria agallocha Daphnane and tigliane [68] Excoecaria agallocha Excoecarins [69] Excoecaria agallocha Phorbol ester [70] Excoecaria agallocha Excogallochaols [71] Excoecaria agallocha Pentacyclic triterpenoids [72] Lythraceae Penicillium sp. Meroterpenoid [73] Pemphis acidula Galloyl flavonol glycosides [74] Sterculiaceae Heritiera littoralis Heritonin [75] Lamiaceae Clerodendron spp. Neoclerodane diterpenoids [76] Scutellaria spp. Neoclerodane diterpenoids [77] -
Diatoms (Bacillariophyta phylum) are the most abundant phytoplankton on Earth and play a crucial role as the main producers on the planet. Diatoms can be used as feed for aquatic organisms because they contain various bioactive compounds, such as chrysolaminarin, eicosapentaenoic acid (EPA; C20:5), and fucoxanthin. On one hand, diatoms store carbohydrate by chrysolaminarin, which has anti-tumor, antioxidant, and immune modulatory functions[89]. On the other hand, diatoms contain abundant long-chain fatty acids, such as EPA, which have been commercialized in improving cardiovascular function, relieving depression and reducing blood pressure[15]. Compared to fish oil, algae EPA is a better source of food supplements and pharmaceuticals. In addition, diatoms also contain silica nanoparticles, which had been applied in drug delivery and nanoparticle synthesis[90−92].
Diatoms contain a large amount of carotenoids, such as β-carotene, lutein, zeaxanthin and violaxanthin, providing anti-oxidant, anti-obesity, anti-diabetes, anti-cancer and anti-Alzheimer's disease effects[93]. To be specific, β-carotene, as the most effective natural quencher, is involved in scavenging singlet molecular oxygen, peroxyradicals and reactive oxygen species, thus preventing lipid peroxidation[94]. Meanwhile, β-carotene is also involved in cell growth, differentiation and apoptosis[95]. In addition, the anticancer activity of β-carotene has been demonstrated in lung cancer patients[96]. Lutein and zeaxanthin are responsible for the coloring of the macula in the retina, so these two pigments are very important in ophthalmology. Lutein and zeaxanthin are also filters that destroy blue light and quenchers of excited oxygen, and are therefore also used to prevent age-related macular degeneration[97]. Violaxanthin has strong anti-proliferative activity on breast cancer cells, which makes this pigment an effective drug compound to induce apoptosis[98].
Due to the broad prospects of diatoms in aquaculture, human health foods, medicine and cosmetics, the demand for diatoms derived high-value products in the global market is increasing. Global diatomite market production is as high as 2,640,000 tons in 2021. That is 110,000 tons (or 4.7%) more than the same period in 2020. The market share of Melosira was 42.9%, while Pinnularia and Coscinodiscus account for 31.5% and 16.2%, respectively[99].
-
Dinoflagellates (Miozoa phylum, Dinophyceae class) are highly diverse, flagellated and unicellular algae, with reported-to-date 4,000 living and fossil species, including benthic, epiphytic and planktonic species. Many species live freely, while others live as endosymbionts with marine invertebrates, and remains are parasitic. Symbiotic algae related to c echinocytes, mainly members of the Dinoflagellata superclass, family Symbiodiniaceae, have a high degree of phylogenetic diversity[17]. A number of dinoflagellates can synthesize polyketone compounds[100]. Many of these compounds are potent toxins that have caused public health and ecological problems. On the other hand, many marine toxins, due to their medical and commercial value, can be used as anti-cancer agents or probes of cellular processes, increasingly attracting people's interest[101]. Besides that, PCPs (peridinin-chlorophyll α-proteins) is a water-soluble light-trapping complex, which is only found in dinoflagellates. Its commercial product, peridinin-chlorophyll-protein complex, has been widely used in probe development due to its unique fluorescence properties[102]. However, the challenge in pharmaceutical applications is that most flagellates are usually fragile and slow-growing, making it difficult to provide sufficient amounts of toxins. Therefore, developing large-scale cultivation strategies is critical to produce a large amount of bioactive compounds using flagellates[103]. In addition, research on the functional genomic genomes of flagellate derived polyketone biosynthesis is still limited, partly due to the peculiarities of the dinoflagellate nucleus and the lack of the transformation system. However, a basic understanding of the genetics of flagellate toxin biosynthesis may be helpful for developing several fruitful avenues of research[101].
-
Around 50% of tropical marine algae belong to red algae (Rhodophyta phylum). The Red algal genus Laurencia (Rhodomelaceae family, Ceramiales order) is a prolific producer of halogenated secondary metabolites, such as diterpenes, triterpenes, sesquiterpenes and C15-acetogenins[104]. Kamada et al. had discovered three types of bromoallenes and four types of C15-acetogenins in L. nangii[105−107]. They also isolated two new non-halogenated sesquiterpenes from L. snackeyi[108]. On the other hand, L. dendroidea-derived (−)-elatol and (+)-obtusol can provide inhibitory effects on the larvae of Aedes aegypti, which is the insect vector of Dengue fever[80]. In addition, L. intricata-derived laurenditerpenol was found to inhibit tumor cell growth under hypoxic conditions, without affecting normal cell growth under normoxic conditions[82]. Moreover, four Laurencia sp. were discovered to produce six new compounds, among which omaezol/hachijojimallene showed strong inhibitory activity against the larvae of barnacle Amphibalanus amphitrite[81].
Phycobiliprotein is a light-harvesting complex, which is found in red and blue algae and plays a key role in photosynthesis. In recent years, phycobiliprotein has been widely used in the development of food and cosmetic colorants and fluorescent probes, because of its bright color and good phototrapping activity. In addition, Phycobiliprotein has attracted wide attention in pharmaceutical and biomedical fields due to its antioxidant, anti-inflammatory, anti-tumor and photosensitive properties[109].
As for other red algae, in Rhodosorus sp., 19.6% sulfate and 11.6% uronic acid were identified in polysaccharides, which shows potential as a promising source of antioxidants applied in food, medicine, and cosmetics[110]. Griffithsia-derived griffithsin is a novel anti-HIV protein and a new type of lectin that binds to various viral glycoproteins in a monosaccharide-dependent manner[79]. The polyhalogenated monoterpene, which is isolated from Plocamium hamatum, present bacteriostatic activity[111]. Furthermore, a new lectin KSL was found in Kappaphycus striatus and provide anticancer activity against carcinoma cell lines, such as HT29, Hela, MCF-7, SK-LU-1 and AGS[112]. At the same moment, Vidalia sp.-isolated vidalenolone can inhibit SH2 activity, making it promising for the treatment of proliferative disorders[113].
-
It had been reported that tropical marine brown algal species can produce promising value-added molecules. Brown algae-derived natural polysaccharides, including algin, laminarin and fucoidan, have attracted extensive attention due to their potential application in the field of medicine. The polysaccharides and their derivatives have many biological activities, such as wound healing, anticoagulation, antiviral and anticarcinogenic[114]. For example, sulfated polysaccharides, polyphenols and diterpenes are the most important ones[115]. Lobophora variegata, which is often associated with tropical coral reefs, exerts strong antiprotozoal and antibacterial activities. It is revealed that the extract of L. variegata has a strong inhibitory effect on HIV-1 strains (including drug-resistant/primary HIV-1 isolates), and can even protect primary cells from HIV-1-infection, thereby indicating a promising source for developing novel HIV-1 inhibitors[78]. In addition, the common linear diterpenes in brown algae have high chemotaxonomic and ecological significance. It is reports that a new linear diterpene, named as bifurcatriol, was isolated from Bifurcaria bifurcata[116]. Meanwhile, total extract of L. variegata has shown antiprotozoal activity against Trichomonas vaginalis[117].
Nutritional studies on brown seaweeds indicate that they could be used as an alternative source of dietary fiber and protein[118]. On one hand, dietary fiber in seaweeds is mainly composed of sulfated polysaccharides, which are resistant to human digestive enzymes and therefore have the nutritional properties of cellulose[119]. On the other hand, through the study of the biochemical and nutritional aspects of algal protein, it was found that the degradation of algal fiber by enzyme can prepare bioactive peptides and improve the digestibility of protein. At present, antihypertensive bioactive peptides from seaweeds have received much attention, and these bioactive peptides have many beneficial effects as angiotensin-converting enzyme (ACE) inhibitors[120, 121].
Cancer is the currently the second worst disease worldwide. Natural products extracted from seaweeds with apoptotic activity have attracted widespread attention as medcines for preventing or treating cancer[122]. Methanol extracts from 13 tropical seaweed species in the Northeast of Brazil were assessed as induced apoptosis agents on human cervical adenocarcinoma (HeLa). Among these, the most promising ones, MEDM and MEDC, which were identified from Dictyota ciliolata and Dictyota menstrualis, can inhibit SiHa (cervix carcinoma) cell proliferation (~20%) at higher concentration (200 μg/mL)[83]. Moreover, five new bioactive meroditerpenoids, of which three exhibited moderate cytotoxicity to NCI-H460 human lung cancer cell line (LC50 ranges from 2 to 11 μM), were isolated from Stypopodium flabelliforme in Papua New Guinea[84]. On the other hand, three new terpenoids were isolated from S. zonale, and one methyl ester presents in vitro cytotoxic activity on human lung and colon carcinoma[85].
-
Although the activity of bromoperoxidase is highest among species of red algae (Rhodophyta), a large number of green algae (Chlorophyta) also exhibit bromoperoxidase activities. Furthermore, bromoperoxidase activity can reach maximum levels in some green algae species, and the enzymes responsible for bromoperoxidase activity have been isolated and identified in several cases. These seaweeds as a population are rich source of linear oxygenated and brominated terpenes. The natural products of Avrainvillea nigricans have been investigated and reported to isolate a new diphenylmethane, which possesses moderate antibiotic activity against several human pathogens[123]. Then Chen et al. described the potential anticancer-type activity from A. rawsonii, using a mechanism-based enzyme analysis based on inosine 5'-monophosphate dehydrogenase (IMPDH), which is a rate-limiting enzyme in purine biosynthesis[86].
It is well known that tropical green algae can synthesize large quantities of sulphated polysaccharides, such as Caulerpa-derived sulphated galactans, Monostroma-derived sulphated rhamnans, Codium-derived sulphated arabic galactans, Ulva- and Enteromorpha-derived ulvans, and some special sulphate acidified mannan from different species[43]. In addition, polyphenols, including carboxylic acids, hydroxycinnamic acids, phlorotannins, catechins and flavonoids, had been identified in tropical green algae, such as Chlorella sp. and Rhodophyta sp.[124]. Moreover, green algae can produce high amounts of polyunsaturated fatty acids, such as arachidonic acid, eicosapentaenoic acid, α-linolenic, oleic and linoleic acid[125].
The genus Caulerpa, a marine green algae, is introduced into the Mediterranean Sea from southwestern Australia[126]. The complex composition of secondary metabolites is considered as one of the greatest advantages of the Caulerpa species. The functional groups of these secondary metabolites provide various biological effects, including antifouling, ichthyotoxic, antimicrobial, insecticidal, feeding deterrent, anti-inflammatory, cytotoxic and growth regulatory properties[127−129]. In addition, Paul et al. studied the effects of feeding deterrence on brominated sesquiterpenes, which are produced by Pacific collections of Neomeris annulata in Guam. It is reported that brominated sesquiterpenes provide chemical defenses on herbivory by reef fishes[130]. In addition, considerable β-carotenoids and zeaxanthin were isolated from pigments of Prochloron sp., which was collected from various localities in the tropical Pacific Ocean[131].
-
Seagrasses represent one of the coastal tropical ecosystems of greatest importance, because of their multiple ecological and economic benefits[132]. Since seagrasses have to adapt to changing tropical marine circumstances, their metabolites that could be very useful for therapeutical purposes[133]. The marine angiosperm Thalassia testudinum, usually known as turtle grass, is a dominant seaweed that grows on the Caribbean shelf and is related to Syringodium filiforme. The hydroalcoholic extract of T. testudinum contains abundant polyphenols, among which the richest metabolite in this extract is thalassiolin B, a glycosilated flavonoid with properties such as repairing skin damage and antioxidant activity[134]. In addition, seagrasses form a good, durable and nearly inexhaustible source for polyunsaturated fatty acids, with an (n-6) FA: (n-3) FA ratio of about 1.0, which is much lower than the suggested counterpart from the World Health Organization to prevent inflammation, cardiovascular and nervous system disease. Some marine macroalgae, such as Palmaria palmata (Rhodophyta), which contains a high proportion of eicosapentaenoic acid (EPA, C20:5, n-3). On the other hand, docosahexaenoic acid (DHA, C22:6, n-3) was detected in Sargassum natans (Phaeophyceae)[135]. In addition, the biological characteristics of seagrass and its rich complex sugars indicate its potential as renewable energy. For example, the low lignin content in seagrass makes it easy for the enzymatic hydrolysis of cellulose. Seagrasses does not need to transport nutrients or water internally, which saves energy. Moreover, many seagrasses have a higher biomass productivity (13.1 kg dry weight m−2 over 7 months) than land plants (0.5−4.4 kg dry weight m−2 year−1)[136]. Therefore, it is potential to use seagrass as raw material for ethanol industry[137].
Mangroves provide crucial resources to humanity, including food, fisheries support, coastal protection and carbon sequestration[138]. On one hand, the physicochemical and antioxidant properties of edible mangrove fruits had been identified in many studies. In the Sundarbans mangrove forest of Bangladesh, ten edible fruits were evaluated for their anti-bacterial, anti-diarrheal, and cytotoxic activities. Sonneratia caseolaris (mangrove plant) ethanol: methanol (1:1) extract presents the highest antibacterial activity, thus facilitates the potential of diarrhea treatment and useful nutraceuticals[139]. Budiyanto et al. reviewed the nutritional composition of several edible mangrove fruits, and suggested the usage of Avicennia marina fruit as an alternative food to support the sustainable development goal of eradicating world hunger[140]. On the other hand, mangrove-associated microorganisms, including endophytic fungal and bacterial, as well as microorganisms from soil samples, have been proven to be abundant sources of bioactive secondary metabolites. In the early 2000s, Xylaria sp.-derived xyloketal B and Salinospora tropica (marine Actinobacteria)-isolated salinosporamide A revealed a great potential for drug discovery and inspired studies in-depth on mangrove microbes[141, 142]. Further investigation of mangrove microbes focused on natural product chemistry, biotechnology, bioactivity and chemical synthesis of their bioactive metabolites, thus demonstrating the potential of mangrove microbes as a rich source of lead compounds[143]. In particular, over the last decade, research on these microorganisms has led to characterization of nearly 1,000 new metabolites, of which approximately 850 derived from fungi (most of which obtained as endophytes), and ~120 from bacteria[144]. Therefore, the overall trend in the number of experimental articles dedicated to describing new bioactive mangrove-derived metabolites remains promising.
-
This mini-review concludes current studies of value-added compounds in tropical marine plants, including microalgae, seagrasses and mangroves. So far, tropical marine plant extracts are mostly used for the therapy of the globally threatening diseases, such as acquired immune deficiency syndrome, Dengue fever and cancer. Moreover, many active compounds are isolated and identified from total extracts of tropical marine plants for further research and development of medicines. These findings crucially suggest tropical marine plants as a promising source in the fields of biochemistry, biomass energy resources, and pharmacology.
We gratefully acknowledge funding from the Intergovernmental Project of National Key R&D Program of China (2021YFE0110100), the Key R&D Program of Hainan Province (ZDYF2022XDNY140), the Postdoctoral Natural Science Foundation of Hainan Province (RZ2100007121), the National Natural Science Foundation of China (32060061), and the National Key R&D Program of China (2021YFA0909600).
-
The authors declare that they have no conflict of interest.
-
Received 24 April 2023; Accepted 9 June 2023; Published online 3 August 2023
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Hainan University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Xin Y, Du M, Yu X, Paithoonrangsarid K, Mao Y, et al. 2023. Exploring value-added compounds from tropical marine plants. Tropical Plants 2:10 doi: 10.48130/TP-2023-0010
Exploring value-added compounds from tropical marine plants
- Received: 24 April 2023
- Accepted: 09 June 2023
- Published online: 03 August 2023
Abstract: Marine plants contribute half of the global organic carbon fixation, thus play a key role in global carbon cycles. Tropical biomes are the most diverse communities, which produce numerous value-added compounds. The metabolites from tropical marine algae and plants have been utilized for the therapy of the globally threatening diseases, such as acquired immune deficiency syndrome, Dengue fever, and cancer. However, metabolite treasure underpinned by tropical marine plants is largely untapped. The bioactive nature of more compounds remain to be discovered. This mini-review examines several aspects of value-added compounds from tropical marine plants, such as microalgae, macroalgae, seagrasses, and mangroves. Biotechnological and pharmaceutical applications involving these compounds are also discussed.
-
Key words:
- Exploring /
- Value-added /
- Compounds /
- Tropical /
- Marine