-
Polyphenols, a class of bioactive compounds, are secondary metabolites primarily synthesized by plants. They possess multiple phenol structural units[1]. The antioxidant powers of these substances can protect proteins, lipids, and carbohydrates from oxidative damage, thereby decreasing the risk of various chronic illnesses[2]. In food and organism lines, polyphenols are therefore considered to be a valuable source of nutraceuticals. However, the existence of multiple hydroxyl groups in polyphenol's structure made them highly unstable against heat, light, as well as alkaline conditions. In addition, poor solubility attributes of polyphenols restrict their incorporation into various food products[1]. In recent studies, protein-polyphenol complexes were found to provide polyphenols with physical and chemical stability[3]. Generally, the majority of interactions between proteins and polyphenols are non-covalent (i.e., hydrophobic, van der Waals). Jakobek showed that hydrogen bonds are capable of stabilizing proteins[4]. These interactions can be affected by pH, temperature, and structure of both protein and polyphenol[3]. Aside from changing the physiochemical and functional properties of proteins, the protein-polyphenol complex can also alter the thermostability, solubility, and digestibility of protein. The type of interaction between polyphenols and proteins can also affect protein molecular conformation. Thus, polyphenols and proteins interact in a complex way that needs to be understood[5].
Food systems require polyphenols to be stable before they can be effectively incorporated. Canizales et al. found that encapsulation is a promising technique for achieving stability, which involves the entrapment of compounds in immiscible materials (either solid or liquid)[6]. Nanoencapsulation generally produces nanoscale particles (nanocapsules) that are smaller than 1,000 nanometers[7]. Because of nanoencapsulation's improved stability, solubility, and permeability, it has recently become a popular way to protect and deliver polyphenols[8]. Furthermore, a generally recognized as safe (GRAS) encapsulating material is required for the fabrication of carriers (nanocarriers). Many food and pharmaceutical products are made up of biological polymers used in food, such as proteins, carbohydrates, and lipids[9].
Considering their biodegradability, metabolic feasibility, and ease of manipulation, proteins are promising encapsulating materials. In several studies, protein-based carriers are found to be better compounds for the protection and targeted delivery of polyphenols[10,11]. Commonly, protein can be used as the carrier for polyphenols with different encapsulation methods, such as emulsification, nanohydrogel, and biopolymer complex formation[12,13]. In addition, protein-polyphenol interaction can stabilize and enhance the antioxidant capacity of polyphenols. Therefore, in order to improve the stability of polyphenols, protein can be utilized as a carrier for the molecules[14].
As nanotechnology and protein-encapsulated polyphenols are becoming more popular, proteins play the role of a carrier material which needs to be examined to assess the current status and gaps. Therefore, the present review aims to discuss how proteins serve as carriers for polyphenols and to gain a deeper understanding of molecular chemistry. The interactions between different proteins and polyphenols were also reviewed. Moreover, the focus will also be on the recent advances in nanoencapsulation techniques to encapsulate polyphenols using protein as a carrier molecule. Future recommendations were also made based on gaps in the current studies.
-
Generally, nanoparticles are typically used as carriers (transporter) for bioactive/polyphenolic compounds to improve their stabilization, and bioavailability, prevent them from deterioration, and allow for regulated release at specific locations[15]. However, carriers for food packaging based on bioactive ingredients are limited to edible and inexpensive materials. Food biopolymers like proteins, polysaccharides, and lipids are ideal carrier materials for this purpose[7]. Among them, protein as a carrier has attracted attention due to its advantages over others in terms of higher biocompatibility, biodegradability, non-antigenicity, and low cost. In comparison with other colloidal carriers, protein-based carriers have shown good stability in biological fluids, thus, providing controlled and sustained release of drug molecules in the targeted delivery systems[10]. Previous studies confirmed that due to the interaction with a protein molecule, the bioavailability and bioaccessibility of polyphenols could be increased, and the antiproliferative activity of polyphenols can be preserved[11], which suggests that proteins are good carrier molecules for polyphenols. Additionally, protein is known for various beneficial functional properties, which involve structure formation (the ability to form structure, i.e., spheres, or fibres)[4], surface activity, and antioxidant activity. Moreover, a wide range of functional groups on the surface of protein enables them to interact with various substances, which facilitates the fabrication of carriers (nanocarriers) that can encapsulate bioactive compounds (i.e., polyphenols)[16]. These characteristics have led to increased interest in the utilization of protein as a carrier molecule for polyphenols.
-
Protein-polyphenol interactions are usually driven by non-covalent hydrophobic interactions, which can then be stabilized by hydrogen bonds[11]. Ionic interactions, Van der Waals binding, and hydrogen-bridge binding are all references to non-covalent combination, which is reversible, but considered weaker as compared to covalent interactions because they don't involve sharing of electrons. However, a covalent bond (irreversible) can also be formed between proteins and polyphenols. In such interaction, phenols are converted into quinones, followed by their reaction with nucleophilic groups of protein (i.e., NH2 and –SH)[3]. In phenolic-protein interaction, amino acids (i.e., tyrosine, phenylalanine, tryptophan) can interact with phenolic compounds via hydrophobic binding. In addition, phenolics act as hydrogen donors, which results in the making of hydrogen bondings with the carboxyl group of proteins. Consequently, hydrogen bonding can occur between nitrogen/oxygen molecules of amino acids and the carboxyl group of phenolics. Moreover, phenolics can produce quinone radicals, which mediate the development of a covalent bond[17].
Thus, among the bonding/interactions proposed for protein-phenolics, hydrogen bonds, ions (electrostatic interactions), hydrophobic interactions, and covalent bonds have all been proposed. This interaction, however, appears to be driven primarily by hydrogen and hydrophobic bonding[18]. For these associations to form, there are three steps involved. Initially, after adding polyphenols, various phenolic compounds could interact with proteins. Afterwards, a higher polyphenolic amount leads to the emergence of dimers coated with polyphenol, resulting in precipitation. In the end, the large number of molecules results in the formation of larger soluble or insoluble complexes/aggregates[19]. As a result of interactions between polyphenol's hydroxyl group and protein chains or hydrophobic stacking, aggregates are formed[18].
Moreover, hydrophobic interaction can be formed between two aromatic rings (i.e., hydrophobic amino acids can be among them)[20]. There is a direct relationship between protein concentration and hydrophobic interaction. For instance, lower protein concentration leads to the formation of a hydrophobic monolayer; however, higher concentration results in an increased hydrophobic surface layer, which occurs because of the complexation of polyphenols and protein. A crosslinking process also occurs between protein molecules. As a result of these steps, both aggregation and precipitation occurred. When such interactions occur, a protein residue and a phenol residue form an ionic bond (or electrostatic force)[21]. The large number of interactions between proteins, ligands and solvent molecules makes it difficult to simultaneously distinguish their main combining power between molecular[22].
On the other hand, it is possible for phenolic compounds to interact with proteins in a specific or non-specific manner. Enzymes and proteins that have tertiary structures engage in specific interactions (such as milk immunoglobulin)[14]. Additionally, a weak protein-phenolic interaction could also result from hydrolysis, making peptides and polyphenols extracted from broad beans, cocoa beans, flaxseed, and salivary proteins more accessible to nucleophiles[18].
The detailed interactions between various proteins and specific polyphenols are discussed below.
Protein-flavonoids interaction
-
Flavonoids consisted of a diphenyl propane carbon skeleton (C6-C3-C6), and based on their oxidation level and substitution patterns of C-ring, it is divided into different types. Anthocyanidins, isoflavones, flavones, flavon-3-ols, flavone-3-ols and flavon-3-ols are a few examples of flavonoids[3]. Most flavonoids have three phenolic rings, two benzene rings (A and B) and a heterocyclic pyran ring (C). The unique structure and properties of different flavonoid derivatives are mainly determined by the change of substituent space, character, direction and quantity[23].
Flavonoids have the ability to interact with proteins through both covalent and non-covalent binding mechanisms. In covalent interaction, the association appears between functional groups, e.g., protein's amine and amide group on one side, whereas, quinones, on the other side, are formed by flavonoid oxidation (chemically or enzymatically)[24]. It is possible that proteins and flavonoids interact non-covalently in a chemically specific or non-specific manner. Specific binding is often reported for interaction between flavonoids and proteins having defined globular tertiary structures. Particularly, non-globular proteins, including salivary proline-rich proteins and albumin-type proteins (such as casein, bovine serum albumin, and ovalbumin), tend to demonstrate non-specific interactions with flavonoids[24].
Research has been conducted extensively on the relationship between milk proteins and flavonoids. Most of these studies revealed the domination of non-covalent interactions involving bond-hydrogen, hydrophobic, and van der Waals force between them[3]. The milk proteins (i.e., β-Lg) have gained attention because of their capability to interact and enhance the stability of various compounds (i.e., flavonoids). The binding interaction between (−)-epigallocatechin (EGC) with bovine β-Lg was studied[25], and it was confirmed that EGC was bound to β-Lg, and this binding was mainly due to hydrogen bonding and van der Waals interactions. Pu et al. also studied the interaction between flavonoids and β-Lg. This interaction occurs between 8, 8, 13, 16, 15, and 6 amino acids residues of β-Lg and 6 flavonoids (kaempferol, myricetin, phloretin, epigallocatechin-3-gallate, naringenin, and quercetin), which resulted in 2, 5, 6, 12, 4, and 4 hydrogen bonds with β-LG respectively. In hydrogen bonding (except with naringenin), Thr18 contributed by acting as a hydrogen donor, while flavonoids act as hydrogen acceptors[26]. Moreover, the most significant role in hydrogen bonding between flavonoid-protein interaction is played by Gln and Glu (amino acids). For instance, it was found that Glu44, Glu45, Glu157, and Gln59 acted as hydrogen acceptors to develop hydrogen bonds with epigallocatechin-3-gallate (EGCG). Overall, hydrogen bonding, Van der Waals, and hydrophobic combining were the dominant driving forces[26]. The binding affinity of cyanidin-3-O-glucoside (C3G) towards ovalbumin was found to be greater in neutral as compared to an acidic environment, while this interaction resulted in conformational changes in proteins structure (α-helix structure increase, reduction of β-turn, and total reduction in main secondary structure), which lead to more unfolding of ovalbumin structure[22].
The relevance of bovine serum albumin (BSA) to model biotic interaction with HSA and proteins found in saliva has made it a commonly used example to study flavonoid-protein interactions[24]. Although milk protein like casein was able to improve the stability of anthocyanins and avoid their decomposition effectively, protein concentration could be a primary factor that can influence the protective effect. For instance, when the concentration of casein rises to the critical micelles concentration (about 0.5−2 mg/ml), casein, being monomeric, aggregates through self-association, minimizing its interaction with anthocyanin (from grape skin anthocyanin extract)[27].
The previous research shows that the main driving forces in flavonoid protein interactions are van der Waals interaction, hydrogen bonding, and electrostatic interaction. However, the effect of these interactions depends on protein and flavonoid structures[24]. Also, due to flavonoid-protein interaction, conformational changes occurred in the protein’s structure. Consequently, it suggests that flavonoids like EGCG and ECG would show strong affinities towards proteins.
Protein-phenolic acid interaction
-
Phenolic acids, a class of polyphenols, consist of two major subclasses which are hydroxyl benzoic acids (like gallic acid, vanillic acid, propyl gallate, and methyl gallate) and hydroxyl cinnamic acids (involving chlorogenic acid, ferulic acid, caffeic acid, and cinnamic acid)[5]. The protein-phenolic acid interaction is mainly carried out by hydrogen bonding (between hydroxyl groups of polyphenol and a polar group of proteins) and hydrophobic interaction, different phenolic acids can be related to protein binding sites[4]. Interaction between phenolic acids (coumaric acid, chlorogenic acid, caffeic acid, and ferulic acid) and proteins (α-lactalbumin and β-lg) were examined, and it was revealed that interaction of phenolic acids takes place with the C=O and C-N groups of protein’s structure. As a result of this interaction, rearrangement of the polypeptide carbonyl hydrogen-bonding pattern can occur, representing the interaction between phenolic acids and peptide bonds[28].
Extensive investigations have been conducted on the interactions between proteins and phenolic acids[5]. For example, a study on the interaction between rice glutelin and gallic acid (GA) revealed that binding was dominated by van der Waals force and hydrogen bonding[5]. However, increasing GA concentration also resulted in a decreased hydrophobicity of rice glutelin. Budryn et al. reported the interaction of hydroxycinnamic and CA (from green coffee) with food proteins (including egg white, whey protein isolates, and soy protein isolates)[29]. These researchers observed that a significant proportion of phenolic acids were bound to protein, and binding was mostly dominated by hydrophobic interaction. Similarly, non-covalent bindings have also been reported by Li et al.; however, these researchers concluded that different phenolic acids (i.e., CA, ferulic acid, gallic acid), may bind to different binding sites from protein (i.e., β-casein)[30].
In comprehensive research, Wu et al. examine the correlation between the structure and affinity of β-lactoglobulin and 71 phenolic acids[31]. Similar to other studies, hydrogen bonding was found to be a major force behind phenolic acid-protein interaction. Furthermore, it was revealed that the affinity of phenolic acids for β-lg was enhanced when the hydroxyl group was replaced with the methyl group at the second position. Moreover, hydroxylation at the third position also increases the affinity of phenolic acids. On the other hand, as the result of GA binding, structural changes in protein structure with the reduction in α-helix structure and an increase of β-sheet structure of rice glutelin were recorded[5]. Previously, Zhang et al. studied phenolic acids (CA, caffeic acid) interaction with milk whey protein (α-lactalbumin and β-lg) and observed alteration of whey protein structure with an increase in β-sheet and a significant decrease in α-helix structure[28].
Hydrophobic interaction
-
A stilbene consists of two benzene rings connected by an ethane linker. A variety of stilbene compounds (i.e., resveratrol) are complex with proteins, but this mechanism could potentially modify the configuration and binding sites of proteins, inducing conformational alterations[18]. Using milk proteins (β-lg, β-casein, and BSA) as a model, Gorji et al. investigated the effect of resveratrol on them and found domination of hydrophobicity[32]. In this type of interaction, no significant effect of resveratrol on the secondary structure of β-lg was found; however, it impacts the tertiary arrangement and leads to disturbance through a subtle opening of the hydrophobic pocket, resulting in structural disruption.
Another study on the binding of resveratrol with protein (zein and gliadin) revealed that binding is mainly carried out by hydrophobic interaction when resveratrol binds with gliadin while binding with zein is mainly carried out via hydrogen bonding[33]. Cao et al. observed that hydrophobic interaction was the dominant force during stilbenoids-HSA binding. These authors concluded that the addition of a hydroxyl group also improved the affinity[34]. However, the o-hydroxy derivatives significantly reduced uptake, and the glycosylation of resveratrol significantly reduced uptake. Therefore, the glycosylation process weakens the affinity in these interactions.
Protein-tannins interaction
-
There are two classes of tannins based on their structure, both of which are phenolic compounds. One of them is condensed tannins (proanthocyanidins), and the other is hydrolyzable tannins. Tannins have the ability to bind with proteins predominantly via hydrogen bonds which are formed between phenolic donors and peptide acceptors[18]. The covalent or noncovalent binding affinity between proteins and tannins depends on whether reversible or irreversible binding between molecules occurs. Furthermore, these interactions can lead to the generation of both soluble and insoluble complexes[35]. Additionally, the binding of tannins to globular proteins includes surface exposure of aromatic residues, while the association with proline-rich proteins can involve face-to-face stacking of aromatic groups on proline residues[18]. The binding of proline-rich protein and tannins has shown strong hydrogen bonding[35]. Nevertheless, interactions with proline-rich proteins can be confirmed in a flexible way to enhance hydrogen bonding accessibility[18].
-
Several factors affect protein-polyphenol interactions, including pH, temperature, and structure of both proteins and polyphenols[36,37].
Temperature is an important parameter in protein-polyphenol interaction as it influences hydrogen bond interaction and results in the development of hydrophobic bonding[36]. When proteins are heated, their conformation changes and their hydrophobic sites are exposed, which affects how hydrophobic compounds interact. It is these hydrophobic groups that provide interactions between the phenolic compounds and non-polar groups[37]. In addition, the temperature may affect structure evolution in the solubility of protein molecules and ligands, and these changes may affect interactions between proteins and polyphenols[3]. A previous study by Wang et al. found that higher temperatures increased the affinity of EGCG for BSA[38]. It might be due to hydrophobic ports unfolding and being exposed, causing higher levels of EGCG binding on the BSA surface. Furthermore, heat causes polyphenols to oxidize, leading to the formation of quinone derivatives and the decomposition of polyphenols, resulting in the loss of bioactivity[37]. As a result, temperature is one of the key parameters that determine the interaction between proteins and polyphenols, their bioactivities, and their functional properties.
PH plays a significant role in protein-polyphenol interactions as well, which has been extensively investigated. It is generally known that proteins dissociate at low pH levels (pH < 7.0), which exposes binding sites of phenolic substances by electrostatic interaction[36]. At pH 4.9, the binding affinity of tannic acid with globular protein (i.e., BSA) was higher than at pH 7.8[20]. According to Rawel, Meidtner, and Kroll, GA and BSA have a lower binding affinity (at pH 3.5)[39]. When the pH was altered, the researchers observed no substantial variations in the binding affinity between carbonic anhydrase (CA) and bovine serum albumin (BSA). Nevertheless, With a decrease in pH, the binding sites for ferulic acid and carbonic anhydrase (CA) were modified, primarily due to alterations in the tertiary structure of bovine serum albumin (BSA). As a result, changes in pH are mainly associated with conformational transformation in protein macromolecules, which indicates the indirect effect on protein-polyphenol interaction.
Moreover, protein-polyphenol interaction can be affected by the type of polyphenolic compound and its structure. Different polyphenols vary in their molecular weight, hydroxylation, methylation, hydrogenation as well as glycosylation, which results in a strong influence on interaction with proteins[36]. It is generally believed that polyphenols have a higher binding affinity as their molecular weight increases[3]. An easily exposed combining site among polyphenols like thearubigin (from black tea) and theaflavin, compared to flavanols (catechins), results in a greater affinity between phenolic compounds and milk proteins[36]. Increasing hydroxylation can also affect combining affinities. For instance, higher flavone hydroxylation results in upper BSA combining efficiency[37]. Also, hydrogenation of the C2-C3 double bond of flavonoids can decrease the combining affinities between polyphenol and milk proteins[37]. As well, phenolic acid binding affinity with proteins, such as leucine-rich proteins, is influenced by factors such as hydroxylation, methylation, steric hindrance, and the specific type of phenolic acids[30].
-
Recently, researchers' attention towards nanoencapsulation has increased due to the efficient controlled release of encapsulated compounds/ingredients, effective delivery, and greater stability[8]. For the fabrication of nanoparticles, two basic methods have been used, which are top-down and bottom-up methods. For selected nanoparticle applications, a top-down approach involves using accurate tools to reduce the size and shape of nanoparticles. While, the bottom approach involves self-assembly and self-organization of biomolecules for the construction of materials influenced by different factors, like pH, heat, ionic strength, and concentration. Nanoprecipitation and coacervation are examples of bottom-up methods, while emulsification is an example of a top-down approach[7].
A variety of methods have been used to encapsulate and stabilize different polyphenols using proteins as nano-carriers. Table 1 summarizes the detail of protein-based nano-carrier for the stabilization of various polyphenolic compounds. The different techniques along with their application are discussed below.
Table 1. Use of protein as the carrier for various polyphenols.
Nanoencapsulation techniques Carrier compound Polyphenolic compound Particle size
distribution (nm)Key discoveries Utilizations Citations Nanoemulsion WPC and pectin Olive leaf phenolic extract − Emulsion stability with controlled release of the encapsulated substance Food product fortification Mohammadi et al. (2015)[12] Nanoemulsion WPC and pectin Gallic acid 100−200 emulsion from the WPC-pectin complex had the same resistance (against creaming and sedimentation) as a synthetic emulsifier (Tween-80) Food, pharmaceutical and cosmetic industry Gharehbeglou et al. (2019a)[8] Nanoemulsion WPC and pectin Oleuropein 191 Successful encapsulation with an encapsulation efficiency of 91% Food, pharmaceutical and cosmetic industry Gharehbeglou et al. (2019b)[40] Nanoemulsion WPC Curcumin 141 This method was effective to increase the bioaccessibility of compounds Food and pharmaceutical industry Sari et al. (2015)[41] Nanoemulsion BSA Curcumin 18−30 Stable and controlled drug release was achieved Food and pharmaceutical industry Kaur et al. (2015)[42] Nanoemulsion Chitosan and soy protein Grape and apple pomace phenolic extract 5−300 Successfully encapsulation of encapsulant Food, pharmaceutical and cosmetic industry Gaber Ahmed et al. (2020)[43] Biopolymer nanocomplex formation WPC and pectin D-limonene 100 4% WPC and 1% pectin yielded superior results in complex formation Food products like cakes, muffins, juices and biscuits Food products such as cakes, muffins, juices, and biscuits Ghasemi et al. (2018)[44] Biopolymer nanocomplex formation Complexation of zein, sodium caseinate, and pectin Eugenol 140 Provide better stability to eugenol Food industry Veneranda et al. (2018)[13] Biopolymer nanocomplex formation Protein isolate and Peet pectin Anthocyanin < 200 Ineffective in enhancing antioxidant activity and color stability Application of natural colorants and nutraceuticals Arroyo-Maya & McClements (2015)[45] Biopolymer nanocomplex formation lactoferrin and pectin Curcumin 208 This complex resulted in high encapsulation efficiency (85%) with a 13.4% loading capacity Food, pharmaceutical, and cosmetic sectors Dai et al. (2017)[5] Biopolymer nanocomplex formation Zein and pectin Curcumin 250 More than 86% encapsulation efficiency was achieved Food, pharmaceutical, and cosmetic sectors Hu et al. (2015)[46] Biopolymer nanocomplex formation Zein and pectin Resveratrol 235 Successful encapsulation Bioactive food and beverage items, as well as their integration into nutraceuticals and medicinal preparations Huang et al. (2017)[5] Biopolymer nanocomplex formation Chitosan and β-lactoglobulin Quercetin 170−350 Increased encapsulation efficiency Food industry Ha et al. (2013)[47] Biopolymer nanocomplex formation Zein/sodium caseinate complex Quercetin 130−161 Encapsulated quercetin was stable when exposed to UV light and alkaline pH Food, pharmaceutical, and cosmetic sectors Patel et al. (2012)[48] Biopolymer nanocomplex formation Whey protein isolate and pectin Quercetin 200−500 The encapsulated phenolic compound showed better stability (4 times better) against UV irradiation as compared to free quercetin Food products like beverages Wijaya et al. (2019)[49] Biopolymer nanocomplex formation Casein and pectin Rutin 199−697 Successful encapsulation Food and pharmaceutical industry Luo et al. (2015)[50] Nanoydrogel 'LDL' and 'pectic substances' Curcumin <60 Successful creation, outstanding stability in simulated gastrointestinal conditions, and regulated release of curcumin. Food and pharmaceutical industry Zhou et al. (2016)[51] Nanoydrogel Lactoferrin-glycomacropeptide and chitosan Curcumin and caffeine Bioaccessibility of encapsulants was increased Food and pharmaceutical industry, Drug delivery Bourbon et al. (2018)[52] Nanoydrogel Lactoferrin Curcumin 89.4 Successful encapsulation Food and pharmaceutical industry de Araújo Lopes et al. (2018)[53] BSA = Bovine Serum Albumin, WPC = Whey protein concentrate. Emulsification/nanoemulsion
-
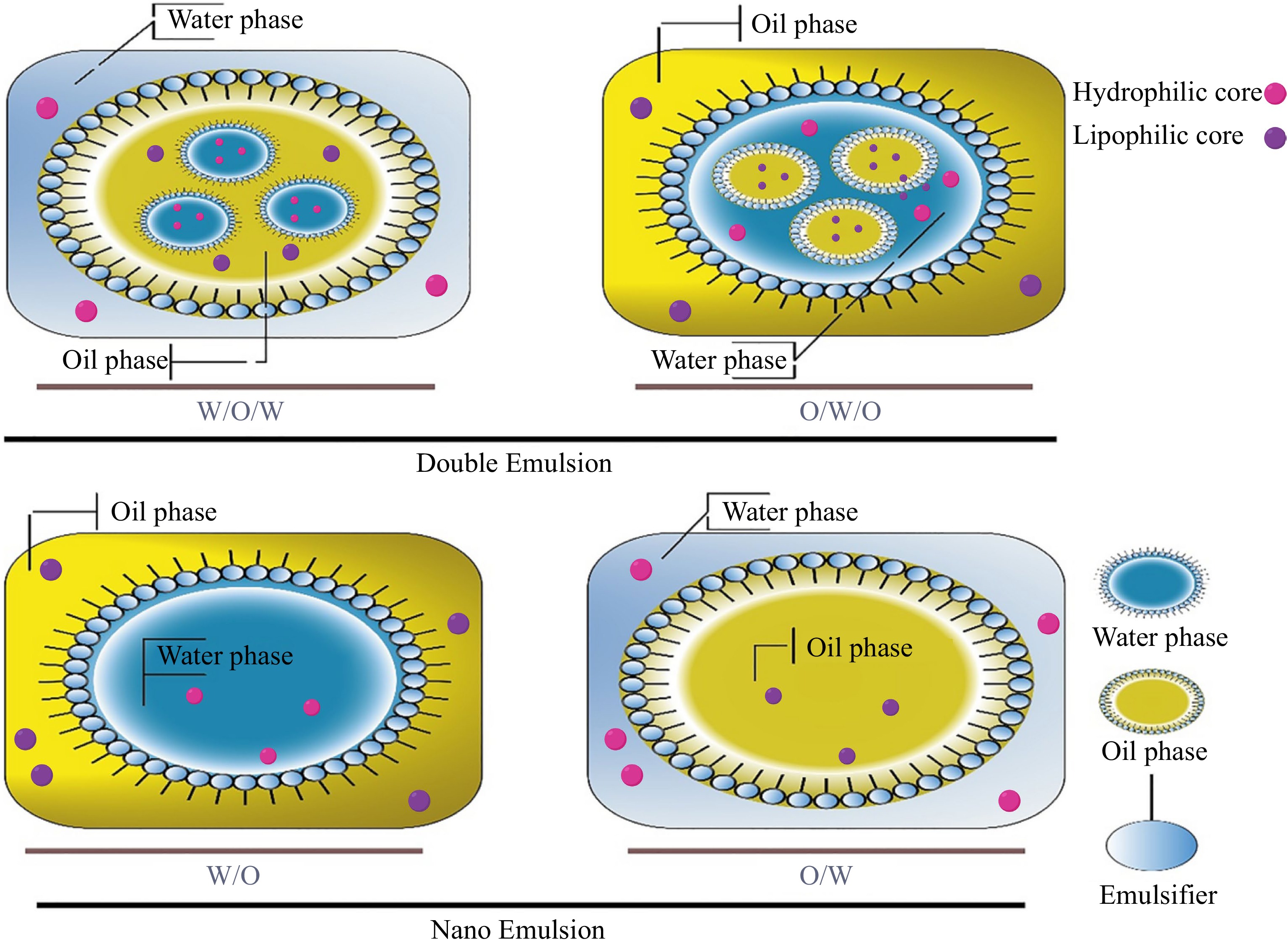
The emulsion is defined as the combination of two immiscible liquids, where one liquid is dispersed into another (e.g., water in oil (W/O), oil in water (O/W)) with the help of an appropriate emulsifier[7,54]. While nanoemulsion is a submicron or mini-emulsion characterized by its smaller mean radii (about 50−500 nm)[9]. Figure 1 shows a schematic diagram for different forms of emulsions. To achieve emulsification, various methods like ultrasonication, homogenization, microfluidization, and high-shear mixing have been used. Generally, the emulsion can be divided into two groups, single emulsion (oil in water, O/W, water in oil, W/O) and double emulsion (oil in water in oil O/W/O, and water in oil in water, W/O/W)[8, 55]. In a double emulsion, a W1/O/W2 emulsion is made of W1/O droplets, which are distributed in the exterior continuous aqueous phase (W2)[7]. In such a type of emulsion, functional compounds (i.e., polyphenols) are carried by the internal water phase (W1)[8]. High or low energy techniques could be utilized to get a double emulsion. High energy techniques include ultrasonication and homogenization, which result in smaller and homogenous droplets[7].
The size of particles plays a crucial role in the stability of emulsions, where smaller particle sizes contribute to enhanced emulsion stability. In the case of nanoemulsion, droplet sizes range from 10−100 nm, and are generally enclosed by a slight emulsifier layer which is distributed evenly in the aqueous phase. Moreover, the stability of the emulsion depends on its viscosity, droplet size in a continuous phase, type and concentration of the emulsifier in a continuous phase, as well as density difference between continuous and dispersed phases[7]. Also, protein is an effective emulsifier for the preparation of emulsion and can lead to generating of protein coated lipid-nanoparticles[16].
In order to enhance the procedure of nano-encapsulation and final product quality, different methods have been used based on emulsification[56]. The emulsion diffusion method is one of the methods which involves emulsion and diffusion steps. In the emulsification step, both the organic and aqueous phases are developed separately, and then the emulsion is formed with the help of mechanical shearing. In this case, encapsulated material can either present in the organic/aqueous phase depending on its polarity. During the later stage, known as the diffusion step, the introduction of water leads to the removal of the organic solvent present in the oil phase. This process results in the separation of oil and biopolymer, accompanied by a reduction in particle size, leading to the formation of a nano-carrier[7]. Another technique that has been used is an emulsification-solvent evaporation method in which polymer and organic solvents are emulsified into an aqueous phase with the help of an emulsifier. In a later stage, the evaporation of solvent results in polymer precipitation as nanospheres. Such methods have the ability to form particles with small diameters (< 100 nm). Das Purkayastha and Manhar report this compound loading efficiency of about 75%−96%[57].
Proteins have been widely used in emulsion for stabilization and control release of polyphenols[58]. For example, Mohammadi et al. successfully used whey protein concentrate (WPC) for nano-encapsulation of olea europea leaf polyphenol extracts with the help of multiple emulsion methods (W/O/W)[12]. These authors concluded that this method has the ability to formulate stable nanoemulsions and controlled the release of the encapsulated compounds. In another study, a double nanoemulsion was prepared from the WPC-pectin complex in order to protect GA[8]. Results indicated that emulsion from the WPC-pectin complex had the same resistance (against creaming and sedimentation) as a synthetic emulsifier (Tween-80). Moreover, it was observed that this complex had greater stability during a long storage period. Thus, protein along with pectin can be used for the stabilization of double emulsion contained with phenolics[8]. In a similar study, Gharehbeglou used double emulsions made up of WPC and pectin to nanoencapsulate oleuropein (a polyphenol found in olive leaf)[8]. Under optimum conditions (8% WPC, 1.97% pectin and pH 6.12), they achieved encapsulation efficiency of 91% with a droplet size of 191 nm.
In another study, Sari et al. prepared a nanoemulsion for the encapsulation of curcumin (a natural polyphenol) with the help of whey protein concentrate[41]. These authors concluded that this method was effective to increase the bioaccessibility of compounds (like polyphenols). Similarly, curcumin-loaded nanoemulsion has also been formulated with the help of BSA, and researchers found that such systems exhibit 99% encapsulation efficiency with a slow and constant release[42]. In a recent study, Ahmed et al. successfully encapsulated grape and apple pomace phenolic extract with the help of nanoemulsion utilizing chitosan and soy protein and observed enhanced antioxidant activity of the phenolic extract[43] These studies show the effectiveness of protein to stabilize phenolic compounds in emulsification systems.
Biopolymer complex (nanocomplex)
-
Nanoencapsulation technique also includes the fabrication of nanoparticles through complex formation between biopolymers (i.e., proteins, carbohydrates)[7,59]. These biopolymer complex nanoparticles could be formed through electrostatic interaction among these biopolymers, for instance, a net positive charged protein can interact with ionic polysaccharides (if the isoelectric point is higher than the pH of the medium)[60]. The interaction between biopolymers can occur by direct interaction of protein/polysaccharide complexes. Moreover, interaction can also begin with the generation of protein nanoparticles followed by the adsorption of ionic polysaccharides on nanoparticles, also known as layer by layer method[7]. In the biopolymer complex, the interaction is mainly affected by pH, ionic strength, concentration and density of proteins and polysaccharides, composition of biopolymers, and order of biopolymer synthesis. Polyphenols could be encapsulated in such complexes by adding the encapsulate in a complex biopolymer solution and homogenizing it[60]. Alternatively, as an alternative approach, the compounds can be dissolved in a solution containing complex biopolymers, and subsequently separated and obtained in solid form through the processes of centrifugation and drying[7].
Limonene is one of the most popular phenolic compounds found in citrus essential oil[61]. Ghasemi et al. extracted D-Limonene from citrus peel and successfully encapsulated it with the help of nanocomplex formation of WPC and pectin[44]. These authors investigate the different concentrations of WPC (4%, 6%, 8%), pectin (0.5%, 0.75% and 1%) and pH (3, 6 and 9) and evaluated product stability. These researchers observed that optimum stability, viscosity, and colors were obtained at 4% protein, 1% pectin and pH 3. They concluded that such nanocomplex encapsulated D-Limonene may have applications in various food products, i.e., juices[44].
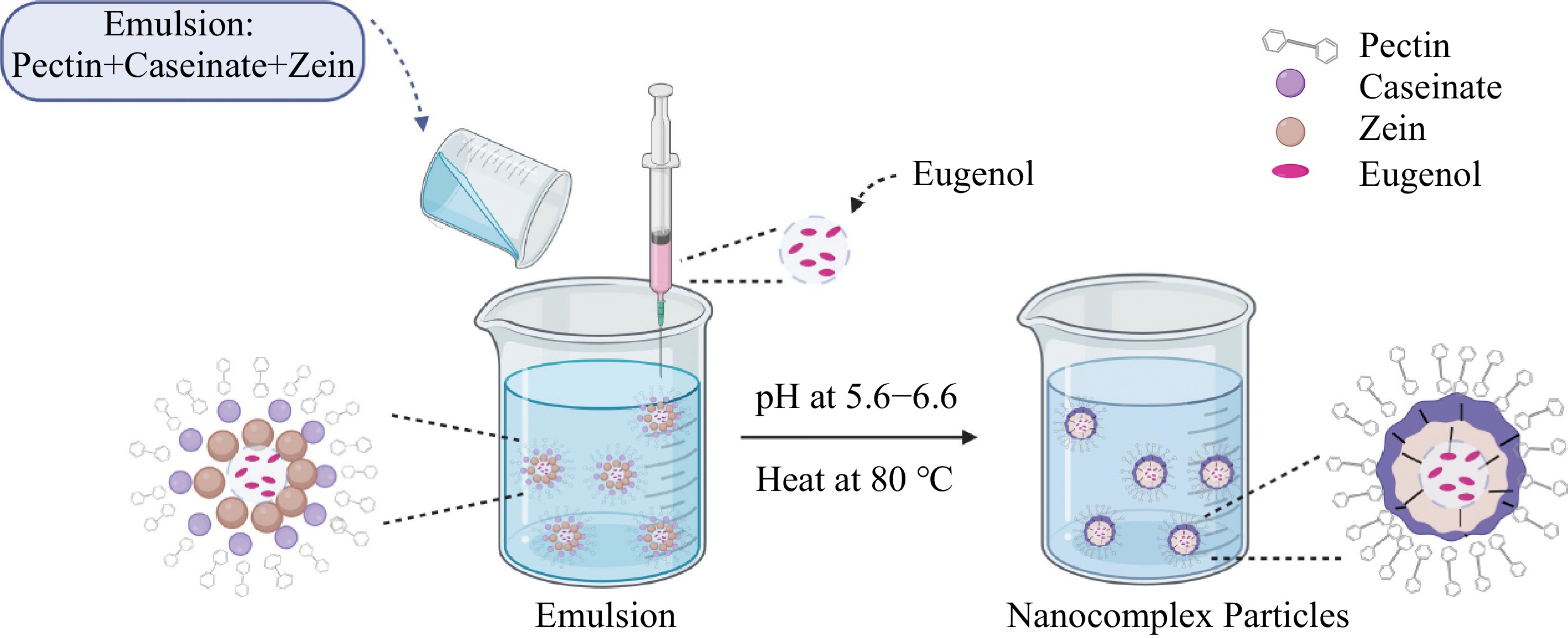
Moreover, the researcher has also encapsulated eugenol (a phenolic compound from clove oil) in zein/sodium caseinate/pectin complex nanoparticles to provide stability to eugenol[13]. The nanocomplex formation using zein/casein/pectin particles is represented in Fig. 2. Anthocyanin was encapsulated by thermal process and electrostatic complexation using a protein isolate and beet pectin[45]. However, these researchers found that this technique does not cause improvement in colour stability as well as antioxidant activity of anthocyanin. Similarly, the interaction between lactoferrin and pectin nanoparticles can also be used to encapsulate curcumin. This complex resulted in high encapsulation efficiency (85%) with a 13.4% loading capacity[62]. In addition, the researchers concluded that the produced nanocapsules could serve as a suitable delivery system for enhanced curcumin stability and controlled release. Additionally, the zein/pectin complex can also be used for the production of nanoparticles loaded with curcumin and higher encapsulation efficiency (86%) can be achieved by this method[46]. In addition, Razi et al. successfully encapsulated curcumin using a caseinate-chitosan nanocomplex[53].
Huang et al. encapsulated resveratrol with the help of the same zein/pectin complex and found the higher antioxidant activity of produced particles than free resveratrol[5]. Thus, the authors suggested that resveratrol-loaded biopolymer complex can have applications in pharmaceutical products and the food industry. Recently, a study demonstrated that tea polyphenol (catechin) can also be stabilized using the complex of BSA and human serum albumin and concluded that these serum proteins are effective as in vitro transport of tea catechins[63].
Previously, a nano-complex of Chitosan oligosaccharide substituted with linoleic acid combined with β-lactoglobulin has been used to encapsulate quercetin, and results showed encapsulation capacity of quercetin was improved by this method[47]. Similarly, quercetin was also encapsulated in zein/sodium caseinate complex nano-particles and was found stable upon exposure to pH (alkaline) and UV light[48]. Similarly, quercetin stability can also be improved using whey protein isolate and pectin nanocomplex particles. These authors found that quercetin was efficiently entrapped with an efficiency of about 97%. Moreover, encapsulated phenolic compound (quercetin) showed better stability (four times better) against UV irradiation as compared to free quercetin[49]. Also, the stability of quercetin can be improved by utilizing pea protein-mesquite gum nanocomplex[63].
Another study demonstrated that the stability and antioxidant activity of EGCG could be enhanced by binding with BSA-i-carrageenan nanocomplex[64]. Similarly, the complex of Lactoferrin-chondroitin has been used for the combined delivery of doxorubicin and ellagic acid for enhanced anti-tumour activity and ultimately improve in vitro/in vivo transport of drugs[65]. According to Luo et al., casein-pectin nanocomplex can be effectively utilized for the targeted delivery of rutin (a flavonoid)[50]. This research confirmed that this method provides stability to rutin in gastric conditions while effectively releasing it in the intestinal environment.
Hydrogels (nanohydrogels)
-
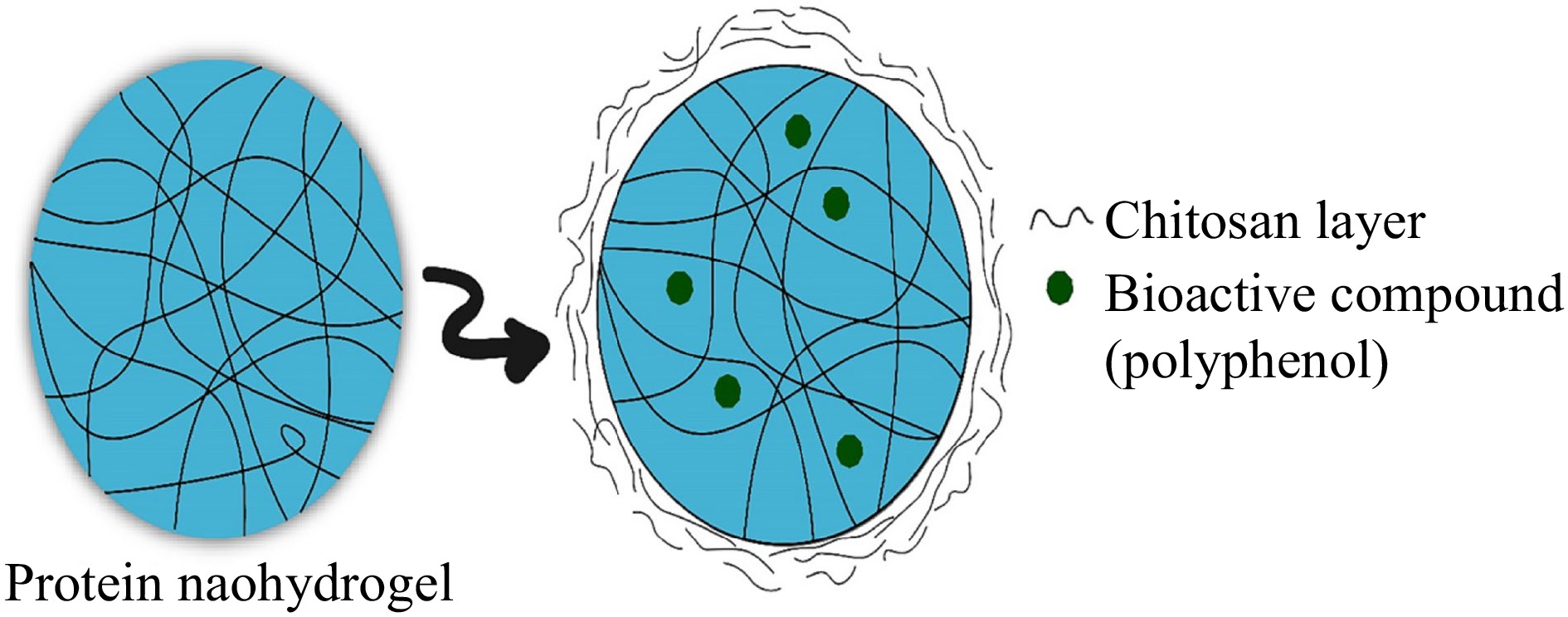
Hydrogels are three dimensional, hydrophilic and cross-linked polymeric structures that have the capacity to take up a large quantity of water/biological fluid inside their network[7]. Figure 3 shows a schematic diagram for protein-based hydrogel and the stabilization of polyphenols in the hydrogel system. Even in severe conditions (such as heat, and pressure), the hydrogel has the ability to hold some absorbed fluid[66]. In the absorption of this fluid, various types of interaction are involved, including osmosis, capillary pressure and biomolecular interaction of polymeric and liquor, which can affect the structure as well as dimension of gels. When hydrogels are produced in nanoparticle size, they are known as nanohydrogels. For hydrogel preparation, protein and polysaccharides are the most commonly used polymers and are generally prepared through the gelation mechanism[7]. Generally, hydrogels can be formed through a single or multiple-step method. Single step technique involves parallel cross-linking of multifunctional monomers, while multiple step methods include the synthesis of polymer molecules, and their subsequent crosslinking[67]. Also, biopolymers with opposite charges could self-assemble into nano-hydrogels via electrostatic forces[57]. During the production of nano-hydrogel, gelled aqueous/fluid phase is achieved through acidification, the addition of multivalent ions or temperature adjustment, or cross-linking agents[67]. Nanohydrogel has the capability to protect both lipophilic and hydrophilic compounds and can provide stability against degradation. Due to their effective performance, hydrogels can be utilized for the targeted release of various compounds (i.e., polyphenols)[55,68].
A study synthesized nanogels with the help of hypo density lipoprotein and pectin in order to encapsulate curcumin for oral drug delivery[51]. They successfully developed nanoparticles with smooth surfaces and uniform size distribution, which could effectively control the release of curcumin. It prepared lactoferrin-glycomacropeptide nanohydrogels (with chitosan coating) for evaluating the stability and the bioaccessibility of two compounds (curcumin and caffeine). In this study, the bioaccessibility of encapsulated compounds was evaluated during gastrointestinal conditions. It was found that the bioaccessibility of curcumin and caffeine in the coated hydrogel was 72% and 63%, while in free form, both compounds showed bioactivity of 66% and 59%, respectively. Moreover, improved antioxidant activity in coated nanogels was also reported[52]. Similarly, it successfully prepared lactoferrin-based nanohydrogel for the encapsulation of curcumin. They observed high loading efficiency and high release rate of curcumin loaded nanohydrogel in a lipophilic food simulant as compared to a hydrophilic one.
-
Protein is a potential carrier material for improving stability, targeted delivery and controlled release of phenolic compounds. The protein-polyphenol interactions can affect the structure and physiochemical properties of proteins as well as polyphenols. Among non-covalent forces (i.e., Van der Waals and hydrogen bridge bindings), Van der Waals is the dominant force, which is always reversible. However, irreversible covalent interactions could also occur between protein-polyphenol. On the contrary, protein can be used as carrier material for stabilization and targeted delivery of polyphenols (curcumin, catechin, flavonoids, etc.) using the nanoencapsulation technique. Different encapsulation methods have been utilized for encapsulation purposes involving emulsion (single and double emulsion), nanohydrogel and nanocomplex formation. It is worth noting that most of the research focused on the use of protein with other polymers (i.e., carbohydrates), which could be an effective strategy to enhance stability and control the release of polyphenolic compounds by nanocomplex formation. Although many studies are presented, studies related to interactions between specific protein-polyphenol are still scarce. Moreover, there is a need to optimize process conditions for the encapsulation of specific polyphenolic compounds and test in vitro and in vivo conditions for effectiveness. Further studies could open new ways to effectively utilize proteins for the nanoencapsulation of phenolic compounds.
The work was financially supported by the National Key Research and Development Program of China, China (2022YFD2100100), National Natural Science Foundation of China, China (32272776; 32001748), Natural Science Foundation of Ningbo, China (202003N4311), Zhejiang Public Welfare Technology Application Research Project, China (LGN21C200018), Key Research and Development Program of Zhejiang Province (2022C02033, 2021C02014), China and 72nd Postdoctoral Foundation (2022M713423). Young Elite Scientists Sponsorship Program by CAST (2022QNRC001).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Xiao Y, Ahmad T, Belwal T, Aadil RM, Siddique M, et al. 2023. A review on protein based nanocarriers for polyphenols: interaction and stabilization mechanisms. Food Innovation and Advances 2(3):193−202 doi: 10.48130/FIA-2023-0021
A review on protein based nanocarriers for polyphenols: interaction and stabilization mechanisms
- Received: 02 May 2023
- Accepted: 01 July 2023
- Published online: 11 August 2023
Abstract: Protein has been used as the carrier for protecting and targeting polyphenols and increasing their shelf-life. Interactions of a protein molecule with polyphenols are important, which change functions and physiochemical properties of the complex and provide protection to polyphenols. Interactions between proteins and polyphenols are largely non-covalent. Factors that affect such interactions include pH, temperature, and the structure of both proteins and polyphenols. Moreover, excellent stability of polyphenols can be achieved by using nanoencapsulation techniques such as emulsion, nanohydrogel, and nanocomplex formation. The use of protein combined with other compounds such as lipids and carbohydrates was found to be the most suitable carrier for polyphenols encapsulation. This review aims to describe the interaction between proteins and polyphenols, focusing on applying nanoencapsulation for increasing stability and targeted delivery of phenolic compounds.
-
Key words:
- Protein-polyphenol interaction /
- Stabilization /
- Nanoencapsulation /
- Nano-carrier