-
Scutellaria is a perennial genus comprising about 350 species that belong to the Lamiaceae family. New species are continually being discovered[1]. It is the second-largest genus in the Lamiaceae after Salvia and is widely distributed in tropical mountains and temperate latitudes[1,2]. Many Scutellaria species are used as medicines and ornamental plants[1]. Most plants of the genus Scutellaria have medicinal values, with extracts or monomer compounds exhibiting antitumor, hepatoprotective, antioxidant, anti-inflammatory, anticonvulsant, antibacterial, and antiviral effects[2]. Over 295 compounds, including flavonoids, diterpenes, triterpenoids, phenylethanoids glycosides, alkaloids, phytosterols, polysaccharides, and iridoid glycosides, have been isolated from Scutellaria[2−4]. Flavones are essential natural active compounds in the genus Scutellaria that has significant pharmacological activities on the human body, including anti-oxidation, anti-cancer, anti-inflammatory, anti-malignant cell proliferation, anti-angiogenesis, and anti-estrogen effects, with no or few toxic side effects from ingestion[5,6]. Baicalin, baicalein, wogonin and wogonoside are the main components with anti-tumor activity[4].
Scutellaria baicalensis Georgi, also known as 'Huang Qin' in China, has been used in traditional Chinese medicine (TCM) for over 2,000 years to treat headaches, infections, cancer, allergies, and inflammation[7]. The roots of S. baicalensis are the major medicinal part, but the total flavonoid extract of leaves and stems are also used for medicines[4]. S. baicalensis is the most studied plant in the genus Scutellaria in terms of pharmacological research and specialized metabolism. Baicalein from S. baicalensis has been shown to inhibit replication of SARS-CoV-2 by binding its 3C-like protease[8]. The biosynthetic pathways of baicalein and wogonin in S. baicalensis have been fully elucidated[6,7,9]. Moreover, SbMYB3 transcription factor was found to activate the biosynthesis of root-specific flavones[10]. The genome of S. baicalensis has been reported[7], providing a basis for the study of specialized metabolism and genome evolution of Scutellaria.
The whole herb of Scutellaria indica L. (Chinese name Han Xin Cao) is used for clearing heat and detoxifying, promoting blood circulation and hemostasis, dispersing depression, and deswelling[11]. Modern pharmacological studies have demonstrated the antiviral activities of S. indica and its therapeutic effects on cardiovascular diseases[12,13]. Sculellaria barbata D. Don (Chinese name Ban Zhi Lian), is widely used in traditional Chinese and Korean medicine. The whole plant of S. barbata clears heat and detoxifies, removes blood stasis, and promotes diuresis in the TCM system[3,14]. S. barbata has pharmacological activities, including anti-microbial, anticancer, anti-angiogenesis, antioxidant, and anti-inflammation[3,15], with neo-clerodane diterpenoids and flavones being its major bioactive compounds[3,14]. S. barbata can also effectively prevent SARS-CoV-2 infection and replication by inhibiting Mpro and TMPRSS2 protease activities[14]. The chromosome-level genome assembly of S. barbata has also been completed[16]. Scutellaria strigillosa Hemsl. is recorded to clear heat and damp, and it accumulates abundant flavones such as baicalin and wogonoside. S. strigillosa extracts also have anti-proliferative and anti-migratory properties[17]. The whole plant of Scutellaria obtusifolia Hemsl. is used to treat colds, bruises, diarrhea, and snakebites. Scutellaria altissima L. is used to treat pneumonia, high blood pressure and upper respiratory infection[18]. It contains scutellarin with an anti-tumor effect[18] and diterpenoids with antifungal and anti-feedants properties[19].
In this study, we elucidated flavone compositions in the roots and leaves of six Sculellaria species, namely S. baicalensis, S. barbata, S. indica, S. strigilosa, S. obtusifolia, and S. altissima. Additionally, we established hairy root cultures of these six species and studied their growth rates and flavone compositions. The flavone accumulation patterns in both natural roots and hairy roots of the six species were compared. Our data showed that hairy roots of S. barbata and S. indica possess advantages over natural roots in specific flavone production.
-
To determine the flavone contents in the roots and leaves of the six Scutellaria species, namely S. baicalensis, S. barbata, S. indica, S. strigilosa, S. obtusifolia, and S. altissima, LC-MS was applied to detect chrysin, baicalein, baicalin, wogonin, wogonoside, scutellarein, scutellarin, apigenin, and oroxylin A. The results revealed that the main flavones are baicalin, wogonoside, baicalein, wogonin, and scutellarin. However, lower levels of apigenin, chrysin, and oroxylin A were found, and they were only present in the roots of a few species. Scutellarein was not detected in any tissue of the species (Table 1, Supplemental Fig. S1 & S2), hence it may exist in the glycoside form, as scutellarin was found in all the samples.
Table 1. Contents of flavones in roots and leaves of six Scutellaria species.
Species Baicalin Wogonoside Baicalein Wogonin Scutellarin Scutellarein Apigenin Chrysin Oroxylin A S. baicalensis Leaves 2.28 ± 1.24 − − − 20.59 ± 7.68 − − − − Roots 124.96 ± 36.59 27.06 ± 2.51 8.68 ± 5.97 1.74 ± 1.05 2.18 ± 0.62 − − − 0.62 ± 0.71 S.barbata Leaves 7.94 ± 1.34 0.12 ± 0.08 − − 29.50 ± 10.30 − − − − Roots 18.44 ± 2.3 10.78 ± 1.96 1.48 ± 0.07 0.68 ± 0.07 2.35 ± 0.63 − 0.57 ± 0.19 0.05 ± 0.02 − S. indica Leaves 0.23 ± 0.04 − − − 35.66 ± 3.02 − − − − Roots 3.12 ± 0.66 4.7 ± 1.92 0.71 ± 0.05 1.34 ± 0.74 2.02 ± 0.56 − − − − S. strigilosa Leaves 97.53 ± 17.60 7.21 ± 1.06 1.38 ± 0.33 − 6.97 ± 4.05 − − − − Roots 15.59 ± 2.16 8.39 ± 2.00 1.15 ± 0.46 − 3.04 ± 1.12 − 0.28 ± 0.18 − 0.42 ± 0.37 S. obtusifolia Leaves − − − − 0.80 ± 0.37 − − − − Roots 6.45 ± 4.05 3.00 ± 0.40 0.89 ± 0.24 2.03 ± 0.20 0.87 ± 0.37 − − − − S. altissima Leaves − 0.07 ± 0.03 − − 30.16 ± 6.89 − − − − Roots 31.41 ± 8.63 9.48 ± 2.44 − 0.29 ± 0.17 0.81 ± 0.08 − − − − Previous studies showed that S. baicalensis roots mainly accumulated baicalein, baicalin, wogonin, and wogonoside[6]. Our results indicated that all the six species had baicalin and wogonoside in their roots, with S. baicalensis roots having the highest levels of baicalin at 124.96 mg/g dry weight (DW), while S. indica roots had the lowest levels. Interestingly, higher concentrations of baicalin and wogonoside were present in the leaves of S. strigilosa, but only small amounts were found in the leaves of other species. This implies that S. strigilosa might have evolved different regulatory mechanism to synthesize flavones to adapt to environmental challenges. Scutellarin levels in the leaves were higher than that in the roots of all species except S. obtusifolia. Among the leaves of the six species, S. indica had the highest levels of scutellarin, while S. obtusifolia had the lowest (Table 1, Supplemental Fig. S1 & S2). These data reveal the different patterns of flavone accumulation in the roots and leaves of these six Scutellaria species.
Establishment of hairy root cultures and comparison of growth rates
-
Hairy root cultures were established for S. baicalensis, S. barbata, S. indica, S. strigilosa, S. obtusifolia, and S. altissima by infecting their leaves with Agrobacterium rhizogenes strain A4. All six species' hairy roots grew successfully in B5 liquid or solid medium (Fig. 1a & Supplemental Fig. S3). The hairy roots were cultured for 40 d to compare their growth rates. S. baicalensis and S. barbata hairy roots had the fastest growth rates, while S. indica, S. strigilosa, and S. obtusifolia hairy roots grew more slowly (Fig. 1b). However, the growth rate of S. altissima hairy roots cultured for 40 d was too slow and not comparable to other species. These results suggest that the six hairy roots have different growth characteristics.
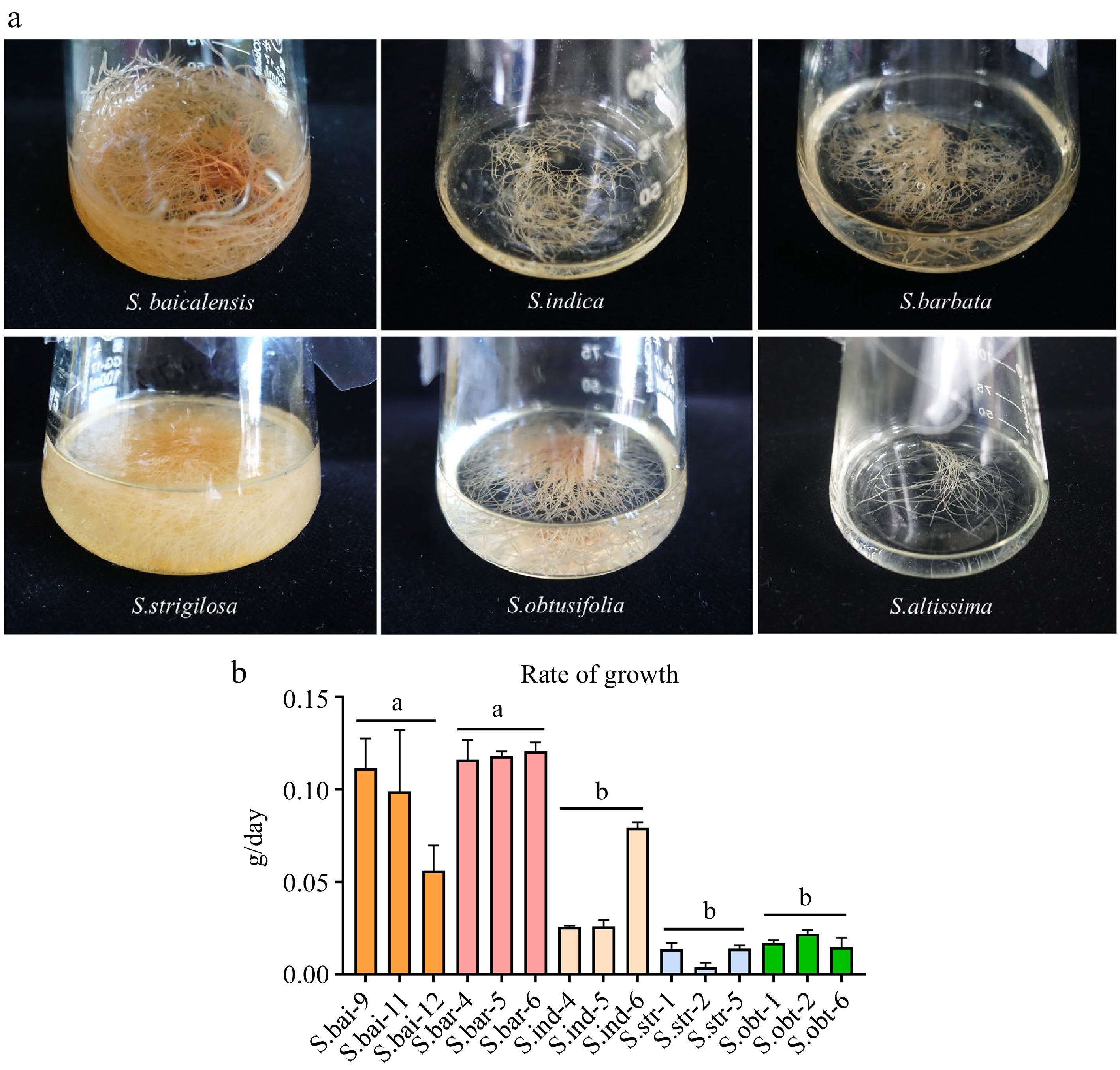
Figure 1.
The growth condition of six Scutellaria hairy roots. (a) Six Scutellaria hairy roots cultured in B5 liquid medium. (b) The growth rate of the five hairy roots. S.bar: S. barbata hairy roots; S.bai: S. baicalensis hairy roots; S.ind: S. indica hairy roots; S.str: S. strigilosa hairy roots; S.obt: S. obtusifolia hairy roots. Five hairy roots were cultured for 40 d. Growth rate (g/day) = (fresh weight of hairy roots cultured for 40 d − fresh weight of hairy roots at initial inoculation)/40. Significance was determined by tukey, and different letters above the bars indicate significantly different values (p < 0.05).
Metabolic profiles of six Scutellaria hairy roots
-
LC-MS was used to analyze the metabolites extracted from the six hairy roots to detect the major flavones. By using retention time of standard substances and mass spectrum data, the presence of these flavones were determined. Among the six hairy roots, S. indica hairy roots were found to have eight flavones, which included scutellarin, scutellarein, baicalin, wogonoside, baicalein, wogonin, chrysin, and apigenin. Both S. baicalensis and S. barbata hairy roots had five flavones: scutellarin, baicalin, wogonoside, baicalein, and wogonin. S. obtusifolia hairy roots also had five flavones: scutellarin, baicalin, wogonoside, wogonin, but very low chrysin content was detected. Scutellarin, baicalin, wogonoside, and wogonin were detected in S. altissima hairy roots, while only baicalin, wogonoside, and wogonin could be detected in S. strigilosa hairy roots. However, oroxylin A was not detected in all hairy roots (Fig. 2, Supplemental Fig. S4, S5, Supplemental Tables S1 & S2).

Figure 2.
Chromatograms of flavones in the hairy roots of (a) S. barbata, (b) S. strigilosa, (c) S. baicalensis, (d) S. obtusifolia, (e) S. indica, and (f) S. altissima. Compounds identified in chromatographic peaks: 1, Scutellarin; 2, Scutellarein; 3, Baicalin; 4, Wogonoside; 5, Apigenin; 6, Baicalein; 7, Wogonin; 8, Chrysin. S.bai: S. baicalensis hairy roots; S.bar: S. barbata hairy roots; S.alt: S. altissima hairy roots; S.ind: S. indica hairy roots; S.obt: S. obtusifolia hairy roots; S.str: S. strigilosa hairy roots.
HPLC was used to quantify the flavones in the six hairy roots. First, the contents of different flavones in the same hairy roots were compared. Hairy roots of S. baicalensis, S. barbata, S. obtusifolia, and S. altissima had the highest levels of baicalin among other flavones, reaching 54.8, 24.28, 12.46, and 7.48 mg/g DW, respectively. However, in hairy roots of S. indica and S. strigilosa, scutellarin and wogonoside had the highest levels, with 19.75 and 17.3 mg/g DW, respectively (Fig. 3). Next, the contents of the same flavone were compared in the hairy roots of different Scutellaria species. Among the six hairy roots, S. baicalensis hairy roots accumulated the highest level of baicalin, followed by S. barbata, while baicalin contents in the other four hairy roots were almost the same. Hairy roots of S. barbata had the highest content of wogonoside, followed by S. strigilosa, S. baicalensis, S. indica, S. obtusifolia, and S. altissima. Scutellarin level in S. indica hairy roots was the highest, whereas S. altissima hairy roots had the lowest. The highest level of baicalein occurred in S. baicalensis hairy roots, followed by S. barbata and S. indica hairy roots. Hairy roots of S. strigilosa produced the lowest level of wogonin, while the highest wogonin content was observed in S. baicalensis hairy roots (Fig. 4).
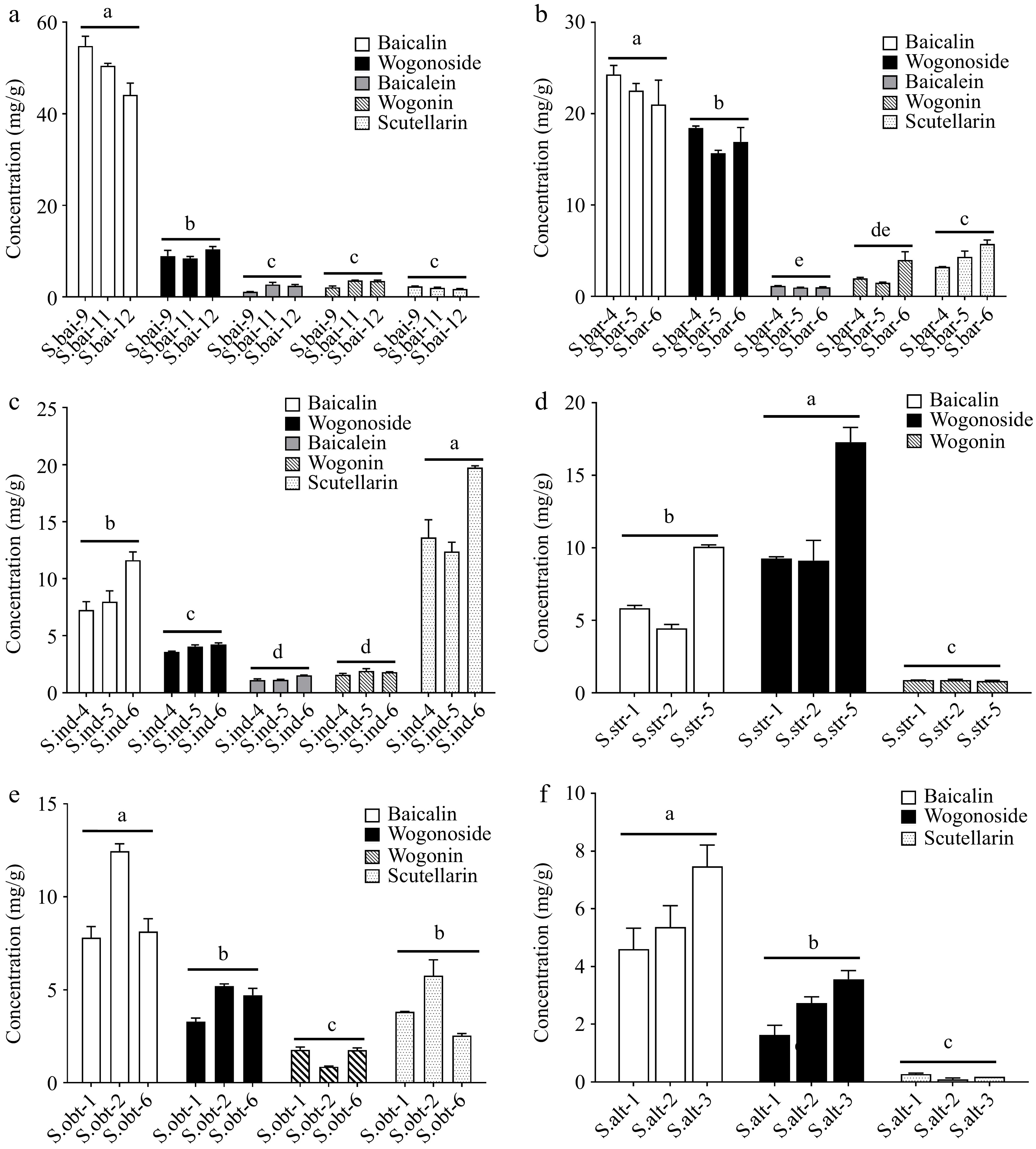
Figure 3.
Comparison of the contents of different flavones in the hairy roots of (a) S. baicalensis, (b) S. barbata, (c) S. indica, (d) S. strigilosa, (e) S. obtusifolia, and (f) S. altissima. S.bai: S. baicalensis hairy roots; S.bar: S. barbata hairy roots; S.alt: S. altissima hairy roots; S.ind: S. indica hairy roots; S.obt: S. obtusifolia hairy roots; S.str: S. strigilosa hairy roots. Significance was determined by tukey, and different letters above the bars indicate significantly different values (p < 0.05).

Figure 4.
Comparison of contents of the same flavones among different hairy roots. Contents of (a) baicalin, (b) wogonoside, (c) baicalein, (d) wogonin, and (e) scutellarin in the different Scutellaria hairy roots. S.bai: S. baicalensis hairy roots; S.bar: S. barbata hairy roots; S.alt: S. altissima hairy roots; S.ind: S. indica hairy roots; S.obt: S. obtusifolia hairy roots; S.str: S. strigilosa hairy roots. 'Bin', 'Bein', 'Wde', 'Win', and 'Sin' represent baicalin, baicalein, wogonoside, wogonin, and scutellarin, respectively. Significance was determined by tukey, and different letters above the bars indicate significantly different values (p < 0.05).
Comparison of flavone contents in six Scutellaria hairy roots and their natural roots
-
To further clarify the patterns of flavone accumulation, we compared flavone levels between the six Scutellaria hairy roots and their natural roots. The roots of S. baicalensis, S. strigilosa, and S. altissima produced higher levels of baicalin than their respective hairy roots. Furthermore, wogonoside levels in the roots of S. baicalensis and S. altissima were also higher than that in their hairy roots. Compared to their natural roots, S. barbata hairy roots produced higher levels of wogonoside and scutellarin, whereas S. indica hairy roots accumulated higher contents of baicalin and scutellarin (Fig. 5). These data suggest that the hairy roots of S. barbata and S. indica have great potential for the rapid production of the bioactive flavones.

Figure 5.
Comparison of flavone contents between the six Scutellaria hairy roots and their natural roots. Flavone levels in the hairy roots and natural roots of (a) S. baicalensis, (b) S. barbata, (c) S. indica, (d) S. strigilosa, (e) S. obtusifolia, and (f) S.altissima. Significance was determined by Student's t test; * 0.01 < p < 0.05 and ** p < 0.01 were considered to indicate significant and highly significant levels, respectively.
-
With the increasing demand for TCM and the degradation of germplasm resources of medicinal plants, it is necessary to use alternative methods for producing bioactive components. Hairy roots are excellent chassis for producing active components, and metabolic engineering can generate more abundant compounds. Flavones are common in Scutellaria species and are important medicinal compounds[2]. While the flavone biosynthetic pathway in S. baicalensis is well elucidated, the pathway in other Scutellaria species remains unclear. Elucidation of the pathways is limited by the difficulty of achieving stable inheritance in these medicinal plants. In this study, we elucidated the flavonoid compositions and flavone accumulation patterns in roots and leaves of S. baicalensis, S. barbata, S. indica, S. strigilosa, S. obtusifolia, and S. altissima. Furthermore, we established hairy root cultures of the six species and investigated their growth rates and different patterns of flavone accumulation.
Previous study have shown that the main components of Scutellaria are flavonoids[2]. Therefore, we analyzed the flavonoid composition of six common Scutellaria species. Although the flavone accumulation patterns in the roots and leaves of the six Scutellaria species were different, baicalin and wogonoside were predominantly accumulated in most of the Scutellaria roots, and scutellarin was mainly accumulated in the leaves (Table 1). This distribution of flavones is similar to that observed in S. baicalensis[6], suggesting that the regulatory mechanism of flavone synthesis in some Scutellaria species may be conservative. Interestingly, baicalin and wogonoside were highly accumulated in S. strigilosa leaves (Table 1), indicating that S. strigilosa may evolve new pathways to regulate flavone biosynthesis that differ from the known mechanisms. Previous reports have also shown that S. strigilosa is rich in wogonoside and baicalin[20,21], and extracts containing flavones from S. strigilosa have demonstrated anti-proliferative and anti-migratory effects[17]. This suggests that S. strigilosa leaves may have great potential to be developed into medicine.
Hairy roots were successfully generated from several Scutellaria plant, including S. baicalensis[22], S. bornmuelleri[23], S. lateriflora[24], S. przewalskii and S. pycnoclada[25]. The hairy roots liquid cultures produced active flavones. In this study, we established hairy root cultures of the six Scutellaria species, all of which were capable of accumulating flavones. Although all the hairy roots produced baicalin and wogonoside, they exhibited different flavone accumulation patterns (Figs 2, 3 & 4). Similarly, there are diverse accumulation patterns of tanshinones and phenolic acids in the two Salvia species[26]. This phenomena may be a combination of environmental and genetic effects. Furthermore, hairy roots of S. barbata and S. indica had a greater capacity to produce specific flavones compared to natural roots (Fig. 5), suggesting that the two hairy roots have the potential to yield specific flavones on an industrial scale.
-
Among the six species, S. baicalensis roots had the highest levels of baicalin and wogonoside, while S. indica leaves accumulated the highest content of scutellarin. Of the six hairy roots, S. baicalensis and S. barbata exhibited the fastest growth rates, followed by S. indica, S. strigilosa, and S. obtusifolia. S. baicalensis hairy roots accumulated the highest contents of baicalin, baicalein, and wogonin, while S. barbata hairy roots produced the highest level of wogonoside. However, S. indica hairy roots accumulated the highest concentration of scutellarin. Compared to natural roots, hairy roots of S. barbata and S. indica showed greater potential to selectively produce specific active flavones. This study provides a foundation for studying the specialized metabolism of Scutellaria species, screening species with high-quality flavones, and metabolic engineering of active flavones.
-
Plants of S. baicalensis, S. barbata, S. indica, S. strigilosa, S. obtusifolia, and S. altissima were grown in the greenhouse of Shanghai Chenshan Plant Science Research Center, Shanghai, China, and their leaves and roots were collected with three biological duplicates for metabolic analysis.
Hairy root cultures
-
The vector pK7WG2R carrying the dsRed marker gene was transformed into Agrobacterium rhizogene strain A4. The positive Agrobacterium transformant was cultured in TY medium overnight until OD600 reached 0.6−0.8. Then TY medium was removed by centrifugation, and Agrobacterium precipitation was re-suspended by MS medium with the equal volume. Acetosyringone was added to the Agrobacterium suspensions to a concentration of 50 μM. The leaves of six Scutellaria species were sterilized with 10% sodium hypochlorite for 8−10 min, and subsequently washed with sterile water three times. On a clean bench, the sterile leaves were cut with a lancet dipped in Agrobacterium solution. These leaves were then cultured in the MS medium with 50 μM acetosyringone for 3 d. After 3 d, these leaves were exchanged into MS medium with 400 mg/L cefotaxime sodium for dark culture. About 2−3 weeks later, hairy roots formed at the infected part of the leaves. When the hairy roots grew to 3−5 cm, a single root was cut and cultured in B5 medium. Positive lines of hairy roots were confirmed by red fluorescence inspection.
Flavone extraction and metabolic analysis by HPLC and LC-MS/MS
-
Based on a previous method[6], freeze-dried powders of the natural roots, leaves, and hairy roots of six Scutellaria species were ultrasonically extracted with 70% methanol for 2 h. Then, flavone extracts were filtered with 0.22-μm filters and analyzed by the Agilent 1260 Infinity II HPLC system. Flavones were detected at 280 nm. The flavone standards (baicalin, baicalein, scutellarein, scutellarin, wogonin, wogonoside, oroxylin A, chrysin and apigenin) were purchased from Sigma-Aldrich. Based on the retention time of flavone standards and standard curves, each flavone was confirmed and measured. Moreover, the retention time of scutellarin, scutellarein, baicalin, wogonoside, apigenin, baicalein, wogonin, chrysin and oroxylin A was 11.94, 16.72, 17.34, 20.96, 22.06, 23.29, 27.71, 28.65, and 29.31 min, respectively.
LC-MS/MS was operated by Thermo Q Exactive Plus, and the procedure is the same as in our previous study[27]. A Phenomenex Luna C18 (2) column (100 mm × 2 mm 3μ) was used to separate flavones. The injection volume was 10 μL. Mass spectra were equipped with a heated ESl source and operated in negative and positive ion modes. The parameters were as follows: aus. Gas flow,10 l/min; aus. Gas heater, 350 °C; sheath gas flow, 40 l/min; spray voltage, 3.5 kV; capillary temperature, 320 °C. For full-scan MS/data-dependent (ddMS2) analysis, spectra were recorded in the m/z range of 50−750 at a resolution of 17,500 with automatic gain control (AGC) targets of 1 × 106 and 2 × 105.
This work is sponsored by Natural Science Foundation of Shanghai (22ZR1479500), Special Fund for Scientific Research of Shanghai Landscaping & City Appearance Administrative Bureau (G212401), Ministry of Science and Technology of China (YDZX20223100001003) and Youth Innovation Promotion Association, Chinese Academy of Sciences. QZ is also supported by SANOFI-SIBS Scholarship.
-
The authors declare that they have no conflict of interest.
- Supplemental Fig. S1 HPLC chromatograms of nine flavones standard. Compounds identified in chromatographic peaks: 1, Scutellarin; 2, Scutellarein; 3, Baicalin; 4, Wogonoside; 5, Apigenin; 6, Baicalein; 7, Wogonin; 8, Chrysin; 9, Oxoylin A.
- Supplemental Fig. S2 HPLC chromatograms of leaves and roots of six Scutellaria species. Compounds identified in chromatographic peaks: 1, Scutellarin; 2, Scutellarein; 3, Baicalin; 4, Wogonoside; 5, Apigenin; 6, Baicalein; 7, Wogonin; 8, Chrysin; 9, Oxoylin A.
- Supplemental Fig. S3 Six Scutellaria hairy roots cultured in B5 solid medium. (a) S.baicalensis hairy roots; (b) S.indica hairy roots; (c) S.barbata hairy roots; (d) S.strigilosa hairy roots; (e) S.obtusifolia hairy roots; (f) S.altissima hairy roots.
- Supplemental Fig. S4 Chromatograms of nine flavones standard analyzed by LC-MS. Compounds identified in chromatographic peaks: 1, Scutellarin; 2, Scutellarein; 3, Baicalin; 4, Wogonoside; 5, Apigenin; 6, Baicalein; 7, Wogonin; 8, Chrysin; 9, Oxoylin A.
- Supplemental Fig. S5 Secondary mass spectrum of nine flavones standard.
- Supplemental Tables S1 Characteristics of standard substance of nine flavones by UPLC-ESI-MS/MS.
- Supplemental Tables S2 Characteristics of flavones from six Scutellaria hairy roots by UPLC-ESI-MS/MS.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zheng M, Fang Y, Zhao Q. 2023. Comparative analysis of flavones from six commonly used Scutellaria species. Medicinal Plant Biology 2:12 doi: 10.48130/MPB-2023-0012
Comparative analysis of flavones from six commonly used Scutellaria species
- Received: 13 June 2023
- Accepted: 09 August 2023
- Published online: 13 September 2023
Abstract: Scutellaria plants have been used for thousands of years for medicinal purposes, and flavones are the main bioactive compounds with properties such as anti-cancer, anti-viral, and anti-inflammatory. Although the pharmacological effects of active components and specialized metabolism in S. baicalensis are well-understood, few studies have been conducted on other Scutellaria species. In this study, we investigated the patterns of flavone accumulations in roots, leaves, and hairy roots of S. baicalensis, S. barbata, S. indica, S. strigilosa, S. obtusifolia, and S. altissima. Among the six species, S. baicalensis roots contained the highest concentrations of baicalin and wogonoside, while S. indica leaves accumulated the highest level of scutellarin. In addition, S. strigilosa leaves were rich in baicalin and wogonoside. Among the six hairy roots, S. baicalensis hairy roots had the highest contents of baicalin, baicalein, and wogonin, while S. barbata hairy roots produced the highest level of wogonoside. S. indica hairy roots contained the highest concentration of scutellarin. Compared to natural roots, the hairy roots of S. barbata and S. indica had stronger ability to selectively produce specific active flavones. Overall, this study provides a foundation for investigating diverse specialized metabolism in Scutellaria species.
-
Key words:
- Scutellaria /
- Flavone /
- Specialized metabolism /
- Accumulation patterns













