-
Tea plant (Camellia sinensis) is a prominent beverage crop broadly cultivated worldwide[1,2]. Tea, processed from the new shoots of tea plants, is highly popular because of its unique flavor and plentiful health functions[3,4]. As the origin of tea plants, China holds diverse tea plant germplasm resources. In recent years, albino/etiolated tea plant cultivars with white or yellow shoots have been widely cultivated because the green teas processed from the albino/etiolated tea shoots are favored by tea consumers for their distinct leaf color, special flavor characteristics and scarcity[5]. Therefore, it is of great significance to study the mechanisms behind the phenotypes of, and special flavor characteristics of tea processed from these albino/etiolated tea plant germplasms.
Albino/etiolated tea shoots contain more free amino acids, especially L-theanine (γ-glutamylethylamine), which imparts a strong umami flavor to tea infusions[5,6]. L-theanine, a distinctive quality-related metabolite in tea plants, is a prominent factor in evaluating the qualities of green tea[7]. In tea plants, theanine is the most abundant free amino acid, accounting for 1%−6% dry weight and over half of the total free amino acids[8]. Theanine has various physiological benefits, including reducing anxiety, promoting sleep quality and immunity, conferring the mood of happiness, etc[9,10]. It also counterbalances the effect of caffeine's stimulatory action[11−13].
Theanine synthetase (CsTSI) catalyzes the biosynthesis of theanine from ethylamine (EA) and glutamate (Glu), a process primarily occurring in the roots of tea plants[14,15]. Glutamine synthetase (CsGSs) can also synthesize theanine using EA and Glu as substrates[16,17]. Glu is synthesized mainly by glutamine synthetase/glutamate synthase (CsGS/CsGOGAT) and glutamate dehydrogenase (CsGDHs) in the roots[18]. EA is biosynthesized from alanine by alanine decarboxylase (CsAlaDC)[19,20]. Root-synthesized theanine is transported to new shoots of tea plant, where it is catabolized into Glu and EA[18,21]. CsPDX2.1, the homolog of Arabidopsis glutamine hydrolase, was shown to have theanine hydrolase activity in vitro[22]. Recently, in tea plants, γ-glutamyl-transpeptidase CsGGT2 was functionally verified as theanine hydrolase (Fig. 1)[23].
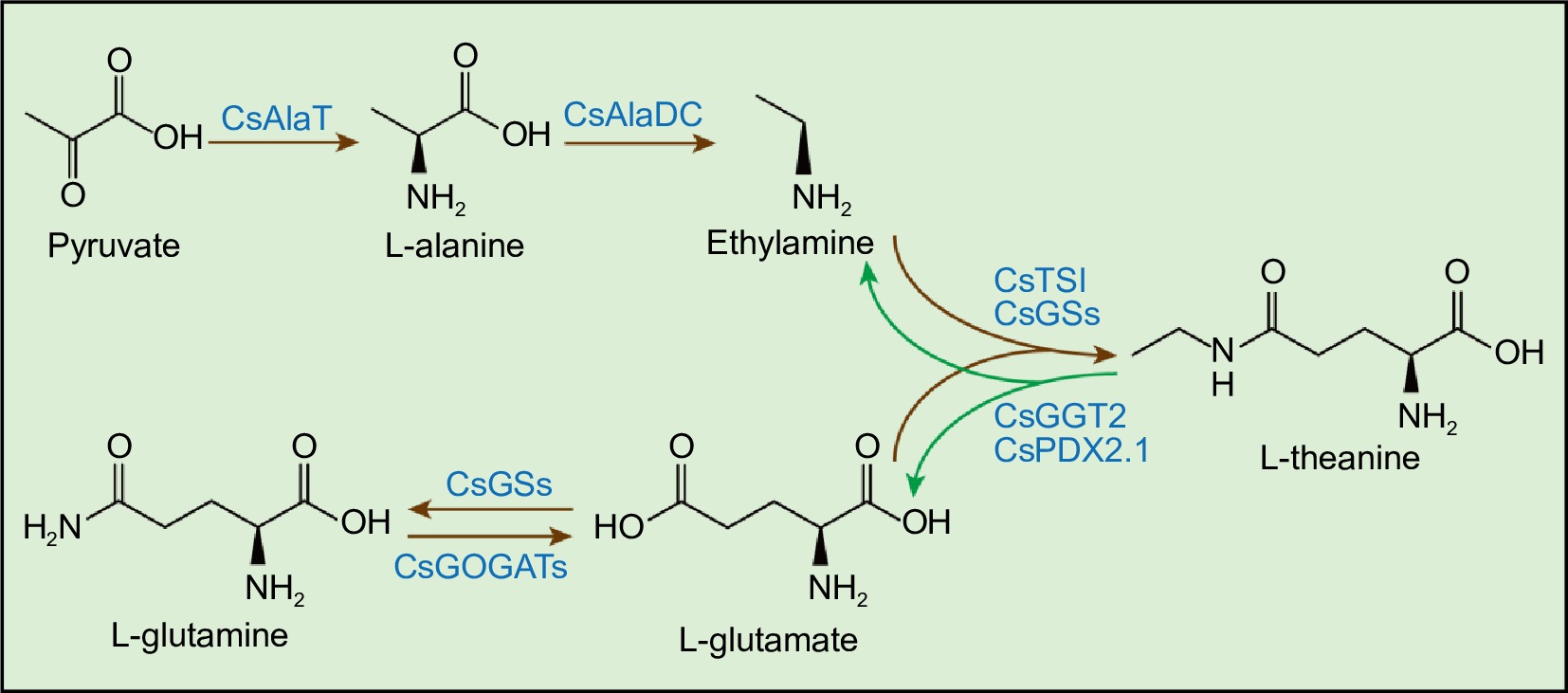
Figure 1.
Theanine metabolic pathway in tea plants. CsGSs, Glutamine Synthetase; CsGOGATs, Glutamate Synthase; CsAlaT, Alanine Transaminase; CsAlaDC, Alanine Decarboxylase; CsTSI, Theanine Synthetase I; CsGGT2, γ-glutamyl-transpeptidase 2; CsPDX2.1, Pyridoxine biosynthesis 2.1. Brown arrows represent the theanine biosynthesis pathway. Green arrows represent the theanine catabolism pathway.
Numerous studies explored the mechanisms of higher theanine accumulation in new shoots of albino/etiolated tea plant cultivars. Camellia sinensis 'Anji Baicha' and 'Huangjinya' are typical albino/etiolated tea cultivars that contain higher levels of theanine compared to Camellia sinensis 'Yingshuang' (a common green tea cultivar)[24]. In these three cultivars, theanine content was correlated with the expression level of genes participating in theanine metabolism, especially CsGS1, CsTS2 and CsGDH2[24]. A study proposed that CsGS1.2-catalyzed theanine biosynthesis contributes the theanine accumulation in the new shoots of an albino tea plant germplasm 'Huabai 1'[17]. However, Cheng et al. proposed that albinism-induced high theanine accumulation is due to the weak theanine catabolism[25]. A recent study demonstrated that the low expression Haem Oxygenase 1 encoding gene CsHO1 contributes to the theanine accumulation in the new shoots of albino/etiolated tea plant cultivars probably by reducing Glu metabolism into chlorophyll[26]. Therefore, there are conflicts on the mechanism underlying the high theanine accumulation in the new shoots of albino/etiolated tea plant cultivars.
In this study, we selected five albino/etiolated tea cultivars as research materials, including Camellia sinensis (L.) O. Kuntze 'Anji Baicha' (AJBC), 'Huangjinya' (HJYA), 'Jinxiangyu' (JXY), 'Huangjinye' (HJYE) and 'Jibai' (JB), with Camellia sinensis (L.) O. Kuntze 'Zhongcha 108' (ZC108, a common green tea cultivar) as the control. By comparing theanine content in the shoots and roots, we found that the high theanine accumulation in new shoots was probably not caused by the strong biosynthesis of theanine in roots. Contrarily, more EA and Glu, were accumulated in the shoots of albino/etiolated tea cultivars. The following correlation analysis between accumulation patterns EA, Glu and the expression theanine metabolic pathway genes suggested that the strong theanine biosynthesis and weak theanine catabolism in new shoots contribute to high accumulation of theanine in albino/etiolated tea cultivars. This study proposed comprehensive insights into the high accumulation of theanine in new shoots of albino/etiolated tea cultivars, and has theoretical significance for the new albino/etiolated tea plant cultivar breeding.
-
Unlignified tea roots and the new shoots (one apical bud with two leaves) were harvested from six tea plant cultivars grown in the Tea Garden of Chaohu Niansheng Agricultural Ecology Co., Ltd (Chaohu, Anhui, China), on December 3, 2020 and April 24, 2021, respectively. These cultivars were AJBC, JXY, HJYE, JB, HJYA, and ZC108. All tea plant cultivars were 2-year-old with similar growth status.
Determination of chlorophyll content in new shoots
-
Chlorophyll was extracted as described by Cheng et al. with minor modifications[25]. Briefly, freeze-dried sample powders (50 mg) were thoroughly homogenized in 80% acetone at 4 °C until the powders became white. The supernatant was determined using UV-Vis spectrophotometer (U-5100, Hitachi, Japan) at the absorbance of 663 nm and 645 nm, after 10 min of centrifugation at 12,000 g. The formula employed in calculating the content of chlorophyll was as follows: Chlorophyll a = 12.7 × A663 − 2.69 × A645; Chlorophyll b = 22.9 × A645 − 4.68 × A663[27].
Determination of theanine and other free amino acid content
-
Theanine was extracted following the method described by Dong et al. with minor modifications[21]. In brief, freeze-dried sample powders (50 mg) were dissolved in 2 mL purified water and heated in a boiling water bath for 0.5 h. After 20 min of centrifugation at 13,680 g, the supernatant was applied to HPLC detector. A reverse-phase C18 column (4.6 mm × 25 cm, 5 µm) was employed in this study. The HPLC parameters and elution gradient used for theanine determination were set according to a previous study[21].
Free amino acids in tea plant samples were extracted according to the description by Zhu et al., with minor modifications[28]. Accordingly, freeze-dried sample powder (50 mg) was completely dissolved using 4% of sulfonyl salicylic acid (2 mL), and then using supersonic extraction for 40 min. After 30 min of centrifugation at 13,680 g, a constant volume (20 µL) of the supernatant was employed for L-8900 Amino Acid Analyzer (Hitachi, Japan) detection. Ion exchange column (4.6 mm × 60.0 mm, 2622, Hitachi, Japan) and reaction column (4.6 mm × 40.0 mm, 855-3523, Hitachi, Japan) were employed in this study. The column temperatures of the ion exchange column and the reaction column were set at 57 °C and 135 °C, respectively. The Amino Acid Analyzer was equipped with buffer solution for elution, the flow rate was set to 0.4 mL/min, and the detection wavelength was 570 nm. The cycle time and reaction time were set to 20 min and 10 min, respectively. A more detailed description is provided in the L-8900 Amino Acid Analyzer User Guide.
Determination of ethylamine content
-
Internal ethylamine in samples was extracted as described with slight modification[29]. Freeze-dried and powered samples (25 mg) were mixed in 1.5 mL purified water, and heated at 85 °C for 5 min. The mixture was centrifuged at 3,500 g for 5 min after stewing for 2 h, and the supernatant was obtained through 0.22-μm membrane filtration. The aliquot (500 μL) was added above the supernatant into the silanized centrifuge tube containing toluene (500 μL), and the solution was modulaed to alkaline pH by affiliating 0.5 M phosphate buffer (500 μL, pH 12.0). Isobutyl chloroformate (25 μL) was added to the mixture as a derivatization reagent. After 10 min of shaking at 250 rpm, the mixture was centrifuged for 5 min at 3,500 g.
The toluene layer (200 μL) was transferred to another silanized centrifuge tube, and 200 μL methanol solution saturated with KOH was added, followed by shaking at 250 rpm for 5 min, and then added 5 M NaOH (600 μL) to the centrifuge tube. The mixture mentioned above was again shaken for another 5 min. The toluene layer was employed for GC-MS detection after centrifugation at 3,500 g for 5 min.
The GC-MS parameters used for ethylamine determination were set according to a previous study[30]. The splitless mode was used and the MS scan was 30−400 m/z and MS were operated under selective ion monitoring (SIM) mode.
RNA isolation and quantitative real-time PCR (qRT-PCR)
-
Total RNA was extracted from samples using the RNAprep Pure Plant Kit (Tiangen, China). The first-strand cDNA was synthesized using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix Kit (TransGen Biotech, China).
Specific primers for qRT-PCR detection are listed in Supplemental Table S1. qRT-PCR was conducted using an ABI QuantStudio 6 Flex detection system. Each reaction reagent (20 μL) contained 6 μL DEPC water, 2 μL cDNA (200 ± 5 ng/μL), 10 μL SYBR Green Supermix (Vazyme, China), and 0.5 μL forward/reverse primers (10 μM). Reaction was performed by a two-step method. CsGAPDH was applied as the internal standardization in each qRT-PCR. The calculation of gene relative expression was employed 2−ΔΔCᴛ method. Each sample was equipped with three independent biological replicates.
Statistical analysis
-
All experimental results originated from three independent biological replicates in this study, and the data are represented as means ± standard deviation. Statistical analysis of the data was conducted employing IBM SPSS Statistics software 19 (SPSS 19), with one-way analysis of variance. Pearson correlation was employed to analyze the correlation coefficients of the two variables by SPSS 19. p-value < 0.05 indicated significant differences via Duncan's multiple-range test.
-
To explore the mechanism of high-level accumulation of theanine in albino/etiolated shoots, five albino/etiolated tea cultivars and a common green tea cultivar were used in this study (Fig. 2a). In order to exclude the differences in geographical and climatic conditions, we grew these cultivars under the same cultivation management. The phenotypes of the spring new shoots of these six tea plant cultivars were observed (Fig. 2a). The leaf color of ZC108 was uniformly dark green, while that of these albino/etiolated tea cultivars varied in the degree of the albinism/ etiolation. AJBC, JB and HJYA leaves were of albinism with slight differences: AJBC had light green vein and jade white leaves; the JB had white vein and pale green leaves; while the HJYA had uniformly jade white color. JXY and HJYE leaves showed slightly yellowish, and JXY tended to be light green compared with HJYE.
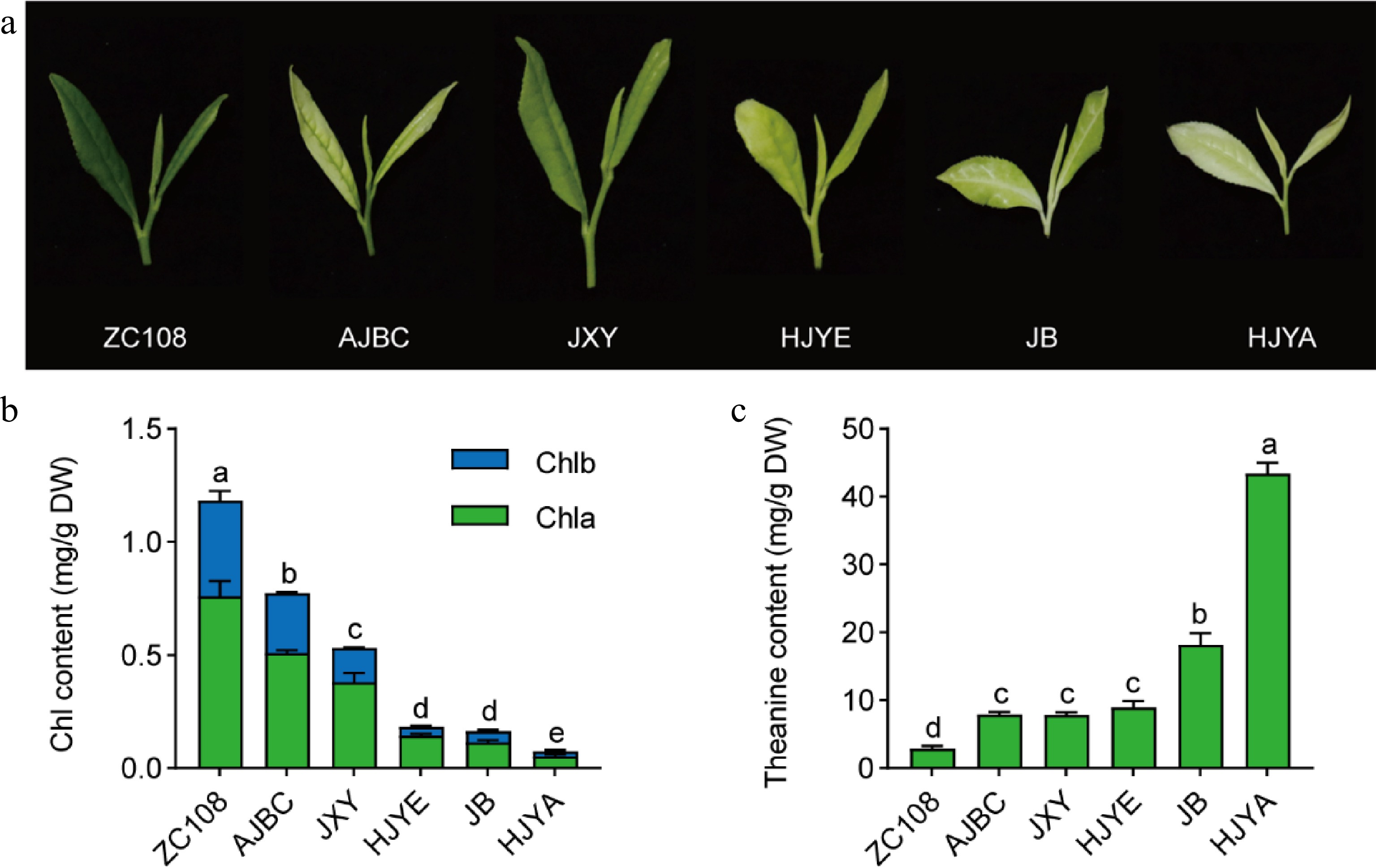
Figure 2.
(a) Phenotype of new shoots picked in April. (b) Chlorophyll contents in new shoots. Chla, chlorophyll a; Chlb, chlorophyll b; Chl, total chlorophyll. (c) The content of theanine in new shoots. Data were denoted as means ± SD (n = 3), statistical significance (p < 0.05) was marked with distinct letters, based on Duncan's multiple range test.
The deficiency of chlorophyll contributes to the albinism/etiolation of tea plant leaves.[31] To further characterize the difference in leaf color of these six tea cultivars, chlorophyll content was measured (Fig. 2b). The chlorophyll content in the shoots of ZC108 was the highest (1.18 mg/g DW), followed by those in AJBC and JXY. The chlorophyll content of HJYE, JB and HJYA were relatively low, especially that of HJYA, at only approximately 0.075 mg/g DW. Obviously, leaf color phenotype and the chlorophyll content of these six tea cultivars were generally consistent.
We then determined the content of theanine in shoots of these six tea plant cultivars (Fig. 2c). Conversely, the content of theanine in ZC108 was the lowest (2.86 mg/g DW), and were higher in AJBC, JXY and HJYE; while the theanine content was the highest in HJYA, at approximately 43.43 mg/g DW. Taken together, the accumulation of chlorophyll in new shoots of these six tea plant cultivars exhibited a generally negative correlation (r = −0.677, p = 0.140) with that of theanine content. These results confirmed that albino/etiolated tea new shoots had higher theanine accumulation and provided the basis of the subsequent investigation.
The roots of albino/etiolated tea plant cultivars contained similar or lower levels of theanine
-
Studies demonstrated that theanine is predominantly biosynthesized and accumulated in the roots during winter[32,33]. To investigate whether the roots of these albino/etiolated tea plants had stronger theanine biosynthesis and accumulation, we detected the content of theanine in the winter roots of these cultivars. Results exhibited that the content of theanine in roots of ZC108, AJBC, JXY and HJYE were high and were not significantly different (Fig. 3a). The theanine content in roots of HJYA was dramatically low, and the theanine content in roots of JB was hardly detected. Therefore, these results suggested that the roots of these albino/etiolated tea plant cultivars synthesized similar or lower levels of theanine. Furthermore, the theanine content in roots showed a slight negative correlation with theanine content in the shoots of these cultivars (Fig. 3b). Taken together, these results implied that the theanine accumulation in these albino/etiolated shoots was not caused by stronger biosynthesis in the roots. This is likely different from that in common green tea cultivars.
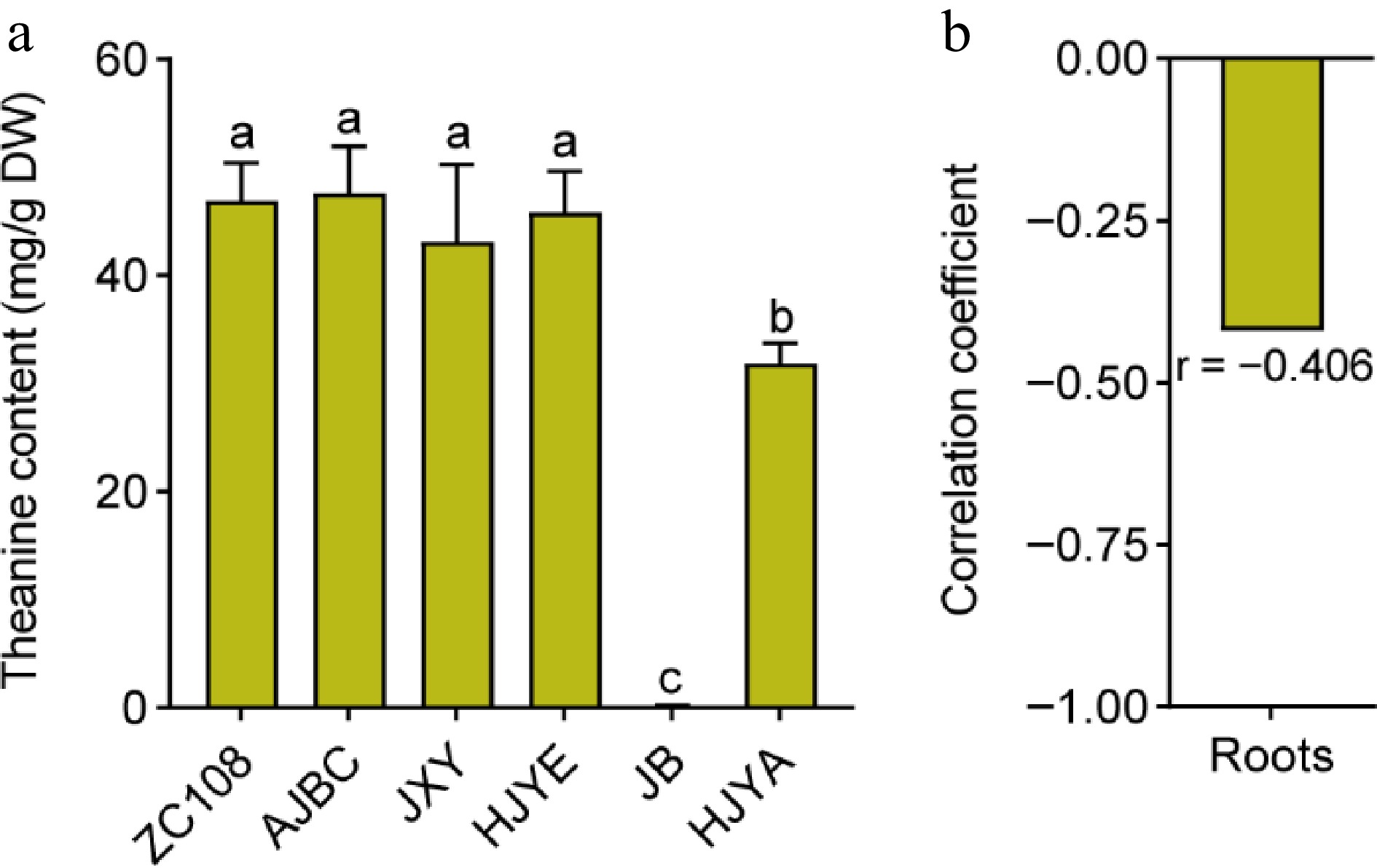
Figure 3.
(a) The content of theanine in roots of common green and albino/etiolated tea plant cultivars. Data were denoted as means ± SD (n = 3), statistical significance (p < 0.05) was marked with distinct letters, based on Duncan's multiple range test. (b) The correlation coefficient of theanine contents in roots with theanine contents in new shoots. Asterisks represent statistical significance determined by Duncan's multiple range test (* p < 0.05, ** p < 0.01.)
Stronger theanine biosynthesis in the albino/etiolated shoots
-
Although theanine is mainly synthesized in the roots, it can also be synthesized in the new shoots[14]. To explore whether theanine accumulation in these albino/etiolated shoots was associated with theanine biosynthesis in new shoots, we first measured the content of EA and Glu, the precursors of theanine biosynthesis, in the shoots of these cultivars (Fig. 4a, b). Interestingly, the results showed that both Glu and EA content in albino/etiolated tea shoots were higher than those in the common green new shoots of ZC108 (Fig. 4a, b). Especially, the content of EA in new shoots of HJYA was around 13.54 fold of that in ZC108. In addition, EA content in shoots of these albino/etiolated tea cultivars were comparable to that in the roots of common green tea cultivars[20]. These results implied strong theanine biosynthesis occurred in these albino/etiolated shoots.
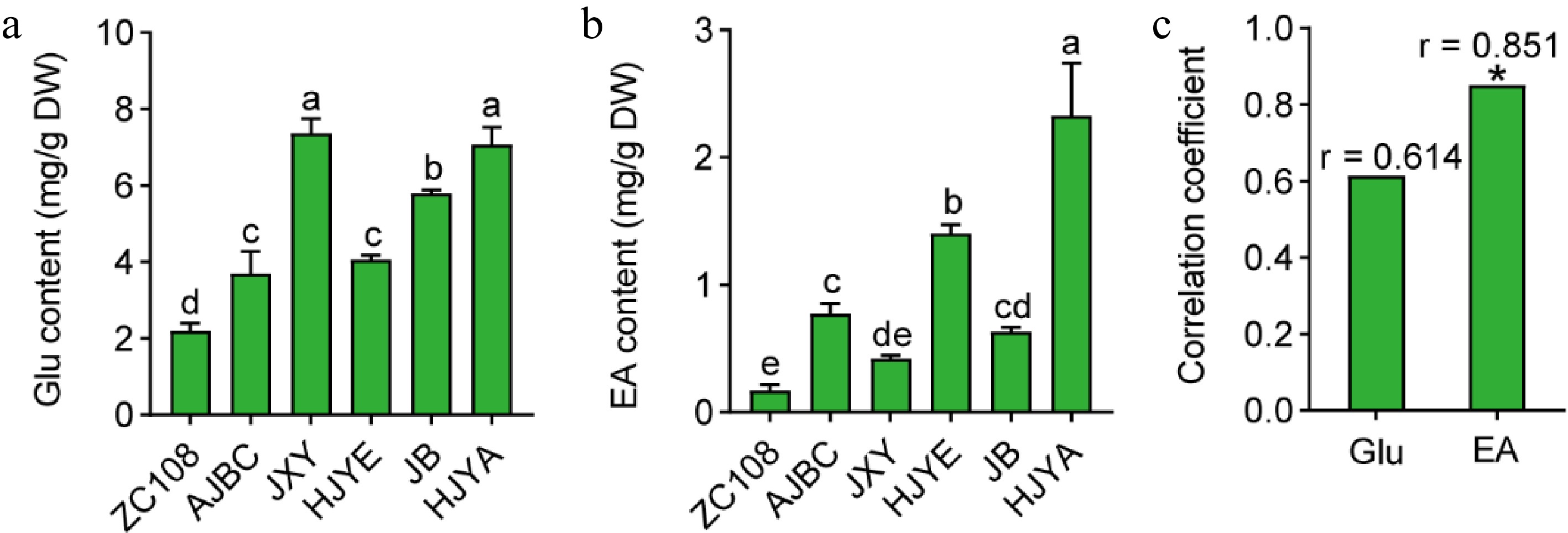
Figure 4.
(a) Glu content in shoots of different tea cultivars. (b) EA content in tea shoots. Data were denoted as means ± SD (n = 3), statistical significance (p < 0.05) was marked with distinct letters, based on Duncan's multiple range test. (c) Correlation coefficient between the contents of Glu and EA with the accumulation of theanine in tea shoots. Asterisks represent statistical significance determined by Duncan's multiple range test (* p < 0.05, ** p < 0.01.)
It was reported that EA content positively correlates with the theanine biosynthesis activity[20,30]. Interestingly, we also noticed that accumulation patterns of Glu and EA were similar with that of theanine in these cultivars (Figs 2c, 4a & b). Further correlation analyses showed that content of Glu and ethylamine in new shoots were positively correlated with that of theanine (Fig. 4c), especially the correlation coefficient of EA content with theanine content reached 0.851 (* p < 0.05). Therefore, these results further suggested that there was stronger theanine biosynthesis in these albino/etiolated shoots.
Expression of theanine biosynthetic genes in the new shoots
-
To further investigate the stronger theanine biosynthesis in these albino/etiolated shoots, we next examined the expression of theanine biosynthetic pathway genes, including CsGOGATs, CsGSs, CsAlaDC and CsTSI (Fig. 1). CsAlaDC was identified to encode alanine decarboxylase which catalyzes EA biosynthesis[19,20]. As depicted below in Fig. 5a, the expression of CsAlaDC was higher in these albino/etiolated shoots, especially in those of HJYE, JB and HJYA. More importantly, the expression levels of CsAlaDC showed a significantly positive correlation (r = 0.995, ** p < 0.01) with the contents of theanine in shoots of these six tea cultivars (Fig. 5d). These results suggested CsAlaDC-catalyzed EA biosynthesis largely contributed to the theanine accumulation in these albino/etiolated shoots.
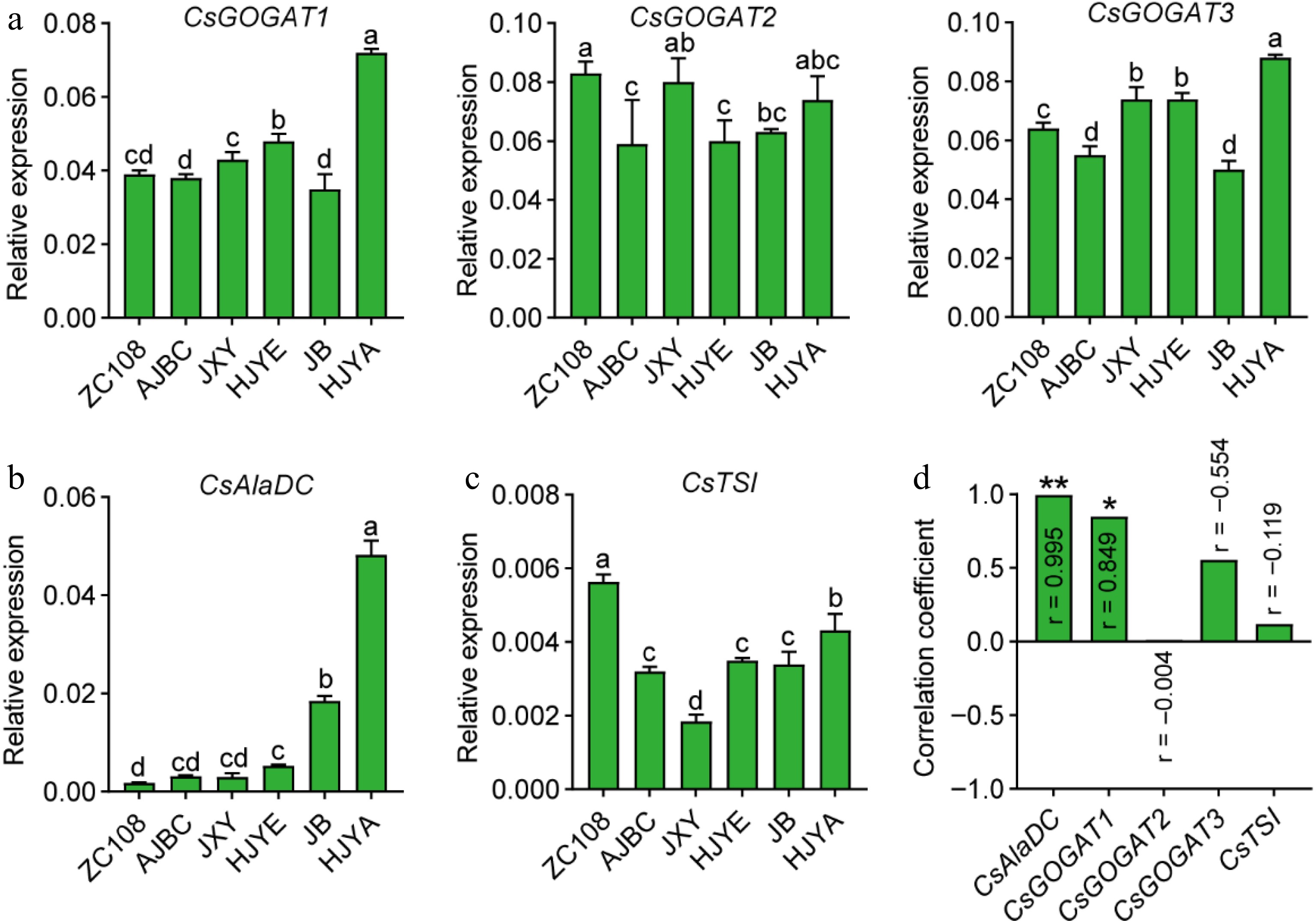
Figure 5.
(a) CsGOGATs expression in tea shoots. (b) CsAlaDC expression in tea shoots. (c) CsTSI expression in normal green and albino/etiolated tea shoots. CsGAPDH was applied as the internal standardization. Data were denoted as means ± SD (n = 3), statistical significance (p < 0.05) was marked with distinct letters, based on Duncan's multiple range test. (d) The correlation coefficient between the expression levels of theanine biosynthesis genes with the content of theanine in tea shoots. Asterisks represent statistical significance determined by Duncan's multiple range test (* p < 0.05, ** p < 0.01.)
Glu was proposed to be synthesized by GOGATs or GDHs in tea plants[18]. However, in plants, the primary role of GDHs is deaminating of Glu to produce α-ketoglutarate and ammonia which then flow into carbon and nitrogen metabolism[34,35]. Our recent work also suggested that CsGDH2.1 catalyzes the deamination of Glu in tea shoots, during late-spring[36]. Consequently, we next determined the expression of CsGOGATs to analyze the Glu biosynthesis in the new shoots. Expression pattern of CsGOGAT1 in the new shoots of these cultivars was generally consistent with that of theanine content (Figs 2c, 5b). A further correlation analysis suggested that the CsGOGAT1 expression was positively correlated with the content of theanine in the new shoots (r = 0.849, * p < 0.05) (Fig. 5d). The expression of CsGOGAT2 and CsGOGAT3 showed inconspicuous correlation (Figs 5b & 4d). These results suggested CsGOGAT1-catalyzed Glu biosynthesis positively contributed to the theanine accumulation in these albino/etiolated shoots.
In tea plants, CsTSI catalyzes the synthesis of theanine from EA and Glu[1,37]. However, CsTSI expression levels were generally not correlated with theanine contents in the roots[20], and CsTSI expression was shown to be likely feedback repressed by the accumulation of theanine in new shoots[38]. Consistently, we also found the CsTSI expression in these albino/etiolated tea shoots was lower than that of ZC108 (Fig. 5c), and the expression was not correlated with the accumulation of theanine in new shoots of these cultivars (Fig. 5d).
CsGSs can catalyze both theanine and glutamine (Gln) biosynthesis[16,17]. We also examined the relative expression of CsGSs (Supplemental Fig. S1). We found that relative expression levels of CsGS1.1 in these albino/etiolated tea shoots were similar with that of ZC108, except that of HJYA was significantly lower (Supplemental Fig. S1a). But the expression showed a down-regulated tendency in the new shoots of JXY, HJYE, JB and HJYA, and the expression levels of CsGS1.1 exhibited a negatively correlation with theanine contents (r = −0.913, ** p < 0.01). The expressions of CsGS1.2, CsGS1.3 and CsGS2 did not showed obvious tendency and were not correlated with the contents of theanine in shoots of these six tea cultivars (Supplemental Fig. S1b). CsGSs expression exhibited a negative correlation with the accumulation of theanine in common green tea shoots[38]. It was reported that CsGS1.2-catalyzed theanine biosynthesis greatly contributed to the higher accumulation of theanine in shoots of an albino tea cultivar 'Huabai1'[17]. However, the expression levels of CsGS1.2 in new shoots of these albino/etiolated tea cultivars were generally similar with that of ZC108. These analyses suggested CsGSs are not regulated or negatively regulated at the transcription levels although they may play a positive role in theanine biosynthesis in the new shoots. Alternatively, these CsGSs probably mainly function in glutamine biosynthesis in tea plants.
Taken together, we found that EA and Glu, the precursors of theanine biosynthesis, accumulated to higher levels in new shoots of these albino/etiolated tea cultivars. Consistently, CsAlaDC and CsGOGAT1, respectively encoding enzyme catalyzing EA and Glu biosynthesis, expressed higher in new shoots of these albino/etiolated shoots. More importantly, the expression levels CsAlaDC and CsGOGAT1 in the new shoots exhibited significant and positive correlation with the theanine accumulation. The above findings strongly suggested theanine biosynthesis in new shoots greatly contributed to the high-level theanine accumulation in these albino/etiolated shoots.
Weak theanine catabolism in these albino/etiolated shoots and the underlying molecular mechanism
-
It was reported that the high-level theanine accumulation in albino/etiolated shoots is mainly determined by the weak catabolism of theanine[25]. CsPDX2.1 was shown to have theanine hydrolase activity, in vitro[22]. We recently found CsGGT2 has theanine hydrolase activity both in vitro and in vivo[23]. More importantly, these studies showed that both CsPDX2.1 and CsGGT2 expression exhibited negative correlation with the accumulation of theanine in new shoots[22,23]. We therefore examined CsPDX2.1 and CsGGT2 expression in the shoots of these cultivars (Fig. 6). However, results suggested that CsPDX2.1 did not show an obvious tendency, and its expression levels was positively correlated with the content of theanine to some degree (r = 0.640, p = 0.171) (Fig. 6a, c). In contrast, CsGGT2 expression in these albino/etiolated shoots was consistently lower than that of ZC108, and exhibited an obviously opposite pattern to the theanine accumulation in shoots (Figs 2c, 6b & c). The above results confirmed the perspective that weak catabolism of theanine contributes to high-level theanine accumulation in these albino/etiolated tea shoots, and the weak catabolism was probably due to the low expression of CsGGT2.
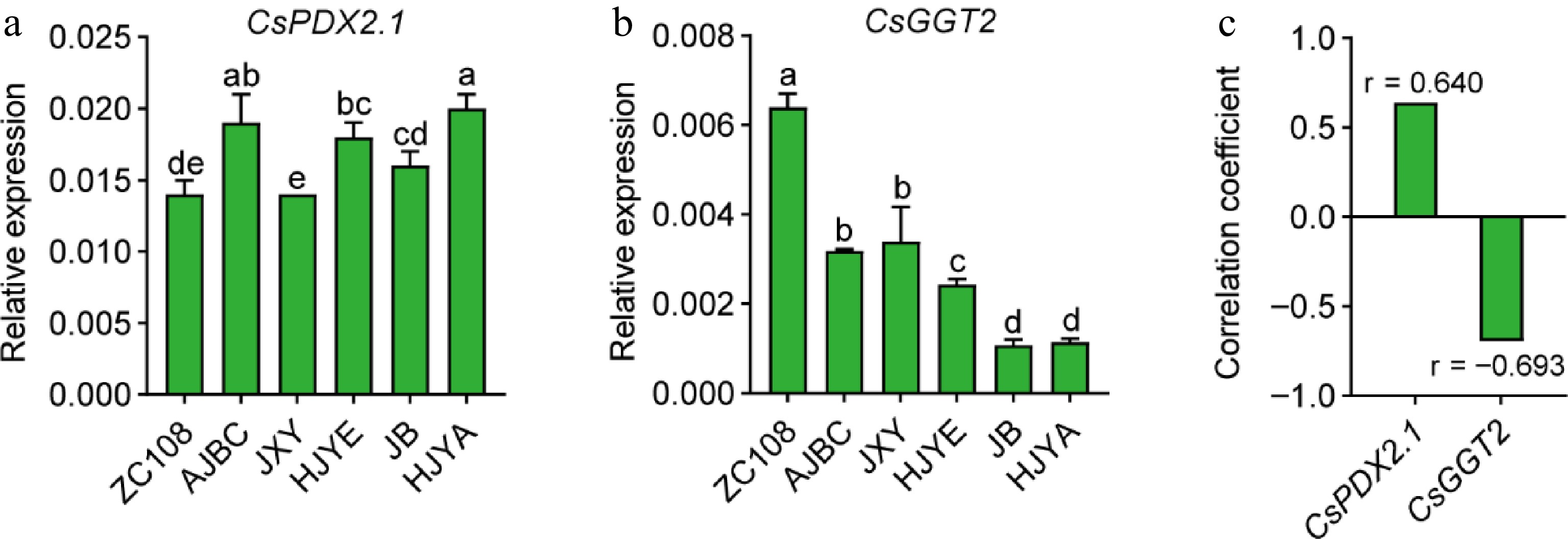
Figure 6.
(a) CsPDX2.1 expression tea shoots, (b) CsGGT2 expression in tea shoots. CsGAPDH was applied as the internal standardization. Data were denoted as means ± SD (n = 3), statistical significance (p < 0.05) was marked with distinct letters, based on Duncan's multiple range test. (c) The correlation coefficient between the CsPDX2.1 and CsGGT2 expression with the content of theanine in tea shoots. Asterisks represent statistical significance determined by Duncan's multiple range test (* p < 0.05, ** p < 0.01.).
-
In the tea plant, theanine is believed to be commonly biosynthesized in roots and then transported to the leaves for degradation[18,22]. However, there is probably a difference in albino/etiolated tea plant cultivars. In this study, we found theanine accumulation was not higher but lower in roots of albino/etiolated tea cultivars (Fig. 3a), suggesting that high-level accumulation of theanine in albino/etiolated tea shoots was likely not caused by stronger theanine biosynthesis in roots. Further analyses suggested that CsAlaDC-catalyzed EA biosynthesis and CsGOGAT1-catalyzed Glu biosynthesis in these albino/etiolated tea shoots were more active. This likely led to stronger theanine biosynthesis and contributed to high-level accumulation of theanine in albino/etiolated tea shoots (Figs 4, 5 & 7). In addition, this study also suggested CsGGT2-catalyzed theanine catabolism was weaker in new shoots of these albino/etiolated tea cultivars, which probably also contributed to the high-level accumulation of theanine in albino/etiolated tea shoots (Figs 6 & 7).
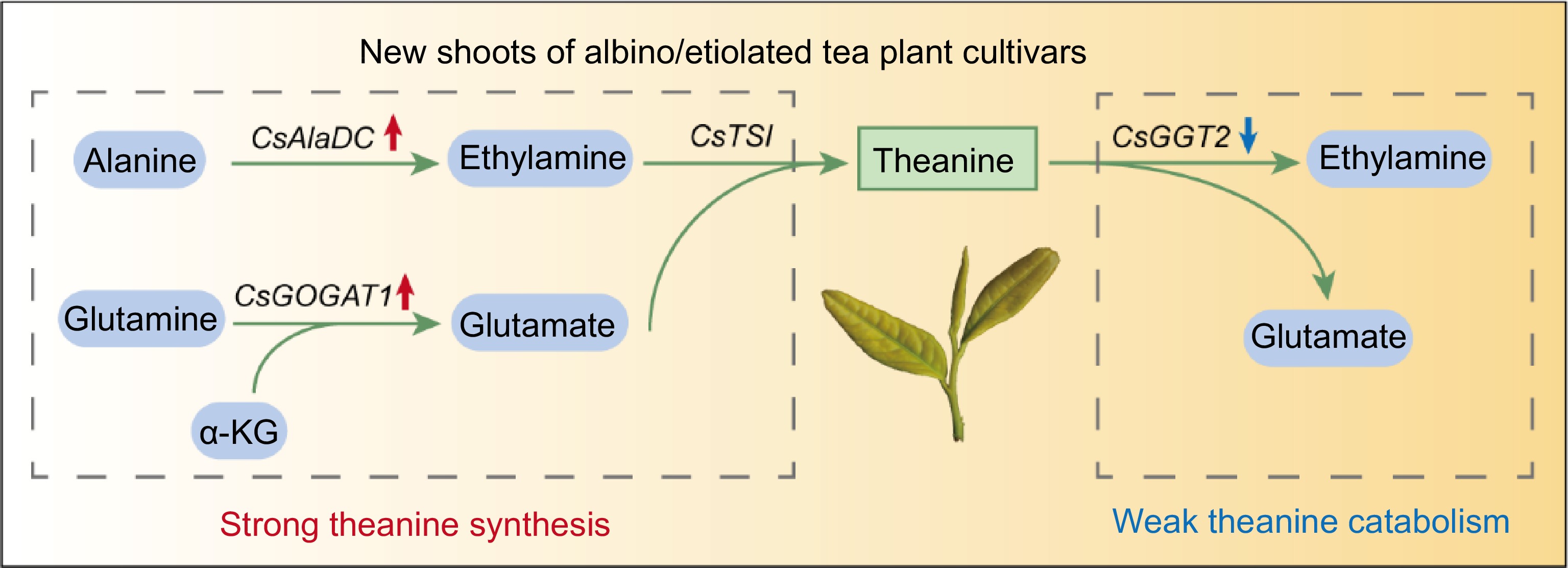
Figure 7.
Proposed model for high theanine accumulation in albino/etiolated tea shoots. α-KG, α-Ketoglutarate. The red and blue arrows indicate up-regulated and down-regulated gene expression in albino/etiolated tea shoots, respectively. In the albino/etiolated tea shoots, CsAlaDC-catalyzed EA biosynthesis and CsGOGAT1-catalyzed Glu biosynthesis were more active, CsGGT2-catalyzed theanine catabolism was weaker. Therefore, we proposed that the strong biosynthesis and weak catabolism of theanine in shoots both contribute to the high accumulation of theanine in albino/etiolated tea shoots.
-
B. Zhu and S. Qiao contributed equally to this work. Z. Zhang, X. Wan and C. Wei conceived the study and designed the experiments. B. Zhu, S. Qiao, M. Li, H. Cheng and Q. Ma carried out the experiments. B. Zhu and Z. Zhang wrote the manuscript. All authors reviewed and approved the final manuscript.
This work was supported by grants from the National Natural Science Foundation of China (32072624, U20A2045), the National Key R&D Program of China (2022YFF1003103, 2021YFD1601101) and the Base of Introducing Talents for Tea Plant Biology and Quality Chemistry (D20026).
-
The authors declare that they have no conflict of interest. Xiaochun Wan is the Editorial Board member of Beverage Plant Research who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and his research groups.
-
# These authors contributed equally: Biying Zhu, Siming Qiao
- Supplemental Fig. S1 Relative expression of CsGSs in tea shoots.
- Supplemental Table S1 Gene-specific primers for 1 qRT-PCR verification.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhu B, Qiao S, Li M, Cheng H, Ma Q, et al. 2023. Strong biosynthesis and weak catabolism of theanine in new shoots contribute to the high theanine accumulation in Albino/etiolated tea plant (Camellia sinensis). Beverage Plant Research 3:23 doi: 10.48130/BPR-2023-0023
Strong biosynthesis and weak catabolism of theanine in new shoots contribute to the high theanine accumulation in Albino/etiolated tea plant (Camellia sinensis)
- Received: 13 July 2023
- Revised: 23 August 2023
- Accepted: 28 August 2023
- Published online: 20 September 2023
Abstract: Tea processed from albino/etiolated tea shoots is favored by consumers because of its high theanine accumulation. To explore why theanine accumulates highly in new shoots of albino/etiolated tea cultivars, we compared theanine content in shoots and roots of albino/etiolated and common green tea cultivars. Results suggested that high theanine accumulation in albino/etiolated tea shoots was likely not caused by higher theanine biosynthesis in roots. Further analyses suggested that CsAlaDC-catalyzed ethylamine biosynthesis and CsGOGAT1-catalyzed glutamate biosynthesis were more active, and CsGGT2-catalyzed theanine catabolism was weaker in new shoots of these albino/etiolated tea plant cultivars. Therefore, the high theanine accumulation in albino/etiolated shoots is probably contributed by the strong theanine biosynthesis and weak catabolism in new shoots. These findings provided more comprehensive insights into the high accumulation of theanine in new shoots of albino/etiolated tea cultivars, and the knowledge can be used in plant breeding for new cultivars with higher theanine accumulation.
-
Key words:
- Camellia sinensis /
- Albino/etiolated tea plant germplasm /
- Theanine /
- CsAlaDC /
- CsGOGAT1 /
- CsGGT2














