-
Silage maize (Zea mays L.) is one of the most important forages in the world, and its yield and quality properties are critical importance for livestock production[1]. Moreover, maize is widely used for silage making around the world due to its richness in sugar content that makes it easy for ensiling. Under natural conditions, microorganisms attached to silage raw materials will result in damage to the dry matter of the silage and protein. In contrast, the ensiling process is a preservation of moist forages for ruminant livestock, which converts water soluble carbohydrates into organic acids like lactic acid in an anaerobic environment[2]. Through ensiling, silage could be well-preserved and supply year-round availability of nutritious and palatable feed for livestock.
As reported, the quality of silage is influenced by many factors such as geographical location, climate, temperature, varieties, cultivation techniques, harvest time and processing level[3]. Of these factors, silage additives are among the most extensively studied technology in ruminant feed preservation over the decades. To date, there have been continuous efforts in searching the most effective inoculants to reach better efficiency of ensiling[4]. Studies have shown that appropriate additives application can effectively improve the fermentation, reduce the consumption of nutrients, and improve the silage quality[5,6]. Interests were raised on cellulase and xylanase as they contained a variety of cell wall degrading enzymes. After degrading the cell wall of plant tissue by cellulase and xylanase, the substrate for microorganisms’ fermentation could be enhanced, which contributes to improvement of the silage quality[7]. According to the studies by Ding et al., adding 0.15% cellulase and xylanase to elephant grass silage reduced the content of cellulose and hemi-cellulose, increased the content of glucose, fructose, sucrose and total water-soluble carbohydrates, and rapidly produced lactic acid, reduced the pH value and ammoniacal nitrogen content[8]. Moreover, enzyme mixture containing cellulase, xylanase and cellobiase reduced silage pH, concentrations of xylose, total sugars and proportion of cell-wall arabinose[9]. However, there were some inconsistent results among studies. Although cellulase improved the quality of oat silage, no correlation with its added concentration was observed[10]. No significant effects on silage quality and digestibility were found when employing fibrolytic enzymes combined with LAB inoculants[11−13]. These discrepancies may be due to difference of plant materials and additive type used. Thus, quantifying the effect of incorporating enzymes on specific type of plants is important.
To our knowledge, presently, there is limited information available about the effects of cellulase and xylanase on fermentation quality of silage maize. Our objectives were to determine the effects of cellulase and xylanase at different levels acting alone or combined on fermentation quality and chemical composition of silage maize.
-
Silage maize (variety: Quchen No. 9) were planted at the experimental farm of Yunnan Agricultural University (N 25°8'12", E 102°45'20", 1,978 m) with a row spacing of 40 cm and seedlings spacing of 25 cm from May 25 to September 15, 2021 in Kunming, Southwest China. When planted, there are three seeds per hole and the sowing depth was 2~3 cm. At the 3-leaf period, only two plants were kept in each hole. During the experiment, field management such as watering, weeding, and pest control was consistent with field production. The local area is a north subtropical monsoon climate. The rainfall is concentrated from May to September each year, and the annual average temperature is about 15.1 °C. The pH of the cultivated soil layer is 6.46, the organic carbon content is 2.06%, the organic matter content is 3.55%. Total nitrogen, the available phosphorus and potassium contents in the topsoil were 135.7 mg·kg−1, 16.2 and 98.6 mg·kg−1, respectively.
Experimental design
-
The silage maize was harvested at the stage of wax ripeness, when the grains became hardened. Then, whole plants were chopped into 1−3 cm pieces with a straw kneading machine (Mingchuan, Dalian Mingchuan Agricultural Machinery Co. Ltd, China). Through the process of squishing, cutting, kneading, stalks and leaves were easy to compress and ferment.
Prior to ensiling, the chemical composition of the silage maize were as follows (% DM): water content 67.04, crude protein content 8.79, ether extract content 4.34, crude ash content 3.56, neutral detergent fiber content 55.6 and acid detergent fiber content 30.99.
Different concentrations of cellulose (No.9012-54-8, 10,000 U·g−1) and xylanase (No.9025-57-4, enzyme activity 100,000 U·g−1) were applied in the experiment. The silage treatments were designed as follows: (a) no additive (CK); (b) cellulase additive at a rate of 0, 0.25, 0.5, 1.0 g·kg−1; (c) xylanase additive at a rate of 0, 0.25, 0.5, 1.0 g·kg−1; (d) combination of cellulase and xylanase at different rates. There are 16 treatments in the study (Table 1). For each treatment, there were three replications. After thoroughly mixing the enzyme additives with the silage maize, the material were put into a silage plastic barrel (12 cm in diameter, 18 cm in height) and pressed as tightly as possible while filling it. The total weight of each barrel is about 2 kg. In the end, barrels were sealed with polyethylene plastic bags and kept indoors avoiding sunshine. After ensiling 60 d at ambient temperature, barrels were opened and sensory evaluation, fermentation quality and nutrition determination were carried out.
Table 1. Cellulase and xylanase experiment design.
Treatments Xylanase (g·kg−1) Cellulase (g·kg−1) C0-X0 X : 0 C : 0 C0.25-X0 C : 0.25 C0.5-X0 C : 0.5 C1.0-X0 C : 1.0 C0-X0.25 X : 0.25 C : 0 C0.25-X0.25 C : 0.25 C0.5-X0.25 C : 0.5 C1.0-X0.25 C : 1.0 C0-X0.5 X : 0.5 C : 0 C0.25-X0.5 C:0.25 C0.5-X0.5 C : 0.5 C1.0-X0.5 C : 1.0 C0-X1.0 X : 1.0 C : 0 C0.25-X1.0 C : 0.25 C0.5-X1.0 C : 0.5 C1.0-X1.0 C : 1.0 Cellulase and xylanase were provided by Shanghai Yien Chemical Technology Co. Ltd. (Shanghai, China). Fermentation quality and nutritional components analysis
-
The fermentation quality were evaluated by the parameters such as pH value, water soluble carbohydrates (WSC), ammonia nitrogen/total nitrogen (AN/TN) and organic acids like lactic acid (LA), acetic acid (AA), propionic acid (PA), butyric acid (BA). The pH was measured with a glass electrode pH meter (Shanghai Leici Instrument Factory, China). WSC was determined using sulfuric acid anthrone colorimetric method[14]. Ammonia nitrogen content was determined using the phenol-hypochlorite sodium colorimetric method[15]. The content of organic acids (lactic acid, acetic acid, propionic acid, butyric acid) was analyzed via Agilent 1100 HPLC (the chromatographic column used was KC-811, 8 mm × 300 mm)[16].
Nutritional components measured include water content (WC), crude protein (CP), Ether extract (EE), crude ash (Ash), neutral detergent fiber (NDF), Acid detergent fiber (ADF). For WC, 10 g of pulverized silage material was dried at 105 °C for 30 min, and then dried at 65 °C to constant weight. CP content was determined according to the procedure of Kjeldahl method[17]. Ash content was determined according to ignition method[12]. EE was determined according to the Soxhlet extraction method[18].
For NDF and ADF content in the maize silage, 0.5 g samples were precisely weighted after drying, grinding and sieving (40 mesh). Then, they were put into prepared neutral detergent reagent or acidic detergent reagent, respectively following the procedure of Van soest method[19].
Data analysis
-
Analysis of variance (ANOVA) was performed using SPSS 25.0 for windows statistical software package. Duncan's method was used for multiple comparisons within cellulase or xylanase. Two-way ANOVAs were used to separate the effects of cellulase, xylanase and their interaction. Differences were considered significant at p < 0.05 level.
The fuzzy mathematical membership function method was used to comprehensively evaluate the effects of cellulase and xylanase on fermentation quality and nutritive parameters[20]. Two calculation formulas were introduced as follows:
$\rm R(_{Xi}) = (X_{i}-X_{min}) / (X_{max}-X_{min}) $ (1) $\rm R(_{Xi}) = 1-(X_{i}-X_{min}) / (X_{max}-X_{min}) $ (2) In formulas (1) and (2): R(Xi) represents the membership function value of an index, Xi is the measured value of the index, Xmax is the maximum measured value of the index, and Xmin is the minimum measured value of the index. When the measured index is positively correlated with silage quality, formula (1) is used. However, when the measured index is negatively correlated with the silage quality, formula (2) is used for calculation.
-
The pH values of silages were not significantly affected by cellulase or xylanase additive, however, all pH values of silage were below 4, indicating the silage maize were well preserved (Table 2). The WSC content was affected by cellulase rather than by xylanase. When the cellulase was applied alone, the WSC content of C0.25, C0.5 treatment increased by 7.95% and 23.5%, respectively, compared with CK (p < 0.05). No interactive effect was observed between cellulase and xylanase.
Table 2. Effects of cellulase and xylanase on pH, water soluble content and ammonia nitrogen/total nitrogen of silage maize.
Xylanase Cellulase pH value Water soluble
carbohydrates
(%)Ammonia
nitrogen/total
nitrogen (%)X0 C0 3.71 ± 0.10Aa 3.02 ± 0.03Ab 10.53 ± 0.44Aa C0.25 3.59 ± 0.00Aa 3.26 ± 0.17Aab 9.36 ± 0.46ABa C0.5 3.72 ± 0.06Aa 3.73 ± 0.27Aa 9.69 ± 0.27Aa C1.0 3.71 ± 0.09Aa 3.33 ± 0.07Aab 9.82 ± 0.49Aa X0.25 C0 3.70 ± 0.03Aa 3.32 ± 0.24Aa 9.50 ± 0.48ABa C0.25 3.68 ± 0.02Aa 3.33 ± 0.26Aa 10.45 ± 0.46Aa C0.5 3.67 ± 0.04Aa 3.39 ± 0.12Aa 8.77 ± 0.57Aa C1.0 3.77 ± 0.09Aa 3.42 ± 0.20Aa 8.49 ± 0.73ABa X0.5 C0 3.65 ± 0.01Aa 3.58 ± 0.23Aa 7.68 ± 0.49Cab C0.25 3.73 ± 0.08Aa 3.85 ± 0.28Aa 6.79 ± 0.17Cb C0.5 3.70 ± 0.02Aa 3.58 ± 0.11Aa 8.49 ± 0.70Aa C1.0 3.64 ± 0.01Aa 3.66 ± 0.07Aa 8.23 ± 0.38ABab X1.0 C0 3.61 ± 0.02Aa 3.40 ± 0.23Aa 8.09 ± 0.43BCa C0.25 3.72 ± 0.07Aa 3.78 ± 0.19Aa 8.31 ± 0.44Ba C0.5 3.73 ± 0.06Aa 3.66 ± 0.17Aa 9.27 ± 0.48Aa C1.0 3.69 ± 0.04Aa 3.79 ± 0.51Aa 7.81 ± 0.56Ba Different lowercase letters indicate there are significant differences between cellulase concentration treatments at the same concentration of xylanase (p < 0.05); different uppercase letter indicates that there are significant difference between different xylanase concentration treatments at the same cellulase concentration (p < 0.05). With regards to organic acids, different changes were observed (Table 3). The lactic acid content increased with the increase of the cellulase concentration, particularly the content of C1.0 treatment was increased by 18.9% (p < 0.05). The change of propionic acid was different to lactic acid, which decreased by 22.5%, 30.1% and 31.2% in C0.25, C0.5 and C1.0 treatment, respectively, when compared to control (p < 0.05). When xylanase was applied alone, the production of lactic acid and acetic acid was significantly inhibited (p < 0.05). Moreover, the contents of lactic acid, acetic acid and butyric acid in X0.25-C1.0 treatment were significantly reduced by 22%, 62.2% and 64.2% (p < 0.05) , respectively. In contrast, AN/TN ratio of X0.25, X0.5 and X1.0 treatment were also significantly decreased due to xylanase additive application in comparison with control (p < 0.05). It showed that the addition of xylanase is beneficial to reduce the ammoniacal nitrogen. However, such effects were not observed after cellulase application.
Table 3. Effects of cellulase and xylanase on organic acids of silage maize.
Xylanase
(g·kg−1)Cellulase
(g·kg−1)Lactic acid
(mg·g−1 FM)Acetic acid
(mg·g−1 FM)Propionic acid
(mg·g−1 FM)Butyric acid
(mg·g−1 FM)X0 C0 12.71 ± 0.58Ab 11.99 ± 0.74Aa 4.45 ± 0.08ABa 1.31 ± 0.04Ab C0.25 13.74 ± 0.82Aab 11.31 ± 1.00Aa 3.45 ± 0.18Bb 1.01 ± 0.09Ac C0.5 14.46 ± 0.64Aab 9.57 ± 1.24Aa 3.11 ± 0.36Bb 1.54 ± 0.02Aa C1.0 15.11 ± 0.32Aa 9.83 ± 1.28Aa 3.06 ± 0.31Bb 1.43 ± 0.07Aab X0.25 C0 13.92 ± 0.59Aa 10.33 ± 0.53Ba 3.18 ± 0.16Cb 2.01 ± 0.39Aa C0.25 14.12 ± 0.35Aa 10.71 ± 0.20Aa 3.29 ± 0.13Bb 1.25 ± 0.27Aab C0.5 13.03 ± 0.77Aa 7.95 ± 2.37ABa 3.23 ± 0.15Bb 1.10 ± 0.25Aab C1.0 10.86 ± 0.35Bb 3.91 ± 0.15Bb 4.19 ± 0.13Aa 0.72 ± 0.11Bb X0.5 C0 10.65 ± 0.06Ba 4.50 ± 0.15Ca 5.09 ± 0.11Aa 1.19 ± 0.39Aa C0.25 11.03 ± 0.27Ba 4.30 ± 0.16Ba 5.21 ± 0.33Aa 1.52 ± 0.09Aa C0.5 10.47 ± 0.11Ba 4.29 ± 0.06Ba 5.13 ± 0.16Aa 1.38 ± 0.32Aa C1.0 10.70 ± 0.27Ba 4.37 ± 0.15Ba 4.77 ± 0.08Aa 1.43 ± 0.20Aa X1.0 C0 9.47 ± 0.22Ba 3.80 ± 0.25Ca 4.00 ± 0.40Bab 1.20 ± 0.18Aab C0.25 9.42 ± 0.08Ca 3.84 ± 0.09Ba 3.28 ± 0.17Bb 1.02 ± 0.12Ab C0.5 9.57 ± 0.44Ba 4.20 ± 0.60Ba 4.63 ± 0.09Aa 1.53 ± 0.09Aa C1.0 10.28 ± 0.18Ba 3.95 ± 0.15Ba 4.64 ± 0.24Aa 1.07 ± 0.06ABb Different lowercase letters indicate there are significant differences between cellulase concentration treatments at the same concentration of xylanase (p < 0.05); different uppercase letter indicates that there are significant difference between different xylanase concentration treatments at the same cellulase concentration (p < 0.05). Chemical analyses
-
Cellulase and xylanase had no significant effect on water content (p > 0.05) (Table 4). When xylanase was added at rate of 0.25 and 0.50 g·kg−1, the CP content increased by 10.02% and 14.26%, respectively (p < 0.05). For EE, its content was increased by both cellulase and xylanase. For example, EE was increased by 8.75%, 24.24% and 11.11% (p < 0.05), respectively when cellulase was added at 0.25, 0.50, 1.0 g·kg−1.
Table 4. Effects of cellulase and xylanase on nutritional parameters of silage maize.
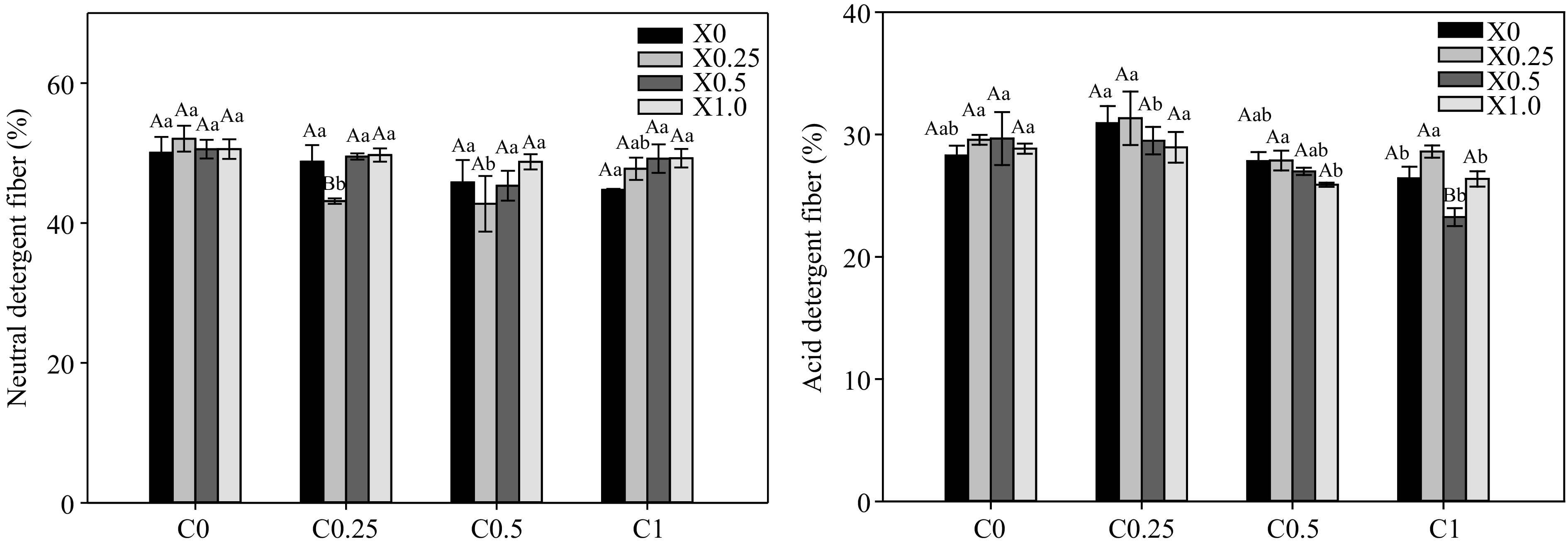
Xylanase (g·kg−1) Cellulase (g·kg−1) Water content (%) Crude protein (%) Ether extract (%) Crude ash (%) X0 C0 74.33 ± 0.33Aa 5.19 ± 0.14Bc 2.97 ± 0.05Bb 50.05 ± 2.27Aa C0.25 74.33 ± 0.33Aa 5.61 ± 0.16Ab 3.23 ± 0.17Aab 48.76 ± 2.38Aa C0.5 75.33 ± 0.33Aa 6.11 ± 0.05Aa 3.69 ± 0.27Aa 45.80 ± 3.21Aa C1.0 74.67 ± 0.33Aa 5.50 ± 0.11Bbc 3.30 ± 0.07Aab 44.74 ± 0.15Aa X0.25 C0 74.67 ± 0.33Aa 5.71 ± 0.04ABab 3.40 ± 0.18ABa 52.06 ± 1.85Aa C0.25 75.00 ± 0.58Aa 5.51 ± 0.14Abc 3.46 ± 0.09Aa 43.14 ± 0.39Bb C0.5 75.33 ± 0.33Aa 5.39 ± 0.10Bc 3.31 ± 0.08Aa 42.76 ± 3.98Ab C1.0 76.00 ± 0.58Aa 5.92 ± 0.07Aa 3.70 ± 0.18Aa 47.76 ± 1.59Aab X0.5 C0 75.33 ± 0.33Aa 5.93 ± 0.02Aa 3.55 ± 0.22Aa 50.55 ± 1.33Aa C0.25 75.33 ± 0.33Aa 5.89 ± 0.14Aab 3.47 ± 0.13Aa 49.51 ± 0.45Aa C0.5 74.33 ± 0.67Aa 5.49 ± 0.20Bbc 3.54 ± 0.11Aa 45.33 ± 2.13Aa C1.0 75.33 ± 0.33Aa 5.37 ± 0.03Bc 3.63 ± 0.07Aa 49.20 ± 2.04Aa X1.0 C0 75.33 ± 0.67Aa 5.38 ± 0.36ABa 3.53 ± 0.11Aa 50.57 ± 1.40Aa C0.25 75.00 ± 0.00Aa 5.72 ± 0.06Aa 3.75 ± 0.19Aa 49.73 ± 0.95Aa C0.5 75.00 ± 0.58Aa 5.57 ± 0.14Ba 3.62 ± 0.17Aa 48.75 ± 1.08Aa C1.0 75.67 ± 0.33Aa 4.63 ± 0.07Cb 3.42 ± 0.17Aa 49.26 ± 1.33Aa Different lowercase letters indicate there are significant differences between cellulase concentration treatments at the same concentration of xylanase (p < 0.05); different uppercase letter indicates that there are significant difference between different xylanase concentration treatments at the same cellulase concentration (p < 0.05). The NDF content in C0.25-X0.25, C0.5-X0.25 and C1.0-X0.25 treatments was significant lower than C0-X0.25 treatment (p < 0.05). When the cellulase was added at 1.0 g·kg−1, the ADF content was significantly reduced (p < 0.05). However, ADF content was not significantly affected by xylanase. The crude ash content in C0-X1.0 treatment was significantly lower than C0-X0.25 (p < 0.05) (Fig. 1).
Interaction between cellulase and xylanase
-
No significant interactions in pH, EE, WC, Ash, NDF and ADF were observed between cellulase and xylanase ( p > 0.05) (Table 5). However, significant effects were on AN/TN, PA, BA, and CP (p < 0.05). The synergistic effect of cellulase and xylanase only exists on the effect on CP (p < 0.01).
Table 5. Interaction of cellulase and xylanase on the fermentation quality and nutritional components
Treatments Cellulase Xylanase Cellulase* Xylanase pH value 0.749ns 0.925 ns 0.503 ns WSC (%) 0.171ns 0.028 * 0.834ns AN/TN (%) 0.826ns 0.019 * 0.028 * LA (mg·g−1) 0.954ns 0.001** 0.000 ** AA (mg·g−1) 0.239ns 0.000 ** 0.005 ** PA (mg·g−1) 0.780ns 0.012 * 0.000 ** BA (mg·g−1) 0.626ns 0.904ns 0.017 * WC (%) 0.425ns 0.316ns 0.335 ns CP (%) 0.638ns 0.584ns 0.000 ** EE (%) 0.606ns 0.241ns 0.152ns Ash (%) 0.025 * 0.017 * 0.142ns NDF (%) 0.044 * 0.227ns 0.335ns ADF (%) 0.003 ** 0.128ns 0.348ns 'ns' indicates that the difference is not significant (p > 0.05); '*' indicates that the difference is significant (p < 0.05); '**' indicates that the difference is very significant (p < 0.01). Comprehensive evaluation
-
The effects of cellulase and xylanase were comprehensively evaluated by the fuzzy mathematical membership function method (Table 6). Generally, the larger the mean value is, the better the silage quality is. After adding different concentrations of cellulase and xylanase, the membership function values of each treatment were higher than the control, and the mean value of the membership function of C0.5 was the largest, followed by C1.0; In the combined treatment, the means of membership functions of C0.5-X0.25 and C0.25-X0.25 are better than other treatments.
Table 6. Analysis of silage maize membership function and comprehensive value ranking.
Treatments R1 R2 R3 R4 R5 R6 R7 R8 R9 R10 R11 R12 Average Rank C0.5 0.28 0.85 0.22 0.89 0.70 0.98 0.36 1.00 0.94 0.85 0.67 0.43 0.68 1 C1.0 0.35 0.37 0.19 1.00 0.74 1.00 0.45 0.59 0.42 0.99 0.79 0.61 0.62 2 C0.5-X0.25 0.57 0.45 0.47 0.63 0.51 0.92 0.71 0.51 0.44 0.15 1.00 0.43 0.57 3 C0.25-X0.25 0.48 0.37 0.02 0.83 0.84 0.89 0.59 0.59 0.64 0.34 0.96 0.00 0.55 4 C0.25 1.00 0.29 0.31 0.76 0.92 0.82 0.78 0.66 0.34 0.17 0.35 0.05 0.54 5 C1.0-X0.5 0.70 0.78 0.61 0.22 0.07 0.20 0.45 0.50 0.85 0.69 0.31 1.00 0.53 6 C0.25-X1.0 0.30 0.92 0.59 0.00 0.00 0.89 0.77 0.73 1.00 0.54 0.25 0.29 0.53 7 C1.0-X0.25 0.00 0.49 0.55 0.25 0.01 0.47 1.00 0.87 0.94 0.43 0.46 0.34 0.48 8 C1.0-X1.0 0.44 0.93 0.73 0.15 0.02 0.27 0.73 0.00 0.58 1.00 0.30 0.61 0.48 9 C0-X1.0 0.89 0.45 0.65 0.01 0.00 0.56 0.63 0.51 0.73 0.79 0.16 0.31 0.47 10 C0.5-X0.5 0.39 0.68 0.55 0.18 0.06 0.04 0.49 0.58 0.74 0.57 0.72 0.54 0.46 11 C0-X0.5 0.65 0.68 0.76 0.22 0.09 0.06 0.64 0.88 0.75 0.24 0.16 0.21 0.44 12 C0.5-X1.0 0.24 0.77 0.34 0.03 0.05 0.27 0.37 0.64 0.85 0.65 0.36 0.67 0.44 13 C0.25-X0.5 0.22 1.00 1.00 0.28 0.06 0.00 0.38 0.85 0.65 0.28 0.27 0.23 0.44 14 C0-X0.25 0.41 0.36 0.27 0.79 0.80 0.94 0.00 0.73 0.55 0.00 0.00 0.22 0.42 15 C0 0.33 0.00 0.00 0.58 1.00 0.36 0.55 0.38 0.00 0.41 0.22 0.38 0.35 16 R1~R12 stand for pH, water soluble carbohydrates, ammonia nitrogen/total nitrogen, lactic acid, acetic acid, propionic acid, butyric acid, crude protein, crude extract, crude ash, neutral detergent fiber and acid detergent fiber. -
In recent years, animal husbandry in China has developed rapidly and faces fodder shortage. Silage maize is an important source of fodder as it has the advantages of high biomass, good fiber quality, and suitable moisture content for ruminants. In order to preserve the nutritional quality and improve the fermentation quality, additives like enzymes and lactic acid are often added to silage materials, which contribute to improvement of the fermentation process directly or indirectly[21,22]. After breaking plant cell walls during the ensiling process, silage fermentation could be improved by providing sugars for the lactic acid bacteria (LAB) and the nutritive value could be enhanced by increasing the digestibility of cell walls[23]. Therefore, interest was raised to use cellulase and xylanase during ensiling as cellulase could convert cellulose into the glucose after hydrolyzing beta-1,4 glycosidic linkages[24] and xylanases help to break down hemicelluloses[25].
The pH value is one of the crucial indicators assessing the silage quality. Lowering pH value in ensiled forage can effectively inhibit proteolysis because plant enzymes are quickly inactivated with a decrease of pH[26]. In contrast, high pH value indicates that the frequent activities of harmful bacteria are not well inhibited. It has been well documented that the optimum pH value for stable silage is below 4.2[27]. In this study, although the pH value of all treatments was not significantly affected by cellulase or xylanase, all values were lower than 3.9, showing that addition of cellulase and xylanase had no adverse effect on the pH value of silage.
Apart from the pH, one of the most useful indicators of silage quality is the percentage of total nitrogen in the silage which is present as ammonia nitrogen. The ammoniacal nitrogen/total nitrogen ratio reflects the degradation degree of protein in the ensiling process. Due to unfavorable microorganisms, the degradation rate of protein and amino acids accelerates, which leads to a higher ratio of ammoniacal nitrogen to total nitrogen. Extensive protein degradation during the fermentation has been documented in some studies[28]. However, in the present study, xylanase reduced the ratio of ammonia nitrogen/total nitrogen by 27.1%, thus it suggests an enhancement of protein preservation after the addition of xylanase. This was further proved by the significant increased in CP in silage, which was in agreement with the study by Yang[29].
In the process of ensiling, WSC is used as the basic substance by lactic acid bacteria and other aerobic microorganisms. Through metabolic activities, lactic acid bacteria use WSC to produce lactic acid and acetic acid, which lowers pH value[30]. As a result, decomposition of WSC by aerobic microorganisms will be inhibited. In our study, cellulase increased the water soluble carbohydrate content, which was consistent with the results reported by Albrecht & Muck[28] . The higher WSC content means the quality of silage is well maintained and related to good silage quality. Along with the increase of WSC, lactic acid content increased after cellulase addition, especially at higher adding rate in the study. LA is the most powerful organic acid capable of rapidly decreasing pH[31] as it is 10 to 12 times stronger than acetic acid and propionic acid[32]. The accumulation of lactic acid is the main reason for the pH decrease during anaerobic fermentation[33].
According to Shao et al., cellulase promotes the degradation of fiber, releases soluble carbohydrates, provides additional fermentation substrates for lactic acid bacteria, and rapidly produces lactic acid[34]. A decrease of butyric acid content was also observed when two additives were applied together. It is believable that butyric acid is responsible for reducing silage intake, its decrease is better for fermentation quality[21]. However, the decreasing effect was closely related to the type of enzyme and dose used[35].
NDF is the most effective indicator to reflect the quality of fiber. ADF is the key to indicate the energy of forage grass, the lower its content, the higher the digestibility of forage grass, and the greater the feeding value. In this experiment, the addition of cellulase reduced the content of NDF and ADF, which is consistent with the study of Hou et al.[36]. It suggests that the plant fibers in silage maize could be digested easier after adding additives. The mechanism behind the decrease in NDF with additive treatment may be related to the increase in WSC content because of degrading cellulose, which need further study.
-
Our study showed cellulase and xylanase additives have positive effects on fermentation quality and nutritive value of silage maize silage. Particularly, addition of cellulase increased the LA, WSC, CP and EE content, and decreased ammonia nitrogen and total nitrogen, NDF and ADF while xylanase inhibited the production of AA and PA, contributed to improvement of CP and EE. Interaction between cellulase and xylanase was observed in organic acids and CP. Both contribute to enhancement of CP. According to the comprehensive comparison of membership function analysis, it suggests that addition of 0.5 g·kg−1 cellulase alone or 0.5 g·kg−1 cellulase and 0.25 g·kg−1 xylanase combined could achieve better silage.
This work was supported by the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDA26050301), by YEFICRC project of Yunnan provincial key programs (No. 2019ZG00902) and Eryuan County Forage Industry Science and Technology Mission (No.202304BI090008).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Li S, Wang H, Luo M, Wu B, Duan H, et al. 2023. Effects of cellulase and xylanase additives on fermentation quality and nutrient composition of silage maize. Circular Agricultural Systems 3:8 doi: 10.48130/CAS-2023-0008
Effects of cellulase and xylanase additives on fermentation quality and nutrient composition of silage maize
- Received: 10 March 2023
- Accepted: 01 June 2023
- Published online: 27 September 2023
Abstract: The aim of this experiment was to determine the effects of cellulase and xylanase additives on the fermentation quality, chemical composition of silage maize. In the experiment, cellulase (0, 0.25, 0.5, 1.0 g·kg−1) and xylanase (0, 0.25, 0.5, 1.0 g·kg−1) in different concentrations were applied alone or in combination on silage materials. After 60 d ensiling at room temperature, the results showed that cellulase and xylanase have positive effects on silage quality and chemical composition of silage. Cellulase increased contents of water-soluble carbohydrate, crude protein and crude fat while decreased contents of ammonia nitrogen and total nitrogen, neutral detergent fiber and acid detergent fiber. For xylanase, it increased the crude protein and ether extract content. Interactive effects were observed in CP and organic acids. Therefore, the adding cellulase and xylanase improved fermentation quality and nutrition value of silage maize. According to the comprehensive evaluation of the membership function, the recommended adding concentration of cellulase is 0.5 g·kg−1 alone. When combined with xylanase, the concentration of both cellulase and xylanase were 0.5 g·kg−1 and 0.25 g·kg−1, respectively.
-
Key words:
- Enzymes /
- Silage additive /
- Lactic acid /
- ADF /
- NDF