-
Diabetes mellitus (DM) is a metabolic disorder resulting from compromised insulin action or insufficient insulin secretion[1]. It is characterized by persistent hyperglycemia and glycosuria, accompanied by multisystem and multiorgan complications, such as diabetic nephropathy and diabetic retinopathy[1]. Over the past few years, there has been a rapid increase in the prevalence of DM. According to the latest data released by the International Diabetes Federation, about 537 million adults aged 20−79 suffered DM in 2021, and this number will reach nearly 643 million by 2030 and approximately 783 million by 2045[2]. Currently, there are two main types of DM, type I diabetes (T1DM) and type II diabetes (T2DM), with T2DM constituting roughly 90%−95% of all cases. T1DM is distinguished by a complete absence of insulin due to the autoimmune destruction of pancreatic β-cells, whereas T2DM is characterized by insulin deficiency and reduced insulin sensitivity[3]. As a severe threat to both global economies and human health, DM has reached a crisis level and is one of the foremost public health challenges of the 21st century. Despite the availability of numerous medications for clinical DM treatment, long-term use still causes adverse effects, such as nausea, diarrhea, and weight gain[4]. Thus, there is a great need to develop alternative natural hypoglycemic agents.
Tea (Camellia sinensis) is one of the world's most popular beverages, which originates from China and has been used as a beverage and medicine for more than 5,000 years[5]. According to the differences in the fermentation degree and manufacturing processes, it can be categorized into dark, black, yellow, white, oolong, and green tea[6]. The potential hypoglycemic effects of tea and its active ingredients have been extensively studied in recent years. Epidemiological and clinical investigations have demonstrated an inverse relationship between regular tea consumption and the incidence of DM[7,8]. Furthermore, a large number of in vitro and in vivo researches have illuminated the capacity of tea and its bioactive compounds (e.g., tea polyphenols, tea polysaccharides, and alkaloids) to mitigate the risk of DM and its associated complications to some extent through different mechanisms of action[3,9]. Therefore, the development of antidiabetic medicines from tea and its bioactive components is receiving increasing attention. This review summarizes the recent research progress on tea bioactive compounds and their hypoglycemic effects and discusses the potential hypoglycemic mechanisms of tea. It will be helpful for further research on the antidiabetic activities of tea and the development of new antidiabetic functional products.
-
The primary objective in managing DM is to attain optimal glycemic control and to stave off or slow down the emergence and advancement of DM complications[10]. A fundamental component of contemporary DM management encompasses the utilization of clinical medications aimed at regulating blood glucose levels. These medications, owing to the multifaceted nature of DM's pathophysiology, operate via a variety of mechanisms to modulate glucose levels[4,11−13], which can be divided into five main categories (as shown in Table 1): (1) In an oral or injection form, insulin can be administered to hormone-deficient T1DM patients and T2DM patients with poor glycemic management. (2) Another approach to manage DM involves the usage of insulin sensitizers, exemplified by thiazolidinediones and biguanides. Thiazolidinediones, like pioglitazone and rosiglitazone, alongside biguanides such as metformin and buformin, generally enhance insulin receptor sensitivity in peripheral tissues like muscle, adipose tissue, and liver, thereby ameliorating insulin resistance. (3) Insulin secretion enhancers such as sulfonylureas are another treatment option. Oral sulfonylureas (e.g., glimepiride, gliquidone, gliclazide, and glipizide) stimulate insulin secretion from pancreatic β-cells. Nevertheless, it's worth noting that sulfonylureas can provoke insulin secretion independently of glucose levels, potentially leading to episodes of hypoglycemia. (4) Therapeutic medications based on incretin hormones, such as dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists, offer additional treatment options. (5) Other drugs, including α-glucosidase inhibitors and sodium-glucose cotransporter-2 (SGLT2) inhibitors, are other treatment options. Notably, commonly used α-glucosidase inhibitors like miglitol, voglibose, and acarbose may induce side effects like gastric flatulence and diarrhea with long-term use[14]. SGLT2 inhibitors increase renal glucose excretion by decreasing renal tubular reabsorption of glucose[14]. Antidiabetic medications may be administered as single agents or combined in dual or triple therapy regimens to effectively manage hyperglycemia. However, despite their efficacy in treating DM, these hypoglycemic medications are highly susceptible to a variety of adverse effects with long-term use, including weight gain, hypoglycemia, nephron- and hepatotoxicity, allergies, and abdominal discomfort (diarrhea, vomiting, and nausea)[4,11−13]. Moreover, they are also expensive. Therefore, there is a high demand for alternative natural hypoglycemic drugs for the management of DM.
Table 1. Summary of the categories of hypoglycemic medicines, common clinical drugs, hypoglycemic mechanisms, and adverse effects.
Categories of hypoglycemic medicines Common clinical drugs Hypoglycemic mechanisms Adverse effects Insulin Insulin Enhance glucose uptake and utilization by systemic tissues and cells while inhibiting glycogenolysis and glycogen isogenesis. Hypersensitivity, lipodystrophy, and lipohypertrophy Thiazolidinediones Rosiglitazone; Pioglitazone Increase insulin sensitivity in liver, muscle, and adipose tissue. Abnormal liver function and weight gain Biguanides Pioglitazone; Buformin; Metformin Inhibit hepatic glucose output, improve the insulin sensitivity of peripheral tissues, and increase glucose uptake and utilization. Loss of appetite, nausea, abdominal discomfort, and diarrhea Sulfonylureas Glimepiride; Gliquidone; Gliclazide; Glipizide Reduce blood glucose levels by stimulating β-cells insulin secretion. Hypoglycemic reaction, loss of appetite, nausea and vomiting, diarrhea, and increased risk of cardiovascular disease Dipeptidyl peptidase-4
(DPP-4) inhibitorsLinagliptin; Saxagliptin; Vigliptin Reduce glucagon and hypoglycemia. Nasopharyngitis, headache, and upper respiratory tract infection Glucagon-like peptide 1
(GLP-1) receptor agonistLixisenatide; Albiglutide; Dulaglutide; Semaglutide Reduce blood glucose levels by increasing insulin secretion and inhibiting postprandial glucagon secretion. Nausea, vomiting, diarrhea, injection-site inflammation, and pancreatitis α-Glucosidase inhibitors Acrobose; Miglitol; Voglibose Hinder the decomposition and absorption
of dietary carbohydrates by inhibiting pancreatic α-amylase and intestinal α-glucosidase.Gastric flatulence and diarrhea Sodium-glucose-cotransporter type-2 (SGLT-2) inhibitors Canagliflozin; Ertugliflozin; Dapagliflozin; Empagliflozin Inhibit glucose reabsorption in the kidney and eliminate glucose from the urine. Urinary tract infection -
Tea contains various bioactive components, some of which remain unchanged in the tea leaves, while others undergo transformations during processing. The potential hypoglycemic compounds in tea predominantly comprise tea polyphenols, tea polysaccharides, and alkaloids[5], and the contents of these main compounds of teas are highly dependent on tea categories[15].
Tea polyphenols
-
Tea polyphenols are a group of polyhydroxy phenolic compounds, which constitute a major group of biologically active components, comprising around 25% of the dry weight of tea and are mainly composed of flavanols (catechins and dimeric catechins) and flavonoid glycosides[5,14].
Catechins
-
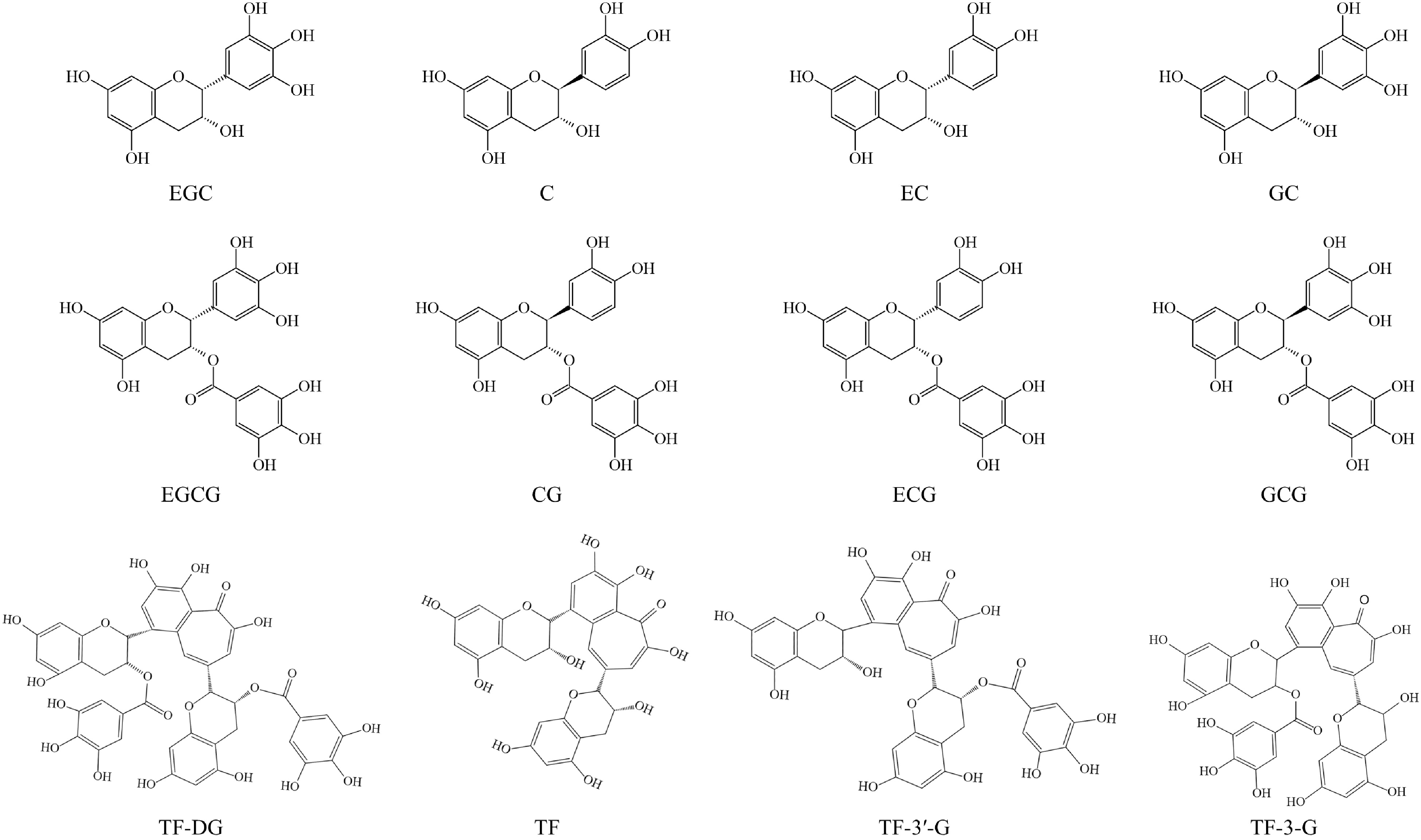
Catechins, a member of the flavanols family, account for over 70% of tea polyphenols and are the major chemical contributors to tea flavor and bioactivities[5,14]. As shown in Fig. 1, catechins mainly include nonester-type catechins, such as epigallocatechin (EGC), catechin (C), epicatechin (EC), and gallocatechin (GC), and ester-type catechins, such as epigallocatechin-3-O-gallate (EGCG), catechin-3-O-gallate (CG), epicatechin-3-O-gallate (ECG), and gallocatechin-3-O-gallate (GCG). Among these compounds, ester catechins are present at higher levels than nonester-type catechins[16]. Due to the processing methods used, the catechin contents in different tea categories can vary greatly. For example, white, green, and yellow teas contain higher catechin levels compared to oolong, black, and dark teas[17]. Furthermore, the contents of catechins can also be influenced by variety and geographic factors; for example, the contents of EGC can be 10 times different in the major black tea producing countries[18].
Dimeric catechins
-
Theaflavins (TFs) and theasinensins (TSs) are two important types of dimeric catechins, which primarily form through enzyme-catalyzed reactions during the fermentation process and are commonly found in fermented teas like oolong, black, and white teas[19]. TFs, mainly include theaflavin-3,3′-digallate (TF-DG), theaflavin (TF), theaflavin-3′-gallate (TF-3′-G), and theaflavin-3-gallate (TF-3-G), constituting approximately 3%−5% (w/w) of the solid extract in black tea and are a compound class of benzotropolone derivatives (Fig. 1)[5]. In our previous study, we found that theasinesins (theasinesin A and theasinesin B) exhibited higher levels in white tea (slightly fermented) and reached their peak in black tea (fully fermented), while they were lowest in green tea (unfermented), indicating that the formation of theasinesins is related to the fermentation degree[20].
Flavonoid glycosides
-
Flavonoid glycosides are the main polyphenols in tea and have potential antidiabetic bioactivity, which comprises about 3%−4% of the dry weight of tea[21]. The flavonoid glycosides in tea differ in their aglycones and conjugated sugars. In terms of aglycones, they can be classified into quercetin, myricetin, kaempferol, and apigenin glycosides; in terms of the number of glycoside moieties, they can be classified into mono-, di-, and tri-glycosides; in terms of conjugated sugars, they can be categorized into glucoside, galactoside, rutinoside, and arabinoside[21].
Tea polysaccharides
-
Tea polysaccharides consist of a diverse array of water-soluble polysaccharides, pectin, and proteins extracted from various parts of the tea plant, including leaves, flowers, and seed peels[22]. These heteropolysaccharides have garnered significant attention due to their numerous health benefits, such as their potential to regulate lipid metabolism, act as antioxidants, and offer antidiabetic effects[23]. The content and composition of tea polysaccharides are influenced by factors like tea tree variety, leaf maturity, and processing technique. It has been shown that fresh leaves with higher maturity have a higher content of tea polysaccharides[24]. In addition, the hypoglycemic activity of polysaccharides have been linked to their molecular weights and structures, with higher molecular weight generally exhibiting weaker hypoglycemic effects[24].
Alkaloids
-
Tea plants contain primarily purine alkaloids, notably caffeine, theobromine, and theophylline, which not only contribute to tea's flavor but also possess significant physiological and biological functions[25,26]. Caffeine, the most abundant purine alkaloid in tea, typically constitutes 1.5%−5% of the dry weight of tea leaves, though it varies with the variety and the growing environment of tea plants[26]. Additionally, there is a considerable disparity in caffeine content between mature and young tea leaves, with new shoots being reported to accumulate significantly higher levels compared to mature leaves[27].
-
Extensive research has been conducted on the impact of tea on DM and its associated complications. In recent years, epidemiological investigations and clinical trials have demonstrated an inverse relationship between regular tea consumption and the occurrence of DM. Additionally, both in vitro and in vivo experimental studies have underscored the beneficial effects of tea in the prevention and management of DM.
Effects of tea against DM and its complications: epidemiological investigations
-
Given the global prevalence of tea consumption, numerous epidemiological studies have been conducted to explore the hypoglycemic properties of tea. For example, a European cohort study found that individuals who consumed a minimum of four cups of tea daily had a 20% lower risk of developing T2DM compared to nondrinkers (hazard ratio (HR) = 0.84, 95% CI (0.71, 1.00))[28]. Another study tracked 5,823 British subjects over an 11.7-year period and revealed a significant negative correlation (HR: 0.66; 95% CI: 0.61−1.22; p value < 0.05) between tea consumption and DM risk after adjustment for age, sex, ethnicity, and social status[29]. Similarly, a prospective cohort study involving 500,000 Chinese adults reported HRs of 0.90 (95% CIs: 0.83, 0.97) for all-cause mortality and 0.88 (95% CIs: 0.78, 1.00) for the risk of microvascular complications among daily tea drinkers compared to non-drinkers[7]. A 10-year follow-up study of 40,011 Dutch people found that tea consumption has an inverse association with T2DM, with an HR of 0.63 (95% CI: 0.47−0.86), and consumption of three or more cups of tea per day were 42% less likely to develop T2DM than nondrinkers[30]. In a retrospective cohort study, individuals in Japan who consumed more than six cups of green tea daily had a multivariable odds ratio (OR) for DM of 0.67 (95% CI, 0.47−0.94), whereas there was no significant association with the incidence of DM for those consuming black or oolong teas[31]. The effects of tea on DM were also investigated in several meta-analyses. For instance, a meta-analysis involving 608 patients with T2DM in China showed that tea consumption led to a reduction in fasting blood insulin levels by 1.30 U/L (95% CI: 0.36−2.24) over an intervention period lasting more than eight weeks[32]. Another meta-analysis comprising 27 studies with 2,194 participants found that green tea significantly lowered fasting glucose (1.44 mg/dL, 95% CI: −2.26 to −0.62 mg/dL; p value < 0.001)[33]. In addition, another meta-analysis showed that people who drank more than four cups of tea per day reduced the risk of T2DM by 20% (relative risk (RR), 0.82 (95% CI, 0.73−0.94)) than those who had never consumed tea[34]. Nonetheless, some epidemiological investigations have yielded contradictory results. A review of nine cohort studies involving a total of 324,141 participants and 11,400 incident DM cases, followed for 5 to 18 years, found no association between tea consumption and the incidence of T2DM (RR, 0.96; 95% CI: 0.92−1.01)[35]. In addition, the multivariable-adjusted HR for the development of DM was 1.64 (95% CI: 1.11−2.40) for those who consumed more than two cups of oolong tea every day than nondrinkers[36]. These inconsistent findings may be attributed to the differences in the sensitivities to tea in different populations, individual differences in subjects, the kinds of tea consumed, and the drinking manner of teas.
In conclusion, a variety of epidemiologic evidence supports the positive role of tea consumption in mitigating DM and its complications, although there are instances of conflicting findings. These discrepancies may be attributed to factors such as the tea dosage, categories, and frequencies of tea consumption, as well as the tested individual differences. In addition, there is still a paucity of studies on the association between tea consumption and DM complications, which needs to be further investigated.
Effects of tea against DM and its complications: clinical trials
-
Numerous clinical trials have delved into the potential of tea as a therapeutic agent for DM. For instance, a randomized, double-blind, placebo-controlled, and parallel trial revealed that a one-year intervention using flavan-3-ols (major compounds in tea) improved biomarkers of cardiovascular disease (CVD) risk by reducing insulin resistance and improving insulin sensitivity[37]. Another clinical trial observed a preventive effect of long-term green tea consumption on diabetic retinopathy, with regular green tea drinkers experiencing a roughly 50% reduction in the risk of diabetic retinopathy compared to nondrinkers (OR = 0.49, 95% CI (0.26–0.90))[38]. Multiple randomized controlled trials have indicated that the daily intake of polyphenols and green tea extract has the capacity to improve tolerance to oral glucose among individuals possessing a clean bill of health while simultaneously decreasing fasting plasma glucose and glycosylated hemoglobin levels[39,40]. Hence, it is plausible that green tea may serve a valuable purpose in augmenting glycemic control and reducing insulin resistance among individuals with T2DM. In addition, a clinical trial involving Taiwanese patients with diabetes who were taking hypoglycemic drugs revealed that oolong tea served as an effective adjunctive oral hypoglycemic agent for treating T2DM[8].
In short, clinical trials have shown that tea helps prevent and control DM and its complications primarily through the improvement of insulin resistance and reduction of postprandial glucose levels.
Effects of tea against DM and its complications: in vitro and in vivo experimental studies
-
The effects of tea on DM and its complications have been extensively studied in teas of different categories through in vitro and in vivo experimental studies in recent years.
Several experimental animal studies have provided evidence of the potential of green tea extract to enhance insulin sensitivity and reduce blood glucose levels in diabetic mice[41,42]. According to the leaf morphology and sensory evaluation, Lu'an guapian green tea (LGGT) can be categorized into five grades (ranging from low to high): summer grade (SuG), third grade (TG), second grade (SG), first grade (FG), second premium (SP), and first premium (FP)[43]. In addition, it was found that higher grades were associated with improved glucose tolerance and insulin sensitivity in mice fed with a high-fat diet[43]. Zhou et al. found that roasted yellow tea could reduce fasting blood glucose levels and improve insulin sensitivity by strongly inhibiting α-glucosidase activity[44]. Additionally, the administration of large-leaf yellow tea was observed to improve impaired insulin resistance and glucose tolerance in mice with diet-induced diabetes[45]. Black tea, known for its anti-inflammatory and antioxidant effects, proves beneficial for T2DM by inhibiting various radicals, leading to reduced blood glucose levels[46]. In addition, black tea extract significantly lowered glycosylated hemoglobin and fasting glucose levels in diabetic rats while increasing plasma insulin levels in a dose-dependent manner[47]. White tea showed antidiabetic activity through the reduction of insulin resistance, hyperlipidemia, and oxidative stress[6]. Furthermore, prediabetic rats that consumed white tea for two months showed higher insulin sensitivity and lower glucose intolerance than control rats[48]. In the realm of Pu-erh tea, extracts from ripened Pu-erh tea were found to improve gut microbiota composition, particularly enhancing the abundance and diversity of beneficial bacterial species like Alloprevotella, Prevotellaceae NK3B31 group, and Lactobacillus in rats with streptozotocin-induced diabetes[49]. Notably, these effects were more pronounced in rats treated with ripened Pu-erh tea than in those treated with raw Pu-erh tea[49]. Similarly, Fu brick tea extract was demonstrated to have positive effects on gut dysbiosis in T2DM, by reducing the Firmicutes/Bacteroidota (F/B) ratio and promoting the proliferation of beneficial genera like Parabacteroides, Bifidobacterium, and Roseburia[50]. It has been reported that Liubao brick tea has an excellent hypoglycemic effect and significantly ameliorates metabolic disorders caused by hyperglycemia, mainly by enhancing intestinal flora diversity, including increasing beneficial bacteria, decreasing harmful bacteria, and improving metabolic disorders and insulin resistance[51].
-
The mechanisms by which the bioactive components of tea (tea polyphenols, tea polysaccharides, and alkaloids) help prevent and treat DM and its complications mainly include improving insulin resistance, inhibiting carbohydrate digestion and absorption (inhibiting α-amylase and α-glucosidase activity), regulating gut microbiota, inflammatory cytokines, and gene and protein expressions in the insulin signaling pathway, as well as ameliorating DM complications (as shown in Fig. 2 & Table 2).
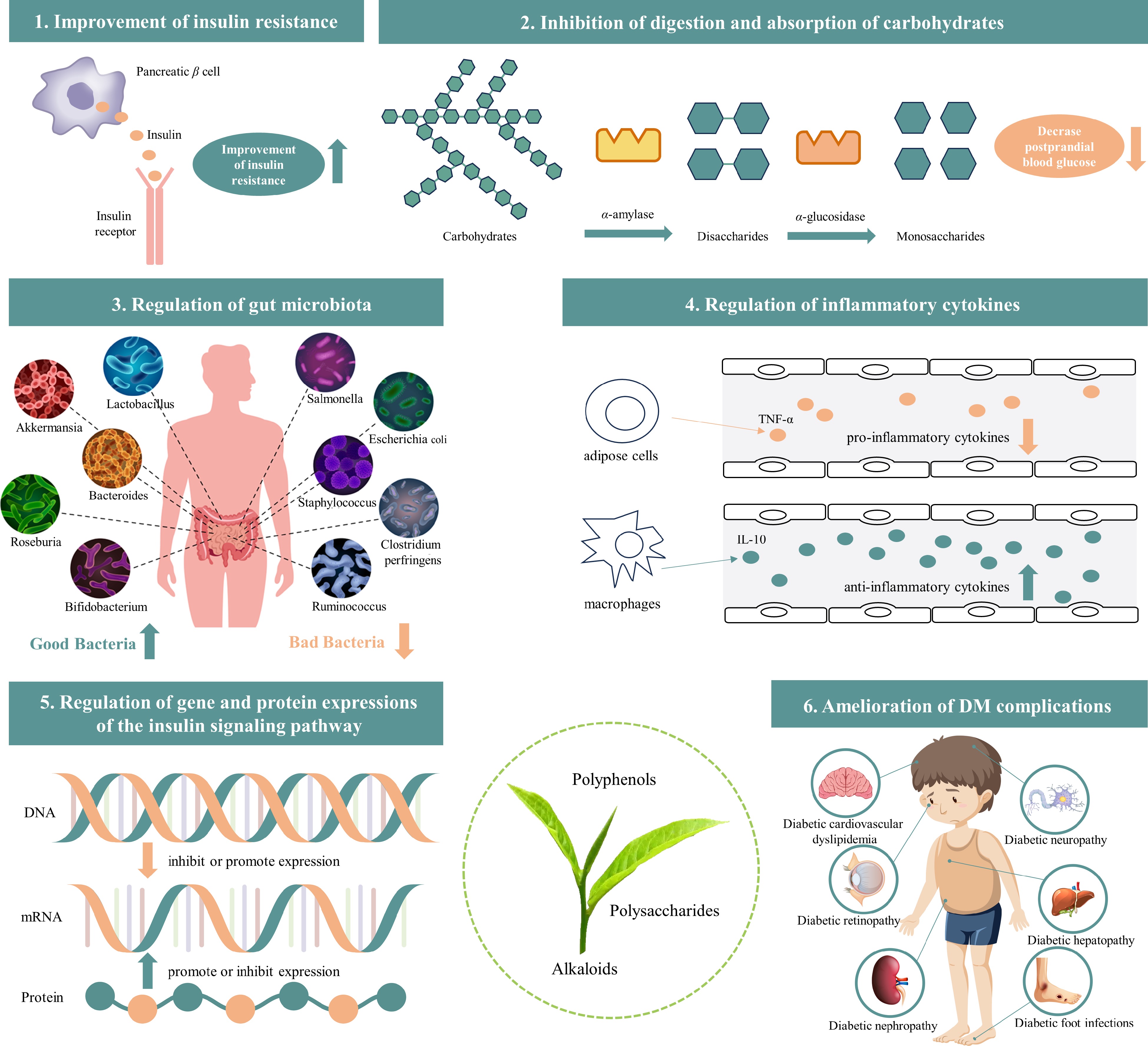
Figure 2.
The mechanisms of tea bioactive components (tea polyphenols, polysaccharides, and alkaloids) help prevent and treat DM and its complications (image source from Freepik.com).
Table 2. The effects and mechanisms of tea and its bioactive components on DM based on in vitro and in vivo studies
Tea/bioactive components Animal models Doses (day) Duration (weeks) Effects and mechanisms Ref. Yellow tea extract C57BL/6J mice 60 or 120 mg/kg 10 Improves impaired glucose tolerance, pyruvate tolerance, and insulin resistance. [45] Black tea extract Wistar rats 25, 50, or 100 mg/kg 30 d Decreases the level of glucose, glycated hemoglobin and increases the levels of insulin. [47] White tea extract Wistar rats / 8 Improves glucose tolerance and insulin sensitivity. [48] Pu-erh tea extract Wistar rats 120, 160, 600, or
800 mg/kg6 Increases the abundance of the beneficial bacteria. [49] Fu brick tea extract C57BL/6J mice 100, 200, or
400 mg/kg8 Ameliorates the T2DM-induced gut dysbiosis by decreasing the Firmicutes/Bacteroidota ratio at the phylum level. [50] Liubao tea extract C57BL/6J mice 834 or 1,667 mg/kg 10 Increases the diversity of intestinal flora. [51] Tea polyphenols Wistar rats 200 mg/kg 6 Improves insulin sensitivity and decreases the inflammatory factors. [55] Kaempferol Sprague–Dawley rats 50 or 150 mg/kg 10 Enhances insulin sensitivity. [56] Theaflavins Spontaneously diabetic torii rats 25 mg/kg 20 Improves impaired glucose tolerance. [57] Tea polysaccharides ICR mice 150, 200, or 300 mg/kg 4 Improves insulin resistance. [60] EGCG; Epiafzelechin-3-gallate; ECG / / / Exhibits inhibitory effects against α-glucosidase. [64] Oolong tea polyphenols; EGCG; EGCG3″Me / / / Exhibits inhibitory effects against α-amylase. [67] Quercetin-3-O-(6″-O-galloyl)-β-galactopyranoside; Quercetin-3-O-(3″-O-galloyl)-β-glucopyranoside / / / Exhibits inhibitory effects against α-glucosidase. [68] Tea polysaccharides ICR mice 1 or 5 mg/kg / Exhibits inhibitory effects against α-glucosidase. [69] Tea polysaccharides ICR mice 50 mg/kg / Exhibits inhibitory effects against α-glucosidase. [70] Tea polysaccharides / / / Exhibits inhibitory effects against α-amylase and α-glucosidase. [71] Tea polysaccharides / / / Exhibits inhibitory effects against α-glucosidase. [72] Tea polysaccharides C57BL/6 mice 200, 400, or
800 mg/kg8 Increases the phylogenetic diversity of HFD-induced microbiota. [76] EGCG Db/db mice 10, 50, or 100 mg/kg 8 Increases the abundance of beneficial bacteria. [78] Tea polysaccharides Wistar rats 100, 200, or
400 mg/kg/ Modulates gut microbiota diversity and increases the abundance of beneficial genera. [79] Quercetin Wistar rats 50 mg/kg 3 Exhibits anti-inflammatory activity. [82] Caffeine KK-Ay mice 250 mg/L 5 Reduces inflammatory cytokine expression (TNFα, IL-6, and MCP-1). [83] EGCG Db/db mice 2.5, 5.0, or 10.0 g/kg 7 Decreases the PEPCK mRNA expression in the adipose and liver tissues. [87] Tea polyphenols Wistar rats 200 mg/kg 6 Upregulates the insulin signaling protein levels. [55] Tea polysaccharides Kunming mice 200, 400, or
800 mg/kg4 Upregulates the expressions of the critical proteins in the PI3K/Akt signal pathway including GLUT4, p-Akt, and PI3K. [90] Tea polyphenols; Tea polysaccharides; Caffeine Sprague–Dawley rats 400 or 800 mg/kg 6 Reduces rat serum leptin levels, inhibits the absorption of fatty acids, and reduces the expression levels of the IL-6 and
TNF-α genes.[91] EC Sprague–Dawley rats 50 or 100 mg/kg 2 Improves advanced glycation end products-induced retinal vascular injury. [94] Improvement of insulin resistance
-
Insulin resistance, a fundamental characteristic of T2DM, arises when liver, adipose, and muscle cells improperly utilize the insulin produced by islet β-cells[13]. Impaired glucose homeostasis and insulin resistance lead to the development of cellular hyperglycemia and hyperinsulinemia[52].
Experimental evidence has indicated that green tea extract possesses the potential to ameliorate insulin resistance and enhance glycemic control[53]. For example, it improved insulin resistance in human HepG2 liver cancer cells by activating the 5'-adenosine monophosphate-activated protein kinase pathway[54]. And this activation mitigated the obstruction of insulin stress signaling pathways caused by the phosphorylation of insulin receptor substrate-1[54]. Furthermore, green tea polyphenols exhibited the capacity to enhance insulin sensitivity in insulin-resistant rats via upregulating insulin signaling protein levels[55]. A previous study showed that kaempferol ameliorates insulin resistance in T2DM rats by modulating hepatic IKK/NF-κB signaling[56]. Spontaneously diabetic Torii rats elevated fasting glucose levels to 139 ± 23 mg/dL at 28 weeks of intervention, and the ingestion of TF significantly reduced the fasting glucose level to 74 ± 11 mg/dL, which may be due to the induction of the increase in incretin secretion by TF[57]. Moreover, it has been demonstrated that TF-DG could effectively improve glucose uptake in insulin-resistant HepG2 cells and regulate glucose levels in diabetic zebrafish[58]. Additionally, it has been speculated that the richness of tea polysaccharides and polyphenols in yellow tea may contribute to the improvement of insulin resistance and the imbalance of glucolipid metabolism[59]. Among the polysaccharides from black (fully fermented), green (unfermented), oolong (semi-fermented), and dark (post-fermented) teas, black tea polysaccharides exhibited superior efficacy in controlling blood glucose levels and improving insulin resistance, indicating that the fermentation degree significantly increases the hypoglycemic effect of tea polysaccharides[60]. An in vitro experiment reported that pu-erh tea polysaccharides exhibit similar properties to glucose transporter type 4 and peroxisome proliferator-activated receptor γ by improving insulin resistance and reducing blood glucose levels, which could enhance adipocyte differentiation and glucose uptake[61].
Inhibition of digestion and absorption of carbohydrates (inhibit of α-amylase and α-glucosidase activity)
-
Carbohydrates constitute the primary exogenous sugars for the body, and starchy foods contribute significantly to overall energy intake. Upon ingestion, starch undergoes initial hydrolysis by salivary and α-amylase, resulting in the production of reducing sugars like maltose and maltotriose. Subsequently, α-glucosidase further hydrolyzes these intermediates to yield glucose[62]. Thus, the two enzymes mentioned above both play crucial roles in regulating starch digestion, and inhibiting their activity has been proposed as a strategy to impede starch digestion and attenuate the rapid increase in postprandial blood glucose levels[63].
Notably, the inhibitory impact on α-glucosidase was significantly augmented through the galloylation of polyphenols[64]. Nongalloylated polyphenols, such as EGC, epiafzelechin, and EC, showed a weaker inhibitory activity than their corresponding galloylated polyphenols EGCG, epiafzelechin-3-gallate, and ECG, respectively[64]. In addition, in the cases of tea polyphenols, typically comprising catechins and TFs, it has been reported that the presence of 3- and 3'-galloyl moiety on the C ring enhances the affinity of catechins and TFs for α-amylase, thereby heightening the inhibitory activities of both polyphenols against the enzyme[65]. Among four different tea extracts (black tea, green tea, oolong tea, and dark tea extracts), black tea extract exhibited the most pronounced hypoglycemic activity, presumably due to the rich content of TFs in black tea, which inhibited sucrase-isomaltase activity and thus delayed the hydrolysis of isomaltose, maltose, and sucrose, leading to lower postprandial blood glucose[66]. Another investigation revealed that oolong tea polyphenols, 3′′-methyl-epigallocatechin gallate (EGCG3″Me) and EGCG, exhibited inhibitory effects against α-amylase with IC50 values of 0.375, 0.572, and 0.350 mg/mL, respectively[67]. In the realm of quercetin glycosides, quercetin-3-O-(6"-O-galloyl)-β-galactoside (IC50 value: 1.35 ± 0.06 μM) and quercetin-3-O-(3"-O-galloyl)-β-galactoside (IC50 value: 0.97 ± 0.02 μM) emerged as the most potent α-glucosidase inhibitors among the tested compounds. Their inhibitory potency surpassed that of the positive control acarbose (IC50 value: 50.58 ± 0.25 μM) by approximately 37 and 52 times, respectively[68]. Pu-erh dark tea polysaccharide has been reported to lower blood glucose levels in starch-fed mice by inhibiting α-glucosidase activity[69]. A study by Xu et al.[70] showcased that polysaccharides isolated from pu-erh tea possess anti-α-glucosidase activities comparable to or even superior to the clinically used antidiabetic drug acarbose. Interestingly, the anti-α-glucosidase activities demonstrated an increase with the fermentation time of pu-erh tea, suggesting that longer fermentation may lead to the polymerization of proteins and polysaccharides, thereby altering their configuration and conformation and enhancing biological activity. Furthermore, it was reported that tea polysaccharides with smaller molecular weights exhibit better biological activities, such as lowering blood glucose and antioxidant activity, andα-amylase inhibition was significantly and positively correlated with arabinose or galactose content[71]. Moreover, a recent study highlighted the pu-erh tea polysaccharides, which was characterized by the highest total protein and phenolic contents, and displayed the strongest α-glucosidase inhibitory and antidiabetic activities compared to polysaccharides from the other five tea categories
Regulation of gut microbiota
-
Numerous studies have shown that excessive consumption of foods rich in sugar and fat may disturb the natural balance of gut flora and contribute to the onset of DM[24]. Bioactive compounds in tea could increase probiotic levels by modulating the gut flora, thereby improving gut health to alleviate DM.
Nie et al. observed a decreased ratio of the major phyla Firmicutes/Bacteroidetes and a significant reduction of Bifidobacteria in patients with DM[73]. Another study also confirmed the former finding that the genera Fusobacterium, Blautia, and Ruminococcus were positively associated with DM, while the genera Bifidobacterium, Akkermansia, Roseburia, Faecalibacterium, and Bacteroides were negatively associated with DM[74]. Flavonoids modulate the intestinal flora, increase the diversity and abundance of beneficial bacterial species, and improve intestinal barrier function, thereby reducing insulin resistance[75]. Black tea contains high levels of water-soluble dietary fiber, such as the oxidative polymerization products of tea polysaccharides and polyphenols (e.g., catechins and TFs). These components have the potential to improve lipid and glucose metabolism by regulating the intestinal flora[76]. Huang et al. found that bound polyphenols could modulate diabetic rats' imbalanced microbial community by significantly decreasing harmful bacteria, such as Proteobacteria[77]. A previous animal study demonstrated that the oral administration of EGCG at a dose of 100 mg/kg daily for eight weeks improved glucose homeostasis by increasing glucose tolerance in diabetic mice[78]. Additionally, this diet intervention led to an elevation in the population of bacteria from the Christensencelaceae family while concurrently decreasing the prevalence of Enterobacteriaceae in the intestine of diabetic mice[78]. In addition, polyphenols, such as EGCG, EGC, GC, and other catechins, demonstrated the capacity to hinder the proliferation of numerous pathogens, such as Staphylococcus, Salmonella, Clostridium perfringens, Escherichia coli, and certain gram-negative mimics of the genus Bacillus[73]. These actions have the potential to contribute to the enhancement of intestinal microecology, which may be beneficial for managing DM[73]. It has been shown that tea polysaccharides might exhibit hypoglycemic effects through the regulation of gut microbiota diversity and the augmentation of the relative prevalence of beneficial genera[79]. The antidiabetic effects of Liupao dark tea may be attributed to the increased amount of beneficial bacteria, such as Bacteroides, S24-7, Lactobacillus, Clostridiales, Prevotella, and Ruminococcaceae, in the gut microbiota, which might be modulated by the high contents of polyphenols[80]. These results suggest that the intake of bioactive components from teas may lead to a more balanced environment to promote gut microbiota diversity and maintain a healthy state.
Regulation of inflammatory cytokines
-
Inflammatory cytokines are linked to the development of DM, and inflammatory responses may lead to insulin resistance, which in turn contributes to the development of DM[81]. Medicine targeting inflammatory cytokines is considered as one of the strategies for preventing and managing DM.
Tumor necrosis factor α (TNF-α) is a proinflammatory cytokine generated by adipose cells. It can cause an increase in the release of fatty acids by adipocytes and resulted in increased levels of free fatty acids which deteriorate insulin signaling and decrease insulin secretion[81]. In contrast, IL-10 is an anti-inflammatory cytokine produced by type 2 macrophages and lymphocytes, which play key roles in suppressing pro-inflammatory cytokines production like TNF-α and IL-6[81]. Various studies have demonstrated that bioactive components in teas exert hypoglycemic effects by lowering the concentrations of pro-inflammatory cytokines and elevating the levels of anti-inflammatory cytokines. For example, the anti-inflammatory effect was observed in quercetin by increasing plasma adiponectin and reducing TNF-α levels in diabetic rats[82]. Moreover, tea polysaccharides may increase immunoreactivity by boosting immune cell activity via enhancing the levels of anti-inflammatory cytokines (e.g., IL-10, IgM, IgG, IL-4, IL-2, and IgA ), and decreasing the levels of pro-inflammatory cytokines like TNF-α and IL-6[22]. Additionally, the beneficial impact of caffeine in KK-Ay mice primarily resulted from its ability to diminish the expression of inflammatory cytokine expression (TNF-α, IL-6, and MCP-1) and ameliorate fatty liver conditions[83]. In addition, the anti-inflammatory effect of TFs should also be considered as they are the major phenolic compounds of black tea and have been reported to inhibit nitric oxide synthase by downregulating the activation of NF-κB in macrophages[84].
Regulation of gene and protein expressions of the insulin signaling pathway
-
The bioactive compounds present in tea contribute to the regulation of blood glucose levels by promoting/inhibiting the expressions of genes and proteins involved in glycometabolism and the insulin signaling pathway[85].
It has been reported that EGCG promoted the tyrosine phosphorylation expressions of the insulin receptor and insulin receptor substrate-1, and inhibited the expression of phosphoenolpyruvate carboxykinase gene for the treatment of DM[86]. In addition, it also lowered the mRNA expression of the phosphoenolpyruvate carboxykinase in H4IIE cells, as well as in the liver tissue and adipose of db/db mice[87]. In addition, it was reported that a supplement of tea polyphenols could improve insulin sensitivity by upregulating the insulin signaling protein levels in insulin-resistant rats[55]. Another study showed that the hypoglycemic mechanism of theasinensin A and B involved the facilitation of glucose transporter 4 translocation to the plasma membrane, thus enhancing glucose intake in rat skeletal muscle cells, which was mediated by the CaMKK/AMPK signaling pathway[88]. Following treatment with tea polysaccharides, there was a notable augmentation in insulin secretion under high glucose conditions (25 mM). In addition, this treatment resulted in the upregulation of gene transcriptions for GLUT2, PKA, INS-2, INS-1, GLP-1R, GCK, and PDX-1 at the mRNA level, alongside increased expression of PDX-1 at the protein level[89]. Furthermore, the antidiabetic effects of tea polysaccharides were counteracted by PKA and AC inhibitors but not by PLC inhibitors, suggesting that tea polysaccharides enhanced antidiabetic activity through the cAMP-PKA signaling pathway[89]. Li et al. evaluated the hypoglycemic effect and possible mechanism of tea polysaccharides in T2DM mice model[90]. It was found that tea polysaccharides enhanced the expression of pivotal proteins within the PI3K/Akt signaling pathway, encompassing GLUT4, p-Akt, and PI3K, which indicated the participation of the PI3K/Akt signal pathway in the hypoglycemic mechanism of tea polysaccharides[90]. Moreover, polyphenols, polysaccharides, and caffeine exhibited the capacity to enhance blood lipid and antioxidant levels, effectively reducing rat serum leptin levels and the expression levels of the IL-6 and TNF-α genes[91].
Amelioration of DM complications
-
DM is often associated with many complications, including diabetic cardiovascular dyslipidemia, diabetic retinopathy, diabetic foot infections, diabetic nephropathy, diabetic neuropathy, and diabetic hepatopathy. Some reports suggesting that these complications can be improved by the bioactive compounds in tea.
TFs and catechins effectively mitigated the disruption of insulin signaling caused by high glucose levels[92]. In addition, they also curbed lipid accumulation, suppressed fatty acid synthesis, and promoted fatty acid oxidation through the activation of the LKB1-AMPK pathway[92]. The lack of insulin deficiency and insulin resistance can trigger exaggerated vascular constriction, leading to an increased risk of diabetic cardiovascular dyslipidemia[93]. Another study also confirmed the above finding that black tea polyphenols enhance vasoconstriction by modulating the PI3K-Akt pathway and endothelial nitric oxide synthase phosphorylation[93]. EC destroyed glycated human serum albumin in a dose-dependent manner and decreased the accumulation of advanced glycosylation end products in the retina, thereby having beneficial effects on diabetic retinopathy[94]. It has been shown that patients with diabetes treated with EGCG or green tea polyphenols can sustain normal levels of apoptosis in podocytes, but the proportion of apoptotic podocytes were significantly increased in untreated diabetes patients[95]. Additionally, the potential effect of old tree white tea on diabetic nephropathy is attributed to its high contents of polyphenols (particularly EGCG) and polysaccharides[96].
This agrees with the findings presented by Xu et al.[97] and Yi et al.[98], which indicated that the hypoglycemic and antioxidant properties of tea polysaccharides and tea polyphenols have the potential to significantly ameliorate and prevent diabetic kidney injury. An in vivo investigation revealed that tea polyphenols could potentially inhibit autonomic dysfunction by preventing alterations in arterial pressure variability[99]. Additionally, another study proposed that tea polyphenols could exert significant effects on diabetic liver injury through their antioxidant activity[100].
-
DM and its associated complications have emerged as a significant public health issue. Current clinical medicines usually produce some toxic side effects; thus, developing new, safe, and effective hypoglycemic drugs from natural products is necessary. Tea exhibits antidiabetic activity due to its richness in various active components, such as tea polyphenols, tea polysaccharides, and alkaloids. Epidemiological and clinical investigations have demonstrated an inverse relationship between regular tea consumption and the incidence of DM. Furthermore, in vitro and in vivo experiments have showcased the beneficial effects of tea in preventing and managing DM and its associated complications. The bioactive components in teas protect against DM and diabetic complications via several possible mechanisms, including the improvement of insulin resistance, inhibition of digestion and absorption of carbohydrates (inhibit α-amylase and α-glucosidase activity), regulations of gut microbiota, inflammatory cytokines, and gene and protein expressions of the insulin signaling pathway, as well as amelioration of DM complications.
The potential antidiabetic properties of tea are intricately linked to its bioactive compounds. However, it's important to note that the chemical composition of tea can vary significantly based on factors such as the tea plant cultivar, degree of fermentation during processing, and tea preparation methods (including variables like the quantity of tea used, brewing time, and water volume). Consequently, this variation can lead to inconsistent antidiabetic effects observed in studies using tea from different sources. Hence, it is crucial to isolate and purify individual bioactive compounds to evaluate their specific antidiabetic effects, in addition, further structural modifications (e.g., hydroxy methylation, sulfation, acetylation, etc.) of compounds may improve its hypoglycemic effects. Such an approach could help elucidate the primary antidiabetic components present in tea and have practical implications for optimizing tea processing methods. However, their clinical applications encounter impediments similar to those of other natural products, such as limited solubility and oral bioavailability, thus hindering the therapeutic delivery of tea and its bioactive components, notably tea polyphenols. Furthermore, variations in the bioavailability of tea constituents due to diverse physiological conditions among individuals and populations warrant consideration. Consequently, a critical focus of future research lies in enhancing the bioavailability of tea. In recent years, nanotechnology has witnessed significant advancements, offering a promising avenue for addressing these issues. Therefore, developing novel and sustainable techniques such as nanotechnologies, including metal-based, carbohydrate-based, lipid-based, protein-based, and polymer-based nanoparticles, to enhance the therapeutic delivery of these invaluable natural compounds is a crucial necessity.
-
The authors confirm contribution to the paper as follows: Study conception and design: Gao J, Lin Z, Dai W; data collection: Gao J, Chen D, Lin ZY, Peng J, Yu S, Zhou C, Jiang H, Sun R; analysis and interpretation of results: Gao J, Chen D, Dai W; draft manuscript preparation: Gao J, Dai W. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
This work was supported by the Zhejiang Province Natural Science Foundation (LR23C160002), the Science and Technology Innovation Project of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2023-TRICAAS), and the National Natural Science Foundation (32172630 and 31972467).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Gao J, Chen D, Lin Z, Peng J, Yu S, et al. 2023. Research progress on the antidiabetic activities of tea and its bioactive components. Beverage Plant Research 3:32 doi: 10.48130/BPR-2023-0032
Research progress on the antidiabetic activities of tea and its bioactive components
- Received: 19 July 2023
- Revised: 28 September 2023
- Accepted: 13 October 2023
- Published online: 04 December 2023
Abstract: Diabetes mellitus (DM) is a pressing global public health issue with a high incidence of morbidity and mortality due to its complications. Although there are many medicines available for the treatment of DM, long-term use causes various adverse effects, such as diarrhea, vomiting, and nausea. Tea, owing to its richness of diverse bioactive components including tea polyphenols, tea polysaccharides, and alkaloids, has displayed promising antidiabetic properties. Screening antidiabetic bioactive compounds derived from teas is receiving increasing attention. Epidemiological and clinical investigations have demonstrated an inverse relationship between tea consumption and the incidence of DM. Both in vitro and in vivo experiments have substantiated the hypoglycemic effects of tea and its bioactive components through several possible mechanisms, including improvement of insulin resistance, inhibition of carbohydrates digestion and absorption (inhibit α-amylase and α-glucosidase activity), regulations of gut microbiota, inflammatory cytokines, and gene and protein expressions in the insulin signaling pathway, as well as amelioration of DM complications. This comprehensive review provides an up-to-date overview of the hypoglycemic properties associated with tea and its bioactive components. It also delves into their potential mechanisms, offering a theoretical foundation for further research into tea's antidiabetic properties and for the development of innovative antidiabetic functional products.
-
Key words:
- Tea /
- Bioactive components /
- Diabetes mellitus /
- Mechanisms