-
Huangqin tea (HQT, Fig. 1), also called Huangjin tea in Chinese, has a long history of consumption in China[1]. It is a healthy herbal tea crafted from the aerial parts of Scutellaria baicalensis Georgi, S. scordifolia Fisch, S. amoena C. H. Wrigh, and S. viscidula Bung[2−4]. Unlike traditional tea made from Camellia sinensis (L.) O. Kuntze, HQT lacks stimulating components and is renowned for clearing heat and dry dampness, eliminating toxins, promoting digestion, and soothing fire[1,5]. It can be enjoyed both as a hot beverage and as a cold drink, and modern research suggests that HQT exhibits several pharmacological properties, such as anti-inflammatory, chemopreventive effects of colorectal cancer[6], anti-aging[7], cardiovascular protection, hypoglycemic effect, hypolipidemic effect, anti-tumor, anti-bacterial, anti-influenza virus, and enhance human resistance[8−12]. The predominant compounds found in HQT are flavonoids and essential oils and its potential health benefits are likely attributed to the presence of distinctive flavonoids, including isocarthamidin-7-O-β-D-glucuronide, carthamidin-7-O-β-D-glucuronide, apigenin-7-O-β-D-glucopyranside, chrysin-7-O-β-D-glucuronide, scutellarin, baicalin, wogonoside, and chrysin[13−18].
HQT is made using simple ingredients and has a straightforward preparation method. Traditionally, the above-ground portions of Scutellaria species have been utilized for crafting HQT, with the differentiation between stems and leaves remaining unessential. During the hot summer season (July to August), the aerial parts (stem, leaves, flowers) of these species of Scutellaria from a single source are collected and cut into small sections, which are then directly dried for later use. Alternatively, freshly harvested branch and leaf sections may undergo a transformative process through multiple rounds of steaming and subsequent drying within a steamer. Once suitably conditioned, they find their place within sealed containers, poised for extended storage periods. The brewing ritual commences by incorporating 7−8 g of this tea into 2 L of water, with subsequent replenishments of water at intervals 2−3 times. As the brewing ritual concludes, HQT graces the senses with a resplendent golden infusion—earning it another name, 'Huangjin tea'.
HQT boasts a range of health advantages and is frequently gathered from the upper sections of Scutellaria species plants cultivated in the vicinity, intended for tea consumption. In recent years, large planting bases have been established in several regions, such as Beijing, Inner Mongolia Wuchuan, Yakeshi, Hebei Chengde, Shanxi Province, and Shandong Province, to comprehensively develop S. baicalensis resources. HQT has gained more attention and is now produced by specialized operating companies in various grades, such as bulk tea, bagged tea, and unique tea culture[12].
To provide up-to-date information on the chemical composition, bioactivity, and safety aspects of the Scutellaria genus from HQT, this review paper has collected related literature from various databases, such as PubMed, Web of Science, Sci Finder, Scopus, Baidu Scholar, China National Knowledge Internet (CNKI), Wanfang and Weipu Data. This paper aims to draw attention to the need for further research and application of HQT in preventing and managing certain chronic diseases.
-
HQT can be derived from several Scutellaria species. S. baicalensis is the most extensively cultivated, with a significant biomass in its aerial parts. Consequently, the primary source of HQT is the aerial part of S. baicalensis, and research focused on these aerial parts is also the most extensive.
The aerial part and the root of S. baicalensis share similarities in their primary constituents, notably the significant presence of flavonoids, which are regarded as the main active components. However, there are specific chemical differences between the above-ground and underground parts of S. baicalensis. Our preliminary experiments have revealed the disparities in chemical composition between these two parts[18]. Notably, the root of S. baicalensis finds its primary use in medicinal applications, characterized by notably higher expression levels of specific 4′-deoxyflavones compared to the aerial organs. In contrast, the above-ground parts of the plant are employed to prepare tea. This differentiation in utilizing these plant components reflects the accumulated wisdom of generations cultivated through extensive periods of tasting and experience.
This section provided an in-depth review of the chemical composition of the plant from which HQT is derived. Additionally, a specific emphasis on reviewing the aerial parts of S. baicalensis, which serve as the primary source of HQT has been placed.
Flavonoids
-
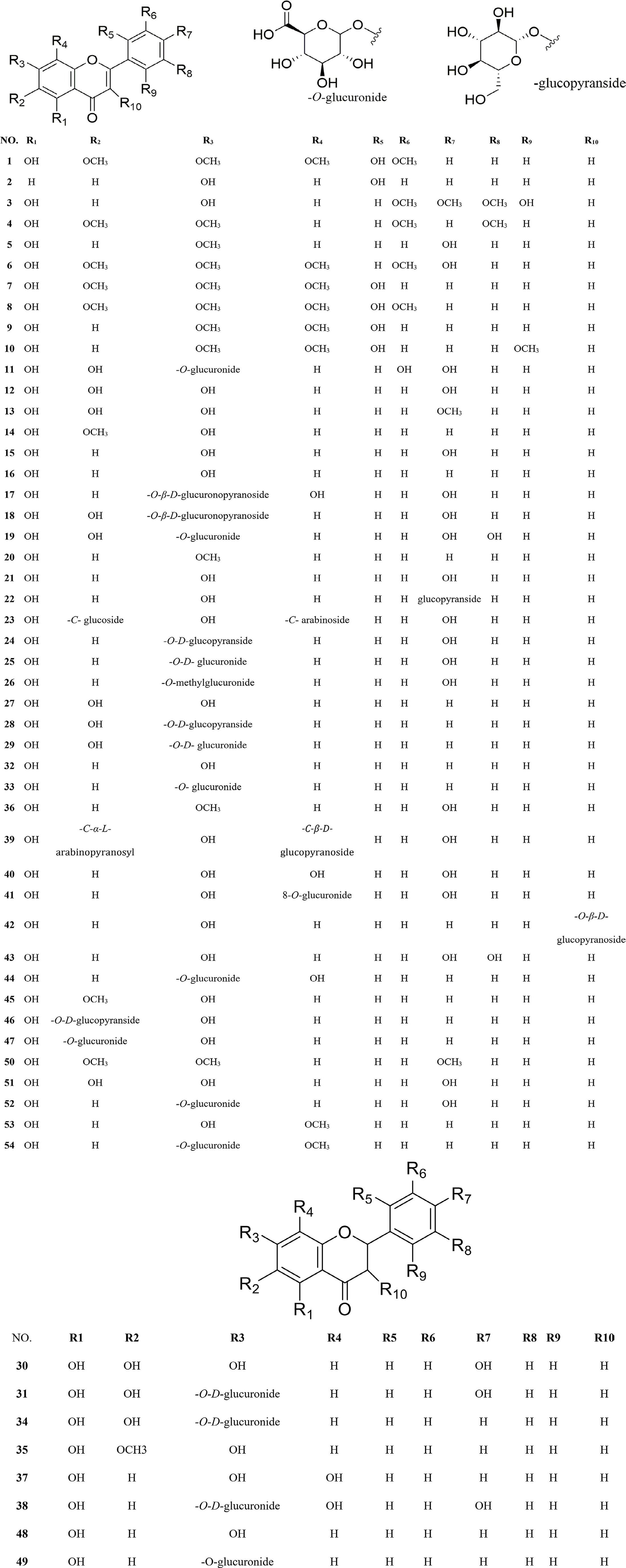
In the 1970s, researchers began to study the chemical composition of the aerial parts of S. baicalensis. The flavonoids are the most abundant chemical components in the aerial parts (flowers, stems, and leaves), and about 54 flavonoid compounds were identified (Table 1, Fig. 2). Most of them are flavonoids, dihydro flavonoids, and glycosides. The main glycoside-forming sugars are arabinose, glucose, and glucuronide, with the most abundant glucuronide glycosides. The optimization of extraction processes can yield a remarkable total flavonoid content of up to 5% in the stems and leaves of S. baicalensis[19].
Table 1. Flavonoids of HQT.
Number Name Formula Species Ref. 1 2',5-Dihydroxy-3',6,7,8-tetramethoxyflavone C19H18O8 S. baicalensis [17] 2 2',6-Dihydroxyflavone C15H10O4 S. baicalensis [16] 3 3',4',5,5',7-Pentamethoxyflavone C20H20O7 S. baicalensis [16] 4 4',5-Dihydroxy-3',5',6,7-tetramethoxyflavone C19H18O8 S. baicalensis [22,27] 5 4',5-Dihydroxy-7-methoxyflavanone C16H14O5 S. baicalensis [16] 6 5,4′-Dihydroxy-6,7,8,3′-tetramethoxyflavone C19H18O8 S. baicalensis [17] 7 5,2′-Dihydroxy-6,7,8-trimethoxyflavone C18H16O7 S. baicalensis [17] 8 5,2′-Dihydroxy-6,7,8,3′-tetramethoxyflavone C19H18O8 S. baicalensis [17] 9 5,2′-Dihydroxy-7,8-dimethoxyflavone C17H14O6 S. baicalensis [17] 10 5,2′-Dihydroxy-7,8,6′-trimethoxyflavone C18H16O7 S. baicalensis [17] 11 5,6,7,3',4'-Pentahydroxyflavone-7-O-glucuronide C21H20O13 S. baicalensis [13] 12 5,6,7,4'-Tetrahydroxydihydroflavone C15H12O6 S. baicalensis [17] 13 5,6,7-Trihydroxy-4'-methoxyflavone C16H12O6 S. baicalensis [15] 14 5,7-Dihydroxy-6-methoxyflavanon C16H14O5 S. baicalensis [17] 15 5,7,4′-Trihydroxy-6-methoxyflavanone C16H14O6 S. baicalensis [22] 16 5,7,4'-Trihydroxyflavanone C15H12O6 S. baicalensis [13] 17 (2S)-5,7,8,4'-Tetrahydroxyflavanone 7-O-β-D-glucuronopyranoside C21H20O12 S. baicalensis [21] 18 (2S)-5,6,7,4'-Tetrahydroxyflavanone 7-O-β-D-glucuronopyranoside C21H20O12 S. baicalensis [21] 19 6-Hydroxyluteolin-7-O-glucuronide C21H18O13 S. baicalensis [13] 20 7-Methoxychrysin C16H14O4 S. baicalensis [16] 21 Apigenin C15H10O5 S. baicalensis, S. amoena,
S. scordifolia, S. viscidula[17,21−25] 22 Apigenin-4'-glucopyranside C21H20O10 S. baicalensis [17] 23 Apigenin-6-C-glucoside-8-C-arabinoside C26H28O14 S. baicalensis [13] 24 Apigenin-7-O-β-D-glucopyranside C21H20O10 S. baicalensis [17] 25 Apigenin-7-O-β-D-glucuronide C21H18O11 S. baicalensis, S. amoena,
S. scordifolia[13,23,24] 26 Apigenin-7-O-methylglucuronide C22H20O11 S. baicalensis [28] 27 Baicalein C15H10O5 S. baicalensis, S. amoena,
S. scordifolia, S. viscidula[22−25] 28 Baicalein-7-O-D-glucopyranside C21H20O10 S. baicalensis [22] 29 Baicalin C21H18O11 S. baicalensis, S. amoena,
S. scordifolia, S. viscidula[16,23−25] 30 Carthamidin C15H12O6 S. baicalensis [13,20] 31 Carthamidin-7-O-β-D-glucuronide C21H20O12 S. baicalensis [22] 32 Chrysin C15H10O4 S. baicalensis, S. amoena,
S. scordifolia[13,21−24] 33 Chrysin-7-O-β-D-glucuronide C21H20O9 S. baicalensis, S. amoena [23,29] 34 Dihydrobaicalin C21H20O11 S. baicalensis [22,28] 35 Dihydrooroxylin A C21H20O11 S. baicalensis [13] 36 Genkwanin C16H12O5 S. baicalensis [16] 37 Isocarthamidin C15H10O6 S. baicalensis [20,28] 38 Isocarthamidin-7-O-β-D-glucuronide C21H20O12 S. baicalensis [28] 39 Isoschaftside C26H28O14 S. baicalensis [13,16] 40 Isoscutellarein C15H10O6 S. baicalensis [21,30] 41 Isoscutellarein 8-O-β-D-glucuronide C21H18O12 S. baicalensis [21] 42 Kaempferol 3-O-β-D-glucopyranoside C21H20O11 S. baicalensis [13,28] 43 Luteolin C15H10O6 S. baicalensis [22,30] 44 Norwogonin-7-O-glucuronide C21H18O12 S. baicalensis, S. amoena [13,17,23] 45 Oroxylin A C16H12O5 S. baicalensis, S. amoena [17,23] 46 Oroxylin A-7-O-D-glucopyranside C22H22O10 S. baicalensis [22,28] 47 Oroxylin A-7-O-β-D-glucuronide C22H20O11 S. baicalensis, S. amoena [13,23] 48 Pinocembrin C16H12O5 S. baicalensis [13] 49 Pinocembrin-7-O-glucuronide C21H20O11 S. baicalensis [13,16,22] 50 Salvigenin C18H16O6 S. baicalensis [21] 51 Scutellarein C15H10O6 S. baicalensis [28] 52 Scutellarin C21H18O12 S. baicalensis, S. amoena,
S. scordifolia, S. viscidula[23−25,29] 53 Wogonin C16H12O5 S. baicalensis, S. amoena,
S. scordifolia, S. viscidula[21−25] 54 Wogonoside C22H20O11 S. baicalensis, S. viscidula [13,25] In 1976, Takido et al.[20] isolated two flavanone derivatives, carthamidin and isocarthamidin, for the first time as natural products from the leaves of S. baicalensis. Later, Yukinori et al.[21] identified two new flavanones, (2S)-5,7,8,4'-tetrahydroxyflavanone 7-O-β-D-glucuronopyranoside and (2S)-5,6,7,4'-tetrahydroxyflavanone 7-O-β-D-glucuronopyranoside), in the leaves of S. baicalensis. Eight compounds of chrysin, wogonin, apigenin, salvigenin, scutellarein, isoscutellarein, apigenin 7-O-glucuronide, and isoscutellarein 8-O-glucuronide were also isolated. Wang et al.[15] used column chromatography to isolate seven flavonoids (wogonin, chrysin, 5,6,7-trihydroxy-4'-methoxyflavone, carthamindin, isocarthamidin, scutellarein, and chrysin 7-O-β-D-glucuronide) from a water extract of the leaves of S. baicalensis. Liu et al.[13] identified 21 flavonoids in the stems and leaves of S. baicalensis by HPLC-UV/MS and NMR, and found one flavonone (5,6,7,3',4'-Pentahydroxyflavone-7-O-glucuronide) was a new compound. Zhao[17] firstly isolated 5,6,7,4′-tetrahydroxyflavanone 7,5,7-dihydroxy-6-methoxyflavanone, oroxylin A, 5,4′-dihydroxy-6,7,8,3′-tetramethoxyflavone, 5,2′-dihydroxy-6,7,8,3′-tetramethoxyflavone, 5,2′-dihydroxy-7,8,6′-trimethoxyflavone, 5,2′-dihydroxy-7,8-dimethoxyflavone, 5,2′-dihydroxy-6,7,8-trimethoxyflavone, apigenin 4'-β-D-glucopyranoside, and apigenin-7-β-D-glucopyranoside from the aerial parts of S. baicalensis. Ma[22] firstly isolated 5,7,4'-trihydroxy-6-methoxyflavone, 5,4'-dihydroxy-6,7,3',5'-tetramethoxyflavone, from stems and leaves of S. baicalensis. Wang et al.[16] isolated 5,4'-dihydroxy-7-methoxyflavanone, genkwanin, 7-methoxychrysin, 3',4',5,5',7-pentamethoxyflavone from 60% ethanol extracts for stems and leaves of S. baicalensis for the first time. Also, the compounds of carthamidin-7-O-β-D-glucuronide, oroxylin A-7-O-β-D--glucuronide, and chrysin were isolated from this plant for the first time.
The concentration of these chemical components in HQT varies depending on the plant part utilized. Employing the HPLC-DAD method, Shen et al.[18] established that the aerial parts (stems, leaves, and flowers) of S. baicalensis are rich in flavonoids, resembling the roots in composition but exhibiting significant disparities in content. The contents of isocarthamidin-7-O-β-D-glucuronide (106.66 ± 22.68 mg/g), carthamidin-7-O-β-D-glucuronide (19.82 ± 11.17 mg/g), and isoscutellarein-8-O-β-D-glucuronide (3.10 ±1.73 mg/g) were the highest in leaves. The content of apigenin-7-O-β-D-glucopyranoside (18.1 ± 4.85 mg/g) and chrysin-7-O-β-D-glucuronide (9.82 ± 5.51 mg/g) were the highest in flowers. HQT has a high content proportion of flavone glycosides, which is closely related to the activity of HQT. The concentrations of the nine main flavonoids in HQT infusions were measured using HPLC. The content of isocarthamidin-7-O-β-D-glucuronide (52.19 ± 29.81 mg/g) was the highest; carthamidin-7-O-β-D-glucuronide (31.48 ± 6.82 mg/g), chrysin-7-O-β-D-glucuronide (10.65 ± 0.40 mg/g) and apigenin-7-O-β-D-glucopyranside (5.39 ± 0.92 mg/g) were found at moderate levels in HQT samples. As for flavone aglycones, scutellarin (12.77 ± 1.14 mg/g), baicalin (1.88 ± 0.48 mg/g), isoscutellarein-8-O-β-D-glucuronide (2.84 ± 0.60 mg/g), wogonoside (0.23 ± 0.02 mg/g) and chrysin (0.03 ± 0.01 mg/g) has lower content in HQT[6].
Although there are few studies on the chemical constituents of the aerial parts of S. amoena, S. scordifolia, and S. viscidula it has been shown that the compounds of the aerial parts are similar to S. baicalensis. The aerial parts of S. amoena contain the compounds of baicalein, baicalin, oroxylin A, oroxylin A-7-O-β-D-glucuronide, wogonin, chrysin, chrysin-7-O-β-D-glucuronide, norwogonin, 5,7-dihydroxy-6,8-dimethoxyflavone, scutellarin[23]. Zhang et al.[24] identified compounds of chrysin, wogonin, baicalein, apigenin, apigenin-7-O-β-D-glucoside, baicalin, and scutellarin in whole plants of S. scordifolia. The stems and leaves of S. viscidula all contain compounds of wogonoside, apigenin, baicalein, wogonin, baicalin, and scutellarin. The contents of baicalein, wogonoside, wogonin, and apigenin in the stem of S. viscidula were higher than those in the stem of S. baicalensis. In the leaves of the two species, the content of scutellarin was higher, while the content of other compounds was lower[25]. The content of scutellarin in S. viscidula was stem (2.30%) > leaf (1.78%) > flowers (0.38%)[26].
Essential oils
-
The aerial parts of S. baicalensis are rich in essential oils, and the taste of HQT is closely related to this. The flowers of S. baicalensis are thought to have a Concord grape aroma, while HQT has a bitter flavor with distinctive herbal notes. Extensive analysis has identified 145 components in the essential oil obtained from the aerial parts of S. baicalensis. These components span various chemical classes, such as alkanes, carboxylic acids, fatty acids, monoterpenes/oxygenated monoterpenes, sesquiterpenes triterpenoids and Vitamins (Supplemental Table S1), which have demonstrated their efficacy in combatting bacteria, reducing inflammation and inhibiting tumor growth[27−31]. Among these, major constituents include germacrene D (5.4%−39.3%), β-caryophyllene (29.0%), caryophyllene (18.9%), eugenol (18.4%), caryophyllene (15.2%), caryophyllene oxide (13.9%), (E)-β-caryophyllene (11.6%), 5-en-3-stigmasterol (11.3%), carvacrol (9.3%), thymol (7.5%), vitamin E (7.4%), neophytadiene (7.3%), γ-elemene (6.2%), 1-octen-3-ol (6.1%), allyl alcohol (5.5%), bicyclogermacrene (4.8%), myristicin (4.7%), acetophenone (4.6%), α-amyrin (4.6%), β-amyrin (4.4%), germacrene d-4-ol (4.3%), spathulenol (4.2%), β-pinene (4.1%), α-humulen (4.0%), 1-vinyl-1-methyl-2-(1-methylvinyl)-4-(1-methylethylidene)-cyclohexane (4.0%) are found in the aerial parts of S. baicalensis from different places[27−31].
Takeoka et al.[27] identified 64 components in volatile components of S. baicalensis flowers by solid-phase microextraction and analyzed them by GC and GC-MS. These flowers were collected at San Francisco State University (USA). Among the flower volatiles, the content of β-caryophyllene, germacrene D, δ-cadinene, γ-muurolene, and γ-cadinene were more than 3%. The essential oil obtained from the stem of S. baicalensis is mainly composed of diphenylamine, 2,2-methylenebis (6-tert-butyl-4-methylphenol), bornyl acetate, β-caryophyllene, germacrene D and 1-octen-3-ol.[32]. Gong et al.[28] analyzed and identified the specific chemical constituents of the aerial parts of S. baicalensis by using GC-MS technology and identified 37 compounds in total, such as allyl alcohol, acetophenone, caryophyllene, α-humulene, germacrene D, and γ-elemene. The plant material was collected in the Qinling Mountains in China. Lu et al.[29] found a big difference in essential oil components between the aerial and root of S. baicalensis from Kunming Botanical Garden, Yunnan Province (China). The aerial part of S. baicalensis mainly contained enols and sterols such as neophytadiene and vitamin E. However, it has the same compounds as the roots, such as nerolidol, hexadecanoic acid, 1,2-benzenedicarboxylic acid, squalene, stigmast-4-en-3-one, and partial alkanes. Recently, Wang et al.[31] found the essential oil level of the aerial parts of S. baicalensis was 0.09% (v/w, based on fresh weight) while its density was 0.93 g/mL, and obtained 31 components accounting for 97.64% of the crude essential oil, including sesquiterpenoid, monoterpenoids, phenylpropanoids, and others. It is also reported that the major components of the essential oil from the aerial parts of S. baicalensis were myristicin, eugenol, caryophyllene, caryophyllene oxide, germacrene D, spathulenol, and β-pinene, with eugenol as the most abundant. The sample of the aerial parts were harvested from Tangshan City (China). The composition of S. baicalensis essential oils varies according to the plant part used, geographical location, and growing conditions.
Others
-
Zgórka & Hajnos[33] identified the phenolic acid compounds of aerial parts of S. baicalensis by solid-phase extraction and high-speed countercurrent chromatography: p-coumaric acid, ferulic acid, p-hydroxybenzoic acid, and caffeic acid. Chirikova & Olennikov[34] found that the aerial part of S. baicalensis contains 11 kinds of saturated fatty acids and nine kinds of unsaturated fatty acids, among which the palmitic acid content is the highest. Chlorogenic acid, fernlic acid, protocatechuic acid, vanillic acid, rosmarinic acid, caffeic acid, p-hydroxybenzoic acid, and p-coumaric acid were also detected.
Zhao [17] isolated four sterol compounds: β-sitosterol-3-O-β-D-glucoside, α-apinasterol, β-sitosterol, and four ester compounds: methoxyphaeophorbide, p-hydroxybenethyl ethanol hexadecanoic methyl ester, ethoxyphaeophorbide, and n-octadecanol, lutein from the aerial parts of S. baicalensis.
It is reported that flavonoids and diterpenes are the two main groups of active constituents in the genus Scutellaria. However, only one diterpene (scutebaicalin) was identified in the stems and leaves of S. baicalensis[35].
By atomic absorption spectrophotometry, Yuan et al.[36] determined the contents of 11 metal elements in different parts of S. baicalensis. It was found that the leaves and stems of S. baicalensis were rich in Mg, K, Cr, Ni, Co, Fe, Mn, and Pb. Meanwhile, Yan et al.[37] developed an inductively coupled plasma mass spectrometry method and determined 23 kinds of inorganic elements in the stems and leaves of S. baicalensis from eight regions. Although there were no differences in the types of inorganic elements in the stems and leaves of S. baicalensis from the different areas, the content of these elements varied significantly. Among these elements, Fe, Zn, Cu, Mn, Cr, Co, Ni, Sr, B, and Ni were essential human body elements. The content of Al (516.83 μg/g) and Fe (700.62 μg/g) was the highest, while the content of B (31.54 μg/g), Ti (23.10 μg/g), Mn (65.64 μg/g), Sr (62.27 μg/g), and Ba (89.68 μg/g) was relatively high.
Olennikov et al.[38] studied the water-soluble polysaccharides from the aerial parts of S. baicalensis from Russia and found that the polysaccharides from S. baicalensis gradually accumulated before flowering and progressively decreased after flowering.
Yan et al.[39] found that the stems and leaves of S. baicalensis were rich in amino acids, and there was no difference in the kinds of amino acids among different producing areas, but there was a significant difference in the contents of amino acids. The content of proline, threonine, glutamic acid, lysine, glutamine, and arginine was higher, and the content of methionine, hydroxyproline, and citrulline was low.
-
Several studies have focused on the functional properties of HQT, with increasing attention given to the aerial parts of S. baicalensis as the main raw material for HQT production. S. baicalensis stems and leaves flavonoids (SSF) are considered the functional components of HQT. Modern pharmacology has shown that the flavonoids extracted from the stem and leaf of S. baicalensis have been found to possess anti-inflammatory, anti-bacterial, antiviral, antipyretic and analgesic, anti-tumor, hepatoprotective, antioxidant, hypoglycemic, hypolipidemic, detoxification, myocardial ischemia protection, brain injury protection, and immunomodulatory effects. However, few studies have been conducted on the individual flavonoid compounds in the total flavonoid extract from S. baicalensis. Therefore, this paper aims to summarize and supplement the current research on the functional properties of HQT and its primary raw material (S. baicalensis) extract.
Anti-inflammatory activity
-
Injury and infection could lead to inflammation, which plays a key role in the accelerated pathogenesis of immune-mediated disease[40]. Tong et al.[41], Zhou et al.[42], and Zhao et al.[43] found that S. baicalensis stem-leaf total flavonoid (SSTF) could inhibit acute exudative inflammation caused by xylene, glacial acetic acid, and egg white and also have a significant inhibitory effect on chronic inflammatory of granulation tissue hyperplasia. Wang et al.[44] observed the effect of SSTF on the aerocyst synovitis of the rat model and found that it could reduce capillary permeability, reduce the aggregation of neutrophils and basophils in tissues, reduce histamine, bradykinin, and other substances that increase vascular permeability, which is conducive to the recovery of vascular permeability in inflammation. Studies have shown that the SSTF significantly inhibits specific and non-specific inflammatory responses and can regulate the body's cellular and humoral immune functions. The mechanism of action is closely related to the effective reduction of capillary permeability, inhibition of PGE2 and NO synthesis in vivo, reduction of TNF-α expression, and reduction of inflammatory exudation[45,46]. SSTF (200 mg/kg) could balance the
${\text{CD}^+_4} $ Based on the studies, S. baicalensis stem-leaf extract shows promising anti-inflammatory properties. These effects are likely mediated through a combination of factors, including the modulation of immune responses, reduction of inflammatory mediators, and potential interactions with signaling pathways like TRPV1. However, it's important to note that while these studies provide valuable insights, further research, including clinical trials, is needed to establish the full extent of its benefits and its potential for therapeutic applications in humans.
Anti-bacterial and antiviral activity
-
Scutellaria baicalensis stem and leaf aqueous extract exhibit different degrees of inhibition of 36 strains from 13 kinds of bacteria, such as Staphylococcus aureus, Staphylococcus, Streptococcus pneumoniae, alpha-hemolytic streptococcus, beta-hemolytic streptococcus and Escherichia coli. This shows that anti-bacterial activity against Staphylococcus aureus is strong (MIC50 0.94 g/L, MBC 0.94 g/L). In vivo (217 mg/kg), it protects against the death of mice infected by Staphylococcus aureus and shows a certain dose-dependence[53]. Zhang et al.[54] found that the stem and leaves of S. baicalensis against Staphylococcus aureus and Shigella dysenteriae with MIC values of 1 and 4 mg/mL, respectively. Besides, it is reported that the water extract of the aerial part of S. baicalensis could inhibit the growth of several common pathogenic bacteria in aquacultures, such as Aeromonas hydrophila, Edwardsiella tarda, Vibrio alginolyticus and V. harveyi[49].
Zhao et al.[55] found that the active part of the stem and leaf of S. baicalensis could inhibit the cytopathic effect caused by 10 kinds of viruses such as Coxsackie B virus, influenza virus, parainfluenza virus, adenovirus, respiratory syncytial virus, and herpes simplex virus. It is suggested that the active parts of the stem and leaf of S. baicalensis can be used for the prevention and treatment of influenza virus, parainfluenza virus, coxsackievirus, and other related infectious diseases.
These findings indicate that S. baicalensis stem and leaf extract possess anti-bacterial and antiviral properties, making it a potentially valuable natural resource for combating infections caused by various pathogens. However, while these results are promising, further research, including clinical trials, would be necessary to fully establish the effectiveness and safety of using S. baicalensis extract for preventing or treating bacterial and viral infections, including COVID-19.
Antipyretic and analgesic effect
-
In a series of studies, Tong et al.[41] demonstrated that SSTF at a dosage of 20 mg/kg significantly reduced body temperature in rats with fever induced by subcutaneous injection of a 10% dry yeast suspension. Zhang et al.[56] conducted research on the antipyretic effect of scutellarin, an extract from S. baicalensis stems and leaves, in febrile rabbits and observed an antipyretic substantial impact induced by pyrogen. Yang et al.[57] conducted several animal experiments, where they discovered that intraperitoneal injection of effective doses of SSTF (42.2 and 84.4 mg/kg) exhibited a specific inhibitory effect on infectious fever in experimental animals. Moreover, intraperitoneal injection of appropriate SSTF doses (30.1, 60.3, and 120.6 mg/kg), as found in Yang et al.'s experiments[58], effectively inhibited the pain response in experimental animals. Furthermore, Zhao et al.[59] noted that SSTF exhibited a certain inhibitory effect on the pain response in experimental animals subjected to chemical and thermal stimulation.
These findings suggest that S. baicalensis stem and leaf extract may have antipyretic and analgesic effects, particularly its total flavonoid component. These effects could be beneficial for managing fever and providing pain relief. However, as with any natural remedy, further research, including controlled clinical trials in humans, is necessary to fully understand the effectiveness, safety, and optimal dosing of S. baicalensis extract for these purposes.
Neuroprotective effect
-
Amyloid protein (Aβ) has been widely recognized as the initiator of Alzheimer's disease (AD)[60]. The SSTF can improve cognitive function and delay the process of dementia. SSTF has been found to exert neuroprotective effects in AD animal models through various mechanisms. Ye et al.[61] demonstrated that oral administration of SSTF (50 mg/kg) could effectively improve cognitive function and reduce neuronal injury in Aβ25-35-3s -induced memory deficit rats. The underlying mechanisms may involve inhibiting oxidative stress and decreasing gliosis[62,63]. Furthermore, SSTF was shown to reduce Aβ-induced neuronal apoptosis by regulating apoptosis-related proteins Bax and Bcl-2[64]. Subsequent studies further validated the neuroprotective effects of SSTF. Cheng et al.[65] found that SSTF treatment inhibited neuronal apoptosis and modulated mitochondrial apoptosis pathway in composited Aβ rats. Ding & Shang[66] found that the SSF improves neuroprotection and memory impairment in rats due to its inhibition of hyperphosphorylation of multilocus Tau protein in rat brains. SSTF has also been found to exert neurogenesis-promoting effects by regulating BDNF-ERK-CREB signaling[12] and activating the PI3K-AKT-CREB pathway[67].
In further studies, Ding et al.[11] proposed that the effect of SSF on promoting neurogenesis and improving memory impairment may be related to the regulation of abnormal expression of Grb2, SOS1, Ras, ERK, and BDNF molecules in the BDNF-ERK-CREB signaling pathway. Zhang et al.[68] found that SSF (25, 50, and 100 mg/kg) could significantly modulate okadaic-induced neuronal damage in rats, which provides a basis for evaluating SSF as a means to reduce tau hyperphosphorylation and Aβ expression in Alzheimer's disease. Cao et al.[69] found that SSTF (100 mg/kg, 60 d) may alleviate tau hyperphosphorylation-induced neurotoxicity by coordinating the activity of kinases and phosphatase after a stroke in a vascular dementia rat model. Gao et al.[70] demonstrated that the stems and leaves of S. baicalensis (SSF, 25, 50, and 100 mg/kg/d, 43 d) could inhibit the hyperphosphorylation of tau in rats' cerebral cortex and hippocampus induced by microinjection of okadaic acid, which may be related to the activities of protein kinase CDK5, PKA and GSK3β. Furthermore, Liu et al.[67] demonstrated that SSF (35, 70, and 140 mg/kg/d, 43 d) improved composited Aβ-induced memory impairment and neurogenesis disorder in rats through activated the PI3K-AKT-CREB signaling pathway and up-regulated the mRNA and protein expression of TRKB, PI3K, AKT, CREB and IGF2. More recently, a new study demonstrated that SSF (35, 70, and 140 mg/kg) alleviated myelin sheath degeneration in composited Aβ rats, potentially modulating sphingomyelin metabolism[71]. Collectively, these findings suggest that SSTF holds therapeutic potential for AD by targeting multiple Alzheimer's pathogenesis-related processes.
Li et al.[72] confirmed that SSTF (5 mg/kg) could improve the behaviors and the numbers of dopaminergic neurons in the substantia nigra in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease in mice, and these beneficial activities appear to be associated with the reduction of the level of serum malondialdehyde.
These studies suggest that S. baicalensis stem and leaf extract have the potential to exert neuroprotective effects in various neurodegenerative conditions, including Alzheimer's disease and Parkinson's disease. These effects could be attributed to its ability to modulate oxidative stress, apoptosis, signaling pathways, and protein hyperphosphorylation. However, as with any potential therapeutic agent, further research is needed to establish the full extent of its benefits, optimal dosages, and mechanisms of action, as well as its potential applications in human patients.
Effects on cardiovascular and cerebrovascular diseases
-
In recent years, ischemic cerebrovascular disease has seriously threatened human health. Cerebral ischemia is one of the leading causes of death. It can occur in focal or global ischemia, with most cases associated with ischemic stroke[73]. Neuronal protection against oxidative damage has been proposed as a potential therapeutic strategy to avoid damage during ischemic stroke[74]. It is reported that SSTF can reduce neuronal apoptosis and free radical damage caused by heart and brain ischemia. Zhao et al.[75] have found that the pretreatment of SSTF (100 mg/kg/d) can protect the ischemia-reperfusion myocardium by enhancing the activity of the anti-oxidative enzyme, inhibiting lipid peroxidation and attenuating the oxygen-free radicals-mediated damage to the myocardium in rats. In further studies, Zhao et al.[76] proposed that SSTF (50, 100, or 200 mg/kg/d, 7 d) pretreatment could alleviate the neuronal damage incurred by ischemia-reperfusion, demonstrating a neuroprotective effect in focal ischemia-reperfusion rat model, which may involve the prohibition of the apoptosis of the neurons. Yu et al.[77] confirmed that SSTF (17.5, 35, and 70 mg/kg/d, 7 d) could attenuate cardiomyocyte apoptosis during ischemia reperfusion injury by down-regulating the protein expression of the JAK2 gene. Qin et al.[78] found that SSF (17.5, 35, and 70 mg/kg/d, 38 d) can decrease the expression of the NMDAR in hippocampus, and increase the expression of VEGF in the cerebral cortex of chronic cerebral ischemia rats.
Focal cerebral ischemia-reperfusion can result in neuronal loss but strongly promotes activation and proliferation of hippocampal glial cells. Losing hippocampal neurons is considered one of the basic pathological mechanisms of cognitive impairment[79]. Zhao et al.[79] found that the pretreatment with SSTF (100 and 200 mg/kg) could improve neurological function after focal cerebral ischemia-reperfusion injury, with preventive and protective effects. Shang et al. found flavonoids from S. baicalensis (35−140 mg/kg) could attenuate neuron injury and improve learning and memory behavior in rats with cerebral ischemia/reperfusion[80]. In further studies, Kong et al.[81] found that the mechanisms of the protective effects on the brain against cerebral ischemia/reperfusion injury of SSTF may involve decreasing the content of brain water, increasing microvascular recanalization, reducing the apoptosis of hippocampal neurons, and attenuating free radical damage. Bai et al.[82] proposed that SSTF (100 mg/kg/d, 7 d) could protect the neurological function in rats following I/R injury by alleviating the damage to the ultrastructure of cerebral cortex neurons and synapse. Yan et al.[83] found that SSTF (100 mg/kg/d, 7 d) pretreatment can exert preventive, protective effects on cerebral tissue by relieving brain edema, decreasing neural damage, promoting microvascular repatency, and increasing enzyme activity. It has been reported that the SSTF may protect neurons and their synaptic structures in multiple ways, but whether this mechanism enhances the resistance of neurons to damage or increases the repair function remains to be further explored.
Essential hypertension is a common chronic cardiovascular disease, which can lead to multiple target organ damage, such as heart, brain, and blood vessels. It is a risk factor for coronary heart disease, heart failure, and other cardiovascular diseases. It is reported that SSTF (17.5, 35.0, and 70 0 mg/kg, 8 weeks) can inhibit myocardial remodeling in primary hypertensive rats, and the medium dose exerts the best inhibitory effect, and the mechanism may be related to inflammatory response induced by inhibiting the NF-κB signaling pathway[84].
S. baicalensis stem and leaf extract have the potential to provide neuroprotective effects in conditions related to ischemic cerebrovascular disease and hypertension. Its ability to modulate oxidative stress, inflammation, and apoptotic pathways appears to contribute to its beneficial effects. However, further research is needed to fully understand the mechanisms and optimal usage of SSTF for these therapeutic purposes.
Anti-aging effects
-
Aging is associated with the deterioration of physiological function and the decline of cognitive ability[85]. It is reported that the alcohol extracts from roots, stems, leaves, and flowers of S. baicalensis (400 mg/kg, 7 weeks) could regulate the content of differential metabolites in urine samples of D-gal-induced aging-model rats to different degrees and play a certain role in improving the metabolic disorders of aging rats[7]. A further study investigated the anti-aging effects and potential mechanisms of S. baicalensis leaves and flower extract. S. baicalensis leaves (400 and 800 mg/kg, 7 weeks) have an anti-aging effect, which can improve the acquired alopecia, slow response, and other characteristics of aging rats, increase the spontaneous activity of aging rats, and reduce the damage of lipid peroxidation and glycosylation induced by D-galactose[86]. The S. baicalensis flowers extract (400 and 800 mg/kg, 7 weeks) could effectively reverse the cognitive decline and oxidative stress injury and alleviate liver pathological abnormalities in the D -galactose-induced aging rats, which are involved in the glutamine-glutamate metabolic pathway[85].
S. baicalensis extracts, particularly those from leaves and flowers, may have anti-aging properties by regulating metabolic disorders, improving cognitive function, reducing oxidative stress, and alleviating aging-related physiological abnormalities. However, further research is necessary to fully understand the mechanisms underlying these effects and to determine the potential of these extracts for human applications in addressing age-related issues.
Others
-
The SSTF (200 mg/kg d, 35 d) can reduce the joint damage of collagen-induced arthritis mice and balance the
${\text{CD}^+_4} $ ${\text{CD}^+_4} $ It is reported that the essential oils from the aerial parts of S. baicalensis showed toxicity against booklice (Liposcelis bostrychophila) with an LC50 of 141.37 μg/cm2. The components of myristate, caryophyllene, eugenol, and caryophyllene oxide displayed dramatic toxicity against the L. bostrychophila, with LC50 values of 290.34, 104.32, 85.75, and 21.13 μg/cm2, respectively[31].
Guo & Xu[90] have found that SSTF could inhibit the proliferation of Hela cells obviously (p< 0.01). Tang et al.[10] have found that SSTF could play an anti-colon cancer role by up-regulated the expressions of Cleaved Caspase-3 and the ratio of Bax/Bcl-2 (p < 0.05 and 0.01) and significantly down-regulated the expressions of MMP-2 and MMP-9 in HCT116 cells (p < 0.05 and 0.01). Recently, Shen et al.[6] studied the chemopreventive effects of HQT against AOM-induced preneoplastic colonic aberrant crypt foci in rats and found HQT inhibits AOM-induced aberrant crypt foci formation by modulating the gut microbiota composition, inhibiting inflammation and improving metabolomic disorders.
Cardiovascular disease is one of the most important threats to human health. Hyperlipidemia is a major risk factor for atherosclerosis, which can cause various cardiovascular and cerebrovascular diseases. Different doses of SSTF (50, 100, and 200 mg/kg) can effectively reduce the body weight increase of rats with hypertriglyceridemia, reduce the serum levels of triglyceride(TG), total cholesterol(TC), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C), which indicate that SSTF has the effect of regulating blood lipid[91].
Oxidative stress is important in developing tissue damage in several human diseases[92]. The antioxidant capacities of separated organs (flower, leaf, stem, and root) of S. baicalensis were conducted by DPPH, ABTS+, and RP methods, respectively. The results showed that the antioxidant activity of the root (66.9 ± 0.3, 121.6 ± 0.5, and 80.2 ± 0.4 μg/mL) was the highest, followed by the leaf (68.4 ± 1.3, 128.2 ± 2.1, and 135.8 ± 2.0 μg/mL), stem (127.8 ± 3.1, 199.2 ± 1.7, and 208.2 ± 8.3 μg/mL) and flower (129.8 ± 6.3, 285.7 ± 4.7, and 380.3 ± 14.2 μg/mL)[93]. The antioxidant activities of the extracts of the aerial part of S. baicalensis and separated organs were conducted by DPPH assay and reducing power method with the antioxidant ability 7.73–8.83 mg TE/g DW and 51.48–306.09 mg TE/g DW, respectively. The content of total polyphenols and total flavonoids were significantly positively correlated with the reducing power[94]. Liu et al.[95] found that the flavonoids extracted from the stems and leaves of S. baicalensis (SSF, 18.98, 37.36, and 75.92 μg/mL) could protect rat cortical neurons against H2O2-induced oxidative injury in a dose-dependent manner. Cao et al. found that SSTF could alleviate the damage of human umbilical vascular endothelial cells injured by H2O2 and reduce their apoptosis, which may be related to the increasing level of Bcl-2[96].
Preventive treatment with SSTF (50, 100, and 200 mg/kg) could significantly inhibit the blood glucose increase induced by alloxan in mice, and SSTF treatment could reduce the blood glucose level in diabetic mice. Both the prevention group and the treatment group could increase the activity of serum superoxide dismutase and decrease the content of malondialdehyde[97]. Liu et al.[98] found that SSTF (75 and 150 mg/kg) could significantly reduce blood glucose and blood lipid and improve insulin resistance in type 2 diabetic rats with hyperlipidemia.
Yang et al.[99] found that SSTF (35 mg/kg/d, 8 weeks) could resist hepatic fibrosis by inhibiting the expression of α-smooth muscle actin in Hepatic Stellate Cells. In vivo, it is reported that SSTF (50, 100, and 200 mg/kg) could significantly reduce alanine transaminase activities in serum, increase the expression of superoxide dismutase and reduce the content of malondialdehyde in acute hepatic injury mice induced by carbon tetrachloride and ethanol[100].
These findings suggest that S. baicalensis stem and leaf extract hold promise in promoting various aspects of health, including immune modulation, antioxidant activity, anti-tumor effects, cardiovascular health, oxidative stress protection, and more. However, further research, including clinical studies, is necessary to better understand the full therapeutic potential and safety of these effects in human applications.
-
Flavonoids are considered the main active components in HQT. As the active substance basis of HQT, the safety of SSTF has also been investigated. After 90 d of oral administration of SSTF (0.5, 1, and 2 g/kg) to rats, no abnormal changes were observed in all indexes, and no delayed toxic reactions or obvious toxic reactions were observed, indicating that the toxicity of SSTF is low[101]. The LD50 value of SSTF was 14.87 g/kg is equivalent to 68.5 times the maximum dose in the pharmacodynamic test of mice, and the experiment confirms the safety of oral administration of the SSTF. Intraperitoneal injection of SST showed certain toxicity in mice, with an LD50 value of 732.11 mg/kg[102]. Liu et al.[103] conducted a systematic safety assessment experiment on the aqueous extract of S. baicalensis stem and leaves based on the China National Standard 'Guidelines for the Safety Evaluation of Food Toxicology (GB15193-2014)'. The results indicated that S. baicalensis stem and leaves are non-toxic, non-teratogenic, and non-mutagenic. Acute toxicity tests in mice revealed a Maximum Tolerated Dose of 15.0 g/kg. A 90-d feeding trial showed no changes in toxicological damage in animals, even at a high dosage of 8.333 g/kg (equivalent to 100 times the recommended human daily intake), suggesting the safety and non-toxicity of consuming S. baicalensis stem and leaves. In addition, HQT has long been used in folklore, and no toxicity has been reported.
These findings collectively indicate that HQT is generally safe for consumption. However, as with any herbal product, it's important to follow recommended dosages and consult healthcare professionals, especially for individuals with pre-existing health conditions or medications.
-
In recent years, HQT, a non-Camellia tea with a long history in China, has attracted attention due to its diverse pharmacological activities. Among various Scutellaria species, S. baicalensis is the most extensively studied and cultivated source for HQT. The aerial parts, including flowers, stems, and leaves, serve as the principal source of HQT preparation. HQT are rich in flavonoids and volatile components with various beneficial effects. To date, about 295 compounds have been identified from HQT, including approximately 54 flavonoid compounds and 145 volatile components identified online. The current research on the activity of HQT primarily focuses on flavonoid compounds, with limited studies on the larger quantity of volatile oil compounds.
Additionally, the processing and brewing techniques used to prepare HQT may influence the bioactivity of its flavonoid content, although few studies have investigated this. More research is necessary to optimize the processing and brewing techniques to maximize the health benefits of HQT. Comparative studies reveal that the aerial parts of S. baicalensis, while sharing similarities with the roots, contain varying flavonoid compositions. Limited research on S. scordifolia, S. amoena, and S. viscidula, indicates the presence of comparable flavonoid compounds in their aerial parts. Although individual flavonoids like baicalin, wogonin, and scutellarin have demonstrated various therapeutic properties, it is essential to consider the synergistic effects of these compounds when consumed together in the form of tea. These findings contribute to laying the groundwork for quality assessment of HQT and offer insights into potential health benefits.
HQT is mainly derived from the aerial parts of S. baicalensis. Recent studies have increasingly recognized the pharmacological value of the aerial parts of S. baicalensis. Preliminary pharmacological studies have shown that the aerial parts of S. baicalensis may possess beneficial activities in antioxidant, anti-tumor, antiviral, anti-bacterial, protection of ischemia-reperfusion injured neural function, neuroprotective effects against brain injury, and blood lipid regulation. These findings suggest that the value of using HQT may be attributed to these pharmacological activities. Although HQT is generally safe for consumption, further investigation is required to understand its safety profile, particularly in special populations such as pregnant or lactating women, children, and individuals with pre-existing medical conditions. Additionally, potential interactions between the flavonoids in HQT and conventional medications must be examined to ensure their safe and effective use with pharmaceutical treatments. In addition, although the safe dose of HQT on rodents has been studied, the safe dose for humans has yet to be determined.
In conclusion, these initial research results support the potential health benefits of HQT and encourage more in-depth studies on its raw materials. Further studies are necessary to elucidate the synergistic effects of the flavonoids in HQT, optimize the processing and brewing techniques for maximum bioactivity, and investigate the safety profile and potential interactions with conventional medications. A comprehensive understanding of HQT will contribute to developing evidence-based recommendations for promoting health and well-being.
-
The authors confirm contribution to the paper as follows: Conceptualization and writing: Quan Y, Li Z, Meng X, Li P, Shen J; Figure and table modification: Quan Y, Li Z, Meng X, Li P; review and editing: Wang Y, He C, Shen J. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This research was funded by the Shandong Provincial Natural Science Foundation, China (ZR2022QH147, ZR2022QH165) and the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-D-202005).
-
The authors declare that they have no conflict of interest.
- Supplemental Table S1 Chemical composition of the essential oil of HQT.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Quan Y, Li Z, Meng X, Li P, Wang Y, et al. 2023. A comprehensive review of Huangqin (Scutellaria baicalensis Georgi) tea: chemical composition, functional properties and safety aspects. Beverage Plant Research 3:31 doi: 10.48130/BPR-2023-0031
A comprehensive review of Huangqin (Scutellaria baicalensis Georgi) tea: chemical composition, functional properties and safety aspects
- Received: 22 August 2023
- Revised: 11 October 2023
- Accepted: 13 October 2023
- Published online: 04 December 2023
Abstract: Huangqin tea (HQT), derived from the aerial parts of various Scutellaria species, in particular S. baicalensis Georgi, has a long history of traditional use in China. Its significance has grown in recent years due to its potential anti-aging, colon cancer chemopreventive, and cardiovascular protective properties. Huangqin tea source plants have identified over 295 chemical constituents, including flavonoids, essential oils, phenolic acids, sterols, diterpenes, polysaccharides, and amino acids. Pharmacological research has underscored the diverse beneficial effects of Huangqin tea and flavonoid extracts. These effects encompass anti-inflammatory, antiviral, anti-bacterial, antipyretic, and analgesic properties, along with neuroprotective effects and protection against cardiovascular and cerebrovascular diseases. Safety studies indicate that HQT is generally safe within recommended dosages and historical use. HQT presents multifaceted potential health benefits, though comprehensive research is necessary to ensure its effectiveness and safety in human applications.
-
Key words:
- Bioactivity /
- Chemical composition /
- Huangqin tea /
- Safety /
- Scutellaria