-
Brassica encompasses a diverse array of essential crops in agriculture and horticulture, each valued for its distinct edible components. These plants find extensive cultivation as vegetables, sources of oilseeds, and flavor-enhancing condiments. Notably, the by-product of rapeseed oil extraction, known as oil cake, has emerged as a superior feed for both cattle and poultry. Brassica is distinguished by the existence of three genomes labeled A, B, and C, which are all traceable back to a common ancestor as mesohexapolyploids. The existence of these genomes in pairwise combinations distinguishes the different Brassica species. B. oleracea is characterized by the C genome (CC; n = 9), while B. nigra is dominated by the A genome (AA; n = 10) in B. rapa (BB; n = 8). Remarkably, these diploid genomes can also combine in pairs to give rise to amphidiploid allotetraploid species like B. napus (AACC; n = 19), B. juncea (AABB; n = 18), and B. carinata (BBCC; n = 17)[1]. Plants in the Brassicaceae family generate glucosinolates (GSLs), which are a type of plant secondary metabolite, in order to protect themselves from herbivores and plant defense mechanisms against environmental stress. Each group of GSLs is produced by a separate metabolic pathway that shares a similar set of enzymes involved in the genetically controlled production of the core structure of GSLs. When disruption causes harm to plant tissue, glucosenolates are rapidly digested by built-in myrosinase to yield D-glucose, sulfate ions, and distinctive breakdown products, including isothiocyanates. Many scientists that investigate plant physiology, plant breeding, plant genetics, and food functioning have focused on the beneficial isothiocyanates, or glucosinolates, which help promote human health. When stress is applied, the accumulation of each individual GSL is influenced by the abiotic stress duration, intensity, developmental stage of the plant, genotype, climate, and cultivation circumstances, including fertilization, harvest time, and plant position. GSL accumulation in Brassicaceae increased through optimized pre-harvest and post-harvest management in response to the potential health benefits of glucosinolates and their derivatives and the increasing demand for health-promoting vegetable products in ordinary life[2]. Plant breeding, biotechnology, metabolic engineering, plant cell culture, hairy root culture, and microbial host-based engineering are among the numerous methods that can be utilized to increase GSLs in Brassicaceae.
-
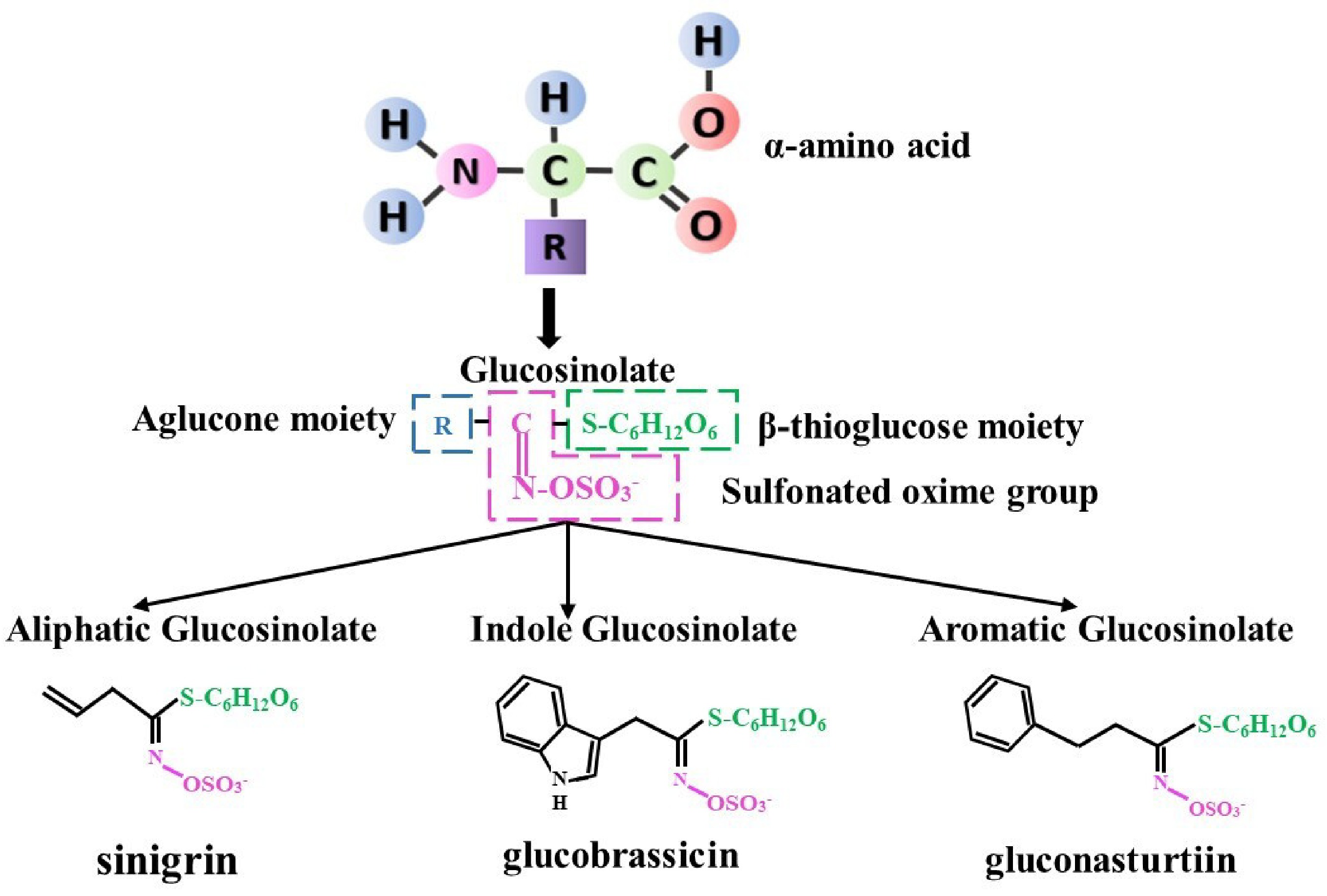
Glucosinolates (GSLs), also known as β-thioglucoside-N-hydroxysulfates, represent a category of secondary metabolites found abundantly in nearly all Brassica species[3]. These compounds have been the subject of extensive study, with the number of identified GSLs in plants ranging from 88 to 137[4]. Of these, 17 have been extensively documented in common Brassica crops[5,6]. Naturally existing GSLs are S-bound glucosides with the cis-N-hydroximinosulfate ester structure that defines them. Glucosinolates (GSLs) consist of three functional groups: a variable α-amino acid-derived aglycone side chain, a β-thioglucose unit, and a sulfonated oxime group. These GSLs share a core structure that consists of a glucose moiety joined to a sulfated oxime at the C-atom via an S-glycosidic bond (Fig. 1). These compounds are nearly categorized into three primary groups based on their precursor amino acids: aromatic GSLs (derived from phenylalanine and tyrosine) like gluconast urtiin, indolic GSLs (derived from tryptophan) like glucobras sicin, and aliphatic GSLs (consisting of isoleucine, alanine, methionine, and leucine) like sinigrin (Fig. 1[7]).
The document outlines the principal positive health effects of GSLs and their bioactive derivatives and provides an index of GSLs reported in various Brassicaceae plants across various parts, including the leaf, flower, root, and stem, with sprouts containing the maximum concentrations[8]. Species abundant in sinigrin, such as white and red cabbage, cauliflower, kale, and kohlrabi, possess strong and fiery flavors of condiments constantly consumed by large numbers of consumers and have many health benefits like anticarcinogenic, anti-inflammatory, wound-healing properties, antioxidant, and antimicrobial activity (Table 1[9−13]). The compound glucoiberverin, found in cauliflower, kale, and white and red cabbage have the potential to be used in antimicrobial activity (Table 1[9,11−14]). Progoitrin, which is present in mustard spinach, cauliflower, broccoli, rapeseed, turnip, and Chinese cabbage, inhibits thyroid hormone production and has antiviral activity; it also has the goitrogenic consequence of a protein-rich seed diet on livestock effects (Table 1[9,11−13,15−17]). Chinese cabbage, turnip, turnip green, and swede contain glucobrassicanapin, which exhibits antibacterial effects (Table 1[9,11−13,18]). The presence of glucobrassicin and neoglucobrassicin in Chinese cabbage, broccoli, green cabbage and red cabbage has been shown to effectively suppress the growth of prostate cancer (Table 1[11−13,19,20]).
Table 1. Major glucosinolates and their health benefits in Brassicaceae.
Common name Chemical name
(side chain R)Molecular weight (g·mol−1) Prominently observed in Benefits to health or toxicity Ref. Aliphatic glucosinolate Three carbon chain length Sinigrin 2-Propenyl GSL 359 Mustard green, white and red cabbage, cauliflower, kale and kohlrabi Anticarcinogenic, antimicrobial, anti-inflammatory, wound-healing properties, and antioxidant activity [9−13] Glucoiber verin 3-Methylthio propyl GSL 407 White and red cabbage,
cauliflower and kaleAntimicrobial activity [9,11−14] Glucoiber in 3-Methylsulfi nylpropyl GSL 423 Cabbage, broccoli and cauliflower Antimicrobial activity [9,11−14] Four carbon chain length Gluconapi n 3-Butenyl GSl 373 Chinese cabbage, mustard spinach, cabbage, broccoli, cauliflower and rapeseed Anticarcinogenic activity, prevention of postprandial hypertriglyceridemia and antimicrobial activity [9,11−13,
18,21,22]Progoitrin (2R) 2-Hydroxy- 3-butenyl GSL 389 Chinese cabbage, mustard spinach, turnip, broccoli, cauliflower and rapeseed Antiviral activity, effect of goitrogens, and suppression of the synthesis of thyroid hormones [9,11−13,
15−17]Glucoeruc in 4-Methylthio butyl GSL 421 Garden rocket Anticarcinogenic activity, antioxidant activity and anti-obesity activity [9,11−13,
23−25]Glucorap hanin 4-Methylsulfi nylbutyl GSL 437 Broccoli, garden rocket, cauliflower and kohlrabi Anti-inflammatory activity, recovery and prevention of muscle atrophy and alcohol intolerance preventive [9,11−13,
26−28]Five carbon chain length Glucobras sicanapin 4-Pentenyl GSL 387 Chinese cabbage, turnip, turnip green and swede Antimicrobial activity [9,11−13,18] Glucoalyssin 5-Methylsulfi nylpentyl GSL 451 Ethiopian mustard Anticarcinogenic activity and antimicrobial activity [11−14,29,30] Aromatic glucosinolate Gluconast urtiin 2-Phenethyl GSL 423 Watercress and horseradish Anticarcinogenic activity, cardioprotective and neuroprotective activity [11−13,22,
31,32]Glucotrop aeolin Benzyl GSL 409 Red cabbage Antidiabetic, neuroprotective activity and prevention of
multiple sclerosis
[11−13,22,33]Indole glucosinolate Glucobras sicin 3-lndolyl methyl GSL 448 Green cabbage, broccoli, chinese and red cabbage Impediment to cancer in prostate [11−13,19,20] Neogluco brassicin 1-Methoxy-3- indolymethyl GSL 478 Red cabbage, Chinese cabbage, broccoli, and green cabbage Inhibition of cancer in prostate [11−13,19,20] Glucosinolate synthesis
-
Over the past few decades, researchers have described several genes for GSL production in the model plant Arabidopsis thaliana[34]. Amino acids like phenylalanine (Phe), methionine (Met), tryptophan (Trp), or tyrosine (Tyr) are the biosynthetic sources of the majority of glucosinolates in Brassica crops[6]. Aliphatic, indole and aromatic GSLs are the three forms of GSLs that are produced from methionine, phenylalanine, and tryptophan, respectively (Fig. 2).
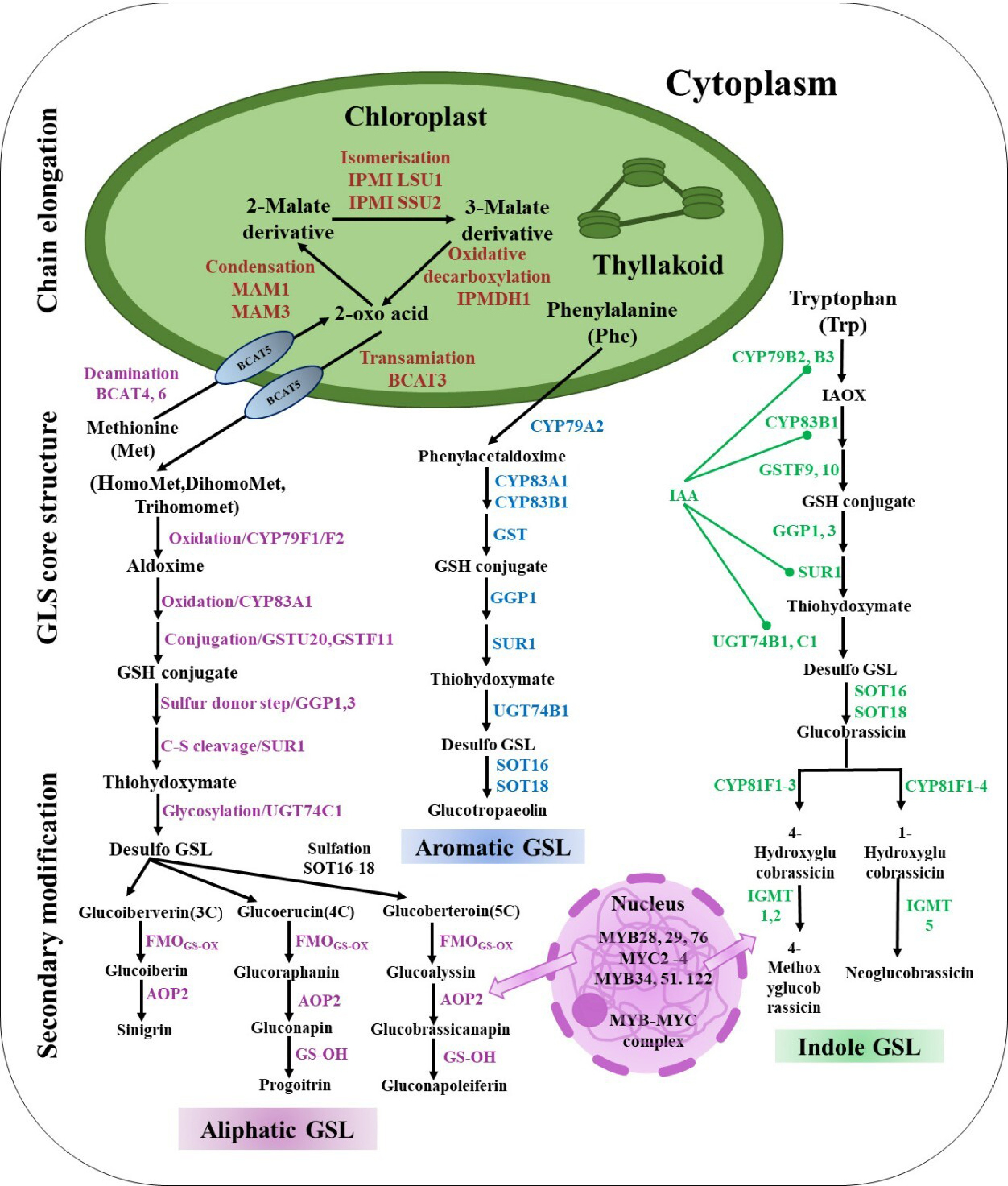
Figure 2.
Mechanisms in Brassicaceae synthesize GSLs: The biosynthesis of GSLs consists of three phases, including chain elongation, GSL core structure, and secondary modification. Met, methionine; Phe, phenyl alanine; Trp, tryptophan; BCAT, branched-chain amino acid aminotransferase; MAM, methylthioalkylmalate synthase; IPMI, isopropylmalate isomerase; IPMDH, isopropylmalate dehydrogenase; CYP79F1, cytochrome P450 79F1; CYP79F2, cytochrome P450 79F2; CYP79A2, cytochrome P450 79A2; CYP83A1, cytochrome P450 83A1; CYP83A2, cytochrome P450 83A2; CYP83B1, cytochrome P450 83B1; GSTU20, glutathione-S-transferases TAU 20; GSTF11, glutathione-S-transferases F11; GGP1, gamma-glutamyl peptidases 1; GGP3, gamma-glutamyl peptidases 3; SUR1, super root 1; UGT74C1, UDP-glycosyl transferase 74C1; UGT74B1, UDP-glycosyl transferase 74B1; SOT16, sulfotransferase 5a; SOT16, sulfotransferase 5c; SOT16, sulfotransferase 5b; FMO, flavin monooxygenase glucosinolate S-oxygenase; AOP2, alkenyl hydroxalkyl-producing 2; GS-OH, 2-oxoglutarate-dependent dioxygenase; CYP81F1, cytochrome P450 81F1; CYP81F2, cytochrome P450 81F2; CYP81F3, cytochrome P450 81F3; CYP81F4, cytochrome P450 81F4; IGMT1, indole GSL O-methyltransferase 1; IGMT2, indole GSL O-methyltransferase 2; IGMT5, indole GSL O-methyltransferase 5.
The process of aliphatic GSL biosynthesis involves several enzymes. It starts with the deamination of amino acids like methionine to 2-oxo acids by BCAT (branched-chain amino acid aminotransferase). These 2-oxo acids then undergo a series of transformations, among which are condensation, isomerization, and oxidative decarboxylation, which elongate the chain by a solitary methylene group with the help of MAM and IPMDH methylthioalkylmalate synthase (MAM) and isopropylmalate dehydrogenase (IPMDH). This molecule is capable of undergoing transamination or further elongation. Cytochrome P450 79F (CYP79F) converts amino acids like homoMet, dihomoMet, and trihomomet into aldoximes, which cytochrome P450 83A (CYP83A) then oxidizes to reactive nitrile oxides. These reactive nitrile oxides are then conjugated with glutathione by glutathione-S-transferases (GSTs), resulting in GSH conjugate, then sulfur donated by gamma-glutamyl peptidases (GGPs), and cleaved into thiohydroximates by super root 1 (SUR1), which are then S-glucosylated by glucosyltransferases, or UGTs of the 74 family, to create desulfo glucosinolates. Three genes that encode cytosolic sulfotransferases (SOT16–18) are in charge of the sulfonation of desulfo GSLs to produce aliphatic GLSs after the core structure assembly process (Fig. 2). After the formation of the glucosinolate structure, oxygenation, hydroxylation, alkenylation, benzoylation, and methoxylation alter the side chains. The S-oxygenation of glucoiberverin, glucoerucin, and glucoberteroin by flavin monooxygenases (FMOGS-OXs) creates S-oxygenated aliphatic glucosinolates, such as gluciberin, glucoraphanin, and glucoalyssin, respectively. Ultimately, gluciberin, glucoraphanin, and glucoalyssin were converted into sinigrin (3C GSL), gluconapin, and glucobrassicanapin by using 2-oxoglutarate-dependent dioxygenases (AOPs), respectively. GS-OH (2-oxoglutarate-dependent dioxygenase) converts gluconapin to progitrin (4C GSL), and glucobrassicanapin is transformed into gluconapoleiferin (5C GSL) by GS-OH (Fig. 2). Targeting MYB regulatory elements, including MYB28, MYB29, and MYB76 in Brassicaceae species, where they are essential regulators of GSL biosynthesis (Fig. 2[35−37]).
Arabidopsis thaliana synthesizes aromatic glucosinolates from phenylalanine without involving the chain elongation process (Fig. 2). Phenylalanine goes through a series of reactions to create GSH conjugate. The end result of CYP79A2's conversion is phenylacetaldoxime. The process of core structure assembly involves the conversion of GSH conjugate into thiohydroximates by gamma-glutamyl peptidases (GGP1) and super root 1 (SUR1), which are subsequently S-glucosylated via glucosyltransferases to yield desulfo-glucosinolates, which are ultimately converted into aromatic (glucotropaeolin) GSLs (Fig. 2).
Indole-3-acetaldoxime (IAOx), which is made from tryptophan, is a key link between the primary and secondary metabolic pathways and the production of auxin. Thiohydroximate is produced through the reaction of glutathione conjugates, a key step in GSL biosynthesis likely involves super root 1 (SUR1) and gamma-glutamyl peptidases (GGP1) in cabbage[38]. UGT74 converts thiohydroximate into desulfo GSLs, which then undergo sulfonation by cytosolic SOT16–18 genes to produce glucobrassicin. Other genes, like CYP81F and IGMT (indolic glucosinolate O-methyltransferase), modulate various indole GSL structures and ultimately form 4-methoxyglucobrassicin and neoglucobrassicin. The MYB34, MYB122, and MYB51 transcription factors are key regulators of GSLs, influencing their production and IAOx derivative accumulation. Aldoximes, thought to be universal precursors, synthesize IAA, an essential plant growth regulator. Changes in the GSL biosynthetic genes UGT74B1, CYP79B2, CYP79B3, SUR1, and CYP83B1 have an indirect effect on IAA homeostasis through their effect on IAOx. Two more genes, indole GSL O-methyltransferase (IGMT1) and cytochrome P450 81F (CYP81F), manage the change from glucobrassicin to 4-methoxyglucobrassicin. Glucobrassicin is changed by enzymes CYP81F and indole GSL O-methyltransferase (IGMT5) to make neoglucobrassicin. Important regulators of GSLs, transcription factors MYB34, MYB51, and MYB122, affect both their production and the accumulation of IAOx derivatives (Fig. 2). Researchers believe that Brassicaceae crops share the same biosynthetic framework as Arabidopsis.
Glucosinolate distribution and hydrolysis
-
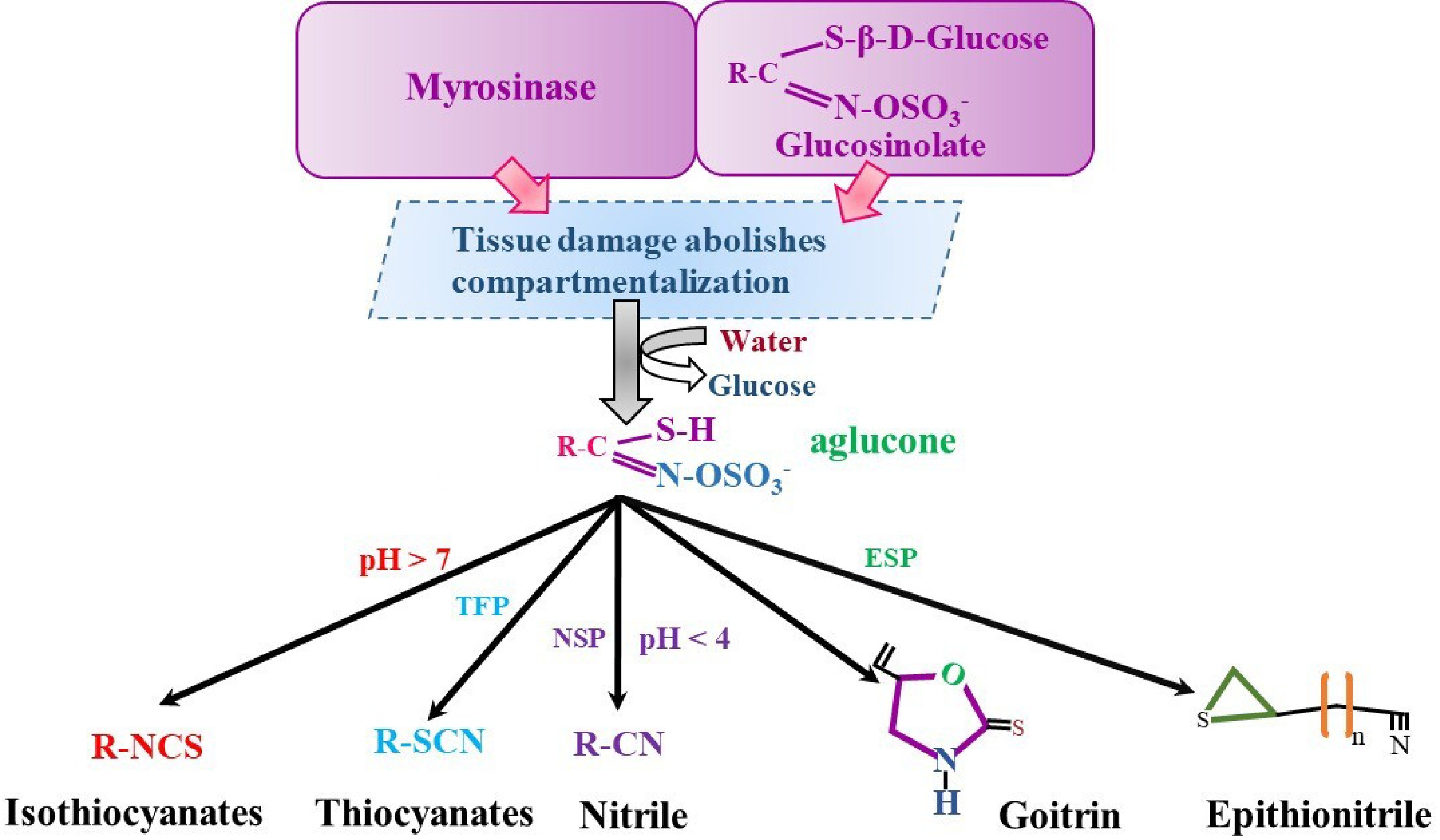
Disruption of plant tissue in response to bacteria or insects leads to rapid hydrolysis of glucosinolates by myrosinase. Glucosinolates exhibit a heterogeneous distribution in cells and are predominantly stored in specialized S-cells, which are rich in sulfur and located adjacent to the phloem[39−41]. Myrosinases, or β-thioglucoside glucohydrolases, reside in distinct compartments, which are myrosin cells and sentinel cells. Unaltered glucosinolates are typically biologically inactive. This activation results from myrosinases enzymatically cleaving the thioglucosidic bond, producing an unstable aglucone. Epithionitrile, thiocyanates, goitrin, isothiocyanates, and nitriles are among the compounds generated by this biochemical reaction (Fig. 3[19,42]). The various forms of GSL derivatives result from a combination of factors, including the instability of thiohydroxamate-O-sulfonate aglycone and GSL substrate, in addition to the effects of particular proteins such as ESPs, NSPs, and TFPs. The prevalence of ferrous ions (Fe2+), pH levels, and interactions with proteins that interact with the enzyme thioglucoside glucohydrolase also influences these outcomes (Fig. 3[43,44]). Furthermore, the glucosinolate-myrosinase system has been identified as being associated with interactions between plants and insects, as well as between plants and pathogens. Multiple studies have linked the breakdown products of glucosinolates to the protection of plants against insects, diseases, and herbivores. Isothiocyanates (ITCs) have functional effects such as anti-inflammatory, antimicrobial, and anti-carcinogenic activities[45]. Furthermore, Arabidopsis thermotolerance was increased, and heat shock protein (HSP) gene expression was boosted by the exogenous application of isothiocyanates. These findings imply that isothiocyanate is a signaling molecule that helps plants become thermotolerant. Thiocyanates possess anticancer, antifungal, antibacterial, and anti-parasitic properties. The ability of epithionitrile to stimulate phase-II enzymes, which help to prevent cancer, including allyl ITC. Nitriles mediate a substantial reduction in anticancer activity.
-
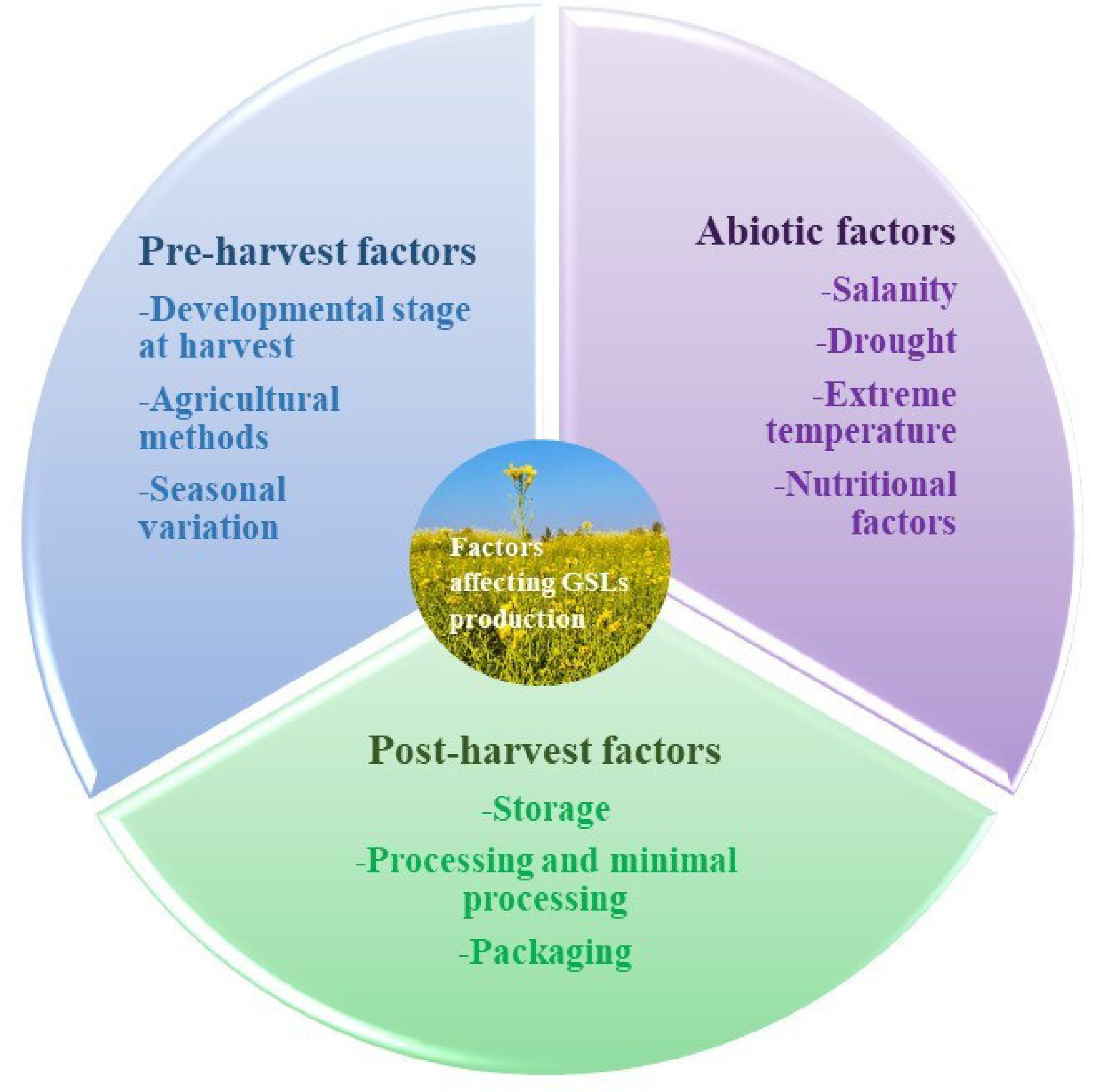
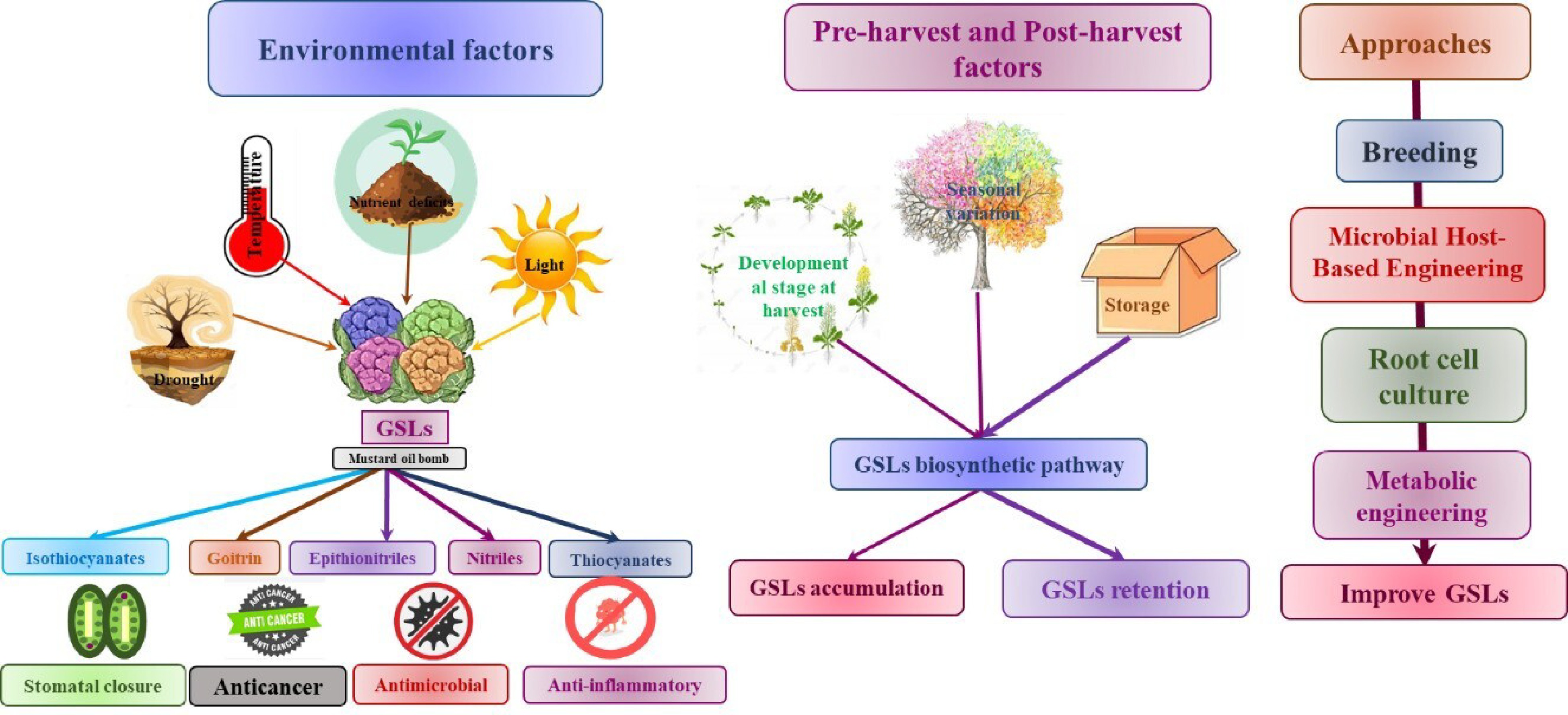
Previous studies have provided evidence that the GSL content of Brassica cultivars is subject to variation based on genetic and environmental factors. Plants utilize defense mechanisms in response to harmful environmental circumstances such as drought, salt, severe temperatures, and excessive UV radiation. These responses lead to the accumulation of specific metabolites or phytochemicals, including GSL[46]. Several factors are recognized like pre-harvest factors including the maturation stage, genotype, growing environment, and cultural practices. Post-harvest activities include harvesting, packaging, and storage. These determinants influence the profiles of GSL in Brassica.
Abiotic factors
-
Plants are exposed to various abiotic stressors during their growth, which leads to the biochemical changes and gene upregulation, which ultimately improve the synthesis of primary and secondary metabolites (Table 2). Plants have developed several stress tolerance systems, which encompass physiological and biochemical alterations leading to morphological changes. Additionally, it has been discovered that plants react to abiotic stress by either increasing or decreasing their accumulation of GSL. Previous research has demonstrated that environmental factors, including temperature, salt content, water loss, and light, have can alter the composition of specific GSLs, which depend upon the plant's developmental stage as well as the intensity and duration of abiotic stress (Fig. 4[47−52]). Understanding how plants use metabolites to resist stress is tough due to complex regulation. For instance, the interconnection of pathways such as GSL synthesis with numerous others complicates their direct involvement in stress resistance. Despite some studies, there's still a lot we don't know about these mechanisms. We urgently need research to uncover the hidden workings of plant stress resilience.
Table 2. Impact of abiotic stress, pre-harvest, and post-harvest factors on Brassicaceae glucosinolate levels.
Variable Plant species Change in glucosinolate level Ref. Abiotic factors Soil salinity Brassica oleracea var. italica (broccoli) and B. rapa var. pekinensis (Chinese cabbage) Increase total GSLs (40, 80 mM) [44] R. sativus (radish), Brassica oleracea var. botrytis
(cauliflower) and B. oleracea var. italica (broccoli)Increase total GSLs with reduced water [56] Water availability B. oleracea var. capitata (cabbage), Brassica napus (rapeseed) and Brassica oleracea var. italica (Broccoli) Increase total GSLs with severe drought [44] Brassica carinata (Ethiopian mustard) and B. oleracea
var. gemmifera (Brussels sprouts)No effect under mild drought [44,56] B. rapa var. rapa (turnip) Increase total GSLs with mild drought [44] Brassica oleracea var. capitata (cabbage) and
Arabidopsis thaliana (thale cress)Decrease total GSLs under mild and severe drought [44] Temperature stress B. oleracea var. italica (broccoli), B. oleracea var.
botrytis (cauliflower)At a moderate temperature of 14 °C and increase total GSLs [56] B. oleracea var. capitata (cabbage), B. oleracea L. var. gemmifera (Brussels sprouts) and B. oleracea var.
acephala (kale)Total GSLs diminish as temperature rises [61] B. napus (swede) Progoitrin and glucoberteroin should be increased to 21 °C [113] B. rapa var. pekinensis (Chinese cabbage) Vary the total GSLs from 21 to 34 °C [44] B. oleracea var. italica (broccoli) and B. oleracea var.
botrytis (cauliflower)Increase overall GSLs under bright conditions
(450 μmol·m−2·s−1)[56] B. oleracea var. italica (broccoli) and B. oleracea var.
botrytis (cauliflower)Reduce glucoraphanin levels during harvest using high light [44] Light intensity B. oleracea var. italica (broccoli) Diminish overall GSLs with light [44] B. oleracea var. capitata (cabbage) and B. oleracea var. gemmifera (Brussels sprouts) Variation in total and indole GSLs with respect to light intensity and duration [61] Cardamine fauriei (Ezo-wasabi) Total GSLs increase; red + blue luminescence [68] Brassica oleracea var. acephala (kale) Change in total and indole GSLs with respect to light intensity and day length [61] Arabidopsis thaliana (thale cress) Light increases total GSLs, while darkness reduces total GSLs [44] Sulfur application B. oleracea var alboglabra (Chinese kale) Blue light diminishes gluconapin levels [114] B. oleracea var. italica (broccoli) Enhance indole and alkyl GSLs (60 mg of S plant−1) [56] Raphanus sativus (radish) Boost alkenyl GSLs (30 mg·plant−1) [56] Brassica rapa var. rapa (turnip) Promote overall GSLs (60 kg S ha−1) [44] Potassium application Brassica rapa var. rapa (turnip) Decrease total GSLs with K+ deficiency [44] Arabidopsis thaliana (thale cress) Increase overall GSLs when K+ is lacking [44] Nitrogen application B. oleracea var. botrytis (cauliflower), B. oleracea var.
italica (broccoli) and Raphanus sativus (radish)Increase total GSLs by decreasing nitrogen [56] Brassica oleracea var. italica (broccoli) Increase average GSLs while decreasing N (1 g of
N plant−1)[44] Brassica oleracea var. italica (broccoli) Raise the overall GSLs by 5.2 mM Se [44] Selenium application Raphanus sativus (radish) Enrich the soil with total GSLs and glucoraphanin [81] Pre-harvest factors Brassica oleracea var. italica (broccoli) Increase indole GSLs in immature florets, increase glucoraphanin between transplanting and harvest [61,115] Developmental stage
at harvestB. oleracea var. capitata (cabbage), B. oleracea var. gemmifera (Brussels sprouts), B. oleracea var. botrytis (cauliflower) and B. oleracea var. acephala (kale) Increase glucoraphanin between transplanting and harvest [61] Seasonal variation B. oleracea var. italica (broccoli) and Brassica oleracea
var. botrytis (cauliflower)Enhance overall GSLs in spring and autumn [56] B. oleracea var. capitata (cabbage) and B. oleracea var. gemmifera (Brussels sprouts) Increase glucoiberin and glucobrassicin in spring [84] Agricultural practices B. oleracea var italica (broccoli) The levels of 3-indolylmethyl-GSL were considerably elevated in crops grown organically [91] Post-harvest factors Storage B. oleracea var. italica (broccoli) The content of 4-methylsulfinylbutyl-GSL in florets exhibited an increase subsequent to a 6-d storage period, which was followed by a decrease in temperature [94] Processing and minimal processing B. oleracea var. italica (broccoli) An increase of 490% in total GSLs, 4,200% in 4-hydroxy-3-indolylmethyl-GSL, and the rise of 1,300% in 1-methoxy-3-indolylmethyl-GSL was reported after 24 h of incubation at a temperature of 20 °C [103] Packaging Brassica oleracea var. italica (broccoli) Compared to fresh florets, storage in a regulated atmosphere consisting of 0.5% O2, 20% CO2 and air. resulted in a 21%–42% increase in total GSL [112] Salinity
-
A major abiotic factor is salinity, which affects plant development, plant physiology, and secondary metabolism, which includes signal molecules, oxidative stress, and intermediary processes. Plants respond to this salt stress in several ways, either by adjusting their osmotic balance or by building up secondary metabolites like glucosinolates. Salinity has a differential effect on the biological breakdown of GSLs in plants, which is influenced by various environmental conditions such as heat, radiation, nutritional management, the exact type of GSL being generated, and the plant's genotype. Glucosinolate levels increased marginally when salinity levels reached 80 mM, contrary to previous results, which demonstrated a substantial increase in the number of total glucosinolates in broccoli and Chinese cabbage at a salinity level of 40 mM (Table 2[44]). As an alternative study showed, the halophyte species Thellungiella has different glucosinolate compositions in different stages of development and organs because it is exposed to salt[53]. In B. oleracea L. var. italica, the GSL content increased when exposed to 40 and 80 mM NaCl for two weeks, which was comparable to the effect observed in B. rapa L. subjected to NaCl solutions at concentrations of 20, 40, and 60 mM for 5 d[54]. Broccoli inflorescences exhibited a substantial increase in total glucosinolate content when exposed to a salinity of 40 mM[55]. However, when salinity levels attained an increase of 80 mM, the glucosinolates were significantly reduced.
Drought
-
A drought occurs when a plant does not have access to enough water, which is necessary for healthy growth and development. Inadequate groundwater and poor precipitation are two of the main causes of drought in agricultural areas. Drought has varying effects on the accumulation of particular GSLs, depending on the stage of plant development, the length of the drought stress, and its severity. A greater accumulation of glucosinolates was observed in Brassica species subjected to water stress, including broccoli, radish, and cauliflower (Table 2[56]). Glucosinolate levels within Arabidopsis thaliana rosette leaves and cabbage decreased significantly in response to water scarcity, both under mild and severe drought conditions[44]. Total GSLs are increased in broccoli, cabbage, and rapeseed by acute drought[44]. Brussels sprouts and Ethiopian mustard were unaffected by a moderate drought (Table 2[44]). Several Brassica species, including Nasturtium officinale L., B. oleracea L. var. capitata, B. oleracea L. var. italica, B. napus L., B. rapa ssp. rapifera L., and Brassica carinata L., exhibited an increase in the accumulation of glucosinolates in response to water stress. Levels of GSLs in B. oleracea L. var. capitata increase after two weeks of drought stress[52]. Glucosinolate concentration in B. oleracea L. var. italica is increased by a two-week drought[57]. An overt stress condition that persisted for a duration exceeding one week resulted in the proliferation of Brassica napus. A 25% reduction in available water, which can be classified as mild stress, led to the proliferation of Brassica rapa ssp. Rapifera[58]. Brassica carinata L. exhibited a range of responses when subjected to severe and moderate stress (at 40%, 23%, 17%, and 15% of the total water supply), with some instances demonstrating an increase in growth and others exhibiting no change[59]. In contrast, Brassica oleracea L. var. gemmifera did not demonstrate any notable impact when exposed to moderate stress at a 30% water stress level[60].
Extreme temperatures
-
Exceptionally high temperatures in warm weather are another form of abiotic stress. The duration and intensity of a high temperature determine its effect. Heat stress can harm plants in a variety of ways, including physiological, biochemical, and physical, which lowers their yield. It is well established that elevated daily mean temperatures stimulate myrosinase activity, leading to an increase in GSL degradation after harvest[56]. Researchers have found that high summer field temperatures during the commercial harvest of salad rockets negatively impact some GSL concentrations. However, this does not reliably indicate the concentrations after harvest, as they have been observed to increase during shelf-life storage. Warmer temperatures possess a connection with reduced concentrations of GSL in cabbage, Brussels sprouts, and kale (Table 2[61]). Reduced growth temperatures induce a significant increase in sinigrin content in kale[62]. Broccoli grown at different temperatures produced more aliphatic GSLs at low temperatures and more indolic GSLs at high temperatures. Both temperature extremes (32 and 12 °C) increased the concentrations of aliphatic GSL in kale[62]. Research has demonstrated that Brassica rapa glucosinolate levels increase in response to elevated temperatures (21–34 °C)[63]. Glucosinolate content in Brassica rapa L. is reduced when subjected to temperatures that vary from 15 to 27 °C[63]. Researchers have recognized specific factors involved in MYB transcription as the cause of Brassica rapa glucosinolate accumulation at elevated temperatures[63]. The concentration of GSLs in B. oleracea L. increases at 32 °C[61,64].
Light intensity
-
Research has shown that UV-B radiation can promote the buildup of GSL in A. thaliana[65]. Intense illumination (450 μmol·m−2·s−1) causes an increase in GSLs in broccoli and cauliflower[56]. Research has demonstrated that elevated light levels can result in reduced levels of glucoraphanin in harvested crops, such as cauliflower and broccoli[44]. GSL concentrations in broccoli sprouts increased significantly 24 h after exposure to intense UVB therapy. As a consequence, glucobrassicin increased by 78%, glucoraphanin by 45%, and 4-methoxyglucobrassicin by 170%. Prolonged periods of darkness tend to accumulate more GSLs in plants. The Chinese cabbage seedlings had GSL concentrations that were 6.9-fold greater when exposed to light ten days after sowing[66]. The study's findings showed that plants first cultivated in darkness and then exposed to light significantly expressed genes related to GSL production in A. thaliana, compared to plants only exposed to the usual day-night cycle[67]. The utilization of various light wavelengths on Brassicaceae crops may induce variations in the concentrations of GSLs. For instance, it has been observed that blue light increases the overall GSL content in Ezo-wasabi leaves and turnip roots (Table 2[68,69]). In Brassica oleracea, glucosinolate concentrations diminished during the day and increased at night[70,71]. The broccoli sprouts grown in the presence of light contained more total glucosinolates than those grown in the absence of light[72].
Nutritional factors
Sulfur application
-
The application of commercial fertilizers to Brassicaceae crops is a common procedure; nevertheless, it has the potential to impact the composition of GSL. Applying high sulfur concentrations to crops can achieve significant enhancements in glucoraphanin and other GSLs with established health benefits[73]. It has been shown that increasing concentrations applying 600 mg of S plant−1 to increase concentrations (Table 2[56]). Researchers have determined that 108 mg of S plant−1 can effectively increase glucoraphasatin concentrations in radish. Elevated levels of sulfur have additionally been associated with rises in glucobrassicanapin, progoitrin, gluconapin, and sinigrin[74]. It has been demonstrated that an elevated S supply increases the concentrations of specific glucosinolates, including glucobrassicanapin, progoitrin, gluconapin, and sinigrin in Brassica juncea L. and total glucosinolates in Brassica rapa[75−77]. Interestingly, when all other parameters were met, other studies did not find any appreciable changes in total glucosinolates, aliphatic, or indolic glucosinolates in various cultivars and breeding lines between weak (15 kg·ha−1) and extremely high (150 kg·ha−1) S fertilization[78].
Potassium application
-
An increase in oxylipins and glucosinolates is the result of a potassium (K+) deficiency in Arabidopsis plants[44]. The roots contained more glucosinolates than the stems, and the K+ deficiency did not significantly impact the glucosinolates in the roots. It was hypothesized that glucosinolates serve distinct purposes in the roots and shoots and that jasmonic acid (JA)-signaling mediating glucosinolate synthesis in response to K+ deprivation in the shoot is only marginally involved. Based on similarities with the JA pathway set off by herbivores, it's possible that glucosinolates can help plants fight off K+ deficiency (Table 2[44]). Early-induced potassium deficiency in Brassica rapa seeds resulted in reduced glucosinolate content and plant growth and reproduction[54].
Nitrogen application
-
It has been observed that GSLs for broccoli, cauliflower, and radish increase as nitrogen administration decreases (Table 2[56]). It has been demonstrated that increased nitrogen fertilization (80–320 kg·ha–1) in conjunction with sulfur fertilization (60 kg·ha–1) is ineffective at increasing overall GSL concentrations in turnips; however, it can alter the ratio in favor of greater indolic GSL production. On the contrary, the levels of aromatic and aliphatic GSLs decrease in response to nitrogen levels increasing and sulfur applications at modest levels (10–20 kg·ha–1)[75]. For optimal development and the production of high-quality inflorescences, Brassicaceae necessitate a relatively high nitrogen content[79].
Selenium application
-
Selenium is an essential micronutrient for human beings. A robust association exists between the consumption of selenium through the diet and the likelihood of developing ailments such as cardiovascular disease, cancer, and immune system disorders[80]. Research has employed selenium to enhance the nutritional composition of crops, such as broccoli[81]. Studies indicate that excessive selenium application can reduce GSL content by 90%[82]. The application of selenium to radish plants resulted in an elevation of glucoraphanin levels in the roots (Table 2[81]). Moderate treatment can result in increased sulforaphane (SFN) concentrations in broccoli; nevertheless, alternative research has yielded inconclusive findings regarding sprouts, suggesting that the optimal effects of application are contingent upon the growth stage[80,83].
Pre-harvest and post-harvest factors
-
Numerous pre-harvest and post-harvest variables influence the synthesis, accumulation, and profile of GSLs in Brassica, as demonstrated. The aforementioned factors comprise the harvest stage of development, environmental and seasonal fluctuations, as well as the majority of agricultural practices, processing, storage, and packaging. Table 2 presents an overview of the current state of research and noteworthy discoveries.
Developmental stage at harvest
-
The development of broccoli heads is a critical factor in determining their phytochemical composition. Despite this, we have observed substantial variations in GSL metabolism among broccoli cultivars throughout the maturation process. The developmental stage (ontogeny) of the plants at harvest significantly influences the nutritional value that consumers will consume[84]. Peak GSL accumulation and crop maturity do not invariably coincide from a culinary perspective, as the former is subject to change throughout the life cycle. The broccoli heads exhibited their highest levels of glucoraphanin concentrations 180 d after sowing, and a decline in these concentrations occurred as flowering commenced[85]. Total aliphatic GSL concentrations diminish while indolic GSL concentrations increase over 7 d of sprouting, according to studies on cabbage, cauliflower, and broccoli[74]. Between transplanting and harvest, Brussels sprouts, cabbage, cauliflower and kale should increase their glucoraphanin levels (Table 2[61]).
Seasonal variation
-
The dynamic change in GSLs is highly associated with temperature and duration of daylight, and thus with seasonal variation. Light-induced gene expression in GSL biosynthesis also leads to the observation of diurnal rhythms; however, transcriptional profiling analysis reveals distinct genotype responses to temperature. However, there is only a limited connection between the expression of genes related to GSL and the actual amounts of GSL[72,75,86,87]. The levels of 4-methylsulfinylbutyl-GSL in broccoli florets were significantly higher at 12 °C compared to 18 °C when the photoperiod was long[86]. However, a brief photoperiod did not yield statistically significant differences. 3-methylsulfinylpropyl-GSL, on the other hand, showed the exact opposite pattern. Its concentrations were much higher at 18 °C compared to 12 °C during a short-day photoperiod, but the differences weren't important during a long-day photoperiod. Overall, the concentration of total aliphatic GSL was 33% greater during the long-day photoperiod at a temperature of 12 °C in comparison to the temperature of 18 °C; however, no substantial difference was observed during the short-day photoperiod. During the spring and autumn, augment the overall GSL content of broccoli and cauliflower (Table 2[56]).
Agricultural methods
-
The nitrogen-sulfur balance in the soil can be influenced by irrigation and fertilization, which likely has implications for the concentration and composition of GSLs and other secondary metabolites in plants[88,89]. Different studies have come to different conclusions about how water supply affects the GSL content of Brassica. This effect also seems to be species-specific and depends on other environmental factors. The amounts of methylsulphinylbuty-GSL and methylsulphinylbuty-isothiocyanate rose in most Brassica vegetables but fell in red radish roots because they didn't precipitate enough. Direct application of biochar from olive tree prunings significantly increased the GSL content of broccoli florets compared to treatments using conventional fertilizers. Notably, these treatments contained the lowest concentrations of methylsulphinylbuty-GSL and 1-methoxy-3-indolylmethyl-GSL[90]. An analogous result was observed where broccoli grown organically exhibited a significantly higher concentration of 3-indolylmethyl-GSL compared to conventionally grown broccoli (Table 2[91]). Conversely, 240 and 6% greater levels of 3-indolylmethyl-GSL and 1-methoxy-3-indolylmethyl-GSL were observed in conventional farming compared to organic farming[92].
Storage space
-
Due to its accelerated respiration rate, broccoli is considered an exceptionally perishable crop. This leads to significant economic losses and presents a problem for storing, transporting, and managing the supply. We observed an elevated range of GSLs in broccoli florets and crowns across diverse storage conditions. The florets of broccoli (cultivar 1997), preserved in a regulated environment and maintained at three distinct temperatures: 5, 10, and 18 °C, showed an increase in the concentrations of 4-hydroxy-3-indolylmethyl-GSL and 4-methoxy-3-indolylmethyl-GSL[93]. After 6 d of storage, the 4-methylsulfinylbutyl-GSL content of broccoli florets increased, then decreased regardless of the temperature (0, 5, and 10 °C); at 0–5 °C, the content can be maintained for 12 d (Table 2[94]). Levels of 1-methoxy-3-indolylmethyl-GSL in broccoli heads stored at 1–2 °C and 85%–90% RH for nine days increased by a factor of ten[95]. Broccoli florets (cultivar marathon) exhibited a reduction in total GSL content of up to 80% following a pre-storage period of 1 week at a temperature of 1 °C and 3 d at a temperature of 15 °C[96]. Broccoli florets subjected to 1-methylcyclopropene treatment and kept at 20 °C for 5 d exhibited a substantial decrease in total GSLs[97].
Further studies were conducted to investigate the effects of different storage conditions on broccoli florets. These conditions, which included storing the florets in cold storage at a temperature of 4 °C for 5 d, 4–8 °C for 7 d, or at a temperature of 25 °C for 2 d, followed by fluorescent light exposure did not observe any significant changes in the content of GSLs[98−101]. The feasibility of utilizing the essential oils of sage, fennel, basil, thyme, and caraway to maintain the GSL levels and horticultural qualities of broccoli sprouts at 4 °C and 95% RH during storage[102].
Processing methods
-
A variety of industrial and domestic processing techniques endanger Brassica vegetable crops. Reports indicate that heating, microwaving, dicing, cooking, steaming, and microwaving have a substantial impact on the GSL content of broccoli. Physical injury to plant tissue, such as frosting/defrosting, heating, grinding, slicing, mixing, juicing, and slicing, naturally activates the myrosinase-GSL system, leading to the accumulation of isothiocyanates and GSL decomposition products. After cutting broccoli florets into florets, incubating them at 20 °C for 24 h increased the concentrations of total GSLs by 490%, 4-hydroxy-3-indolylmethyl-GSL by 420%, and 1-methoxy-3-indolylmethyl-GSL by 1,300% (Table 2[103]).
After dicing and storing broccoli florets, 4-methoxy-3-indolylmethyl-GSL and 4-hydroxy-3-indolylmethyl-GSL were raised by a factor of 3.5 and 2 times, respectively, while the majority of glucosinolates decreased significantly[104,105]. We detected the increase in glucosinolate (GSL) production due to wounds, triggered by ethylene (4-hydroxy-3-indolylmethyl-GSL and 1-methoxy-3-indolylmethyl-GSL) and reactive oxygen species (ROS). Combining a treatment involving 250 ppm MeJA and ultrasound (20 min, 24 kHz, 100 m amplitude), the following concentrations increased: 232% for 1-methoxy-3-indolylmethyl-GSL, 187% for 4-hydroxy-3-indolylmethyl-GSL, and 112% for 4-methylthiobutyl-GSL[103,106]. The study discovered that boiling onion and broccoli florets together significantly increased the preservation of GSLs (glucosinolates) in a broccoli/onion system compared to broccoli alone. This finding suggests that such systems may have the potential to enhance the bioactivity of processed broccoli products in a manner that promotes health[107]. Under sous-vide conditions, the GSL content of kalian-hybrid broccoli decreased by 80%[104]. Overall, hydroxylated GSLs exhibit reduced stability at elevated temperatures (100–130 °C) compared to aliphatic GSLs. Furthermore, they undergo accelerated degradation in basic media, as opposed to neutral or mildly acidic media[108].
Packaging
-
Along with modified atmosphere packaging and controlled atmosphere storage, these methods can effectively extend the shelf life of Brassica vegetables without significantly reducing their GSLs. This is in contrast to the harmful effects of traditional canning, which lead to GSL loss and thermal degradation[105]. Previous studies have documented that cauliflower florets from various cultivars, including Parthenon, Aishwarya, and Lord F1, preserved aliphatic, aromatic, and polypropylene GSLs and nutritional quality more effectively when modified atmospheric pressure (MAP) was applied[100,109−111]. Despite the relatively higher temperatures, you could store these florets for up to three weeks. Total GSLs increased by 21%–42% compared to fresh florets following storage in an atmosphere containing air, 0.5% O2, and 20% CO2 (Table 2[112]). Broccoli stored for a duration of seven days at 10 °C under conditions of 0.5% O2 + 20% CO2 or 20% CO2 exhibited a 35% reduction in individual GSL concentrations, including 3-indolylmethyl-GSL. On the other hand, levels of 4-methoxy-3-indolylmethyl-GSL increased while it was stored in an atmosphere with low oxygen and continued to increase after it was moved to air.
-
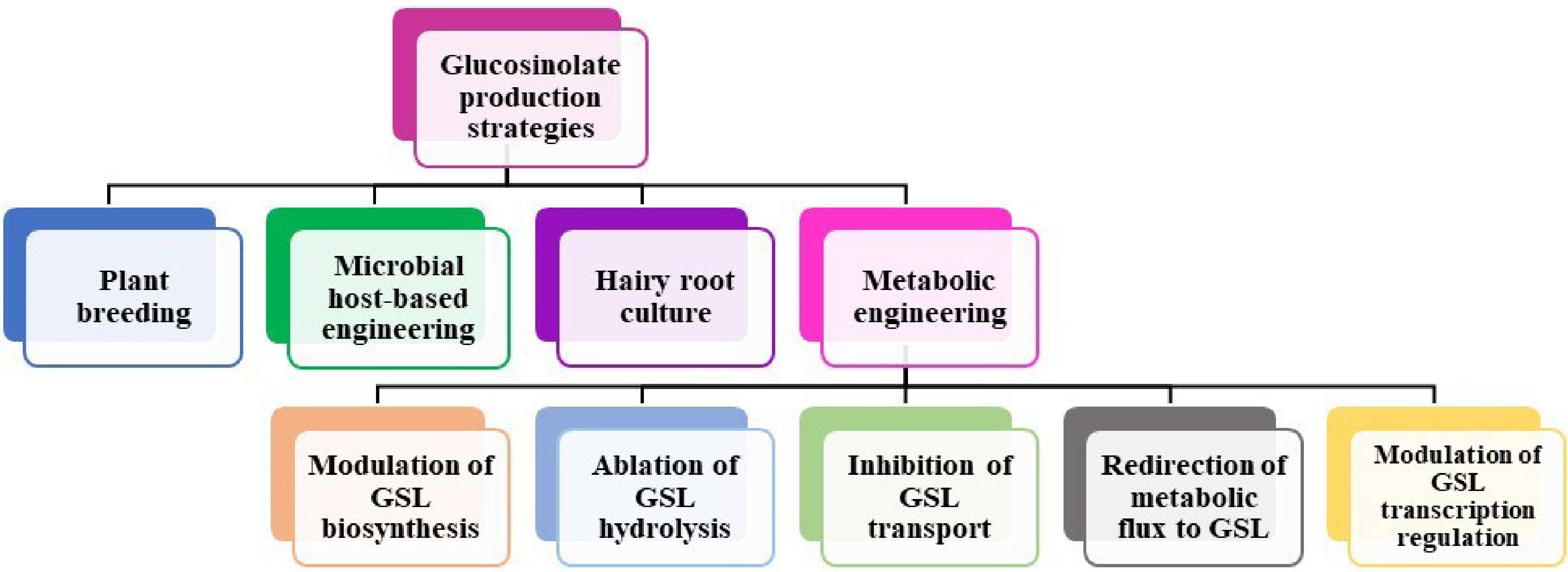
Existing literature indicates that as awareness of the health benefits for both plants and humans has increased, the emphasis has shifted to increasing GSL production. A growing body of knowledge regarding the health benefits of GSL has generated an increasing interest in increasing GSL consumption[116]. A multitude of methodologies have been implemented in order to attain substantial quantities of GSL production. Among these are normal breeding methods, transgenic methods, growing root and plant cells, metabolomics engineering, and microbial host-based engineering (Fig. 5). This section comprehensively discussed each of these approaches and made comparisons concerning the achieved production levels.
Plant breeding and cultures of hairy root
-
The private sector has well-established molecular breeding for certain Brassicaceae crops, but it's unclear how much of these efforts have gone toward enhancing the presence of GSL in commercially marketed types suitable for human consumption. The well-documented health advantages of GSL hydrolysis products (GHP) like glucoerucin, glucobrassicin, and glucoraphanin contribute significantly to the overall benefits[117]. These benefits could potentially be enhanced through selective breeding. When conducting reproduction for modified GSL profiles, it is imperative to consider a multitude of factors. In order to establish analogous resources for particular Brassicaceae commodities, it is imperative that breeders and researchers possess an extensive comprehension of the breeding history of plants and the GSL and ITC varieties that have been cultivated in diverse environments. Brussels sprouts (B. oleracea var. gemmifera) GSL profiles have been effectively modified through breeding to decrease bitterness and increase palatability[19,118]. Breeding Brassica oleracea var. italica, a commercially cultivated broccoli variety, and B. villosa, a wild variant exhibiting naturally elevated levels of 4-methylsulfinylbutyl glucosinolate (4MSB) and a 10-fold augmentation in the overall glucosinolate (GSL) concentration, resulted in the creation of BeneforteTM, an industrially accessible broccoli variety containing elevated concentrations of 4-methylsulfinylbutyl isothiocyanate (4MSB)[119]. We significantly more efficiently induced the conversion of glucosinolates (GSLs) to isothiocyanates (ITCs) compared to other metabolites[120]. So, adding the MYB28 gene as a transgene through a genetically modified organism (GMO) method might make it possible to grow a type of broccoli that has higher levels of 4-methylsulfinylbutyl glucosinolate (4MSB). An average decrease of 7% in S-methyl cysteine sulfoxide (SMCSO) levels is observed in plants harboring the B. villosa MYB28 allele; this decrease is associated with an increase in glucoraphanin[73]. Introgression of markers from Bacillus villosa into broccoli that are linked to genes that regulate the ratios of glucoraphanin to glucoiberin[121]. Selection for such alleles may have long-term implications for the health benefits experienced by consumers. An additional domain that could potentially be influenced via breeding is the alteration of the hydrolysis product pathway. A. thaliana possesses the epithiospecifier modifier 1 (ESM1) gene, responsible for encoding a protein that inhibits the conversion of glucosinolates (GSLs) to nitriles by the epithiospecifier protein (ESP). It would be critical to identify, select, and propagate these genes into Brassicaceae cultivars to improve the predictability of hydrolysis product formation. Since nitriles have considerably lower bioactivity than ITCs, reducing their production would be beneficial. This would lead to a greater availability of ITCs and the potential health advantages they offer. Hence, relying exclusively on GSL accumulation for the production of improved variety is insufficient; it is imperative to also take into account the abundance ratios of ITC, which vary among species, variations, and genotypes[122]. Potential candidate genes within the areas of the quantitative trait locus (QTL) influencing glucosinolate production were identified by comparative genomic analysis. QTLs for seed and leaf glucosinolates were found in B. rapa. Based on synteny with Arabidopsis and the identification of putative orthologous genes in B. rapa, comparative genomic analyses were used to identify the genes involved in the glucosinolate biosynthesis pathway that may account for the QTL. By crossing B. rapa with B. oleracea and employing marker-assisted selection, Chinese cabbage lines with modified glucosinolate profiles were produced. Recently, the complete genome sequence of B. rapa and cDNA/BAC libraries were used to identify the genes involved in the biosynthesis of glucosinolate. Comparative genomic research has established high collinearity in the glucosinolate biosynthesis pathway between A. thaliana and B. rapa. Candidate genes were co-mapped with the glucosesinolate QTLs identified by using the DNA sequences of Arabidopsis and B. oleracea for genes involved in glucosinolate production. The development of super broccoli is the best-known example, as described above. A unique mutant lacking dehydroerucin was found in radish, a popular vegetable in Japan. With the use of HPLC tests and a thorough evaluation of the glucosinolate profiles in numerous genetic resources, this mutant plant was identified as a local variety grown in Japan. In 2012, a new cultivar known as 'Daikon Chukanbohon Nou 5' was produced from the dehydroerucin null mutants. It is anticipated that using this cultivar as a breeding material will result in the creation of new cultivars with superior agronomic performance for the manufacturing of innovative processed foods. In the near future, to increase beneficial GSLs such as glucoraphanin in B. rapa, the development of molecular markers using sequenced genome information will promote marker-assisted selection (56 Ishida, Hara, Fukino, Kakizaki, and Morimitsu) of glucosinolate breeding.
The cultures of hairy roots can stimulate specialized metabolism[123]. The white mustard plant had the maximum glucosinolate concentration in its leaves, measuring about 60 mmol·g−1 DW. In contrast, the culture of the hairy root and the plant's roots contained only 10−15 mmol·g−1 DW of GSLs. The use of elicitors resulted in the observation that the white mustard plant exhibited the greatest GSL content after a period of 14 d following treatment with an estimated 100 mg of jasmonic acid (JA), equivalent to 20 mmol·g−1 of dry weight. Enhancement of beneficial glucosinolates (glucoraphanin for anticarcinogenic compounds in broccoli, dehydroerucin for pungent condiments in radish) and reduction of precursor glucosinolates of antinutritional compounds (progoitrin in B. oleracea, which includes cabbage) are the main breeding goals for vegetables in the Brassicaceae family.
Microbial host-based engineering of glucosinolates
-
Microbial hosts exhibit advantageous features for facilitating massive-scale manufacturing. At this moment, two microbial hosts, namely Saccharom yces cerevisiae and Escherichia coli, have been employed for the synthesis of GSL. In a study conducted by Mikkelsen et al.[124], the researchers achieved the creation of the fundamental indolic GSL, known as I3M, in the yeast species Saccharomyces cerevisiae. This was achieved through the integration of genes from the fundamental indolic GSL pathway of the plant species Arabidopsis thaliana into the genome of Saccharomyces cerevisiae. Three scholarly studies have been published about the field of GSL engineering in Escherichia coli. The synthesis of benzyl isothiocyanate (ITC) occur through the utilization of β-glucosinolates (β-GSL). This was accomplished by introducing into multiple strains of Escherichia coli a combination of four genes (CYP79A2, CYP83B1, UGT74B1, and SOT18) from the aromatic core structure pathway of Arabidopsis thaliana[125].
Metabolic modification of GSL in Brassica plants
-
Through molecular approaches, various attempts have been made to increase GSL diversity in the Brassicaceae family[35]. Engineering GSL in Brassica crops involves the utilization of various techniques that rely on enzyme and regulator genes (transcription factors). These techniques primarily involve manipulating GSL biosynthesis, preventing GSL hydrolysis, impeding GSL transport processes, and redirecting metabolic flux towards GSL (Table 3). It is important to acknowledge the intricate nature of metabolic engineering, as it involves connections among glucosinolate, phytoalexins, phytohormones, and metabolic pathways that influence the development and growth of the plant[41,126−128]. This enables us to assess the potential and feasibility of employing these genes as targets for future GSL engineering.
Table 3. Metabolic engineering of glucosinolate in Brassicaceae.
Approach Invoved process Strategy Gene name Target species Source species Effect on GSLs Ref. Modulation of GSL biosynthesis Side chain elongation KO MAM1 Chinese cabbage Arabidopsis thaliana Increased aliphatic GSL (3-butenyl-GSL, 4-pentenyl-GSL) [129] Glucone formation KO CYP79F1 Chinese cabbage Arabidopsis thaliana Increased levels of 2-hydroxy-4-pentenyl-GSL, indol-3-ylmethyl-GSL, 4-methoxy-indol-3-ylmethyl-GSL, decreased concentrations of 3-butenyl-GSL and 4-pentenyl-GSL, and increased concentration of 4 hydroxyindol-3-ylmethyl-GSL [140] Glucone formation KO CYP83A1 Chinese cabbage Arabidopsis thaliana Increased aliphatic GSL [129] Secondary
modificationOE FMOGS-OX Brassica rapassp. rapa Brassica rapa
ssp. rapaElevated aliphatic GSL [130] Glucone formation OE CYP79B2, CYP79B3, CYP83B1 Brassica rapa Arabidopsis thaliana Increased indolic GSL [131,141] Glucone formation OE UGT74B1 B. napus Brassica napus Elevate aliphatic GSL and indolic GSL [132] Ablation of GSL hydrolysis GSL hydrolysis Co- expression Myr1.Bn1 B. napus Brassica napus Seeds' myrosinase storing idioblasts were eliminated [133] Inhibition of GSL transport GSL transport KO GTR B. rapa Brassica rapa Lowered GSL content in seedlings [135] Redirection of metabolic flux to GSL Engineering metabolic flux Mutation TDC Brassica napus Catharanthus roseus Diminished indolic GSL in both seedlings and entire plants [136] Transcriptional regulation GS MYB28 Brassica oleracea var. alboglabra Brassica oleracea var. alboglabra Raised in aliphatic GSLs [137] Modulation of GSL transcription regulation Transcriptional regulation OE MYB28 Brassica rapa Brassica rapa Aliphatic GSL and indolic GSL are elevated [138] Transcriptional regulation OE MYB29 Brassica oleracea Brassica oleracea Enhanced aliphatic GSL concentration (2-propenyl glucoraphanin GSL) [139] The synthesis of GSL involves a series of enzymatic reactions, making it possible to control GSL accumulation by manipulating the expression of specific genes. In Chinese cabbage, metabolic engineering was employed to enhance aliphatic GSL production by overexpressing Arabidopsis CYP83A1, CYP79F1, and MAM1 genes, respectively (Table 3[129]). The results showed that the A1-1 transgenic line of CYP83A1 exhibited increased levels of all aliphatic GSLs, while the MAM1 transgenic line M1-1 only showed elevated contents of 3-butenyl GSL and 4-pentenyl GSL. Interestingly, the three CYP79F1 transgenic lines (F1) displayed inconsistent changes in GSL levels. F1-1 demonstrated increased levels of 4-methoxyindol-3-ylmethyl GSL indol-3-ylmethyl GSL, and 2-hydroxy-4-pentenyl GSL, whereas F1-2 and F1-3 lines showed higher levels of 4-hydroxyindol-3-ylmethyl GSL along with reduced levels of 3-butenyl GSL and 4-pentenyl GSL, compared to the wild type. In the hairy roots of turnips, overexpression of the FMOGS-OX genes elevates the quantity of aliphatic glucosinolates (Table 3[130]). The overexpression of CYP79B1, CYP79B2, and CYP79B3 genes in Chinese cabbage from Arabidopsis increases indole GSLs (Table 3[131]). There was no modification in the profile of GSLs when there was a single gene transformation, CYP79B1 or CYP79B3, but when co-expression of CYP79B2 or CYP79B3 with CYP79B1 elevated the level of 4-methoxyindol-3-ylmethyl GSL, 4-hydroxyindol-3-ylmethyl GSL, and indol-3-ylmethyl GSL were acquired in transgenic plants. In B. napus, overexpression of BnUGT74B1 elevates the accumulation of indolic and aliphatic GSL and also increases the resistance of Botrytis cinerea and Sclerotinia sclerotiorum in transgenic plants (Table 3[132]). Co-expression of barnase under the power of the Myr1.Bn1 promoter in the seed myrosin cell with barstar (an inhibitor of barnase) under the power of the 35s promoter of the cauliflower mosaic virus in Brassica napus and eliminate idioblasts storing myrosinase from the seeds without causing any adverse effects on the overall plant (Table 3[133]). The role of GTR proteins is to transport GSL from mother tissue to seeds[134]. In Brassica rapa, targeting non-transgenic induced lesions in genomes (TILLING) produces GTR orthologs. This approach particularly reduced the GSL concentration (60%–70%) in seeds (Table 3[135]). Other methods, such as metabolic flux engineering, involve the transformation of the tryptophan decarboxylase (TDC) gene into Brassica napus, which changes tryptophan to tryptamine instead of indolic glucosinolates (Table 3[136]). Transcription factors also control the metabolic pathway of glucosinolate, and many biosynthetic genes are regulated by one transcription factor. In B. oleracea, the MYB28 transcription factor was overexpressed, then aliphatic glucosinolate was raised (Table 3[137]). But in Brassica rapa, overexpression of MYB28 increases both indole and aliphatic glucosinolates (Table 3[138]). Another transcription factor, BoMYB29, its overexpression in Brassica oleracea raised the accumulation of glucoraphanin and 2-propenyl GSL (aliphatic GSL) (Table 3[139]). As stated in 'Metabolic engineering of GSL in Brassica crops', many biotechnologies have been used to carry out metabolic engineering of GSL in Brassica plants to precisely alter the overexpression, RNA interference, and antisense RNA GSL patterns. Continuous improvements in the metabolic engineering of GSL have led to the identification of more regulators, such as transcription factors and signal transduction components. While MYB28 and MYB29 have been successfully engineered in Brassica crops for efficient GSL modulation, other regulators like MYB34 hold promise. MYB34, a core transcription factor, regulates the biosynthesis of indolic GSL and has shown potential for increasing indolic GSL content and promoting vegetative growth in Arabidopsis. Engineering MYB34 in Brassica vegetables, whose vegetative parts are edible, could enhance GSL accumulation and yield benefits.
-
There is hope for the future of breeding Brassicaceae crops rich in glucosinolates and isothiocyanates (GSLand ITC). Breeders have a wide range of options to choose from, and customer demand for healthier products is rising. Such crops are in demand as consumers become more conscious of the advantages of eating healthily. The viability of this technique is demonstrated by the success of Beneforte broccoli, but it necessitates a comprehensive plan that takes into account several variables, including varietal development, production, agronomy, environment, and consumer preferences. There's a plan in place, even though it might take some time to complete. The culture of hairy roots have shown improvement but remains economically challenging and often result in complex mixtures requiring downstream purification. Microbe engineering represents a promising alternative, requiring significant optimization through strategies such as directed evolution, metabolic flux analysis, and protein engineering. High-throughput screening platforms enabled by computational software and robotic laboratory equipment contribute to the feasibility of these approaches. In the near future, new biotechnology tools are looking forward to high production of glucosinolates. In the future, advancements in metabolic engineering of glucosinolates (GSLs) will continue, with a focus on identifying and manipulating regulators of GSL metabolism. While MYB28 and MYB29 have been successfully engineered in Brassica crops, there's potential for enhancing GSL accumulation by targeting other regulators like MYB34[35]. In Brassica crops, heterologous expression of GSL-related genes offers a feasible strategy for introducing new GSL profiles and enhancing nutritional value. The use of CRISPR/Cas9 technology to knock out genes that negatively control GSL metabolism. It is a promising approach for increasing beneficial GSL content without incorporating outside genetic material[35]. Although the complexity of duplicated GSL metabolic pathways in Brassica crops presents challenges, the identification of conservative core signaling components simplifies manipulation. Omics approaches such as transcriptomics, metabolomics, proteomics, and phenomics contribute to the identification of new constituents and essential controllers of GSL metabolic pathways. Manipulating GSL content based on conserved components proves to be more convenient and efficient. Beyond GSL-containing plants, synthesis in heterologous hosts like tobacco, Saccharomyces cerevisiae, and Escherichia coli is explored. While levels are lower than in Brassicaceous plants, the advantage lies in harvesting single GSL without downstream purifications[35]. Optimization is crucial for overcoming obstacles to commercialized production.
GSLs belong to a group of sulfur-containing compounds that are primarily synthesized by the Brassicaceae family plant species. According to the amino acids from which they originate, GSLs are classified as aromatic, indole, and aliphatic. The food industry has shown considerable interest in the isothiocyanates (ITCs), sulfate ions, and D-glucose that are produced during the hydrolysis of glucosinolates. These compounds can provide health benefits. The degradation of GSLs via myrosinase enzymes is crucial to enhance stress tolerance. Moreover, due to their chemical makeup, GSLs can serve as reservoirs of essential nutrients during periods of scarcity. In essence, GSLs serve nutritional functions and possess the capacity to augment human health. Furthermore, it highlights the captivating interaction that exists between stress response mechanisms and the metabolism of glucosinolates, implying that glucosinolates might serve as signaling molecules along this interrelated pathway. To retain the benefits of glucosinolates throughout the agro-food chain, these strategies consist of the following: breeding for glucosinolate-biofortified cultivars; metabolic engineering to modify glucosinolate profiles; pre-harvest treatments to enhance glucosinolate accumulation; and post-harvest handling and processing methods (Fig. 6). This exhaustive analysis delves into the multifaceted uses of glucosinolates, specifically examining their potential to improve the overall quality and nutritional value of Brassica crops. The capacity to modify the GSL concentration of these crops present promising prospects for employing genetic and environmental interventions to address health and nutritional deficiencies.
-
The authors confirm contribution to the paper as follows: study conception and design: Lakra N, Bansal S, Ahlawat YK; draft manuscript preparation: Bansal S, Mishra S, Ahlawat YK. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The authors acknowledge support from the Department of Botany and Plant Physiology, Chaudhary Charan Singh Haryana Agricultural University, Hisar and CSIR-UGC for providing fellowship during the research work.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Bansal S, Lakra N, Mishra S, Ahlawat YK. 2024. Unraveling the potential of glucosinolates for nutritional enhancement and stress tolerance in Brassica crops. Vegetable Research 4: e015 doi: 10.48130/vegres-0024-0016
Unraveling the potential of glucosinolates for nutritional enhancement and stress tolerance in Brassica crops
- Received: 31 December 2023
- Revised: 04 April 2024
- Accepted: 06 May 2024
- Published online: 23 May 2024
Abstract: Brassicaceae family plants, including cabbage and broccoli, widely distribute glucosinolates derived from amino acids. An S-β-d-glucopyrano unit is anomerically linked to an O-sulfated (Z)-thiohydroximate moiety to form glucosinolates. The potential biological effects of intact glucosinolates are currently being debated within the scientific community. The action of myrosinase on glucosinolate generates glucosinolate-derived hydrolysis products, which increase tolerance to abiotic and biotic stress and improve human health. Here, we investigate the possible applications of glucosinolate bioactive functions, to harness them for the advancement of sustainable agriculture in the future. We have utilized various methods, such as traditional breeding, transgenic techniques, hairy root and plant cell cultures, microbial host-based engineering, and biotechnological methods on Brassica crops to obtain advanced sources of glucosinolates. We can investigate and evaluate the potential for manipulating underutilized or exploited genes related to the biosynthesis, hydrolysis, and transport of glucosinolates, thereby considering them as potential targets for GSL engineering. The goal of this review is to help us learn more about the complicated relationships between glucosinolate-related processes and how they can be used to help plants deal with stress.
-
Key words:
- Glucosinolate /
- Metabolisms /
- Glucosinolates /
- Postharvest /
- Breeding /
- Postharvest shelf life