-
Palm oil production generates various waste such as press fruit fibers (PFF), empty fruit bunch (EFB), oil palm shell (OPS), palm oil mill effluent (POME), decanter cake (DC), and palm kernel expeller (PKE). Indiscriminate disposal of this waste became an enormous issue in palm oil-producing countries because more than 4 tons of waste are generated from every 100 tons of processed fresh palm fruit bunch[1−4]. Landfill and open burning are the common methods of waste management disposal in several countries. Those methods are hazardous to the environment due to the greenhouse gas emission and contamination of soil and water bodies from the leaching of toxic compounds[5,6]. Several biological technology approaches have been introduced in organic waste management including vermicomposting and composting using effective microorganisms[7]. The approach adopted in this study was to use black soldier fly larvae (BSFL) as the waste degrader agent to ingest DC and PKE mixture and excrete them into larvae frass[8]. The black soldier fly (Hermetia illucens) is in the family Stratiomyidae under the order Diptera[7]. The BSFL has increasingly been used as decomposer agents for waste management due to the ability of the larvae to consume various types of agricultural and domestic waste. The life cycle of the insects is divided into four stages including egg, larval, pupal, and adult stages[9]. The larval stages are the longest phases of their life cycle which consist of six larval instars[10] (Fig. 1).
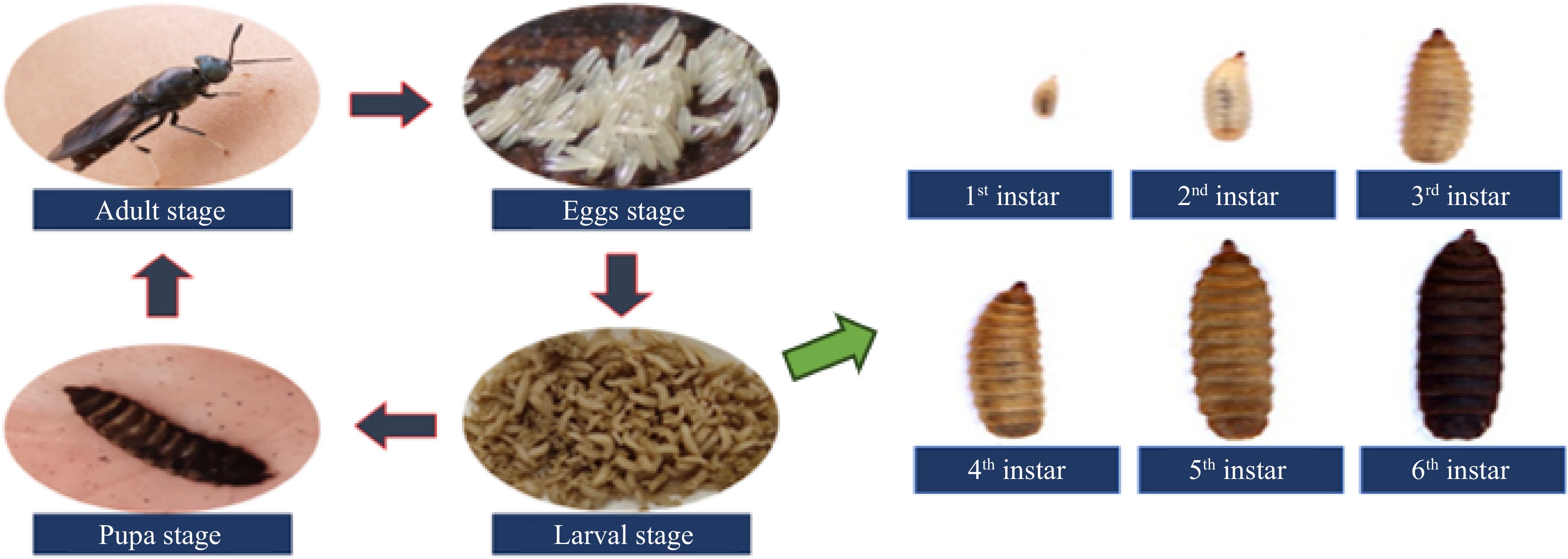
Figure 1.
The life cycle of black soldier flies in four phases and six stages of larvae in different instars.
The average life cycle of a black soldier fly in the tropics, from the egg until adult stage is approximately 40 to 43 d[11,12]. However, the larval stage can last for four months depending on the availability of feed supply and other favourable conditions for the larvae's growth such as appropriate temperature and relative humidity[12]. The duration of six instars during the larval stage is one of the factors affecting the effectiveness degradation of organic waste. Raga et al.[13] and Cheng et al.[14] reported the degradation efficiency of BSFL as a decomposer agent for managing residues from landfill mining and food waste. In other related studies also stated the important of BSFL for managing organic waste with 65%−79% degradation efficiency[15,16]. Thus, BSFL has been widely used for managing organic waste[17,18] and the end-product of larvae excretion known as frass is useful and could be used as a soil organic amendment. Previous research related to the impacts of the BSFL frass application on maize production[16] and kale (Brassica oleracea var. sabellica)[19] shows positive effects on crop development suggesting that BSFL frass has become a promising alternative to commercial fertilizers in improving crop productions.
The frass excreted by BSFL contains macronutrients, micronutrients, and organic matter that could be used to improve soil fertility[20,21]. The BSFL frass contains 5% total nitrogen (N), 2% total phosphorus (P), and 2% total potassium (K) which are suitable to be used as organic fertilizer[22]. The alkaline pH of BSFL frass ranging from 7 to 8 had been reported as one of the factors contributing to the soil acidity improvement that favor plant nutrients availability in soils[23]. In addition, chitin (exoskeleton of larvae) in BSFL frass which consists of 3.0%–6.8% N[24] helps to promote plant growth and development to provide plant defenses against pest attacks[23]. In another related study on BSFL, frass produced from water hyacinth by Hassan et al.[25] suggesting that the BSFL frass has a significant amount of organic matter (50.31%), organic carbon (29.18%), total N (2.18%), ammonium (1,270 mg kg−1), nitrate (1,370 mg kg−1), and available P (1,400 mg kg−1) with a C/N ratio of 13:1. Chiam et al.[26] reported that the application of BSFL frass as an organic fertilizer increased the yield of lettuce compared with conventional fertilizers. However, the quality and nutrient composition of the BSFL frass depends on the type of feeding substrates used for rearing BSFL for frass production[27,28] and the stabilization of frass during the rearing process. For instance, frass produced from BSFL digested on vegetable wastes had a significantly higher value of N, P, and K compared with BSFL frass produced from fruit wastes or starches as feeding substrates for BSFL[29]. In our previous study, we used a similar weight of BSFL to digest the mixture of DC and PKE as feeding substrates for frass production[3]. In this study, the attempt was to use different treatment weights of BSFL to degrade similar amounts of DC and PKE mixture. Also, the attempt focused on the potential of BSFL frass produced from different treatment weights of BSFL as a source of alkaline material to buffer the pH changes of the Bekenu series soil.
Bekenu series soils are acidic soils and lack basic cations due to the high accumulation of sesquioxide (formation of Al and Fe oxides-hydroxides)[30]. Sesquioxide are non-silicate clay mineral or colloid found in soils. The colloid consists of iron (Fe) and aluminum (Al) rich silicates that form through oxidations by chemical weathering. More accumulation of oxides in soil affects the degradation of soil organic matter and soil nutrient such as phosphorus becomes unavailable for crops because it is fixed by sesquioxide[30]. Additionally, excessive inputs of fertilizer, such as ammonium-based fertilizers in Bekenu series soil results in soil acidification because each of the ammonium (NH4+) molecules are nitrified to nitrate (NO3−) and generates two H+ ions in the soil. The common types of lime materials used to neutralize soil acidity are calcium carbonate (CaCO3) and dolomite, but the costs of those lime materials are expensive for farmers in certain developing countries[31]. Well-buffered soil following the application of organic amendments such as sago bark ash, charcoal,[32] and composts[33] have been reported to neutralize soil acidity due to the high cation exchange capacity (CEC) of organic matter from those organic amendments[34]. Therefore, it is expected that the application of BSFL frass could increase the Bekenu Series soil buffering capacity due to high organic matter content and availability of the negative surface sites in the BSFL frass to adsorb H+ ions. It was hypothesized that the frass produced from different treatment weights of the BSFL feed on similar amounts of DC and PKE mixture would differ in degradation efficiency, consist of different physico-chemical properties, and be phytotoxicity free. It is also expected that the BSFL frass application could buffer pH changes in Bekenu series soil regardless of the BSFL weights used during the frass production. The information on using different weights of the BSFL in degradation efficiency, BSFL frass production, and its effect on soil pH buffering capacity are scarcely explored. Therefore, the objectives of the study were to: (i) assess the waste reduction rate (WRR) and waste reduction index (WRI) of BSFL frass produced from DC and PKE mixture as feeding substrates using different treatment weights of BSFL, (ii) determine selected agronomic properties of the BSFL frass, and (iii) evaluate the BSFL frass application effect on the Bekenu series soil pH buffering capacity.
-
The DC and dried PKE were collected from BSF Farming and Trading Bintulu, Sarawak, Malaysia. The DC was air-dried for 7 d at a drying yard of Putra Agriculture Centre, Universiti Putra Malaysia Bintulu Campus (UPMKB), Malaysia. The DC and PKE were mixed as feeding substrate for different treatment weights used in rearing BSFL. The initial characterization of the DC and PKE mixture as feeding substrates was analyzed at Soil Science Laboratory, UPMKB. Selected chemical properties including pH in water, CEC, total organic matter (TOM), total organic carbon (TOC), exchangeable NH4+, available NO3−, total nitrogen (N), and total phosphorus (P). The pH for mixed feeding substrate was determined using a digital pH meter[35]. The CEC was determined using the leaching method[36] followed by steam distillation[37]. The exchangeable NH4+ and available NO3− were determined following the method described by Keeney & Nelson[38]. The Kjeldhal method was used to determine the total N which involved digestion, distillation, and titration procedures[37]. Total P was determined using a single dry ashing method[36] followed by the Molybdenum Blue Method[39]. The TOM and TOC of the mixed feeding substrate were determined using the loss on ignition method[39]. The carbon-to-nitrogen (C/N) ratio of the feeding substrates was calculated by dividing the percentage of C and the percentage of N. The initial characterization of the DC and PKE mixture is presented in Table 1.
Table 1. Selected chemical properties of decanter cake and palm kernel expeller.
Properties Mixture of DC and PKE pHwater 6.33 ± 0.03 CEC (cmol kg−1) 10.1 ± 1.63 Exchangeable NH4+ (mg kg−1) 3.97 ± 0.62 Available NO3− (mg kg−1) 3.27 ± 0.23 Total N (%) 1.72 ± 0.14 Total P (mg kg−1) 2976 ± 87.9 TOM (%) 82.5 ± 2.09 TOC (%) 47.8 ± 1.21 C/N ratio 28:1 The values given are based on a dry-weight basis. Mean value followed by ± standard error. Rearing of black soldier fly larvae for frass production and analyses of frass
-
The BSF eggs were supplied by the BSF Farming and Trading Bintulu, Sarawak, Malaysia, whereas the BSFL rearing was carried out under the drying yard at Putra Agriculture Centre, UPMKB. The larval rearing area was covered with a sunshade netting to maintain the 60% moisture content and ambient temperature of 26 °C. The temperatures of feeding substrates were determined using a Reotemp compost thermometer (Reotemp Instrument Co, Van Nuys, California, USA). Whereas the moisture contents of the feeding substrates were maintained at 60% using the Reotemp compost moisture meter (Reotemp Instrument Co, Van Nuys, California, USA). The rearing process of BSFL were initiated with the BSF eggs laid on a fine net and placed on the top of a plastic tray size of 65 cm length × 37 cm width × 15 cm depth. After the BSF eggs hatched, the juvenile larvae were fed with rejected sago starch as their starter diet until the juvenile larvae reached the second instar (out of six instars at the larvae stage as shown in Fig. 1). Thereafter, the second instar of BSFL was transferred into the feeding trays consisted of five different treatment weights of BSFL (T1, T2, T3, T4, and T5). Each treatment has three replications (feeding trays), and each replicate was supplied with a mixture of DC and PKE in the ratio of 7:3 (700 g DC and 300 g PKE). In two-week intervals, the feeding trays were added with a 3 kg mixture of DC and PKE each until the BSFL reached to prepupae stage at 54 d of rearing. It must be noted that the amount of DC and PKE mixture as feeding substrate for rearing the BSFL was 15 kg for all treatments. However, the mixture of feeding substrates were added in the interval of two weeks to avoid excessive feed and also was based on the degradation capacity of the BSFL.
Treatments with different weights of the BSFL evaluated in this study is summarized as follows:
T1: 15 g of BSFL + 15 kg of DC and PKE
T2: 20 g of BSFL + 15 kg of DC and PKE
T3: 25 g of BSFL + 15 kg of DC and PKE
T4: 30 g of BSFL + 15 kg of DC and PKE
T5: 35 g of BSFL + 15 kg of DC and PKE
The feeding trays were covered with mesh to prevent the oviposition of other flies. The temperatures of the feeding substrates were taken in three-day intervals to avoid the increase in temperature that inhibited the growth of BSFL. The feeding trays were sprayed with water when necessary to ascertain 60% moisture content of the feeding substrates. The BSFL frass was collected manually using a hand sieve to separate the frass from the substrate residues and pupae at 54 d of rearing the BSFL. The BSFL frass was air-dried and was further analyzed for pH, CEC, TOM, TOC, exchangeable NH4+, available NO3−, total N, and total P using standard procedures as previously described.
Black soldier fly larvae as decomposer agent for waste reduction rate
-
The volume of waste reduction by the BSFL was carried out in this study to confirm the efficiency of the degradation rate by incorporating the different treatment weights of BSFL used during the rearing of BSFL. The weight of leftovers of the feeding substrates, chitin, and BSFL frass collected after the larvae have metamorphosed to pupal stages at 54 d of the BSFL rearing was measured using a weighing balance. The effectiveness of BSFL in reducing the substrate was calculated as follows[40]:
$ \rm WRR=[(S-R)/S]\;\times 100{\text{%}} $ (1) $ \rm{WRI=WRR/t} $ (2) where, WRR represents waste reduction rate and WRI refers to the waste reduction index. S is the total quantity of substrate applied throughout the experiment. R is the weight of frass after the bioconversion whereas t is the time of bioconversion.
Phytotoxicity test
-
The germination bioassay was carried out for a phytotoxicity test of BSFL frass under controlled conditions using a method described by Zucconi[41] to ascertain that the BSFL frass is not toxic to the plant. A 10 g sample of air-dried BSFL frass taken from T1, T2, T3, T4, and T5 (different treatment weights) were weighed and mixed with 100 mL of distilled water. The supernatants were shaken for 24 h after which they were centrifuged for 20 min at 10,000 revolutions per minute (rpm). The supernatant of each treatment was filtered through a Whatman No.2 filter paper and labeled as initial extraction (IE). Thereafter, the IE of the BSFL frass of each treatment were diluted into three dilution series 10 times dilutions, 100 times dilutions, and 1000 times dilutions. Diluted BSFL frass extractants from different treatment weights were then labeled according to the dilution series such as dilution 1 (D1), dilution 2 (D2), and dilution 3 (D3), respectively. Ten seeds of Thai Super Sweet hybrid F1 maize (Zea mays) used as a test crop for a germination test were placed in the petri dish and lined with the Whatman No. 2 filter paper. Petri dishes were then filled with 5 mL of four extracted BSFL frass (IE, D1, D2, and D3) representing different treatment weights (T1−T5). A similar procedure was repeated using distilled water which was prepared as a positive control (C) for comparison with the BSFL frass extractants. The Petri dishes were placed in a dark room and maize seeds were germinated for 7 d. Maize seed emergences were monitored daily and the measurement of root and shoot length of the germinated maize seeds was carried out on day 7. It must be noted that if the germination index (GI) values of the BSFL frass extractions fall below 50%, the BSFL frass extractants would be considered highly phytotoxic whereas if the GI values range from 50% to 80%, the GI values suggest that the BSFL frass extractants are moderately phytotoxic. Whereas, if the GI values are more than 80% the values indicate that the extractants of the BSFL frass are not toxic to the plants[3].
The GI, germination (G) and relative root growth (RRG) were expressed in percentage and measured using the following formula:
$ \rm {Germination\;index\;({\text{%}})=[G({\text{%}})\;\times\; RRG({\text{%}})]\;\times \,100} $ (3) where:
$ \rm {G\;({\text{%}})=\dfrac{\rm Number\;of\;seeds\;germinated\;in\;sample}{\rm Number\;of\;seeds\;germinated\;in\;control}\times 100} $ $ \rm {RRG\;({\text{%}})=\dfrac{\rm Mean\;root\;length\;in\;a\;sample}{\rm Mean\;root\;length\;in\;control}\times 100} $ Soil selected chemical properties and buffering capacity analysis
-
The pH buffering capacity of BSFL frass was determined following the method described by Costello & Sullivan[42]. The soil used for the buffering capacity study was Bekenu Series (Typic Paleudults) which was collected from an uncultivated secondary forest of UPMKB that was located at latitude 3°12'20" N and longitude 113°04'20" E. Bekenu Series soil is classified under soil order of Ultisols which is characterized as less fertile and acidic due to high weathering and rainfall. The soil sample was randomly collected with specifications of 1 m length × 1 m width at a depth of 0−15 cm using a shovel after which the soil was air-dried, crushed, and sieved to pass a 2 mm sieve. The initial selected chemical properties of the soil were analyzed for pH in water, CEC, TOM, TOC, exchangeable NH4+, available NO3−, available P, total N, and total P using standard procedures as described previously. The characterizations of the Bekenu series soil are summarized in Table 2.
Table 2. Initial selected chemical properties of Bekenu Series soil for pH buffering capacity determination.
Property Value obtained pHwater 4.67 ± 0.25 CEC (cmol kg−1) 11.67 ± 0.21 Total N (%) 0.37 ± 0.42 Total P (mg kg−1) 5.27 ± 0.31 Available P (mg kg−1) 0.114 ± 0.01 OM (%) 5.03 ± 1.04 TOC (%) 2.91 ± 0.60 Exchangeable NH4+ (mg kg−1) 12.00 ± 2.49 Available NO3− (mg kg−1) 5.20 ± 1.01 The values given are based on a dry-weight basis. Mean value followed by ± standard error. The amount of Bekenu Series soil used in pH buffering capacity analysis was 350 g and labeled as S0 (soil only). It must be noted that the estimation of soil pH buffering capacity was carried out after the insignificant difference found in WRR and selected agronomic properties of BSFL frass. Owing to insignificant effects on those properties, the BSFL frass from all treatments (T1−T5) were mixed with modifications of their weights based on the recommendation of organic fertilizer used in maize cultivation. The amount of the BSFL frass application was based on 5 t ha−1 application in the field and scaled down for experimental purposes which is equivalent to 9 g per container representing the standard recommended for maize cultivation. The BSFL frass only was prepared without the addition of soil and indicated as F1. A similar amount of the BSFL frass (9 g) was used but added with 350 g soil and labeled as F2, whereas the addition of the BSFL frass was reduced by 20% from 100% of recommendation which is equivalent to 7.2 g added with 350 g soil was labeled as F3, and the addition of the BSFL frass reduced by 40% from 100% of the recommendation which is equivalent to 5.4 g added with 350 g soil was set as F4.
Treatments evaluated in soil pH buffering capacity were given as follows:
S0: 350 g of soil only
F1: 9.0 g of BSFL frass only
F2: 350 g soil + 9.0 g BSFL frass
F3: 350 g soil + 7.2 g BSFL frass
F4: 350 g soil + 5.4 g BSFL frass
All of the treatments were weighed separately in plastic vials. Thereafter, 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 mL (1 mL = 0.1 mol H+ kg−1 sample) of 0.25 M H2SO4 were added with the adequate amount of distilled water to bring total liquid addition to 50 mL (1:10 sample: distilled water). The initial pH of samples was taken before the acid was added. The samples were stirred for 10 s after adding acid and equilibration for 72 h at room temperature. Before measurement at 72 h, the suspension was stirred for another 10 s, and the pH of the samples was measured using a digital pH meter. The quantity of acidity needed to reduce pH by one unit was measured as the negative reciprocal of the slope of the linear regression, pH of samples (Y-axis) versus the rate of acid addition (X-axis):
$ \rm{Soil\; pH\; buffering\; capacity\; (mol\; H}^+\ kg^{-1})=-(1/Slope) $ (4) where, the slope is equal to the fitted slope of the linear regression line for each sample.
Experimental design and statistical analysis
-
The experimental design of BSFL rearing for frass production, phytotoxicity test, and soil buffering capacity was laid in a completely randomized design (CRD) with three replications. All of the analyses carried out in the laboratory were replicated three times for data validity and accuracy. Data were subjected to one-way analysis of variance (ANOVA) to test treatment effects whereas the significant means of treatment were compared using Tukey's Studentized Range (HSD) test at p < 0.05. The analysis was done using Statistical Analysis Software (SAS version 9.4).
-
Regardless of the different treatment weights, the BSFL became insatiable feeders which reduced the volume of DC and PKE mixture by more than 50% from the initial 15 kg of the feeding substrates used in all treatments (T1, T2, T3, T4, and T5). All of the BSFL actively digested mixture of DC and PKE started at the third to fifth instar before the larvae reached to prepupal stages. The prepupae migrated from the feeding substrates to the dry area for metamorphosis into pupae before they emerged to the next stage which is an adult phase. At the pupae stage, the pupae were immobile, and their mouthparts were inactive, not feeding, thus the digestive tract was empty. The BSFL frass was collected at the pupal stage and air dried for easier separation due to the clumpy structure of the BSFL frass. The BSFL frass turned into a dark color and had a soft texture (Fig. 2a & b).

Figure 2.
(a) Colour and texture of undigested substrate (DC and PKE mixture). (b) Colour and texture of BSFL frass after being fed on DC and PKE mixture.
The average ambient temperature during the rearing of the BSFL was 26 °C whereas the average substrate temperature in all treatments was 27 °C, which is considered the optimum temperature for bioconversion activity of the BSFL (Table 3). Kim et al.[43] suggested that the optimal temperature of the rearing BSFL was 26 to 27 °C with 60% to 70% relative humidity. If the temperature of the feeding substrates exceeds 30 °C, the BSFL could not survive and is not active in digesting their feed[44]. The present study observed that the BSFL became less active when the substrate temperatures were below the average range such as below 20 °C. The increased temperature in all treatments (feeding trays) was due to the metabolic activity of the BSFL and respiration process in the mixture of feeding substrates. The moisture content of the feeding substrate in all treatments were sprinkled with water when the feeding substrates dried to maintain the temperature at permissible level such as between 26 to 27 °C and maintain the 65% of moisture content. Maintaining the ideal moisture level in the feeding substrate is important because excessive moisture causes the low temperature of the feeding substrates. In addition, BSFL needs a mesophilic environment to convert biowaste efficiently[45,46]. The feeding substrate temperatures in all treatments were similar to the ambient temperature toward the end of the larval stage because of the stabilization stage and maturation phase whereby the larvae turn into pupae and the process of digestion ends when the BSFL reaches into pupal stage (inactive stage) at 54 d of the rearing process (Table 3).
Table 3. Average temperature of ambient and feeding substrates in 54 d of rearing black soldier fly larvae.
Treatment T1 T2 T3 T4 T5 Ambient temperature (°C) 26 ± 0.30 26 ± 0.30 26 ± 0.30 26 ± 0.30 26 ± 0.30 Feeding tray temperature (°C) 27 ± 0.48 27 ± 0.41 27 ± 0.43 27 ± 0.44 27 ± 0.41 Mean value followed by ± standard error. No significant difference among the treatments at p < 0.05. T1: 15 g BSFL + 15 kg DC and PKE; T2: 20 g BSFL + 15 kg DC and PKE; T3: 25 g BSFL + 15 kg DC and PKE; T4: 30 g BSFL + 15 kg DC and PKE; T5: 35 g BSFL + 15 kg DC and PKE. Waste reduction by black soldier fly larvae as a decomposer agent
-
A similar amount of feeding substrates (15 kg of DC and PKE mixtures) used for rearing different weights of BSFL were ingested to 2.43, 2.65, 2.75, 2.66, and 2.42 kg of BSFL frass in T1, T2, T3, T4, and T5, respectively. There was no significant difference in the waste reduction rate (WRR) and waste reduction index (WRI) across treatments suggested that different treatment weights of the BSFL have no effects on the ingestion of 15 kg feeding substrates (Table 4). The feeding substrates supplied in all feeding trays had completely ingested by the BSFL at similar feed conversion efficiency. The assumptions on using different weights of BSFL to ingest similar volumes of DC and PKE mixture would provide different WRR and WRI values were not achieved. According to Jucker et al.[47], WRI is the ability of larvae to reduce feeding substrate whereby the higher the values of WRI, the greater their ability to consume the organic wastes. In this study, different weights of the BSFL seemingly does not affect the number of larvae per individual nor on the rate of feed conversion efficiency or there could be a possibility that not all juvenile larvae (first and second instar) survived until the pupae stage due to competition among larvae for feed ingestion.
Table 4. Bioconversion of feeding substrates into frass excreted by black soldier fly larvae.
BSFL
weights (g)Weight of DC and
PKE applied (kg)BSFL frass
produced (kg)Material reduction WRR (%) WRI (d−1) T1−15 15 2.43 83.79 ± 0.69 1.55 ± 0.01 T2−20 15 2.65 82.36 ± 0.64 1.53 ± 0.01 T3−25 15 2.75 81.69 ± 0.83 1.51 ± 0.02 T4−30 15 2.66 82.25 ± 0.53 1.52 ± 0.01 T5−35 15 2.42 83.84 ± 1.56 1.55 ± 0.03 The weight of DC and PKE are based on a wet-weight basis. The value of the frass given is based on a dry-weight basis. Mean value followed by ± standard error. No significant difference among the treatments at p < 0.05. T1: 15 g BSFL + 15 kg DC and PKE; T2: 20 g BSFL + 15 kg DC and PKE; T3: 25 g BSFL + 15 kg DC and PKE; T4: 30 g BSFL + 15 kg DC and PKE; T5: 35 g BSFL + 15 kg DC and PKE. Selected chemical properties of black soldier fly larvae frass
-
Consistently, pH, CEC, TOM, TOC, exchangeable NH4+, available NO3–, total N, and total P in all BSFL frass had no significant difference despite different treatment weights of larvae (T1–T5) used during the rearing process (Table 5). The pHs of BSFL frass in all treatments were slightly alkaline compared with the initial pH of the rearing substrate which is slightly acidic (Tables 1 & 5). This is because the BSFL can ingest acidic substrates to produce an alkaline frass due to the presence of enzymes such as amylase, lipases, and proteases in the gut of the larvae[48,49]. The finding is consistent with Meneguz et al.[48] whereby the pH of the substrates turns alkaline as affected by protease reactions from BSFL during the digestion process. Besides, the alkalinity of the BSFL frass was attributed to ammonification and mineralization of organic N by microbial activities[50] that produced NH4+ which partly contributes to the increase of pH in the BSFL frass. The higher exchangeable NH4+ and available NO3– in BSFL frass is partly due to the mineralization of organic N into NH4+ and the nitrification process by which the NH4+ is converted to NO3–.
Table 5. Selected chemical properties of black soldier fly larvae frass fed on mixed DC and PKE.
Property Treatments T1 T2 T3 T4 T5 pH in water 7.77 ± 0.1 7.68 ± 0.1 7.46 ± 0.2 7.70 ± 0.1 7.98 ± 0.1 CEC (cmol kg−1) 21.00 ± 0.3 20.63 ±1.5 20.43 ± 1.0 20.37 ± 0.4 20.23 ± 1.2 OM (%) 74.7 ± 2.4 71.3 ± 2.9 73.3 ± 2.7 74.0 ± 0.0 72.7 ± 0.7 Exchangeable NH4+ (mg kg−1) 101.57± 2.5 97.14± 6.4 113.95 ± 3.1 120.72±8.3 114.65 ± 5.3 Available NO3− (mg kg−1) 110.68 ± 4.2 100.17±1.2 84.99 ± 5.5 88.03 ± 9.5 103.44 ± 6.5 Total N (%) 2.51 ± 0.2 2.37 ± 0.2 2.51 ± 0.3 2.55 ± 0.2 2.46 ± 0.3 TOC (%) 43.31 ± 1.4 41.37 ± 1.7 42.53 ± 1.6 42.92 ± 0.0 42.15 ± 0.4 Total P (mg kg−1) 4896.8 ± 56.2 4355.3 ± 158.1 4421.7 ± 236.9 4607.0 ± 332.2 5341.7 ± 299.4 C/N ratio 17:1 18:1 17:1 17:1 17:1 The values given are based on a dry-weight basis. Mean value followed by ± standard error. No significant difference among the treatments at p < 0.05. T1: 15 g BSFL + 15 kg DC and PKE; T2: 20 g BSFL + 15 kg DC and PKE; T3: 25 g BSFL + 15 kg DC and PKE; T4: 30 g BSFL + 15 kg DC and PKE; T5: 35 g BSFL + 15 kg DC and PKE. The CEC of BSFL frass in all treatments was high (Table 5) compared with the initial CEC of the feeding substrates (Table 1). The higher CEC in all BSFL frass regardless of treatment was partly affected by the decomposition and humification process of organic substrates by the BSFL. Tao et al.[51] reported that BSFL degraded the carboxylic, cellulose, and aliphatic components leading to an increase in the oxygen-containing functional group such as hydroxyl (-OH), carboxyl (-COOH), carbonyl (C=O) and aromatic compounds. The higher number of functional groups in BSFL frass known to be negatively charged contributed to the increase in CEC due to the substantial amount of surface sites to adsorb the cation nutrients such as NH4+, K+, Ca2+, and Mg+. The depletion of TOM and TOC in BSFL frass (Table 5) irrespective of treatment compared with the initial values of TOM and TOC of the feeding substrates (Table 1) was related to the stabilization of the BSFL frass which was reflected by the lower C/N values of the frass than the initial C/N value of the feeding substrate. The C/N ratio is one of the parameters to identify the maturity and stability of BSFL frass during the biodegradation process. In this study, the values of the C/N ratio of BSFL frass were between 17:1 and 18:1 (Table 5) compared with the initial C/N ratio of the feeding substrate which is 28:1 (Table 1). The changes in the C/N ratio are associated with the mineralization and degradation of the DC and PKE mixture by BSFL. This is consistent with a study which stated that the recommended C/N ratio of feeding substrate used for BSFL growth should be between 15 to 30 for stabilization and non-phytotoxic frass[52]. The C/N ratio of BSFL frass in all treatments is considered stable and can be decomposed to release plant essential nutrients for crop uptake. The higher total P in all BSFL frass (T1–T5) was because of the high inherent P content of the feeding substrate. This finding is consistent with Lanno et al.[53] who reported that the total P content in frass excreted by larvae is affected by the inherent P content of the feeding substrate.
Phytotoxicity test
-
In this study, the insignificant differences in root length, shoot length, RSG, and GI in all BSFL extractants compared to distilled water showed that the BSFL frass extractants are not toxic to maize seeds germination. The seeds of maize in all BSFL frass extractants were 100% germinated indicating that BSFL frass produced from DC and PKE mixture was free from phytotoxic substances that could inhibit the maize seed germination (Table 6). The use of BSFL frass as germination media is possible because the BSFL frass has sufficient nutrients to support the emergence of maize seeds. In addition, the pHs of all BSFL frass extractants are in the range of optimal pH that suits the requirement of seed germination. The values of RSG in all BSFL extractants are in the range of 80% to 100% which was reported in the other related study on phytotoxicity test to be safe and could be used as germination media because of free from plant toxicity[54]. The maturity of decomposed products is described as the ability to be used effectively for crop development, free from phytotoxicity aspects[55], and the degree of completeness of composting[56]. The maturity degree of BSFL frass in all treatments was corroborated by the values of 100% GI of maize seeds (Table 6).
Table 6. Plant bioassay test of treatments in different concentrations of extractants.
Extractant Concentration Root length (cm) Shoot length (cm) RSG (%) GI (%) C Distilled water 1.26 ± 0.15 2.01 ± 0.13 100.0 100 T1 IE 3.14 ± 0.36 1.54 ± 0.22 80.0 100 D1 5.91 ± 0.45 2.63 ± 0.25 93.3 100 D2 4.29 ± 0.32 2.20 ± 0.20 90.0 100 D3 4.07 ± 0.30 2.19 ± 0.20 93.3 100 T2 IE 5.13 ± 0.43 2.17 ± 0.24 90.0 100 D1 4.69 ± 0.38 2.48 ± 0.23 93.3 100 D2 5.03 ± 0.46 2.14 ± 0.19 86.7 100 D3 3.05 ± 0.36 1.74 ± 0.21 80.0 100 T3 IE 4.03 ± 0.44 1.77 ± 0.24 86.7 100 D1 4.19 ± 0.49 2.42 ± 0.32 80.0 100 D2 4.19 ± 0.38 2.39 ±0.20 93.3 100 D3 3.40 ± 0.41 1.79 ± 0.23 80.0 100 T4 IE 3.36 ± 0.43 1.73 ± 0.28 83.3 100 D1 4.82 ± 0.47 2.29 ± 0.25 86.7 100 D2 3.20 ± 0.21 1.93 ± 0.17 96.7 100 D3 3.20 ± 0.28 2.10 ± 0.18 93.3 100 T5 IE 3.36± 0.42 1.42 ± 0.20 80.0 100 D1 4.02 ± 0.40 2.19 ± 0.25 86.7 100 D2 3.59 ± 0.37 1.74 ± 0.23 83.3 100 D3 3.47 ± 0.25 2.16 ± 0.18 93.3 100 Mean value followed by ± standard error. C is distilled water served as control. IE is initial extraction. D1, D2, D3 are three times dilutions. RSG is relative seed germination. GI is the germination index. pH buffering capacity of soil and BSFL frass
-
Owing to insignificant differences in WRR, WRI, and selected physicochemical properties as described in the previous section, BSFL frass was mixed from all treatments (T1–T5) with modifications of their weights based on recommendation of organic fertilizer used in maize cultivation.
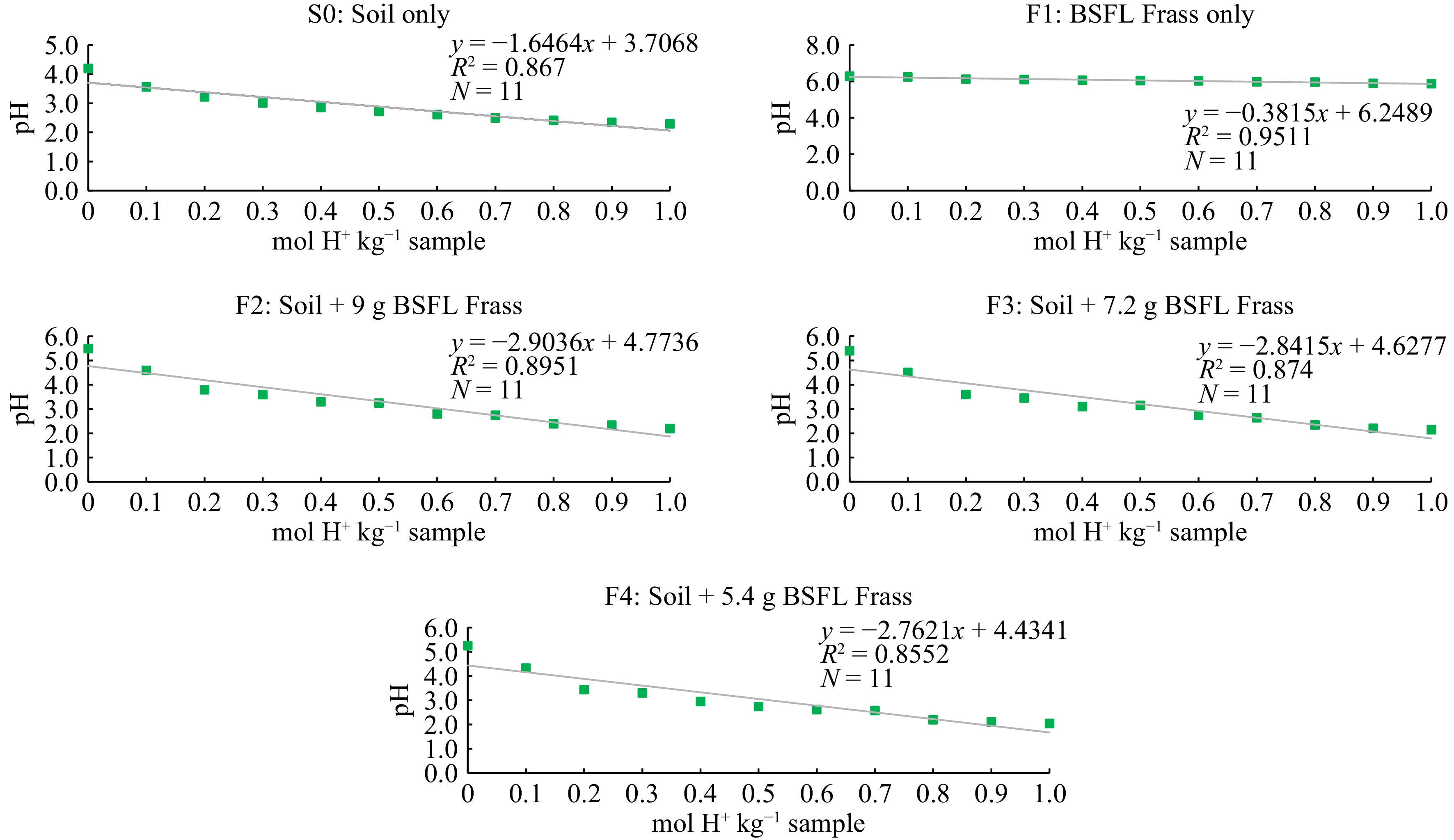
Regardless of treatment, there was a negative relationship between buffering capacity and soil pH in all soils with the regression coefficient (R2) ≥ 0.80 (Table 7, Fig. 3). Among all treatments, the highest pH buffering capacity was observed in BSFL frass only (F1) followed by soil only (S0) compared with soil added with BSFL frass at different amounts (F2, F3, and F4). The buffering capacity of BSFL frass alone (F1) was higher than soil only (S0) due to the high CEC of the BSFL frass. Besides, high TOM and functional groups such as carboxyl and phenols of humic substances in BSFL frass increased the number of negatively charged sites which improved CEC to retain more exchangeable base cation. In other related studies, it was proven that organic amendments such as composts were able to improve the pH buffering capacity of Bekenu Series soil due to high organic matter content[31]. It was expected that in this study, the addition of BSFL frass in soil could improve the pH buffering capacity of the Bekenu Series considering the high TOM content of the BSFL frass. However, the pH buffering capacity of the Bekenu Series soil was not affected by the addition of BSFL frass irrespective of their amount. This could be due to the inconsistent dissolution of organic matter of BSFL frass and the amount of BSFL frass added to the 350 g soil seemingly not sufficient to buffer the changes in pH.
Table 7. Summary of soil pH buffering capacity affected by soil alone, BSFL frass alone and soil with the BSFL frass.
Treatment code Initial pH pH buffering
capacityR2 Soil only (S0) 5.93 ± 0.06 0.6074 0.8670* BSFL frass only (F1) 7.94 ± 0.10 2.6212 0.9511* Soil + 9 g BSFL frass (F2) 6.50 ± 0.04 0.3444 0.8951* Soil + 7.2 g BSFL frass (F3) 6.43 ± 0.02 0.3519 0.8740* Soil + 5.4 g BSFL frass (F4) 6.11 ± 0.04 0.3620 0.8552* * represents a significant difference at p ≤ 0.05. -
Our attempt at using different BSFL weights for frass production with the assumption that the BSFL frass would be different in the WRR, and selected physicochemical properties was not achieved. The DC and PKE mixture as feeding substrates were reduced by 80% from the initial weight of 15 kg suggesting that the BSFL effectively ingested the mixture of DC and PKE and has potential for organic waste reduction regardless of the BSFL weight. The chemical properties of BSFL frass such as pH, CEC, C/N ratio, exchangeable NH4+, available NO3–, total N, total P, TOM, and TOC were not significantly different despite the different treatment weights of BSFL were used to ingest the mixture of DC and PKE. Irrespective of the BSLF weight, all of the BSFL frass showed good chemical properties that could be considered to be used as organic fertilizer or soil-organic amendment. The successful germination of maize in distilled water and all of the BSFL frass extractants showed that BSFL frass was not toxic to the plant and can be used as growing media, soil organic fertilizer, or organic amendment. However, the pH buffering capacity of Bekenu Series soil was not affected by the addition of BSFL frass irrespective of their amount. This could be due to the inconsistent dissolution of organic matter of BSFL frass and the amount of BSFL frass added to the 350 g soil seemingly not sufficient to buffer the changes in pH of Bekenu Series soil. It is recommended that the BSFL farming could be done using the different starter diet ratios at juvenile larvae stage and proceed with the main diet with different larvae density. In addition, different types of feeding substrates for BSFL farming could be considered for frass production because the BSFL frass quality and quantity depend on different substrates.
-
The authors confirm contribution to the paper as follows: study conception and design: David CS, Omar L, Ahmed OH, Roslim MHM, Krishnan K; data collection: David CS; analysis and interpretation of results: David CS, Krishnan K, Omar L; draft manuscript preparation: David CS, Omar L. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed throughout the current study are conducted in Universiti Putra Malaysia Bintulu Sarawak Campus, Malaysia and available from the corresponding author on reasonable request.
-
The authors gratefully acknowledge the Ministry of Higher Education Malaysia and Universiti Putra Malaysia Bintulu Sarawak Campus for providing the research facilities.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
David CS, Omar L, Ahmed OH, Roslim MHM, Krishnan K. 2024. Waste reduction rate, selected agronomic properties, and effect on bekenu series soil pH buffering capacity of black soldier fly larvae frass. Technology in Agronomy 4: e020 doi: 10.48130/tia-0024-0016
Waste reduction rate, selected agronomic properties, and effect on bekenu series soil pH buffering capacity of black soldier fly larvae frass
- Received: 09 November 2023
- Revised: 30 April 2024
- Accepted: 15 May 2024
- Published online: 02 August 2024
Abstract: Black soldier fly larvae (BSFL) were used in this study as decomposer agents to ingest decanter cake (DC) and palm kernel expeller (PKE) mixture into frass (larvae excretion). The objectives of the study were to: (i) assess the waste reduction rate (WRR) of BSFL frass produced from DC and PKE mixture as feeding substrates using different treatment weights of BSFL, (ii) determine the selected agronomic properties and phytotoxicity effect of the BSFL frass, and (iii) evaluate BSFL frass application effect on the Bekenu Series soil pH buffering capacity. The BSFL rearing was conducted in five treatments using a similar amount of DC and PKE mixture (15 kg) as feeding substrates but under different treatment weights of the BSFL. The WRR averages of all treatments were between 81% to 83% suggesting that the BSFL can degrade most of the feeding substrates (15 kg) regardless of the BSFL weight. The average temperature of feeding substrates throughout BSFL rearing was 27 °C irrespective of the BSFL weight. All of the treatments (T1−T5) produced frass on the 54 d of the BSFL rearing process. The BSFL frass has good agronomic properties reflected by the appropriate value of pH (7.4), and significant amount of total nitrogen (2.5%), organic matter (74%), and some of the beneficial elements (calcium, phosphorus, and magnesium) that could be used as organic fertilizer. No phytotoxicity effect detected in maize seed germination suggested that the BSFL frass is safe to be used as soil organic amendment. Although with neutral pH, the BSFL frass did not affect Bekenu soil pH buffering capacity.
-
Key words:
- Organic fertilizer /
- Decomposer agent /
- Insect larvae /
- Palm oil wastes /
- Soil acidity