-
Global warming is a widely recognized climate change event that adversely affects plant growth and development. The rate of increase in global nocturnal (nighttime) temperature (NT) is 1.4 times as much as the rate of rising daytime temperatures (DT)[1,2]. Furthermore, the global temperature has increased at a rate of 0.204 °C per decade in the nighttime and 0.141 °C per decade in the daytime in the latter half of the 20th century[3]. Nighttime temperatures are projected to increase at a faster rate (20%–40% by 2100) than daytime maximum temperatures[4,5]. Each 1 °C increase of NT caused a grain yield decline of 10% in rice (Oryza sativa) during the dry growing season, but yield loss was not related to DT, suggesting that growth inhibition and yield production of crops as a result of global warming may mainly be due to elevated night temperature[6]. High soil temperature at night was found to be more detrimental than high soil temperature in the daytime, and lowering the soil temperature at night was more effective for shoot and root growth of creeping bentgrass (Agrostis stolonifera) than lowering the daytime soil temperature[7]. The physiological and metabolic responses and plant adaptation mechanisms to high nighttime temperature (HNT) are less studied than plant response to high daytime temperature (HDT), which have generally been investigated intensively in various plant species.
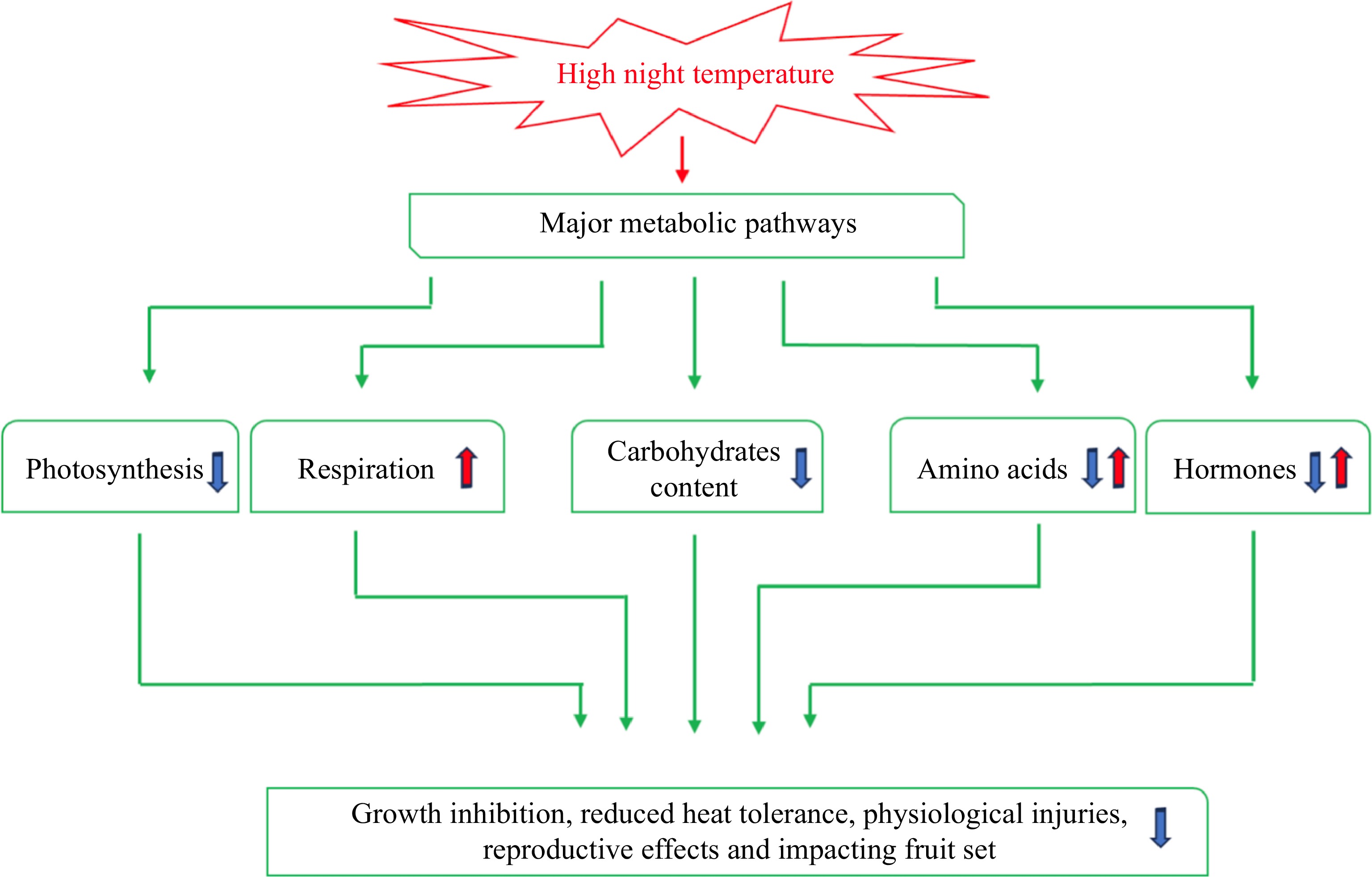
High night temperature hinders plant growth and causes remarkable loss of productivity in various species[8−11]. For example, an earlier study reported that increasing NT from 21 to 29 °C at a constant DT of 29 °C resulted in a 20% reduction in total biomass production of rice plants[12]. A study conducted on wheat (Triticum aestivum) demonstrated that for each 1 °C rise in NT during the time of seed-filling, the grain yield declined by approximately 6%[10]. Other studies on spring wheat and rice showed that an NT increase of 1 °C resulted in a yield reduction of 4%−7%[9,13]. Studies with perennial grass species found that HNT caused a significant decline in turf quality and root dieback due to increased whole-plant and root respiration rate at night, which contributed to a reduction in carbohydrates in creeping bentgrass[14]. The mechanisms associated with the inhibitory effects of HNT on plant growth are still largely unknown, but may involve adjustment, alteration, or interruption of various physiological and metabolic processes, including carbohydrates, amino acids, and hormone metabolism.
This review focuses on the discussion of carbon, amino acids, and hormone metabolism underlying plant response and adaption mechanisms to HNT and provides insight into the current knowledge and future research perspectives for further understanding the effects of NT on the physiological and metabolic processes governing plant growth and development. Understanding the physiological and metabolic effects of HNT and strategies for mitigating the adverse impact of HNT are of great importance for maintaining plant productivity through the current and anticipated future climate change.
-
Photosynthesis and respiration control carbon balance and availability, which are necessary for plant growth and development[15]. Enhancement of dark respiration during and reduction of photosynthesis at night are the foremost physiological responses of plants subjected to HNT[6,16]. Enhanced respiration and limited photosynthesis due to HNT lead to a decline in carbohydrate availability for supporting plant growth and ultimately a loss of plant productivity[8,17−19] (Fig. 1).
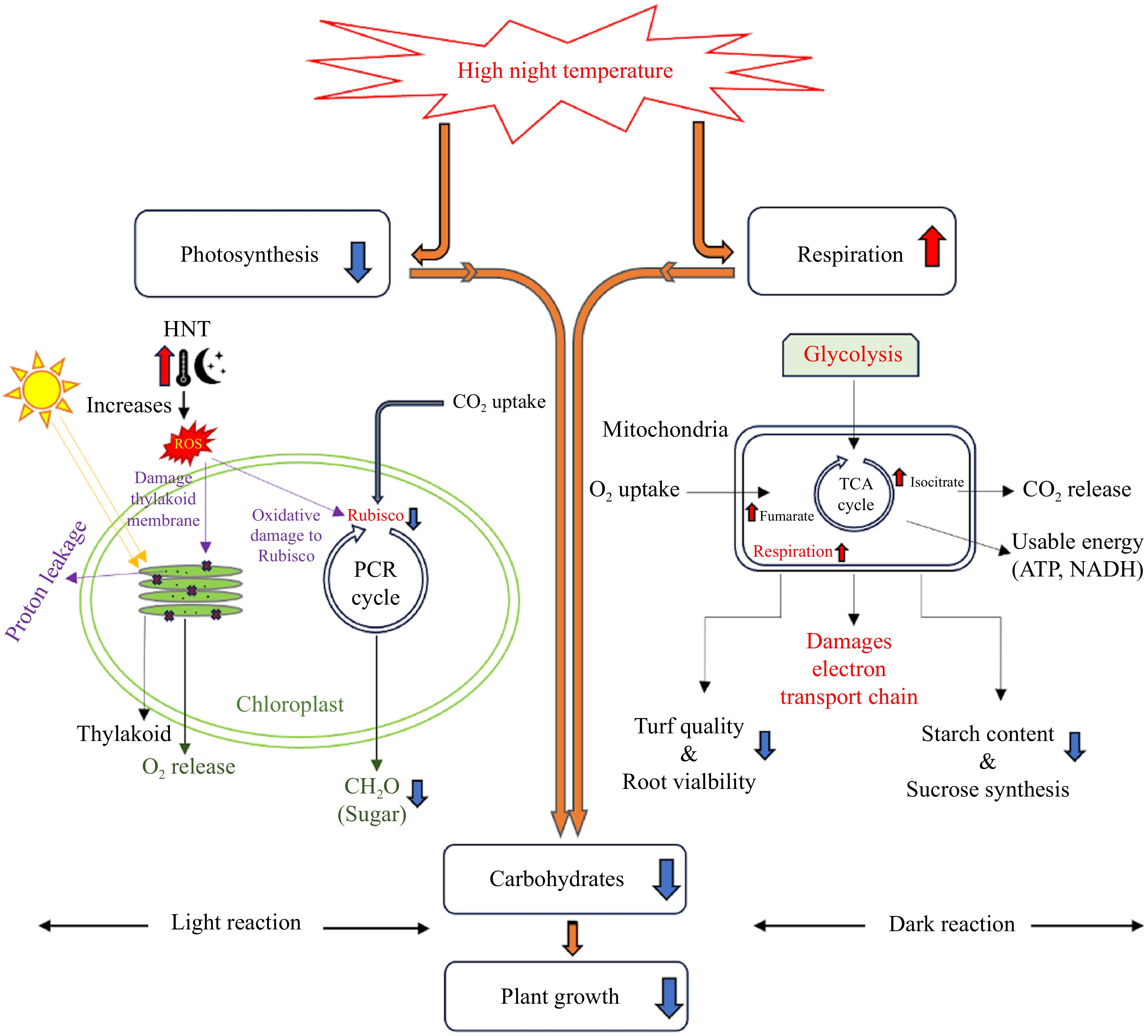
Figure 1.
High night temperature interrupts carbon balance between photosynthesis and respiration, causing a carbon deficit in plants. The upward arrows indicate increase or promotion, and downward arrows indicate decrease or inhibition.
Photosynthesis
-
High night temperature inhibits photosynthesis by interrupting or damaging light reactions (light harvesting, electron transport, and photochemical reactions) and carbon metabolism (carbon fixation, carboxylation, and assimilation). Elevations in NT may adversely affect various activities associated with those processes, which can contribute to an inhibition in photosynthesis and lower production of carbohydrates.
High night temperature accelerates leaf senescence due to loss of chlorophyll and reduces the surface area of leaves that photosynthesize, decreasing the duration of active photosynthesis[16]. It was reported that HNT reduced the number and size of chloroplasts in soybean (Glycine max L. Merr) leaves and caused a decline in the rate of photosynthesis[15]. Inhibition of leaf photosynthesis due to HNT has also been associated with the disruption of chloroplast membranes and an increase in ion leakage and lipid peroxidation[20]. The same phenomenon was observed by[21] where a 4% decrease in the photosynthetic rate was recorded in sorghum (Sorghum bicolor) subjected to temperatures increased from 32/22 °C (day/night) to 44/34 °C (day/night). Enhanced NT caused a decrease in antioxidant capacity and increased the production of reactive oxygen species (ROS) in sorghum and wheat leaves[22,23]. An enhanced level of ROS in plants can result in oxidative damage to the thylakoid membranes and degradation of chlorophyll, resulting in the inhibition of photosynthesis[23,24]. A study on the perennial grass, false wheatgrass (Leymus chinensis), found that long-term nocturnal warming might have the potential to adversely affect crucial metabolic processes, resulting in an increase of mesophyll cell peroxidation[25]. These studies provide evidence for HNT-mediated inhibition of photosynthesis due to reduced light-harvesting capacity and photooxidation.
The damages in thylakoid membranes due to HNT hinder light harvesting, electron transport, and photochemical reactions[26]. High night temperature resulted in enhanced non-photochemical quenching (NPQ)[27]. This alters the efficiency of excitation energy transfer to the photosystem II (PSII) reaction center, affecting the innate quantum efficiency of PSII (F'v/F'm)[28]. This alteration in the photosystem II (PSII) reaction center resulted in a reduced rate of photosynthesis and a slower plant growth[29]. Moreover, HNT also disrupts the photosynthetic electron transport process, further diminishing the overall photosynthetic rate, partly due to an increase in the permeability of thylakoid membranes, which leads to proton leakages[30].
The decrease in photosynthesis in response to HNT has also been associated with the inhibition of photosynthetic enzymes, particularly ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). HNT enhances the production of ROS, which cause oxidative damage to Rubisco[31]. The decline in photosynthetic rate caused by high temperature can be caused by damages or alterations in the various photosynthetic components[32,33], a recent study on rice reported that the decline in the maximum carboxylation rate regulated by Rubisco and the maximal rate of electron transport determined by the thylakoid reactions were the main contributing factors to HNT-mediated decline in photosynthetic rate[34].
Current research has demonstrated the detrimental effects of HNT on photosynthesis and damages in various processes in different studies, but the information is fragmented, as discussed above. Systematic analysis of the critical steps or components of photosynthesis impacted by HNT is lacking. Furthermore, little is known about the mechanisms of plant heat tolerance related to photosynthetic acclimation and adaptation to HNT.
Respiration
-
Increases in respiration rate in response to HNT have been widely reported in various plant species, although the degree of elevated respiration rate in response to HNT may vary based on temperature, growth stage, tissue or organ type, and plant species or cultivar[6,8,14,16,18,35]. For example, three rice cultivars, 'Gharib', 'N22', and 'IR64', were exposed to HNT of 29 °C and their physiological and biochemical properties were compared to those grown under control conditions at 20 °C. The results of this study revealed that HNT caused the leaf respiration rates of 'Gharib' and 'IR64' to increase by 63% and 35%, respectively, while no significant effects were observed for 'N22'. The elevated respiration rate corresponded to a reduction in starch content in the panicles of both 'Gharib' and 'IR64' as well as reduced sucrose synthesis activity in these cultivars, which led to an overall reduction in grain weight and quality[8]. Increases in the content of some intermediates in the tricarboxylic acid (TCA) cycle of respiration, such as fumarate and isocitrate were recorded, which reflects an enhancement in the reparation rate of rice exposed to HNT[36]. The negative effects of elevated respiration rate on crop yield were more pronounced during the exposure of plants in the flowering stage to HNT in comparison to those in the vegetative stage[6,8,35].
The total respiration rate of plant tissues is comprised of maintenance, growth, and ion uptake respiration. It is well known that the maintenance respiration rate of plant tissues increases proportionally with rising temperature, due to a consumption of carbohydrates and reduction in the amount of carbohydrates available for growth and yield[37,38]. However, several studies on maize (Zea mays) and wheat found that yield loss of those plants exposed to HNT was not fully relative to the increased maintenance respiration rate[39]. Interruption of other metabolic processes by HNT may also contribute to the observed growth inhibition and yield reduction. In addition, respiration responses to elevated temperature may exhibit temporal differences. The effects of HNT on daytime respiration vary from those of nighttime respiration in shoots and roots. It was also reported that the nighttime shoot and root respiration rates of creeping bentgrass increased by 20% and 44%, respectively, whereas daytime shoot and root respiration rates decreased by 21% and 26%, respectively, in response to an increase in NT from 19 to 24 °C and concluded that the decline in turf quality and root viability caused by HNT was mainly associated with the increased shoot and root respiration rate at night, particularly root respiration at night. Root respiration accounts for a large proportion of carbohydrate consumption, and therefore, minimizing nighttime root respiration rate to reduce carbohydrate loss could be beneficial for sustaining plant growth in environments with HNT[14].
While respiratory energy production is important for plant growth and development under high temperatures, overconsumption of carbohydrates or loss of carbon due to HNT should be minimized. Effects of HNT on the different components of respiration require further characterization to minimize inefficient consumption of carbohydrates during respiration.
Carbohydrate content
-
Effects of HNT on carbohydrate content vary among specific types of carbohydrates having different roles in the regulation of plant growth and stress response. The nonstructural carbohydrates used in the respiration process, such as sucrose, starch, glucose, and fructose exhibit a declining trend in content due to elevated respiration rate caused by HNT, and this is known to occur particularly when the respiration rate exceeds the photosynthetic rate under various stress conditions in a multitude of plant species[8,14,40−43]. Decreases in the starch content of wheat grains as a result of HNT have been reported and corresponded to the reduction in gene transcript levels of the adenosine diphosphate glucose pyrophosphorylase small subunit, which controls starch synthesis, as well as the increase in isoamylase III, alpha-, and beta-amylase, which regulate starch degradation[16,44]. Total soluble sugar content in the leaves of two rice cultivars decreased when NT was increased from 22 to 28 °C and DT was 30 °C, and the leaves of the heat-tolerant cultivar, 'Nagina22', had significantly higher soluble sugars content than the heat-sensitive cultivar, 'BRS Querencia'[43]. Total nonstructural carbohydrate content decreased significantly in shoots and roots, while shoot and root respiration rate of creeping bentgrass increased when NT was increased from 19 to 24 °C[14]. These studies provide strong evidence that HNT causes a reduction in the availability of carbohydrates, particularly non-structural carbohydrates or soluble sugars, which may lead to suppressed shoot and root growth and accelerated root dieback.
Carbohydrates with roles in stress defense or protection, however, have increased content in response to high night temperatures in certain cases. For example, in a study with rice[45], cultivars tolerant to HNT accumulated more raffinose and stachyose, which are classified as raffinose family oligosaccharides (RFOs)[46]. Plants that experienced HNT also had increased content of myo-inositol[47]. Myo-inositol is a precursor for raffinose and stachyose, and these RFOs are known to play positive roles in plant tolerance to abiotic stresses due to their functions as osmoregulants, antioxidants, or stress signal molecules[48]. The accumulation of RFOs is a mechanism of plant adaption to HNT and could be used as a biomarker for the selection of cultivars that are tolerant to HNT.
-
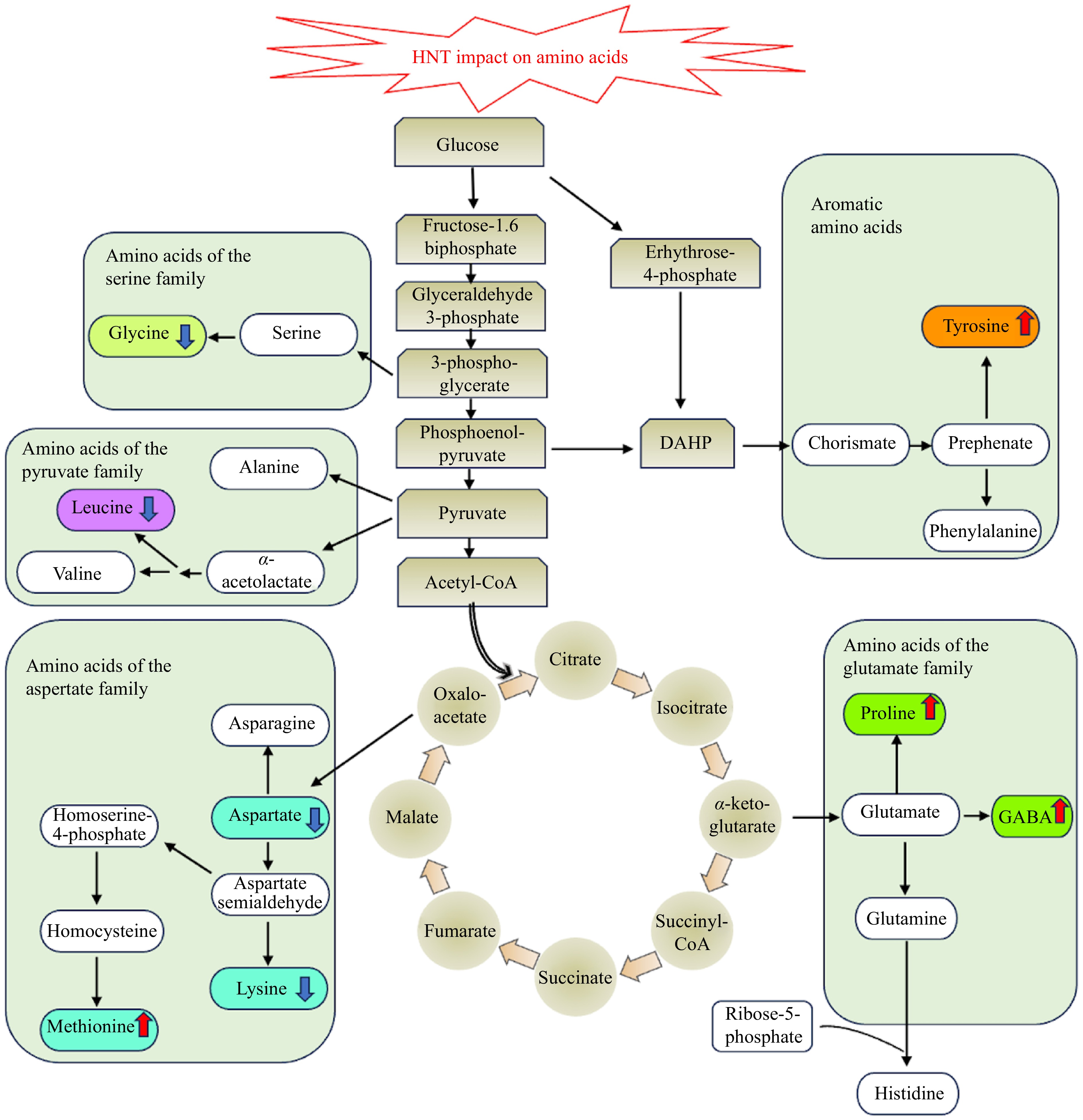
Plant responses to abiotic stresses, including HNT, involve changes in amino acid metabolism; however, proteinogenic amino acids that are biosynthesized into proteins and non-proteinogenic amino acids that comprise a large heterogeneous group of non-coded amino acids and nitrogen-containing metabolites may respond differently to increased temperatures.
Proteinogenic amino acids exhibit both decreases and increases in their content, depending on whether the amino acids are derived from the biosynthesis of amino acids or the degradation of proteins that are affected by heat stress. For example, some proteinogenic amino acids exhibit a decline in content, such as leucine, lysine, glycine, and aspartate under HNT, which could be associated with stress-mediated inhibition of amino acid synthesis[49−51]. Other proteinogenic amino acids, such as tyrosine and methionine increase in content in plants exposed to HNT, which has been associated with accelerated protein degradation due to stress, particularly in heat-sensitive cultivars[41]. The interruption of proteinogenic amino acid metabolism at HNT is mainly associated with metabolic damage and dysfunction.
Many non-proteinogenic amino acids are typically found to play crucial roles in how plants respond to environmental stresses. Non-protein amino acids with roles in stress protection, such as proline, polyamine, and gamma-aminobutyric acid (GABA), have been found to increase in content under HNT in various plant species[45,52−55]. The balancing of the redox potential in the cell cytosol and plastids is achieved by the cyclic process of proline synthesis and degradation, which appears to be essential for antioxidant defense during unfavorable conditions and might be relevant when plants experience stress under HNT[56,57]. Polyamines are related to stress defense due to their roles in osmotic adjustment. The content of polyamines, including spermine, spermidine, and putrescine, increased when plants were exposed to HNT[45,53,54]. Gamma-aminobutyric acid, which functions as a signaling molecule, can exert influence on various physiological processes[58]. Increased content of GABA during stress contributes to tolerance[59]. Increases in the accumulation of stress-protective amino acids play critical roles in plant adaptation to heat stress and could be manipulated through genetic modification or exogenous application to improve plant tolerance to HNT (Fig. 2).
-
Plant hormones are affected by high temperatures, which ultimately alter morphological and physiological traits, as well as plant stress defense systems. Hormones are typically classified as growth-promoting hormones, such as cytokinins, auxins, and gibberellins (GA), or hormones involved in stress response, such as abscisic acid (ABA), ethylene, and salicylic acid (SA), which may respond differentially to HNT (Fig. 3).
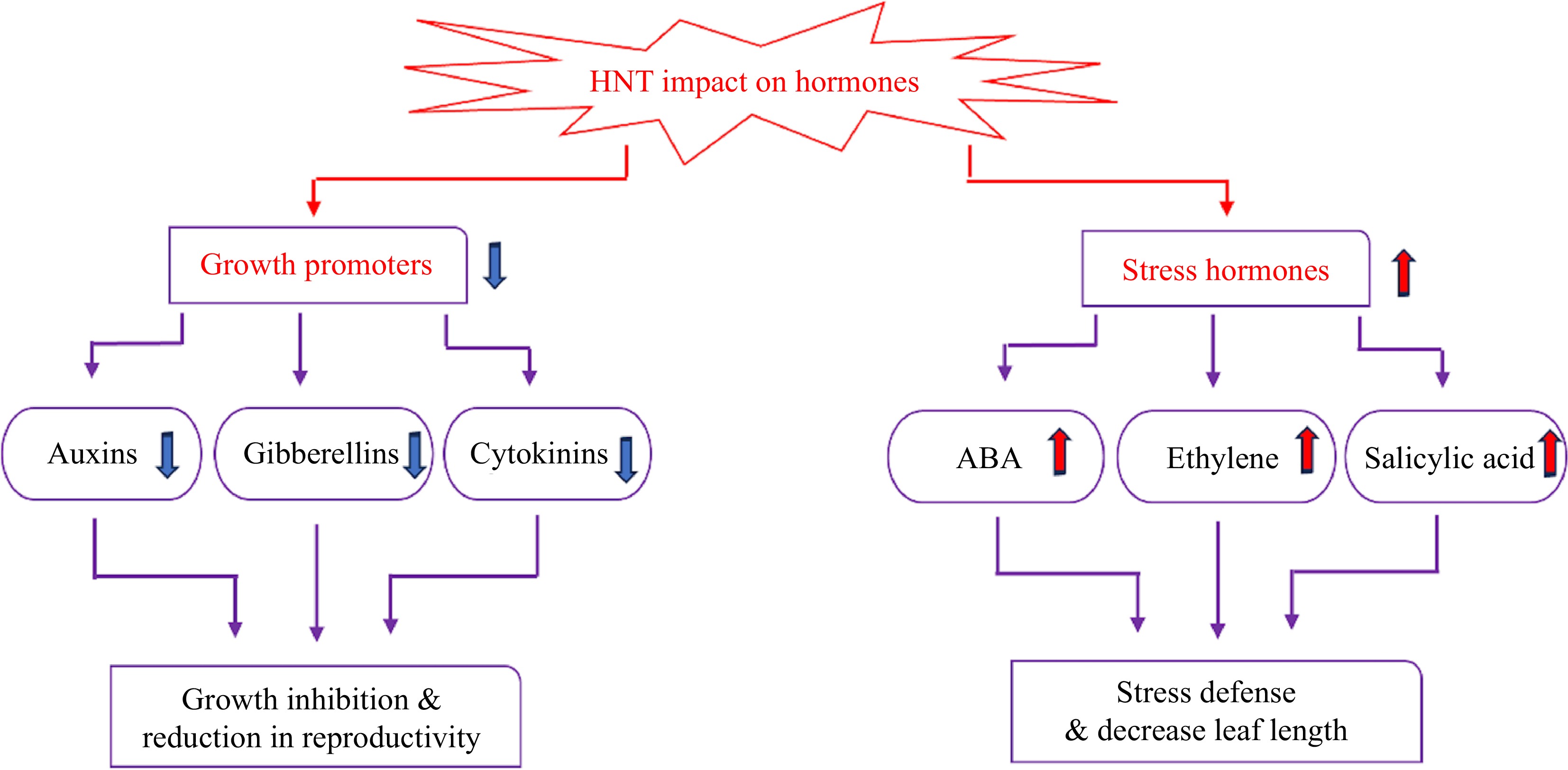
Figure 3.
Effects of HNT on hormone metabolism. The upward arrows indicate increases, and downward arrows indicate decreases in the content of hormones in response to HNT.
High night temperature reduces the content of cytokinins, auxins, and gibberellins[60,61]. The down-regulated expression of GA- and auxin-related genes and the decreased concentrations of GA and IAA by HNT are related to stunted shoot elongation under HNT[62]. High night temperature caused a significant reduction in endogenous IAA, as observed in developing grains of rice and rice panicles[61,63] pistil tissue, and anthers[12,64] and these findings were associated with pollen and spikelet sterility and ultimately grain yield loss. When auxins were applied exogenously, elongation of the pollen tube that was stunted when the plant experienced HNT was restored[12,61]. The interruption of cytokinin metabolism has been associated with reduced kernel filling in cereal crops under high temperatures[65]. Cytokinin levels in developing panicles were reduced in plants subjected to HNT, which resulted in a reduced spikelet number per panicle. This happened due to the inhibition of enzymes involved in cytokinin biosynthesis and a decrease in cytokinin transportation from the root to panicle meristems, and under heat stress, there is an increase in the expression of the cytokinin degradation enzyme, cytokinin oxidase[61,66]. The adverse effects of HNT on the metabolism of growth-promoting hormones largely contribute to growth inhibition and reduction in reproductive capability in various plant species.
The content of ABA, SA, and ethylene exhibits an increasing trend under HNT[61,65]. For example, endogenous ABA levels increased in anthers and young panicles when plants experienced HNT[61,67]. High night temperature caused a reduction in the tiller number during the vegetative stage of growth, which was associated with the alteration of strigolactone and ABA metabolism[68,69]. A study on exogenous application of ABA found increases in grain yield (15%), leaf net photosynthetic rate (6%), and spikelet fertility (6%) and decreases in respiration rate (33%) in rice exposed to HNT, and these findings were linked to a rise in stomatal conductance by 22% and a decrease in nonphotochemical quenching by 29%[70]. An increase in ABA level in response to HNT could be activated for stress defense, although excessive accumulation may cause inhibitory effects on plant growth. The effects of SA may vary depending on the mode of application and environmental conditions. For example, in purple false brome (Brachypodium distachyon) plants, soaking seeds in a sodium salicylate (NaSA) solution or exposing them to 35 °C for 4 h did not significantly increase free SA content. Instead, high SA levels were detected in sprayed leaves[71]. How other stress-related hormones are involved in plant response to HNT is not well documented and deserves further investigation.
-
An increase in night temperatures has been observed and this trend is predicted to continue, as NT is rising at a faster rate than DT[72]. The detrimental effects of rising NT on plant growth, development, and productivity are well known, and some physiological and metabolic impacts of HNT are beginning to be discovered, as reviewed in this paper and exhibited in Fig. 4; however, there is still a lack of insight into why warmer nights are detrimental to plant growth, as explained by effects on physiological and metabolic processes, water and nutrient use and antioxidant metabolism, and how much genetic variability in HNT response exists in various plant species, particularly grass species. Furthermore, the mechanisms of how plants can adapt to this stress remains largely unknown, particularly the key metabolic pathways and molecular factors or networks. Further research addressing these unknown aspects is critically important for improving plant resilience against warmer nights through genetic modification and breeding efforts, as well as through management practices.
-
The authors confirm contribution to the paper as follows: literature collection and writing of the manuscript: Abbas A; editing and revising manuscript: Rossi S; development of outlines and revision of the manuscript: Huang B. All authors reviewed the results and approved the final version of the manuscript.
-
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
The authors would like to thank the Rutgers Center for Turfgrass Science for funding this study.
-
The authors declare no conflict of interest. Bingru Huang is the Editorial Board member of Grass Research who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and the research groups.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Abbas A, Rossi S, Huang B. 2024. Plant metabolic responses and adaptation mechanisms to elevated night temperature associated with global warming. Grass Research 4: e015 doi: 10.48130/grares-0024-0013
Plant metabolic responses and adaptation mechanisms to elevated night temperature associated with global warming
- Received: 09 April 2024
- Revised: 22 May 2024
- Accepted: 03 June 2024
- Published online: 04 July 2024
Abstract: Nighttime temperature has been rising at a faster rate than daytime temperature, and this trend is predicted to continue in the upcoming decades. High night temperatures (HNT) during summer months are particularly detrimental to temperate plant species, including grass species. Elevated night temperature interrupts various physiological and metabolic processes, including carbohydrate, amino acid, and hormone metabolism. The HNT-inhibition of photosynthesis has been associated with accelerated leaf senescence, disruption of cellular membranes and photochemical reactions, as well as restriction of the carboxylation process. Respiration rate increases with HNT, which contributes to the loss of carbon and carbon deficits within plant tissues. The responses of amino acids to HNT vary among amino acids with variable functions, and some non-proteinogenic amino acids or nitrogen-rich compounds having roles in stress protection exhibit increases in content under HNT, suggesting the involvement of these compounds in plant adaptation to HNT. Hormones also vary in their responses to HNT with most growth-promoting hormones, such as cytokinins, auxins, and gibberellic acids, exhibiting a reduction in content, while the content of stress-related hormones, such as ABA and salicylic acid, increases under HNT conditions. The understanding of how HNT limits plant growth is just in its infancy, as many of the physiological and metabolic processes affected by HNT have not yet been investigated and are unavailable in the current literature. The mechanisms of how plants can adapt to this stress remain largely unknown, particularly the key metabolic pathways and molecular factors or networks. Further research addressing these unknown aspects is critically important for improving plant resilience against warmer nights, particularly through genetic modification and breeding efforts, as well as management practices.
-
Key words:
- Metabolic /
- Responses /
- Adaptations /
- Mechanisms /
- Elevateds /
- Temperature