-
Buffalograss (Bouteloua dactyloides) is a member of Chloridoideae subfamily and originates from the semi-arid temperate Great Plains region of the Western United States[1]. Historically buffalograss was used for pastures in the Western United States[2,3]. Due to its strong drought tolerance, heat resistance, disease resistance, low soil nutrient requirements, low height and strong ability for vegetative reproduction, it has received more attention as a low-maintenance lawn grass[4]. As a perennial warm-season turfgrass, it is commonly utilized for urban greening and ecological slope protection, as well as for turf in airports[5].
Buffalograss is a wind-pollinated, highly heterogeneous, and dioecious species with rare occurrences of monoecy. The difference in male and female inflorescence structures of buffalograss is significant enough that plants of different sexes were initially classified into separate genera. It is known that the sex expression of dioecious buffalograss remains stable in natural environments, with a roughly equal sex ratio between males and females[6−9]. Furthermore, when buffalograss is germinated from seeds, it will segregate into female and male plants. In the case of buffalograss that is transplanted or vegetatively propagated, they are typically of the same sex as the mother plant, although on rare occasions, certain plants may alter their sex expression[10]. Buffalograss is dioecious, having an approximate 1:1 female-to-male ratio that is stable[11]. Some cultivars such as 'Sharps Improved' will also exhibit a monoecious phenotype at frequencies up to 13%.
Both female and male plants play distinct roles in the survival and reproduction of the population. The male plants usually allocate all their resources for the effective spread of high-viability pollen, thereby achieving a higher fertilization rate. Conversely, female plants channel more energy into seed production, providing increased resources for their offspring and thereby enhancing the survival rate of seedlings[12]. Generally, the female and male plants have great differences in morphology when flowering. In production, the application and economic value of different sex types varied significantly even within the same species. For example, to harvest seeds, the number of female plants should be maximized under the premise of ensuring pollination. Female plants are also preferred in lawns because its inflorescences are hidden beneath the lawn canopy, while male inflorescences protrude above the lawn canopy, reducing uniformity and overall aesthetics[13]. Therefore, regulating the sex ratio of buffalograss is very meaningful in terms of production and application.
Sex differentiation is a natural phenomenon in the biological world, which is an important process for plants to shift from vegetative growth to reproductive growth. The sex differentiation of plants occurs simultaneously during the process of flower bud differentiation, which is not only regulated by sex determining genes, sex chromosomes, and endogenous signals such as auxin[14], gibberellins[15], carbohydrates[16], but also influenced by environmental factors[17,18] such as temperature[19,20], photoperiod[21,22], water[23−25], and nutrition[26−28]. The complexity of sex-determining mechanisms leads to different genders in plants[29].
There are various methods to control the sex differentiation of plants artificially, among which by spraying exogenous plant hormones and plant growth regulators is undoubtedly the most simple and low-cost method. As early as 1950, some scholars treated cucumbers (Cucumis sativus L.) with 3-Indoleacetic acid (IAA), and the results showed that the proportion of female and male flowers increased significantly[30]. In a study conducted by Rudich et al.[31], three species of Momordica charantia L were treated with ethephon, resulting in the formation of more female flowers. The application of ethylene treatment at an appropriate concentration was found to increase the number of female flowers on each vine of Luffa cylindrica L. Furthermore, it advanced the timing of when female flowers first appeared, increased the female-to-male flower ratio, and significantly reduced plant height, making internodes shorter, and plants more compact[32].
Preliminary research has been conducted on the sex differentiation of buffalograss. Trends of sex expression for buffalograss, showed that conditions of warm temperature, high light, and low N were favorable for female sex expression, whereas conditions of cool temperature, low light, and high N were favorable for male sex expression[6]. Chandra & Huff[33] showed that infection by the pistil smut fungus shifts sex ratios of buffalograss to be nearly 100% phenotypically hermaphroditic by increasing inflorescences, spikes, spikelets, and florets. In addition, increased most seed yield components, and increased pseudosexual reproductive allocation in both sex forms compared to uninfected clones. Unfortunately, plants infected with pistil smut fungus produce smut balls, instead of seed. Exogenous application of IAA, Gibberellin A3 (GA3) and cytokinin (CTK) could significantly increase the number of female flowers and fertile tillers of buffalograss[34]. In this study, a series of experiments were performed on buffalograss in which we manipulated concentrations of NAA, DIECA and measured subsequent female-to-male ratio and seed yield.
-
A field trial was carried out at the China Agricultural University Zhuozhou Experimental Station (39.27'N, 115.51'E), which is located in Zhuozhou, Hebei Province, China. The site has an altitude of 42 m and belongs to the warm temperate continental monsoon climate zone with sufficient sunlight, high temperatures, and rainy summers, but cold and dry winters. The average annual rainfall is 563 mm, the annual average temperature is 11.6 °C, and the cumulative frost-free period is 178 d on average. The experimental site is dominated by sandy loam soil. The physical and chemical properties of the soil are shown in Table 1.
Table 1. Soil physical and chemical properties.
Depth (cm) Bulk density (g/cm3) Field moisture capacity (cm3/cm3) Soil pH Available phosphorous (mg/kg) Available potassium
(mg/kg)Soil organic matter
(g/kg)Alkali-hydrolyzable nitrogen (mg/kg) 0−20 1.67 0.24 7.7 31.76 81.38 7.88 129.24 0−40 1.69 0.20 8.13 13.16 24.36 2.30 128.50 Selection of experimental species, reagents, and instruments
-
Seeds of 'Zhongye No. 1' buffalograss which was bred by China Agricultural University (Beijing, China) were selected as the experimental species. Seeds were harvested on July 10, 2021.
NAA and DIECA were ordered from Rhawn company (Shanghai, China), analytical pure. HP-5890II gas chromatograph (The USA, HP Company) and G2-XS Q-TOF mass spectrometer (The USA, Waters Company) were used to measure endogenous JA content.
Experimental design
-
The experimental arrangement was a randomized complete block design, there were 11 treatments and 1 control. Three replicates were set for each treatment which led to 36 experimental plots, including 33 treatment plots and three control plots (CK). The size of each experimental plot was 2 m × 2 m. Three rows were planted with a row spacing of 60 cm in each plot, and the seeding rate was 20 g per plot.
Our previous experiments showed that 100 mg/L and 150 mg/L of DIECA significantly increased potential seed yield, actual seed yield and female-to-male ratios of buffalograss (Supplemental Table S1). To further investigate DIECA and its interaction with NAA, 2 L of solution were used to spray on the leaves of the test materials in each plot using a manual sprayer in this study. An equal amount of clean water was sprayed in the control group. The reagent concentrations used in different treatments are shown in Table 2.
Table 2. Reagent concentrations in treatment.
Treatment Reagent concentration NAA (mg/L) DIECA (mg/L) A-b-1 – 100 A-c-1 – 150 A-a-2 50 – A-b-2 100 – A-c-2 150 – B-a-1 50 150 B-b-1 100 150 B-c-1 150 150 B-a-2 50 100 B-b-2 100 100 B-c-2 150 100 CK – – If a certain reagent was not used in the treatment, it is marked as '–'. Reagents were sprayed at 10 am after dew dissipated from the buffalograss canopy. Two litters of the corresponding reagents or clean water was sprayed in the plot until the leaves were moist. Spraying operations were carried out every two days for a total of seven times. All test treatments were completed before the materials in the test field reached the initial flowering stage to ensure the test effect.
Experimental fields management
-
Before sowing, compound fertilizers ((N-P-K, 1-0.44-0.91), ET) were uniformly applied to the experimental plots, including 20 kg/hm² of phosphorus, 38 kg/hm² of potassium, and 45 kg/hm² of nitrogen. Before sowing, the seeds were treated at low temperature (−20 °C) for 7 d to break dormancy. Seeds were sowed on May 1st, 2022, and irrigated with punched thin-soft spray tape immediately after sowing. The plots were irrigated every 48 h before emerging, irrigation amount was 24 L per square meter. After emerging, 30 L per square meter of water was irrigated every 48 h to ensure normal growth of the seedlings which facilitated the control of weeds.
Forty-five days after sowing, the natural height of the seedlings reached 5 cm. At this time, the herbicide Prodiamine with a concentration of 800 g/hm² was sprayed to prevent weed seed germination. In the local hot and rainy months of July and August, a mixture of Quinclorac and Amflunolin at a concentration of 500 g/hm² was sprayed every 30 d to control weeds. A gasoline-powered weeder was used to clear weeds in the aisles of the experimental fields. At the end of October of the same year, 30 L per square meter of water was irrigated to ensure that the test materials overwintered successfully.
The next year, after the test materials turned green, they were mowed to a uniform height of 8 cm. An equal amount of compound fertilizer was applied in each plot to ensure normal growth of the test materials. Weed control measures remain consistent with the previous year. Treatments were initialed on May 1st, 2023.
Index measurements and methods
-
During the peak flowering period, a 20 cm × 20 cm quadrat was used and three samples were taken from each plot, with a total of nine samples for each treatment (the same as below) in which the number of female and male fertile tillers in plots were counted. The number of females and spikes in plots were also counted at the same time. The ratio of female-to-male was represented by the ratio of female and male fertile tillers in each treatment plot.
Seed yield was divided into three parts. First, potential seed yield = (female fertile tillers/m2) × (female florets/female fertile tillers) × average weight of a seed. Second, seed yield in experimental plots—Three 50 cm × 50 cm quadrats were placed randomly in each plot, and all mature seeds in the quadrat were harvested manually. Seed that fall to the ground were also collected. Then the harvested mature seeds were dried, cleaned, and weighed. Lastly, actual seed yield—Convert the seed yield per unit area (1 m2) based on the seed yield in experimental plots.
The endogenous JA content in roots and leaves was also measured. Leaf and root samples were obtained from each plot at 6, 12, and 24 h after spraying the reagents. The sampling tool was a root sampler with 10 cm diameter and 30 cm depth. After obtaining fresh plant samples, immediately washed away the soil attached to the roots. After wiping away the water, cut out the roots and the first main-stem leaf from the end, wrapped them separately in aluminum foil, and immediately frozen in liquid nitrogen then stored at −80 °C. Endogenous JA content was analyzed through gas chromatography + mass. The method of Birkemeyer et al.[35] was used with slight modification.
The samples were lyophilized and then ground into a fine powder using a mortar and pestle. The powder (0.1 g) was suspended in a solution of acetone and 50 mM citric acid (70:30, v/v), and 9,10-dihydro-JA (20 ng) was added as an internal standard. To prevent the loss of volatile fatty acids, the extracts were allowed to evaporate overnight at room temperature. The resulting aqueous solutions were filtered and extracted with 3 × 10 mL of diethyl ether. The pooled extracts were loaded onto a solid-phase extraction cartridge (500 mg of aminopropyl sorbent). Once loaded, the cartridges were washed with 7.0 mL of trichloromethane and 2-propanol (2:1, v/v). The bound JA and the relevant standard were eluted with 10 mL of diethyl ether and acetic acid (98:2, v/v). After the solvents were evaporated and the residue was esterified with excess diazomethane, the sample was adjusted to 50 µL with dichloromethane. The extracts were then analyzed by GC-MS (6890N network GC system and 5973 network mass selective detector; Agilent Technologies, Palo Alto, CA, USA). The amounts of endogenous JA were determined by comparing the peak areas of JA with the corresponding standards. Three replicates per treatment were used for JA determination.
Statistical analysis
-
Experimental data were summarized in Excel. The data were subjected to analysis of one-way ANOVA and Duncan's multiple range test (DMRT) by SPSS 26. GraphPadPrism 5 was used to draw graphs.
-
Compared with the control, all treatments reduced the number of male fertile tillers of the experimental plants (Fig. 1a). Among them, the most significant effects were achieved under the treatments of 150 mg/L DIECA + 150 mg/L NAA, 100 mg/L DIECA + 100 mg/L NAA, and 100 mg/L DIECA + 150 mg/L NAA. The number of male fertile tillers decreased by 44%, 42%, and 42% respectively compared with the control group (Fig. 1a). Except for the treatment of 150 mg/L DIECA + 50 mg/L NAA, there were also significant differences between the above three treatment groups and the remaining treatment groups (p < 0.05).
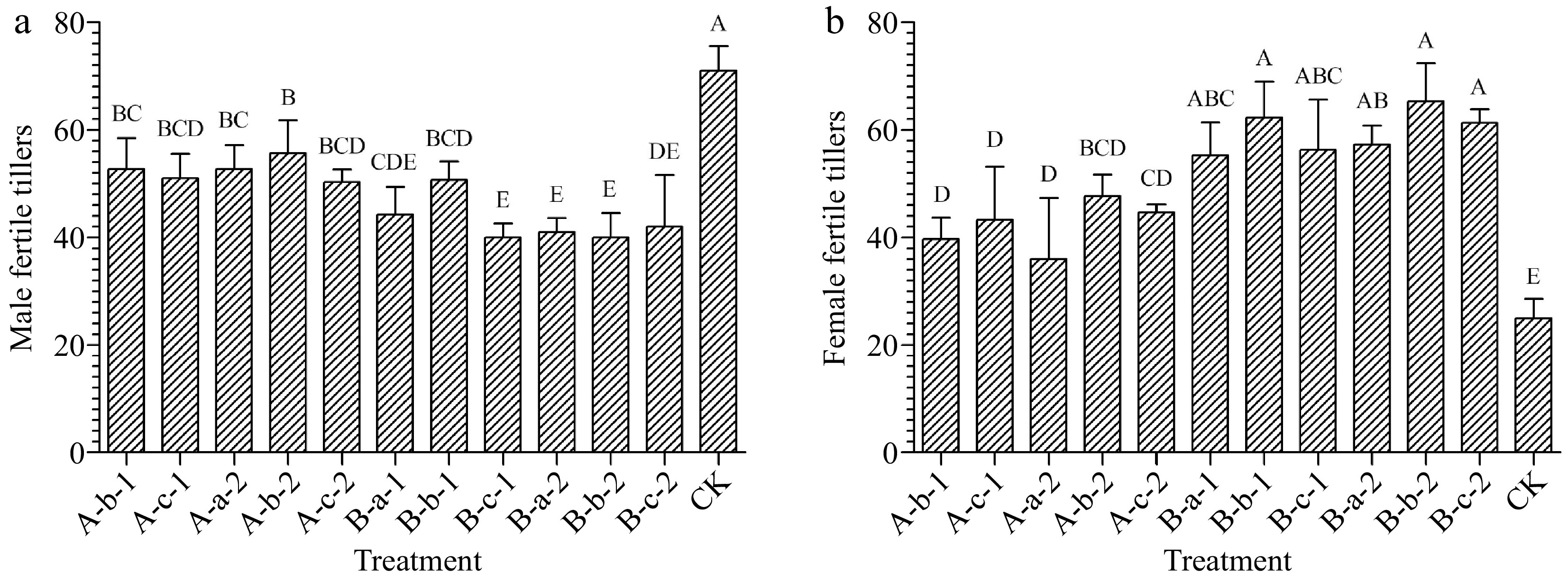
Figure 1.
Effects of spraying reagents on the number of (a) male, and (b) female fertile tillers. The content on the abscissa represents the concentration of reagents used in the corresponding treatment. A-b-1: 100 mg/L DIECA; A-c-1: 150 mg/L DIECA; A-a-2: 50 mg/L NAA; A-b-2: 100 mg/L NAA A-c-2: 150 mg/L NAA; B-a-1: 50 mg/L NAA + 150 mg/L DIECA; B-b-1: 100 mg/L NAA + 150 mg/L DIECA; B-c-1: 150 mg/L NAA + 150 mg/L DIECA; B-a-2: 50 mg/L NAA + 100 mg/L DIECA; B-b-2: 100 mg/L NAA + 100 mg/L DIECA; B-c-2: 150 mg/L NAA + 100 mg/L DIECA; CK: Water.
Compared with the control, all treatments increased the number of female fertile tillers of the experimental plants (Fig. 1b). Among them, the effect was most significant under the treatments of 150 mg/L DIECA + 100 mg/L NAA, 100 mg/L DIECA + 100 mg/L NAA, and 100 mg/L DIECA + 150 mg/L NAA. The average number of female fertile tillers was 249%, 261%, and 245% of the control group respectively (Fig. 1b).
Numbers of spikes and female florets
-
Compared with the control, the four treatments of 100 mg/L DIECA, 150 mg/L NAA, 100 mg/L DIECA + 100 mg/L NAA, and 100 mg/L DIECA + 150 mg/L NAA increased the number of spikes (p < 0.05). Among them, the most significant effect was achieved under the treatment of 100 mg/L DIECA + 150 mg/L NAA (Fig. 2a).
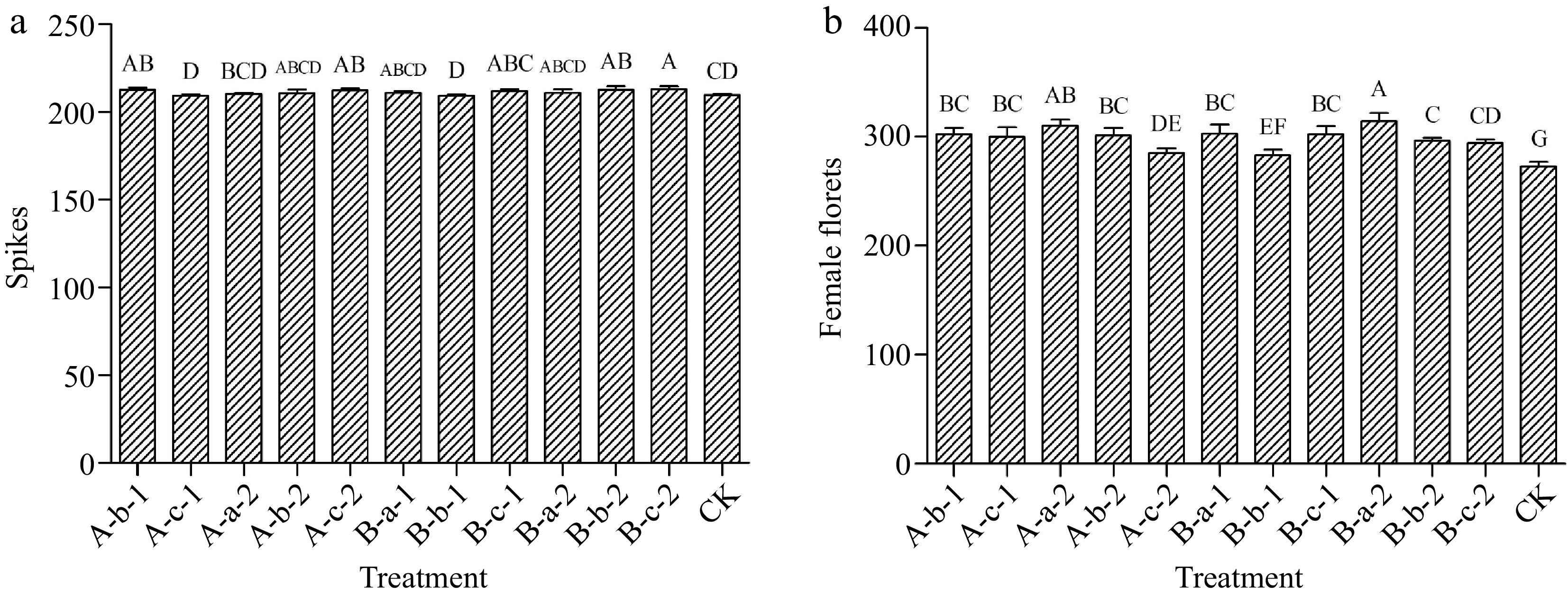
Figure 2.
Effects of spraying reagents on (a) the number of spikes and (b) female florets. The content in the abscissa is the same as Fig. 1.
Compared with the control, all treatments increased the number of female florets in the experimental plants (Fig. 2b). Among them, the most significant effect was achieved under the treatment of 100 mg/L DIECA + 50 mg/L NAA.
Seed yield in experimental plots
-
Compared with the control group treatments of 100 mg/L NAA, 150 mg/L DIECA + 100 mg/L NAA, 150 mg/L DIECA + 150 mg/L NAA, 150 mg/L DIECA + 150 mg/L NAA, 100 mg/L DIECA + 50 mg/L NAA, 100 mg/L DIECA + 100 mg/L NAA and 100 mg/L DIECA + 150 mg/L NAA increased seed yield (p < 0.05) (Fig. 3). The seed yield in experimental plots of treatments mentioned above increased by an average of 57%, 68%, 55%, 57%, 69%, and 77% compared with the control group.
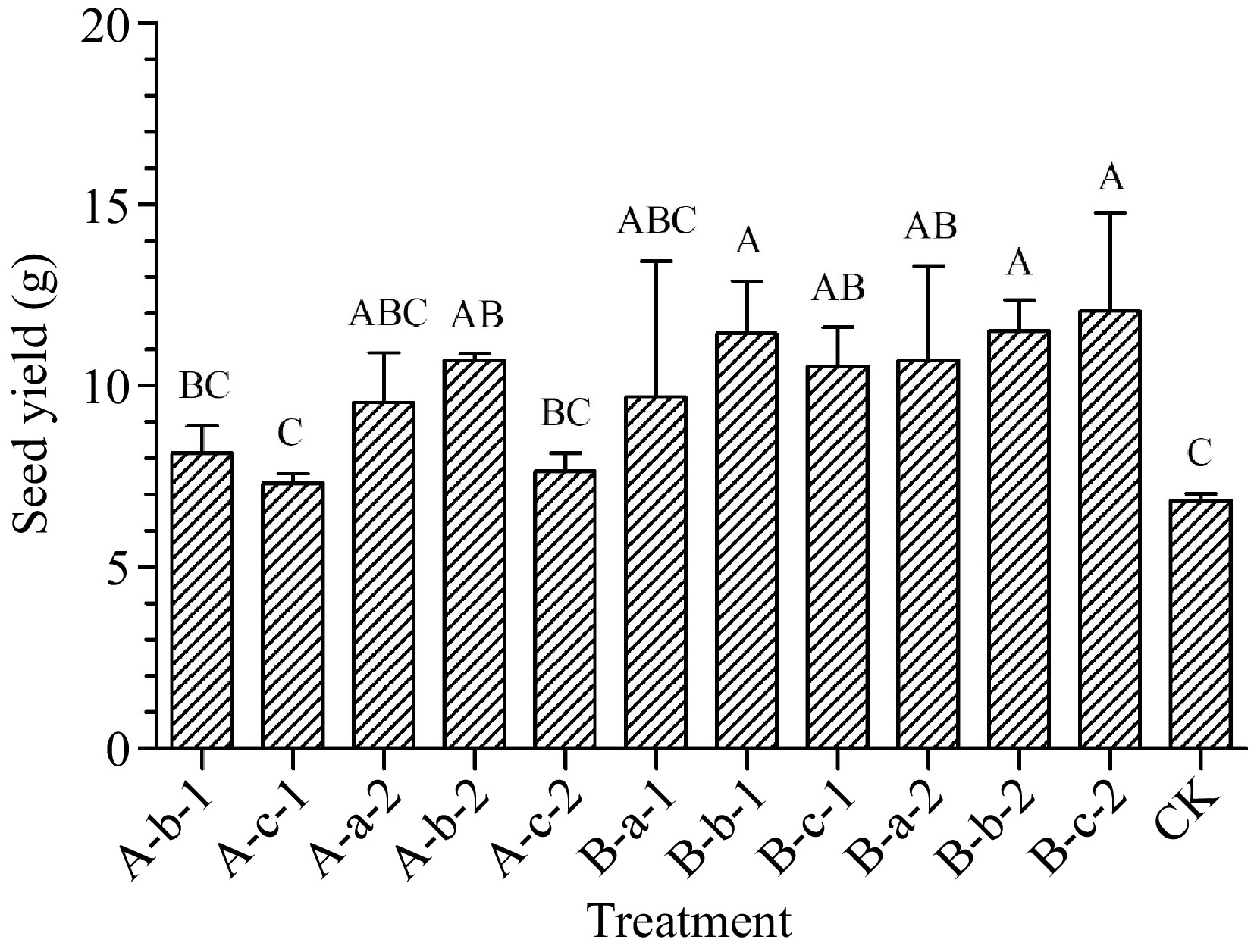
Figure 3.
Effects of spraying reagents on seed yield in experimental plots. The content in the abscissa is the same as Fig. 1.
Potential seed yield
-
All treatments increased potential seed yield compared to the control group (p < 0.05) (Table 3). Among them, 100 mg/L DIECA + 50 mg/L NAA, 100 mg/L DIECA + 100 mg/L NAA, and 100 mg/L DIECA + 150 mg/L NAA have the most significant treatment effects. Potential seed yields of the three treatments increased by an average of 122%, 136%, and 121% respectively. Potential seed yield, actual seed yield, and seed yield coefficient is shown in Table 3.
Table 3. Seed yield in each group.
Treatment Potential seed yield (g/m2) Actual seed yield
(g/m2)Seed yield coefficient A-b-1 44.97c 32.58bc 72% A-c-1 48.70c 22.04c 60% A-a-2 42.15c 38.13abc 90% A-b-2 53.74bc 42.84ab 80% A-c-2 47.44c 30.53bc 64% B-a-1 62.93ab 38.80abc 62% B-b-1 65.96ab 45.78a 69% B-c-1 63.67ab 42.18ab 66% B-a-2 67.71a 42.82ab 63% B-b-2 72.40a 46.00a 64% B-c-2 67.62a 48.22a 71% CK 30.56d 27.29d 89% Values with superscript letters a, b, c and d are significantly different within columns (p < 0.05). Actual seed yield
-
All treatments increased actual seed yield compared with the control group (p < 0.05) (Fig. 4). Among them, three treatments showed the best results. The actual seed yield showed 68% increase under 150 mg/L DIECA + 100 mg/L NAA treatment, 69% increase under 100 mg/L DIECA + 100 mg/L NAA treatment, and 77% increase under 100 mg/L DIECA + 150 mg/L NAA treatment.
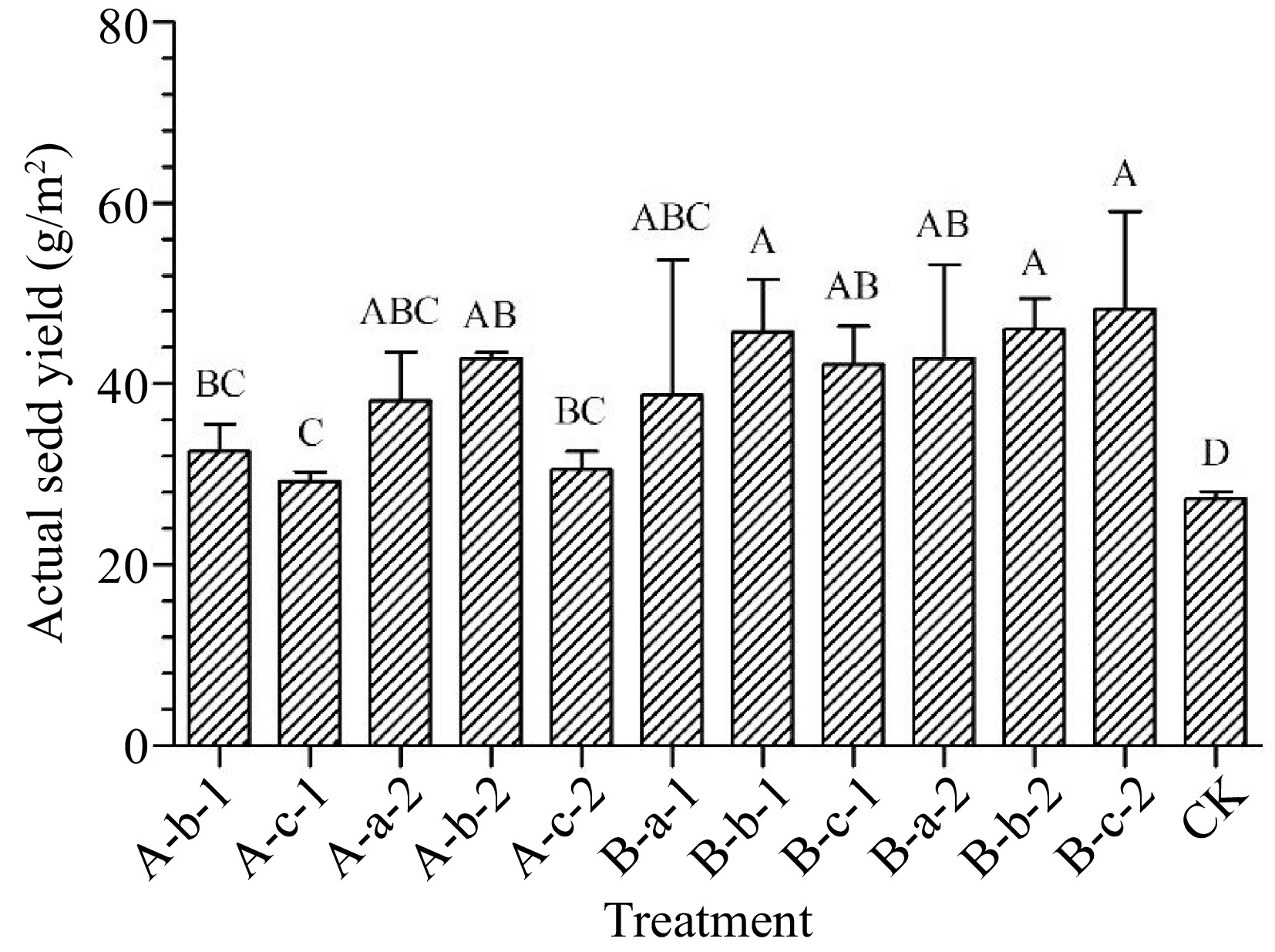
Figure 4.
Effects of spraying reagents on actual seed yield. Note: The content in the abscissa is the same as Fig. 1.
To comprehensively compare the changes in the female to male ratio and seed yield under various treatments and select the treatment with the best effect, Table 4 was created. Under the treatments of 100 mg/L DIECA + 100 mg/L NAA and 100 mg/L DIECA + 150 mg/L NAA, the number of female fertile tillers, male-to-female ratio, potential seed yield, and the actual seed yield of the treatment groups are significantly higher than control groups, even higher than several treatment groups (Table 4). It can therefore be concluded that these two treatments are effective methods to increase the female-to-male ratio and seed yield of buffalograss under field conditions.
Table 4. Sex differentiation and seed yield of each treatment group.
Treatment FFT FF RFM PSY ASY Sum A-b-1 – – – – – 0 A-c-1 – – – – – 0 A-a-2 – – – – – 0 A-b-2 – – – – – 0 A-c-2 – – – – – 0 B-a-1 – A – – – 1 B-b-1 A – – – A 2 B-c-1 – – A – – 1 B-a-2 – A A A – 3 B-b-2 A – A A A 4 B-c-2 A – A A A 4 If the significance of the treatment was marked as 'A' in Duncan's multiple range test, it is marked as 'A' in the table, otherwise it is marked as a '–'. The abbreviations in the column headings represent the following content. FFT: Female fertile tillers; FF: Female florets; RFM: Ratio of female to male; PSY: Potential seed yield; ASY: Actual seed yield; Sum: Total number of 'A'. Female-to-male ratio
-
Compared with the control group, except for the two treatments of 100 mg/L DIECA and 100 mg/L NAA, all other treatments significantly increased the female-to-male ratio of the test materials (p < 0.05) (Fig. 5). Among them, the 150 mg/L DIECA + 150 mg/L NAA, 100 mg/L DIECA + 50 mg/L NAA, 100 mg/L DIECA + 100 mg/L NAA, and 100 mg/L DIECA + 150 mg/L NAA treatments had the most significant effect on increasing the male-female ratio (Fig. 5). Compared with the control group, the female to male ratio of the four treatments increased by an average of 107%, 105%, 130%, and 118% respectively.
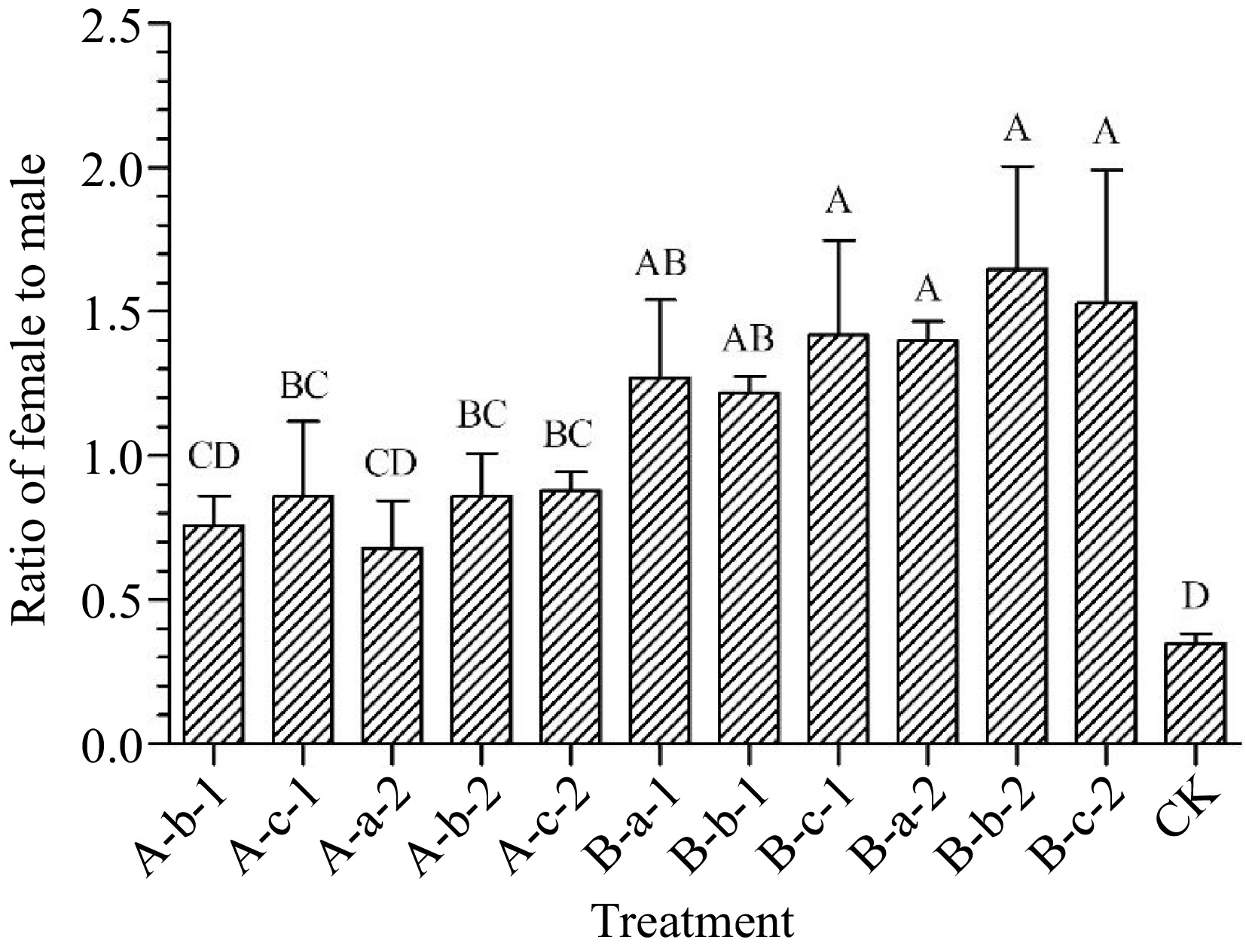
Figure 5.
Effects of spraying reagents on ratio of female to male. Note: The content in the abscissa is the same as Fig. 1.
Endogenous JA content
-
At 6, 12, and 24 h after spraying the reagents, the endogenous JA content in the leaves under all treatments was significantly lower than that of the control (p < 0.05) (Table 5). Six hours after spraying the reagents, the effect was most significant under the treatment of 150 mg/L DIECA and 150 mg/L NAA. The endogenous JA content in plant leaves of treatments mentioned above decreased by 60% and 58% compared with the control. The effect was most significant under the treatment of 150 mg/L DIECA + 50 mg/L NAA 12 h after spraying the reagents. The endogenous JA content in plant leaves decreased by 65% compared with the control. The effect was most significant under 150 mg/L NAA treatment 24 h after spraying the reagents. The endogenous JA content in plant leaves decreased by 57% compared to the control.
Table 5. Endogenous JA content in leaves, 6, 12, 24 h after treatment.
Treatments JA content (ng/g FW) 6 h 12 h 24 h A-b-1 19.02 19.70 26.97 A-c-1 11.15 16.31 21.51 A-a-2 18.87 20.41 25.04 A-b-2 12.86 13.54 12.95 A-c-2 11.67 14.76 12.11 B-a-1 15.74 9.67 20.12 B-b-1 18.13 18.98 14.80 B-c-1 14.46 17.40 20.79 B-a-2 19.96 15.77 19.94 B-b-2 14.44 14.51 18.43 B-c-2 13.50 19.53 17.14 CK 27.90* 27.90* 27.90* '*' indicates that the treatment is significantly different from other groups within column. At 6, 12, and 24 h after spraying the reagents, the endogenous JA content of the plant roots under all treatments was significantly lower than the control (p < 0.05) (Table 6). Six hours after spraying the reagents, the effect was most significant under the treatment of 150 mg/L DIECA + 150 mg/L NAA. The endogenous JA content in plant roots was reduced by 54% compared to the control. The effect was most significant under the treatment of 100 mg/L DIECA + 50 mg/L NAA 12 h after spraying the reagents. The endogenous JA content in plant roots decreased by 44% compared to the control. Twenty four hours after spraying the reagents, the most significant effect was achieved under the treatment of 100 mg/L DIECA + 150 mg/L NAA. The endogenous JA content in the plant roots was reduced by 57% compared to the control.
Table 6. Endogenous JA content in roots, 6, 12, 24 h after treatment.
Treatments JA content (ng/g FW) 6 h 12 h 24 h A-b-1 6.42 6.85 6.78 A-c-1 6.40 8.45 6.40 A-a-2 8.26 8.08 8.22 A-b-2 7.59 7.30 6.18 A-c-2 5.38 8.77 5.75 B-a-1 6.09 5.97 6.64 B-b-1 5.56 6.12 6.40 B-c-1 4.54 7.62 8.16 B-a-2 8.43 5.44 5.14 B-b-2 6.31 5.70 4.22 B-c-2 5.69 9.25 7.30 CK 9.80* 9.80* 9.80* '*' indicates that the treatment is significantly different from other groups within column. -
NAA is a synthetic plant growth regulator that promotes early flowering[36], increases female flowers[37], delays maturity[38] and increases yield[39]. At present, most research on NAA focuses on its effects on plant secondary metabolites using plant tissue culture. The basic research method involves adding NAA and other plant growth regulators to Murashige and Skoog (MS) medium[40,41].
Appropriate concentrations of NAA treatments have been shown to increase the content of monoterpene glycosides in Telekia speciose multi-branch buds[42], terpenoid content in the buds of the South African medicinal plant Sutherlandia Frutescens[43], callus formation in Prunella vulgaris L[44], and flavonoid content in non-regenerating callus of Sterculia urens[41]. NAA enhanced the antioxidant activity of plants, by increasing the activities of peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD) in plants after treatments[44]. Studies have demonstrated that the oxygen free radical antioxidant capacity (ORAC) of soybean sprouts (Glycine max (Linn.) Merr.) is higher in the first three days after NAA treatment compared to the control[45], providing evidence of NAA' s positive effects on plant antioxidant activity. The present study found that spraying 100 mg/L and 150 mg/L NAA significantly increased the female-to-male ratio of buffalograss and significantly increased seed yield. Fifty mg/L and 100 mg/L NAA treatments did not significantly increase the number of spikes but significantly increased the number of female florets. Therefore, it can be inferred that exogenous NAA may have a promoting effect on the reproductive growth of buffalograss plants, but it may have an inhibitory effect on the development of male flowers.
JA plays a role in sex determination in plants
-
JA and its derivatives are widely distributed in various organs of plants[46]. Some studies suggest that JA may play a role in the sex determination process in corn. TS1 and TS2 are key genes for tassel development in maize (Zea mays L.). The loss of any one of them will lead to failure of tassel sex determination, resulting in tassel seeding ts1 (tasselseed1) and ts2 (tasselseed2) mutants[47]. Acosta et al.[48] found that the activity of 13-lipoxygenase (13-LO) disappeared in the developing inflorescences of the ts1 mutant, and the endogenous JA concentration was reduced to one-tenth that of wild-type maize. External application of JA to the developing inflorescences of ts1 and ts2 mutants restored the development of stamens, indicating that the lack of JA is the cause of the mutant phenotype and is closely related to maize stamen development[49]. Furthermore, studies have demonstrated that the expression of the TS2 gene in maize reproductive organs is significantly higher than in vegetative organs. NAA significantly inhibited the expression of TS2 gene. Therefore, the authors propose that the TS2 gene is a plant sex-determining gene that is mediated by JA signaling[50]. A homologous gene, BdTs2, of the maize TS2 gene, is also present in buffalograss. Higher expression of BdTs2 in male inflorescences leads to the abortion of pistils and the development of unisexual male flowers. BdTs2 is specifically expressed in the reduced pistil group of male flowers, but not in other floral organs of the inflorescence[51]. Based on the above results, it is inferred that the expression pattern of the TS2 gene in buffalograss is consistent with that in corn, and its high expression promotes male-oriented sex expression of buffalograss. Additionally, the expression level of the TS2 gene may be influenced by the content of endogenous or exogenous JA.
DIECA likely inhibits the JA pathway by shunting 13 (S)-hydroperoxylinolenic acid to 13-hydroxylinolenic, thereby sharply reducing the precursor pool leading to cyclization and eventual synthesis of JA[52]. In this study, DIECA solution was sprayed on buffalograss plants. The treatment groups showed significantly higher numbers of female fertile tillers, female florets, and seed yield compared to the control group. These results align with previous research and inferences, suggesting that DIECA reduces the endogenous JA content in buffalograss and inhibits the expression of the TS2 gene by inhibiting endogenous JA biosynthesis. This inhibition allows for the development of some female flowers without hindrance, thereby increasing the female ratio and seed yield. The combined use of NAA and DIECA also promotes a higher female-to-male ratio and increases seed yield, with a stronger effect observed when the two are used together.
Taking the treatment with the most significant effect as an example. Two liters of 100 mg/L DIECA + 100 mg/L NAA solution was sprayed in a 4 m2 plot and it was sprayed seven times. The total cost of reagents per hectare equals USD
${\$} $ This study represents the first application of the reagents NAA and DIECA on buffalograss. The effects of these reagents on the number of male and female fertile tillers, the number of male and female florets, and seed yield were statistically analyzed. Additionally, a preliminary investigation was conducted on the effects of NAA and DIECA on the endogenous JA concentration in buffalograss leaves and roots. These findings provide an innovative, simple, and effective method for artificially regulating the female-to-male ratio of buffalograss in field production to increase seed yield.
-
The present results show that spraying NAA, DIECA or a mixture of the two on buffalograss plants under field conditions significantly increased the number of female fertile tillers, the number of female florets, and significantly increased the female-to-male ratio, and seed yield. At the same time, it significantly reduced the number of male fertile tillers and the number of spikes. The results of endogenous JA content showed that spraying these two reagents reduced the endogenous JA content of buffalograss plants. Different reagents have different effects on regulating the endogenous JA content of buffalograss plants at different concentrations and proportions. In addition, the changes in endogenous JA content of buffalograss were also different at 6, 12, and 24 h after spraying the reagents.
-
The authors confirm contribution to the paper as follows: study conception: Zhu K, Wang C; writing – review & editing, writing – original draft, resources, project administration, formal analysis: Zhu K; data curation: Zhu K, Li Y; methodology: Hu QN; data collection: Ren XF; supervision, resources, funding acquisition: Sun Y. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are not publicly available due the fact that the results of this study are the basic data for subsequent studies but are available from the corresponding author on reasonable request.
This research was funded by the China Agriculture Research System (CARS-34-03).
-
The authors declare that they have no conflict of interest.
- Supplemental Table S1 Effects of exogenous DIECA on potential seed yield, actual seed yield and female to male ratios of buffalograss in 2022.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhu K, Wang C, Li Y, Hu QN, Ren XF, et al. 2024. Exogenous spraying of Naphthaleneacetic acid (NAA) and Sodium diethyldithiocarbamate trihydrate (DIECA) increased the female to male ratio and seed production of buffalo grass. Grass Research 4: e014 doi: 10.48130/grares-0024-0012
Exogenous spraying of Naphthaleneacetic acid (NAA) and Sodium diethyldithiocarbamate trihydrate (DIECA) increased the female to male ratio and seed production of buffalo grass
- Received: 21 January 2024
- Revised: 15 April 2024
- Accepted: 18 April 2024
- Published online: 03 June 2024
Abstract: Buffalograss has been extensively used in recent years as a low-maintenance lawn grass. The artificial manipulation of sex differentiation in buffalograss to enhance seed production is important for both cultivation and practical applications. In this study, we applied a specific concentration of synthetic auxin (Naphthaleneacetic acid, NAA) and inhibitors of jasmonic acid synthesis (Sodium diethyldithiocarbamate trihydrate, DIECA) solution on buffalograss under field conditions. The majority of treatments significantly improved the number of female fertile tillers, female florets, the female-to-male ratio, and ultimately, seed yield (p < 0.05). Among all treatments, 100 mg/L DIECA + 100 mg/L NAA, and 100 mg/L DIECA + 150 mg/L NAA, demonstrated the most effective results. Under these two treatments, the number of female fertile tillers increased by 161% and 145% respectively, the number of female florets increased by 8.56% and 7.82% respectively, the female-to-male ratio increased by 130% and 118% respectively, the potential seed yield increased by 136% and 121% respectively, and the actual seed yield increased by 69% and 77% respectively.
-
Key words:
- Buffalograss /
- Sex differentiation /
- Seed yield /
- NAA /
- DIECA













