-
Apple (Malus domestica Borkh.) breeding presents numerous challenges, including a protracted breeding cycle and a scarcity of germplasm resources, impeding progress in the field. Furthermore, the extended juvenile phase, self-incompatibility, and high genetic heterozygosity are considerable obstacles to the conventional breeding methods for dwarfing apple rootstocks[1,2]. Recently, the advent of plant genetic engineering has provided a promising avenue for advancing apple breeding. Among the available techniques, Agrobacterium-mediated transformation has emerged as the preeminent and well-established approach[3]. This technique involves using two distinguished types of Agrobacterium: Agrobacterium tumefaciens and Agrobacterium rhizogenes, each harboring the Ti plasmid and Ri plasmid, respectively[4]. The Ti/Ri plasmids of Agrobacterium encompass two primary functional regions: the T-DNA region and the Vir region. Through interaction between the Vir gene and the T-DNA boundary, the T-DNA undergoes modification and facilitates the formation of a protein complex. Subsequently, this complex gains entry into the cell through the cell membrane. Ultimately, the T-DNA integrates into the nuclear genome of the recipient plants[5].
Transformation of woody plants through A. tumefaciens remains time-consuming and inefficient[6], while an alternative approach using A. rhizogenes offers several advantages in this context[7]. The T-DNA of A. rhizogenes consists of two noncontiguous DNA fragments: TL-DNA and TR-DNA. Notably, TR-DNA encompasses certain genes that exhibit homology to Ti plasmids, including iaaM, iaaH, and genes encoding opine synthesis (Ops), while TL-DNA assumes an essential role in hairy root formation[8,9]. The insertion of TL-DNA has revealed the presence of four loci that are closely associated with hairy root development. These loci, known as rol A, B, C, and D, exhibit no homology to Ti plasmids[10,11]. The TL-DNA fragment appears to enhance rooting ability by potentially modulating cell sensitivity to auxin rather than directly promoting auxin accumulation[12]. Consequently, the genetically modified hairy roots generated through this transformation technique can thrive even in the absence of external hormonal supplementation[13].
Cutting propagation is the primary method for producing apple rootstocks. The ability to form adventitious roots is crucial for successful cutting propagation. However, apple rootstocks like 'M9' often face challenges with rooting and generally do not form roots without the application of exogenous hormones[14]. Auxin is a crucial plant hormone in the formation of adventitious roots. High levels of auxin are required during the induction phase of adventitious roots[15,16]. A. rhizogenes, which contains rol genes, can increase the auxin content in rootstocks, thereby enhancing their rooting ability. Interactions between cytokinin and auxin are known to govern the development of adventitious roots, with a competitive relationship observed between these two hormone classes. Elevated concentrations of cytokinin impede the expression of genes associated with auxin synthesis and transport, thereby reducing endogenous auxin levels and inhibiting the formation of adventitious roots[17]. In the research of rootstocks, Li et al. have reported that modulating auxin levels while decreasing cytokinin concentrations in rootstocks can confer beneficial traits such as enhancing grafting success rates and improving rooting rate and biomass[18]. In Arabidopsis, cytokinin signaling primarily relies on histidine kinases (AHKs), histidine phosphate transfer proteins (AHPs), response regulatory factors (ARRs), and Cytokinin Oxidation/Dehydrogenase protein (CKX)[19,20]. The CKX gene encodes CKX enzymes, and their expression is characterized by tissue-specific and developmental dependencies[21,22]. Introduction of the CKX gene from Arabidopsis thaliana (AtCKX) into tobacco has been shown to increase the number of differentiated cells in root meristems and primary roots of transgenic tobacco[23]. Activation of the AtCKX gene in the lateral root primordium has also been found to promote lateral root formation[24].
In this study, the A. rhizogenes transformation system was successfully established to facilitate the induction of adventitious roots in apple dwarf rootstocks. Remarkably, the introduction of the AtCKX2 gene into A. rhizogenes resulted in the production of dense and robust adventitious roots. These findings hold significant promise in advancing our understanding of adventitious root formation and provide valuable insights to enhance the cultivation of dwarf and densely planted apple orchards.
-
'M9', 'M26', and Malus xiaojinensis plantlets were subjected to a controlled tissue culture environment with a temperature of 25 ± 1 °C and a 16-h light/8-h dark photoperiod. Subculturing of the plantlets was performed at regular intervals of 30 d, ensuring the sustained growth and development of the plants under optimal conditions.
Preparation of Agrobacterium rhizogenes solution
-
The 35S::GUS and 35S::AtCKX2[18] sequences were inserted into the pCAMBIA1305.1 plasmid, resulting in the construction of a recombinant vector. The constructed vector was introduced into two strains of A. rhizogenes, namely K599 and MSU440. Before transformation, the A. rhizogenes were stored at a temperature of −80 °C. Subsequently, they were streaked onto tryptone-yeast extract (TY) solid medium plates and incubated in darkness at 28 °C for 2 d. Single colonies were selected from the plate and cultured overnight in 2 mL of TY liquid medium at 28 °C and 180 rpm. Subsequently, 1 mL of the bacterial culture was transferred into 50 mL of TY liquid medium, followed by cultivation at 28 °C and 180 rpm until OD600 = 0.6 − 0.8. After centrifugation at 5,000 rpm for 10 min, the resulting pellet was resuspended to achieve an OD600 value ranging from 0.4 to 0.6.
A. rhizogenes infected the stem segment of apple stock
-
The terminal buds (~1 cm) from 30-day-old plants of 'M9', 'M26', and M. xiaojinensis were selected for infection. These tips were submerged in a bacterial culture and exposed to cultivation conditions of 28 °C and 180 rpm for 20 min. After excess bacterial culture was removed using sterile filter paper, the shoot tips were transferred to a solid co-culture medium and maintained for three days and moved to a rooting medium containing 250 mg/L Cef and 250 mg/L Tim.
GUS staining of transgenic materials
-
After successful rooting, plantlets of 'M9', 'M26', and M. xiaojinensis were subjected to GUS staining to visualize the expression of the GUS reporter gene. The GUS staining solution comprised 10 mM EDTA, 100 mM NaH2PO4·H2O, 0.5 mM K4Fe(CN)6·3H2O, 0.1% Triton X-100, and 1 mM X-gluc. Plantlets were fully immersed in this solution and incubated for 12–16 h at 37 °C. Subsequently, the plantlets were treated with 95% ethanol at room temperature until complete decolorization. Staining results were observed and recorded.
Determination of hormone content
-
The shoots and roots of transformed 'M26' plants were harvested 80 d after transferring to the rooting media. Untransformed 'M26' plants were employed as controls. Fresh tissues (0.5 g) were carefully ground into a fine powder using liquid nitrogen. Three biological replicates were collected and used for hormone measurements for both the transformed and control plants. Hormone levels were evaluated using Enzyme-linked Immunosorbent Assays (ELISA)[25].
-
'M9' and 'M26' exhibited poor rooting ability, whereas Malus xiaojinensis showed better rooting capability[26]. A. rhizogenes-mediated transformation of 'M9', 'M26', and M. xiaojinensis was first tested on hormone-free 1/2 MS media and it was found that shoot tips of all three cultivars exhibited the capacity to induce adventitious roots. GUS staining was conducted on the A. rhizogenes-induced roots of 'M9', 'M26', and M. xiaojinensis plantlets. The staining results revealed a blue coloration in the roots, indicating successful integration and abundant expression of the GUS gene within the plant genomes (Fig. 1).
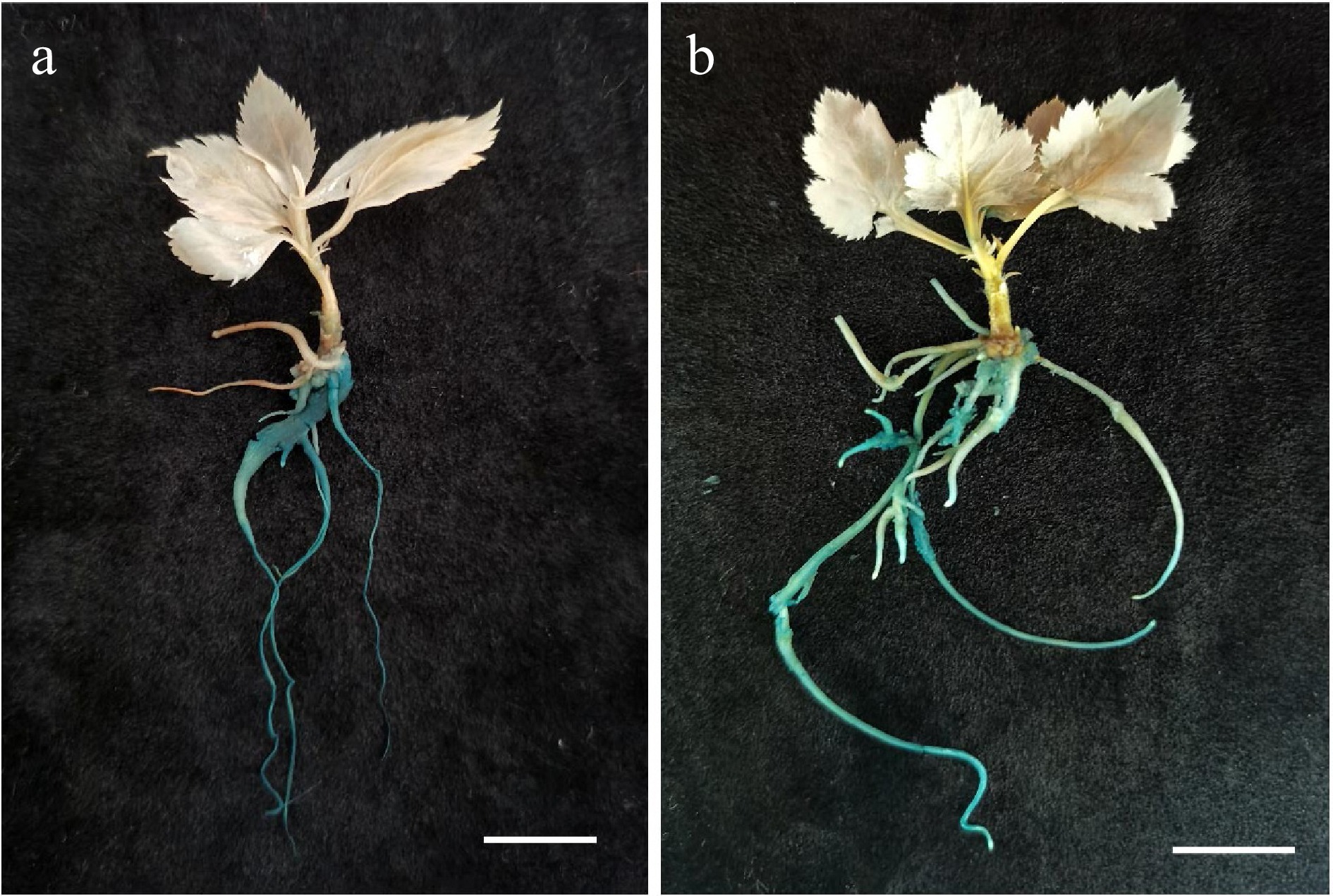
Figure 1.
GUS staining results of (a) 'M9', and (b) Malus xiaojinensis after Agrobacterium rhizogenes transformation. The seedlings were grown for 2 months. Scale bar = 1 cm.
Compared with untransformed plants cultured under hormone-free conditions, the transformed plant materials exhibited significantly higher rooting rates. Specifically, untransformed 'M9' plants displayed a modest rooting rate of 3.33%, while the rate increased significantly to 86.08% with A. rhizogenes strain K599 transformation. Likewise, the rooting rates of 'M26' and M. xiaojinensis showed notable increases compared with untransformed controls (Table 1).
Table 1. Rooting rate of Agrobacterium rhizogenes-transformed rootstocks. The rooting rate was calculated 80 d after transferring to rooting medium.
Rootstocks Strains Total number of
infected plantsRooting rate (%) 'M9' Untransformed 60 3.33 ± 1.67 K599 134 86.08 ± 5.61** MSU440 124 56.68 ± 7.91** 'M26' Untransformed 60 11.67 ± 1.67 K599 142 89.96 ± 4.77** MSU440 146 76.07 ± 2.79** Malus xiaojinensis Untransformed 60 13.33 ± 1.67 K599 158 79.15 ± 5.58** MSU440 213 90.97 ± 1.97** The data are means ± SEM calculated based on three biological replicates. The total number of infected plants represents the combined number of plants from three replicates. Asterisks denote significant difference determined by using a two-tailed Student's t test (*p < 0.05, **p < 0.01). The rooting rate (%) = the number of rooted plants/the total number of infected plants × 100%. Interestingly, the efficiency of root induction varied between the two A. rhizogenes strains utilized in this study. Strain K599 demonstrated a more robust capacity to induce adventitious roots than strain MSU440 for 'M9' and 'M26' rootstocks, with the induced rooting rate observed in the order of 'M26' > 'M9' > M. xiaojinensis. Notably, upon exposure to K599-mediated transformation, 'M9' and 'M26' displayed impressive rooting rates of 86.08% and 89.96%, respectively. Conversely, strain MSU440 exhibited superior performance for M. xiaojinensis, achieving a rooting rate of 90.97%, compared with a rate of 79.15% with strain K599 (Table 1). These findings highlight the strain-specific variations in the ability to induce roots. Specifically, K599 appears better suited for rootstocks with challenging rooting characteristics such as 'M9' and 'M26', while MSU440 is more suitable for M. xiaojinensis, a rootstock showing relatively easier rooting tendencies.
The efficiency of A. rhizogenes-mediated transformation was found to be intricately linked to the concentration of the bacterial growth. At three different concentration levels (0.4, 0.5, and 0.6), the rooting rates of all three rootstock materials exhibited a similar pattern: an initial increase with rising concentrations of bacteria, followed by a subsequent decline. The optimal concentration for achieving a higher rooting rate was determined to be OD600 = 0.5 (Table 2).
Table 2. Rooting rate of transformation with three levels of bacterial culture.
Rootstocks Bacterial concentration (OD600) Total number of infected plants Number of rooted plants Rooting rate (%) 'M9' 0.4 150 115 76.67 0.5 162 141 87.04 0.6 104 68 65.38 'M26' 0.4 137 105 76.64 0.5 165 139 84.24 0.6 124 99 79.84 Malus xiaojinensis 0.4 138 114 82.61 0.5 110 101 91.82 0.6 123 103 83.74 K599 was used to transform 'M9' and 'M26', whereas MSU440 was employed for the transformation of Malus xiaojinensis. The rooting rate was calculated 80 d after transferring to rooting medium. The rooting rate (%) = the number of rooted plants/the total number of infected plants × 100%. Root growth after A. rhizogenes transformation
-
The influence of A. rhizogenes transformation on root growth was thoroughly evaluated by quantifying the number and length of roots under optimal transformation conditions as described above. The control group comprised untransformed plants induced by 0.5 mg/L IBA, while the experimental group consisted of transformed plants from 'M9', 'M26', and M. xiaojinensis rootstock materials. Remarkably, A. rhizogenes-mediated transformation increased the number of lateral and adventitious roots across all three rootstock materials (Table 3).
Table 3. Effects of Agrobacterium rhizogenes transformation on root growth.
Rootstocks Strains Number of
adventitious rootsAdventitious root
length (cm)Number of
lateral rootsLateral root
length (cm)'M9' Untransformed 2.2 ± 0.26 3.39 ± 0.4 1.73 ± 0.93 0.13 ± 0.05 MSU440 3.67 ± 0.61* 3.67 ± 0.73 7.67 ± 2.37* 0.92 ± 0.22** K599 4.53 ± 0.71** 2.43 ± 0.29 13.53 ± 2.65** 1.25 ± 0.23** 'M26' Untransformed 5.94 ± 0.73 5.52 ± 0.65 8.88 ± 2.75 0.47 ± 0.12 MSU440 7.06 ± 1.03 3.88 ± 0.45* 10.75 ± 3.73 1.08 ± 0.27* K599 5.38 ± 0.82 3.74 ± 0.36* 16.38 ± 4.49 1.61 ± 0.27** Malus xiaojinensis Untransformed 3.22 ± 0.27 3.81 ± 0.34 7.07 ± 0.86 0.75 ± 0.14 MSU440 5.89 ± 0.67** 2.85 ± 0.23* 13.07 ± 3.57 1.02 ± 0.21 K599 2.56 ± 0.35 2.08 ± 0.28** 15.7 ± 4.74 0.5 ± 0.12 The growth of GUS-positive roots was evaluated 80 d after transferring to root medium. The data are means ± SEM, asterisks denote significant difference determined by using a two-tailed Student's t test (*p < 0.05, **p < 0.01). Comparative analysis revealed distinct disparities in the effects of MSU440 and K599 strains on lateral root development. 'M9' plants transformed with strain MSU440 exhibited a significant increase, inducing a 4.43-fold increase in lateral roots, while plants transformed with strain K599 demonstrated a 7.82-fold increase in lateral root formation compared with untransformed plants (Table 3). These findings suggest that the two strains possess differential capabilities in promoting lateral root development for the 'M9' rootstock, with K599 potentially exhibiting superior performance in terms of lateral root initiation and growth.
The optimal strain for adventitious root formation is the same for both 'M26' and M. xiaojinensis. In 'M26', the number of adventitious roots increased by 1.19-fold with MSU440 compared with the untransformed control, while in M. xiaojinensis, it increased by 1.83-fold with MSU440. In contrast, K599 resulted in a 2.06-fold increase in adventitious roots for 'M9'. Interestingly, the transformation process exerted an inhibitory effect on the elongation of adventitious roots in 'M26' and M. xiaojinensis. Additionally, it was observed that the K599 strain exerted a stronger inhibitory influence on adventitious root length than MSU440 (Table 3).
Overexpression of AtCKX2 gene promotes adventitious root growth and development
-
Methods to enhance the performance of adventitious roots were further investigated by introducing a 35S::AtCKX2 gene into A. rhizogenes. Transgenic roots lacking the 35S::AtCKX2 gene were utilized as a control group to assess the impact of overexpression of AtCKX2.
While all three rootstocks overexpressing the AtCKX2 gene exhibited comparable rooting rates to the control, the adventitious root length of 'M9', 'M26', and M. xiaojinensis plants transformed with 35S::AtCKX2 gene showed a significant increase by 1.44, 1.54, and 1.24 times, respectively, compared with the controls (Table 4). These findings reflect the promoting effect of the AtCKX2 gene on adventitious root development.
Table 4. Effects of overexpressing AtCKX2 gene on root growth. Analyzing the growth of GUS-positive roots.
Rootstocks Genotype Rooting rate
(%)Number of
adventitious rootsAdventitious root
length (cm)Number of
lateral rootsLateral root
length (cm)M9 Control 72.01 ± 6.21 4.7 ± 0.58 3.17 ± 0.32 11.41 ± 1.93 1.03 ± 0.12 AtCKX2 76.11 ± 5.98 6.39 ± 0.66 4.55 ± 0.47* 19.73 ± 3.48* 1.31 ± 0.11 M26 Control 82.93 ± 2.88 5.74 ± 0.62 4.33 ± 0.34 15.87 ± 2.97 1.71 ± 0.2 AtCKX2 88.68 ± 3.41 8.3 ± 0.83* 6.65 ± 0.63** 26.52 ± 4.08* 3.29 ± 1.65 Malus xiaojinensis Control 86.06 ± 2.90 5.46 ± 0.4 2.97 ± 0.17 12.32 ± 1.96 1.01 ± 0.1 AtCKX2 86.89 ± 2.38 6.54 ± 0.44 3.68 ± 0.2** 19.36 ± 2.96* 1.15 ± 0.1 The data are the means ± SEM, asterisks denote significant difference determined by using a two-tailed Student's t test (*p < 0.05, **p < 0.01). The rooting rate (%) = the number of rooted plants/the total number of infected plants × 100%. Furthermore, overexpression of the AtCKX2 gene increases the number of lateral and adventitious roots in 'M9', 'M26', and M. xiaojinensis compared with the controls. Overexpressing AtCKX2 in 'M9' resulted in an additional 1.69 adventitious roots per plant, with increases of 2.56 and 1.08 adventitious roots per plant observed in 'M26' and M. xiaojinensis, respectively, compared to the control group. Specifically, overexpression of AtCKX2 in 'M26' produced 10.65 more lateral roots per plant, an increment of 7.04 and 8.32 lateral roots per plant in M. xiaojinensis and 'M9', respectively, compared with the controls (Table 4).
Effect of overexpressing AtCKX2 gene on plant hormone content
-
The hormone contents, specifically indole-3-acetic acid (IAA) and zeatin riboside (ZR), were determined in the shoots and roots of 'M26' rootstock plants transformed by A. rhizogenes, as well as control plants without transformation. Noteworthy observations were made regarding the variation in auxin and cytokinin levels among different treatments.
Substantial differences in auxin content were observed in the roots across various treatments, with minor variations detected in shoots. Conversely, significant differences in cytokinin levels were found between treatments in both the shoot and root tissues. A remarkable increase in auxin and cytokinin levels was observed in the roots following A. rhizogenes transformation. However, when the AtCKX2 gene is overexpressed, the auxin and cytokinin content in the roots decreased to approximately the same level as that observed in untransformed plants (Fig. 2).
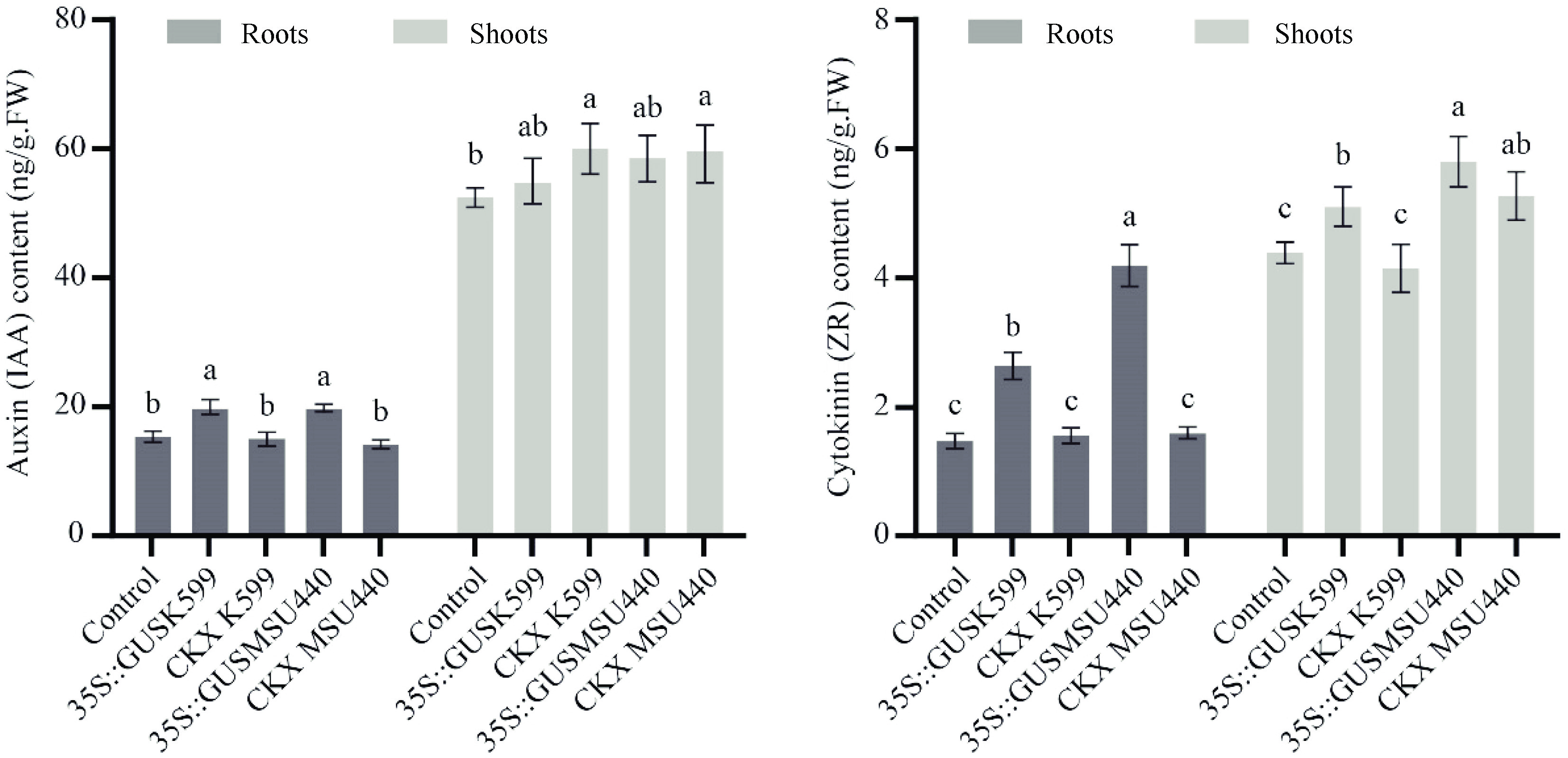
Figure 2.
Hormone contents of Agrobacterium rhizogenes-transformed 'M26' plants, 'M26' untransformed plants were employed as controls. The data are the means ± SD calculated from three biological replicates. Different letters denote significant difference within each tissue type determined by one-way ANOVA (Tukey's test; p < 0.05).
Interestingly, no significant difference was observed between the two strains regarding their capacity to promote auxin accumulation in root tissues. However, a significant disparity was noted in terms of their impact on cytokinin accumulation in both root and shoot tissues (Fig. 2). These findings highlight the distinct effects of the two strains on the regulation of cytokinin levels.
-
In this study, an Agrobacterium rhizogenes transformation system has been successfully established for apple dwarf rootstocks, including 'M9', 'M26', and Malus xiaojinensis. This system has led to significant enhancements in transgenic root induction compared with untransformed plants. A. rhizogenes, renowned for its rapid transformation cycle, demonstrates exceptional efficiency in triggering adventitious root formation[27]. Typically, these roots emerge at the stem segment's base within 1–2 weeks post-transformation, attaining full development within 2–3 months. The successful generation of stable transgenic plants relies on two crucial processes: Agrobacterium-mediated infection of explants and subsequent regeneration of transformed materials. Notably, the choice of A. rhizogenes strains, their concentration, and the selection of suitable explant materials directly affect the overall transformation efficiency[28].
The ability of different Agrobacterium strains to infect the host plant significantly affects rooting efficiency. In the present study, transformation of the 'M9' rootstock using the K599 strain resulted in an impressive rooting rate of 86.08%, whereas the MSU440 strain achieved a lower rate of 56.68% (Table 1). These findings align with prior research that utilized A. rhizogenes strains ARA4, MSU440, C58C1, and K599 for pigeon pea transformation and reported that the K599 strain exhibited a rooting rate of 30%, while the ARA4 strain showed a rate of 8%[29]. These observations highlight the significant differences between Agrobacterium strains in rooting efficiency, emphasizing the importance of strain-specific characteristics in successfully inducing adventitious roots.
The infectivity of different Agrobacterium strains may lead to diverse responses in plants. The rooting capacity of the three rootstocks in this study follows this hierarchy: M. xiaojinensis > 'M26' > 'M9'[26]. The K599 strain showed a superior rooting ability compared with the MSU440 strain for 'M9' and 'M26', which are known for their relatively poor rooting capabilities. Conversely, the MSU440 strain exhibited better efficacy in promoting rooting in M. xiaojinensis. These observations prompt speculation that the K599 strain may possess a greater ability to enhance auxin levels or increase cellular sensitivity to auxin in the plants after transformation. However, it is important to note that for M. xiaojinensis, which inherently possesses easier rooting potential, the K599 strain exhibited a lower rooting rate than the MSU440 strain, suggesting that the concentration of auxin might exceed the optimal threshold for rooting.
Improper concentrations of A. rhizogenes may impair the transformation efficiency. Inadequate bacterial concentration leads to fewer interactions between A. rhizogenes and the wounded explants, which may result in reduced contact and lower transformation rates. Conversely, excessively high bacterial concentration might have negative effects on the explants[30]. In this study, an optimal A. rhizogenes concentration of OD600 = 0.5 was identified for the three rootstocks. High rooting rates of 87.04%, 84.24%, and 91.82% were achieved for 'M9', 'M26', and M. xiaojinensis, respectively, under this optimal concentration (Table 2). These findings align with earlier investigations, which also underscored the significance of maintaining an Agrobacterium concentration of OD600 = 0.5 as optimal for apple materials such as 'GL-3' and 'Royal Gala'[6,31].
The root phenotype induced by A. rhizogenes transformation shares similarities with roots overproducing auxin, characterized by shorter length and increased formation of root hairs[32]. The co-introduction of iaaM and CKX genes has been demonstrated to enhance root length and biomass[18]. Wang et al. showed that initiating AtCKX3 expression using a root-specific PYK10 promoter and a constitutive 35S promoter significantly improved the root development of CKX transgenic tobacco compared with the control plants[33]. In the present study, the introduction of the AtCKX2 gene resulted in increased root length and number across the three apple rootstock varieties.
Analysis of hormone content revealed no significant variation in auxin levels in shoots following transformation with K599 and MSU440 (Fig. 2). Auxin synthesis primarily occurs in young leaves, and buds, with subsequent transport from shoots to roots through vascular tissue[34]. Therefore, an increase in auxin content in roots after transformation may not directly affect auxin levels in shoots. In contrast, cytokinin content after the Agrobacterium infection exhibited substantial changes in shoots, showing a similar trend to that observed in roots (Fig. 2). This is likely attributed to the fact that cytokinin synthesis primarily occurs in the root tip, followed by accumulation and subsequent transport to the shoot[35].
IAA is pivotal in promoting adventitious root formation during the induction phase. However, elevated concentrations of IAA can impede root formation in later stages. A. rhizogenes infection led to heightened auxin levels in transgenic roots compared with untransformed roots, thereby enhancing adventitious rooting rates, and inhibiting root elongation. Moreover, the inhibitory impact of root length and increased cytokinin contents in A. rhizogenes-infected transgenic roots were diminished upon overexpressing the AtCKX2 gene. As the cytokinin level decreased and hormone interactions occurred, the auxin level also decreased to a level similar to that of untransformed roots (Fig. 2). This regulatory mechanism helped to avoid the inhibitory effects of excessive auxin on root length.
-
The present discoveries enrich the comprehension of molecular mechanisms governing root development and propose the potential of employing the A. rhizogenes transformation system for root-related research. This established system offers a convenient and swift approach for examining gene functionality and investigating signal transmission between roots and aboveground components.
-
The authors confirm contribution to the paper as follows: study conception and design: Han Z, Li W; performing the experiments and date analysis: Guo Y, Wang Z, Shu S, Dong J; writing and editing the manuscript: Li W, Han Z, Guo Y, Wang Z, Deng CH, Dong J, Wang Y. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
This work was supported by the National Natural Science Foundation of China (32072529, 32272664, 31801823), the earmarked fund for the China Agricultural Research System (CARS-27), 2115 Talent Development Program of China Agricultural University, the Construction of Beijing Science and Technology Innovation and Service Capacity in Top Subjects (CEFF-PXM2019_014207_000032), and 111 Project (B17043).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Guo Y, Wang Z, Deng CH, Dong J, Shu S, et al. 2024. Improving the root system of apple rootstocks based on Agrobacterium rhizogenes-mediated transformation system. Fruit Research 4: e033 doi: 10.48130/frures-0024-0027
Improving the root system of apple rootstocks based on Agrobacterium rhizogenes-mediated transformation system
- Received: 11 April 2024
- Revised: 03 August 2024
- Accepted: 08 August 2024
- Published online: 08 October 2024
Abstract: Dwarfing and close planting have become the general trend of the apple industry, but the rooting difficulty of dwarfing rootstocks has seriously limited efficient breeding. Agrobacterium rhizogenes can infect plants and induce the formation of hairy roots. In this study, the optimal A. rhizogenes-mediated transformation system was explored for three apple rootstocks: 'M9', 'M26', and Malus xiaojinensis. The results reveal that the best transformation concentration for all three rootstocks is OD600 of 0.5. 'M9' and 'M26' exhibited rooting rates of 86.08% and 89.96%, respectively, upon transformation with A. rhizogenes strain K599. In contrast, M. xiaojinensis attained a rooting rate of 90.97% when strain MSU440 was introduced. Furthermore, a Cytokinin Oxidation/Dehydrogenase (CKX) gene was demonstrated to significantly increase the root length and lateral root density of hairy roots. These results have the potential to enhance the rooting ability of dwarfing rootstocks and contribute to the development of more efficient and productive orchard management strategies.
-
Key words:
- Apple rootstock /
- Agrobacterium rhizogenes /
- CKX /
- Cytokinin /
- Auxin












