-
The Maillard reaction (MR) is known as non-enzymatic browning, which occurs widely during the heating process of food. Melanoidins, the product of the MR, are formed by the condensation of reducing sugar carbonyl and amino group[1]. Melanoidins have been shown to improve food quality, such as color, flavor, and biological activity[2]. For example, melanoidins extracted from bread and biscuits exhibit antimicrobial effects on certain molds and yeasts, which could improve the shelf life and safety of food as a natural antimicrobial agent; melanoidins extracted from bread could serve as a carbon source for Bifidobacterium. Melanoidins from black vinegar significantly suppressed adipogenesis of 3T3-L1[3−5]. Currently, there are many reports on the antioxidant capacity of melanoidins. It is generally recognized that melanoidins have excellent antioxidant activities[6−9]. These excellent properties demonstrate melanoidins have considerable application value in the food industry, which can be used as novel food ingredients.
Lycium barbarum L. has been used as a traditional food and herbal medicine in China, as described in the Compendium of Materia Medica. A large number of bioactive substances, including polysaccharides, phenols, proteins, alkaloids, and vitamins, have been found in Lycium barbarum L.[10,11]. Importantly, the bioactivity of Lycium barbarum L. can be significantly enhanced by the concoction process. It has been reported that this increase in activity can be attributed to the production of melanoidin[12]. Black garlic and black apple, which have been extensively studied, also belong to this category of food products and the presence of melanoidins in the system also increases the functional properties of the products[13−15]. However, the development of novel products with melanoidin from Lycium barbarum L. as a raw material by heat-high humidity or low-temperature-induced processing has not been intensively investigated; in particular, it was carried out with the Ningxia wolfberry as the selective target. Therefore, melanin was extracted and purified from Lycium barbarum L. and a preliminary study was conducted on its physicochemical properties, digestive properties, and functional activities. This study will provide theoretical support for further research on the application of melanoidins extracted from Lycium barbarum L. as functional foods or dietary additives, and provide a new avenue for the utilisation of Lycium barbarum L.
-
Dried Lycium barbarum L. with 20% water content was provided by Zaokang Wolfberry Incorporated Company (Ningxia Province, China). Dried Lycium barbarum L. was placed in a constant temperature and humidity chamber, then treated for 48 h at 60 °C and 50% humidity to obtain black Lycium barbarum L. (BLB). Pepsin (10,000 U/g) was purchased from Hefei Qiansheng Biotechnology Co., Ltd. ( Anhui Province, China). Trypsin (250 U/mg) and α-amylase (10,000 U/g) were purchased from Jiangsu Ruiyang Biotechnology Co., Ltd. (Jiangsu Province, China).
Preparation of melanoidins
-
Melanoidins were extracted according to the method described by Liu et al.[16] with some modifications, as described in the Supplemental File 1. BLB powder (20 g) was extracted with 200 mL of 30% ethanol solution (v/v) in a water bath at 45 °C for 90 min and then filtered to collect the supernatant. The collected supernatant was added to an anhydrous alcohol solution to achieve an ethanol concentration of 70% and then allowed to stand for 12 h at 4 °C to precipitate. Then the supernatant was filtered with filter paper. The filtrate was dialyzed for 48 h to remove compounds with a molecular weight (MW) below 1 kDa and then freeze-dried. Finally, the crude melanoidins were extracted and packed in vacuum-sealed bags for further use.
Isolation and evaluation of melanoidin fractions
-
The isolation of crude melanoidins was conducted using a method previously reported by Tores de la Cruz et al.[17] with some modifications. The prepared crude melanoidin solution was sequentially ultrafiltered three times using ultrafiltration membranes with molecular weight cut-off (MWC) of 50, 10, and 3 kDa to separate the fractions UF1 (ultrafiltration fraction 1, MWC > 50 kDa),UF2 (10 kDa < MWC < 50 kDa),UF3 (3 kDa < MWC < 10 kDa) and UF4 (< 3 kDa) respectively. The ultrafiltration procedure was repeated three times to collect the four melanoidin fractions. The fractions were freeze-dried, weighed and then stored at −20 °C. The chemical composition and antioxidant activity were analyzed to preliminarily evaluate the properties of the four fractions.
Orthogonal experimental design of resin purification
-
According to the preliminary analysis of four fractions, the target fraction was confirmed. The target fraction was further purified according to the method proposed by Zhang et al.[18] with some modifications. Briefly, dynamic adsorption and desorption tests were performed with the pretreated AB-8 resin. Twenty grams of the pretreated AB-8 resin was loaded into the glass column and a sample of the target fraction solution was loaded, and then eluted with 40% ethanol. First, three variables (loading concentration, elution volume, and elution flow rate) that could influence the yield of melanoidin purification were evaluated in single factor experiment. The conditions for the single factor experiment were set as follows: Loading concentrations (2, 4, 6, 8, and 10 mg·mL−1), elution volume (4, 5, 6, 7, and 8 BV) and elution flow rate (1, 2, 3, 4, and 5 mL·min−1). Based on the results of the single factor experiments, an orthogonal L9 (33) test (as shown in Table 1) was performed. The purified melanoidin obtained under the optimal conditions was then analyzed for structure, stability, and antioxidant activity.
Table 1. Analysis and results of orthogonal L9 (33) experimental design.
Number Factors Results a: Loading concentration (mg·mL−1) b: Elution volume (BV) c: Elution flow rate (mL·min−1) Yield (%) 1 6 6 1 0.487 2 6 7 2 0.332 3 6 8 3 0.243 4 8 6 2 0.398 5 8 7 3 0.265 6 8 8 1 0.255 7 10 6 3 0.253 8 10 7 1 0.348 9 10 8 2 0.273 k1 0.351 0.376 0.360 k2 0.306 0.315 0.334 k3 0.291 0.257 0.253 R 0.0594 0.119 0.107 Order of importance b > c > a Optimal level a1b1c1 Determination of the chemical constituents of melanoidin fractions
-
The contents of reducing sugar and total sugar were determined by the DNS method and phenol sulfate colorimetry method, respectively. The total phenolic content was determined using the Folin-Ciocalteu method[19]. The protein content was carried out using the Bradford method using bovine serum albumen as standard[20]. Using rutin as the standard sample, the total flavonoid content was determined. A 0.5 mL sample was mixed with 0.4 mL of 5% NaNO2 and then incubated for 6 min. Afterward, 0.4 mL of 10% Al(NO3)3 was added and reacted for 6 min. Finally, 4 mL of 4% NaOH and 4.7 mL of 70% ethanol were added to the reacted solution. Then the sample solution was incubated for 10 min and the absorbance was determined at 505 nm.
Antioxidant activity determination
Hydroxyl radical scavenging activity
-
The hydroxyl radical scavenging activity was determined using the method described by Wu et al.[21] with some modifcations. Briefly, 1 mL of FeSO4 solution (5 mmol·L−1), 4 mL of salicylic acid solution (5 mol·L−1) and 3 mL of distilled water were mixed with 1 mL sample. Then, 1 mL of H2O2 (5 mmol·L−1) was added and reacted at 37 °C for 10 min. The absorbance of the reacted solution was measured at 510 nm, and the hydroxyl radical scavenging activity was calculated using the following equation:
$ Hydroxyl\;radical\;scavenging\;ability\;\left({\text{%}}\right)=\dfrac{{A}_{0}-({A}_{1}-{A}_{2})}{{A}_{0}}\times 100{\text{%}} $ (1) where, A0: the absorbance of the blank; A1: the absorbance of the control; A2: the absorbance of the sample.
Ferric-reducing antioxidant power
-
The antioxidant power was measured according to the method described by Oracz & Nebesny[22]. One mL of sample (0.5 mg·mL−1) was mixed with 2.5 mL of sodium phosphate buffer (pH = 6.6, 0.2 mol·L−1) and 2.5 mL of potassium ferricyanide (1%, v/v). The mixed solution was incubated in a water bath at 50 °C for 20 min. 2.5 mL of 10% trichloroacetic acid was added when the solution was cooled to room temperature. One mL of ferric chloride (1%, v/v) and 2.5 mL of distilled water were added into 2.5 mL of this mixture. Finally, the absorbance of the mixture was determined at 700 nm after incubation for 10 min.
DPPH radical scavenging activity
-
The DPPH radical scavenging activity of melanoidin was evaluated following previous reports by Kim[23] with slight modifications. Four mL of 95% ethanol solution and DPPH-ethanol mixed solution (2 mL, 0.05 mg·mL−1) were added to 1 mL of sample solution and then allowed to react for 30 min under light protection. The absorbance value of the solution was determined at 517 nm. The DPPH radical scavenging activity (%) was calculated as follows:
$ DPPH\;radical\;scavenging\;activity\;\left({\text{%}}\right)=\dfrac{{A}_{0}-({A}_{1}-{A}_{2})}{{A}_{0}}\times 100 {\text{%}} $ (2) where, A0: the absorbance of the blank; A1: the absorbance of the control; A2: the absorbance of the sample.
Fourier transform infrared spectroscopy (FTIR)
-
The sample obtained above (1 mg) was mixed with dried KBr (100 mg) and pressed into a thin sheet with a tablet press after being ground. Then the sample was analyzed using a spectrometer in the 400−4,000 cm−1 range.
Stability assay
-
The stability of the purified melanoidins was examined according to a previous study[24]. Briefly, the stability was estimated by exposing the samples to different heating temperatures (40, 50, 60, 70, and 80 °C) and different light conditions (sunlight, incandescent light, darkness). The absorbance of the samples exposed to different heating temperatures was determined every 1 h at 420 nm. And the absorbance of the samples exposed to different light conditions were determined every 1 d. The retention rate (R) was calculated using the following equation:
$ R=\dfrac{{A}_{1}-{A}_{0}}{{A}_{0}}\times 100{\text{%}} $ (3) where, R: Retention rate; A0: The absorbance value of the initial sample; A1: The absorbance value of the sample after treatment under different conditions.
Antioxidant activity in vitro simulated digestion
-
The purified melanoidins were digested according to the method described by Peña-Correa et al.[25]. Briefly, in the oral digestion phase, 1.0 g of sample and 20 mL of simulated salivary fluid (pH adjusted to 7.0) were mixed and incubated at 37 °C for 2 min. In the gastric digestion phase, the sample after oral digestion was mixed with 10 mL of simulated gastric fluid (pH adjusted to 1.2) and incubated at 37 °C for 2 h. In the intestinal digestion phase, 10 mL of simulated intestinal fluid (pH adjusted to 7.0) was added to the above gastric digestion mixture and incubated at 37 °C for 2 h. The samples before and after each digestion step were collected and freeze-dried for further analysis of antioxidant activity. Antioxidant activity was analyzed according to the method described above.
Statistic analysis
-
All experimental results were determined in triplicate and the data were expressed as mean ± standard deviation (Mean ± SD). ANOVA accompanied and Duncan’s multiple range tests were performed to examine statistical differences across groups using SPSS software (version 19.0 for Windows, SPSS Inc., Chicago, IL, USA).
-
Four melanoidin fractions with different MW were obtained by ultrafiltration. As shown in Fig 1a, the melanoidin fraction with HMW presented a deeper brown color than in the LMW fractions. The results indicate that the HMW melanoidins were stronger colorants that contribute more to the color of thermally treated foods[21,26]. As shown in Fig. 1b, UF3 showed the highest content (59.37% ± 3.66%), followed by UF4 (24.21% ± 3.01%), UF2 (12.90% ± 1.25%) and UF1 (3.52% ± 0.98%), indicating that melanoidin fractions with LMW were dominant fractions. This result could be due to the low temperature of the reaction system, which leads to the formation of a large amount of LMW melanoidin in the early phase of MR. Melanoidin is the product of the Maillard reaction between proteins and sugars. It has been shown that the reaction time and temperature are related to the size of the molecular weight. When the reaction temperature is low, a large amount of LMW melanoidin is formed in the early phase of MR. LMW melanoidin can be used as a reaction intermediate with other Maillard reaction products to polymerize or crosslink to form HMW melanoidin in the later stage of the reaction with the extension of heating time[27,28]. This finding is consistent with the fact that the molecular weight of melanoidin in ale beers and lager beers increased with the roasting time of the ingredients and the intensity of the heating[28].
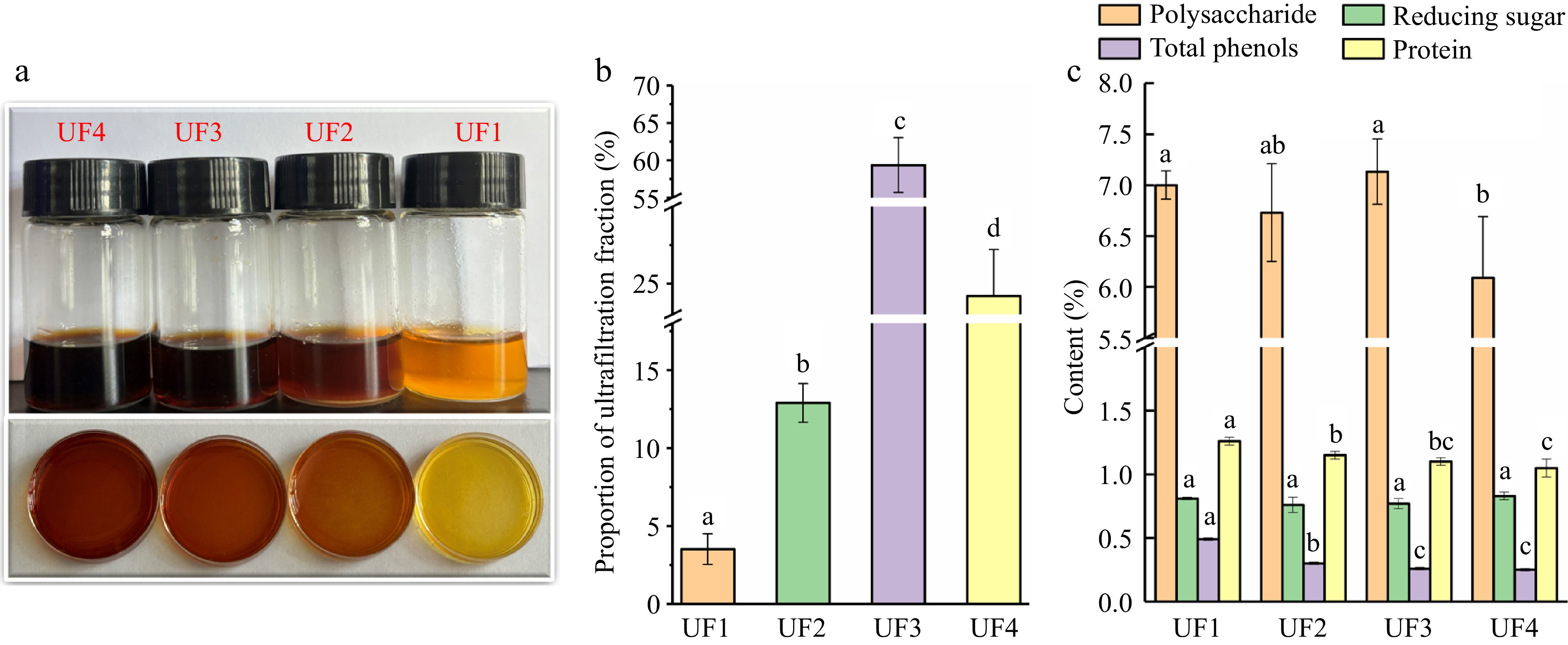
Figure 1.
The physicochemical properties of melanoidin fractions with different MW extracted from induced black Lycium bararum L., including (a) color, (b) proportion of ultrafiltration fraction, and (c) chemical composition. UF1 (Ultrafiltration fraction 1, MWC > 50 kDa), UF2 (10 kDa < MWC < 50 kDa), UF3 (3 kDa < MWC < 10 kDa) and UF4 (< 3 kDa).
Chemical compositions of isolated melanoidin fractions
-
Chemical compositions commonly retained in melanoidins by ultrafiltration membranes include total sugar, reducing sugar, total phenol, and protein[21]. The content of chemical compositions in four melanoidin fractions are shown in Fig. 1c. The total flavonoid, which is abundant in Lycium barbarum L., was not detected in the four melanoidin fractions. The total phenol exhibited a low content of less than 0.5% in four melanoidin fractions. The total phenol content increased with the increase in MW. The findings were similar to those of Naibaho & Korzeniowska[29], who observed that melanoidins in Brewers' spent grain with the higher molecular weight contained more total polyphenol. The reducing sugar and protein also presented low content indicating that the reducing sugar was fully reacted with protein in the Maillard reaction. The total sugar in four melanoidin fractions showed the highest content in the range of 5.91% to 7.14%. And a significant difference in the total sugar content was observed between UF4 and other melanoidin fractions. This is probably the result of the ultrafiltration membrane with MWC < 3 kDa efficiently removing the unreacted high molecular weight sugars.
Antioxidant activities of isolated melanoidin fractions
-
As shown in Fig. 2a, the reducing power of all melanoidin fractions increased with the increase of sample concentration. The reducing power of melanoidin fractions with LMW was found to be significantly higher than those of melanoidin fractions with HMW. The reducing power of UF4 in concentration of 2.0% was about 0.46 times higher than that of UF1. The scavenging activities of four melanoidin fractions with different concentrations against the hydroxyl radical are shown in Fig. 2b. The scavenging activity against hydroxyl radicals of the four melanoidin fractions was positively associated with the concentration. The scavenging activity was enhanced significantly with the decreasing of MW. The scavenging activity against hydroxyl radicals of UF4 at a concentration of 2.0% was 18% higher than that of UF1. The results consistent with a previous report[28] indicated that melanoidin with LMW prepared from beers showed stronger antioxidant activity. It can be seen from Fig. 2c, a positive correlation between the concentrations and radicals scavenging activities of all melanoidin fractions was also observed. The DPPH radicals scavenging activity of UF2 and UF3 was significantly stronger than that of UF1 and UF4. The reason for this result may be that the ability of melanin to scavenge DPPH free radicals is expressed by the donation of hydrogen atoms and UF2 and UF3 may contain more hydrogen to form stable DPPH-H molecules[13,30].
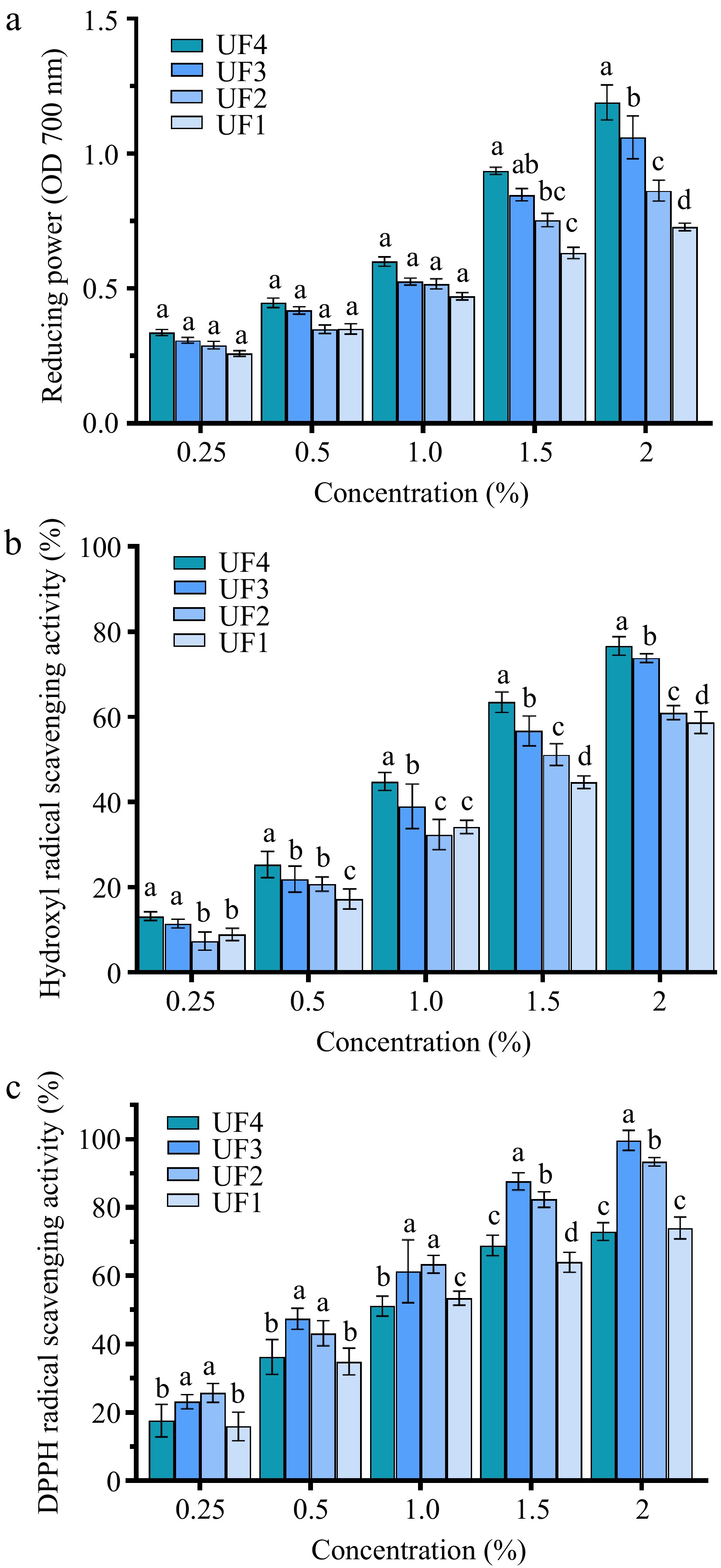
Figure 2.
The antioxidant activities of melanoidin fractions with different MW, including (a) reducing power, (b) hydroxyl radical scavenging activity, and (c) DPPH radical scavenging activity.
Optimization of UF3 purification conditions
-
According to the above analysis of four fractions, UF3 was confirmed as the target fraction for further research and application. Seen from the results of single-factor experiments presented in Fig. 3a−c, the highest yield of melanoidins were 33.5%, 38.5%, and 43.5% at the loading concentration of 8 mg·mL−1, the elution volume of 7 BV and the elution flow rate of 2 mL·min−1 respectively. Based on the single factor experiments, orthogonal experiments with these three variables were performed. Table 1 presents the optimization results. Among these factors that influenced the yield of melanoidins, the elution volume (b) was the most significant influencing factor, followed by the elution flow rate (c), and the loading concentration (a). The optimum parameters for UF3 purification were a1b1c1. The highest yield of UF3 was obtained when the loading concentration, the elution volume, and the elution flow rate were 4 mg·mL−1, 6 BV, and 1 mL·min−1 , respectively.
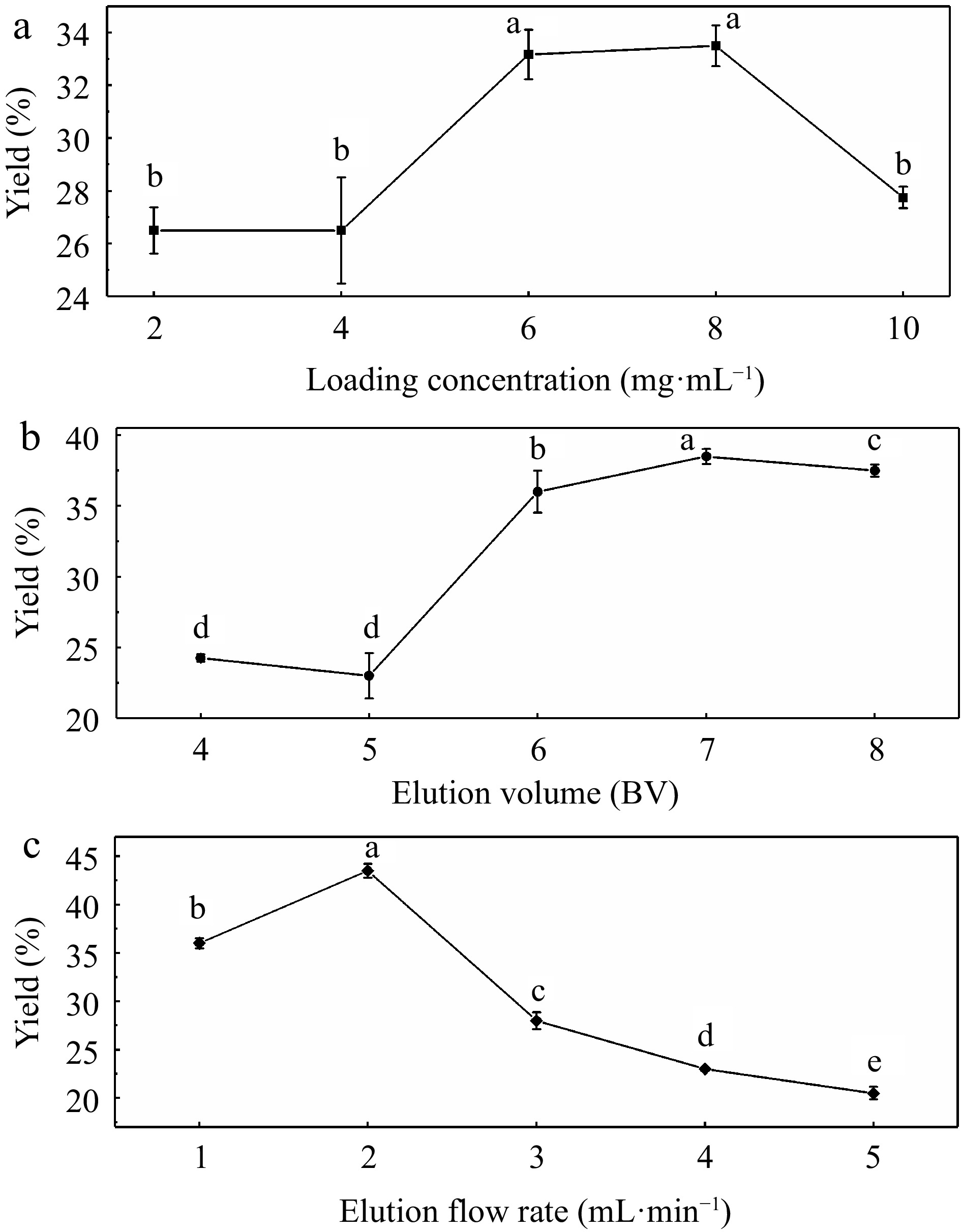
Figure 3.
Impact of (a) loading concentration, (b) elution volume, and (c) elution flow rate on the purification yields.
FTIR analysis of purified UF3
-
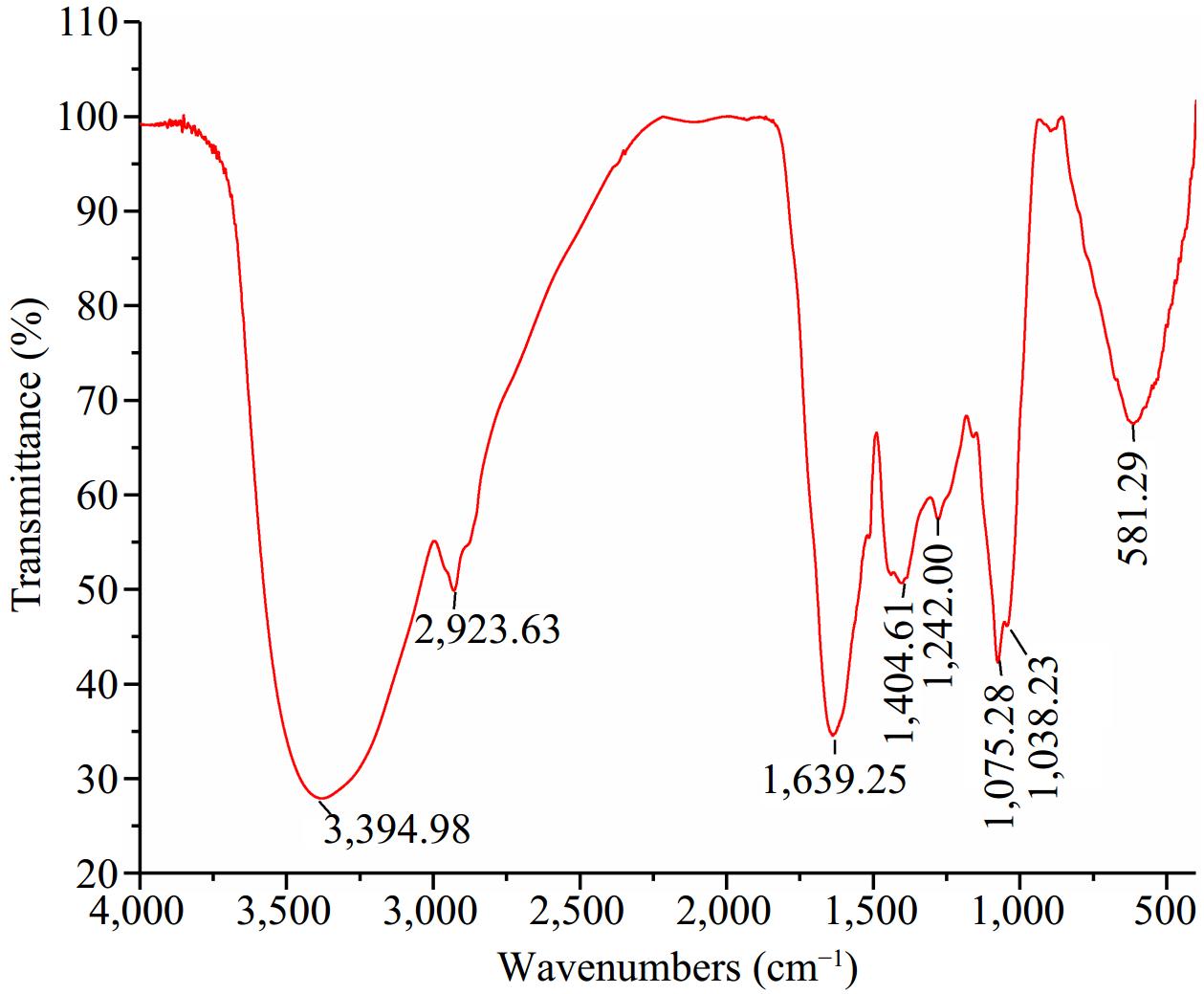
The FTIR spectra of the purified UF3 are shown in Fig. 4. An absorption band at 3,394.98 cm−1 was observed. The absorption bands at 3,600−3,200 cm−1 is assigned to stretching vibration of the hydroxyl group (O-H) or amide group (N-H)[29]. The signal peak presented at 2,923.63 cm−1 could be attributed to the stretching vibration of C-H. The C-H (CH3 and CH2) band are found around 2,943 cm−1[31]. In previous studies, the melanoidin skeleton is rich in O-H, N-H, and -CH functional groups[31−33]. The absorption bands of amide were observed at 1,639.25, 1,404.61, and 1,242.00 cm−1, which was attributed to C=O stretching and C=N stretching modes (amide I, 1,700−1,600 cm−1), N-H bending and C-N stretching modes (amide III, 1,450−1,240 cm−1), respectively[34]. Two absorption peaks at 1,075.28 and 1,038.23 cm−1 can be assigned to C-O-C bending. In addition, a band was observed at 581.29 cm−1 ascribed to the skeletal modes of pyranose rings[35]. In summary, the structure of purified UF3 was close to the typical melanoidins structure, indicating that the UF3 was effectively prepared.
Stability of purified UF3
-
The stability of purified UF3 under different light conditions and temperatures was investigated. Under different light conditions, the retention rate of all melanoidin samples showed a downward trend with increasing exposure time (Fig. 5a). The downward trend of melanoidin samples exposed to sunlight showed a greater decrease than the other two groups. The retention rate of the melanoidin sample was reduced by about 17.23% after 10 d of sunlight exposure. It could be that the ultraviolet rays contained in sunlight accelerated the oxidative degradation of melanoidin. Similarly, melanoidin from black garlic also showed a decreasing trend under ultraviolet conditions[24]. The retention rate of melanoidin treated with incandescent light was similar to that of dark conditions with a retention rate of about 90%, suggesting that the purified UF3 can be stored and used under incandescent light. As shown in Fig. 5b, the retention rate of the melanoidin samples treated with different heating temperatures showed a fluctuating trend, indicating that the heat treatment causes heat polymerization and heat degradation of the purified UF3[28]. Therefore, heat treatment could aggravate the thermal instability of the melanoidin.
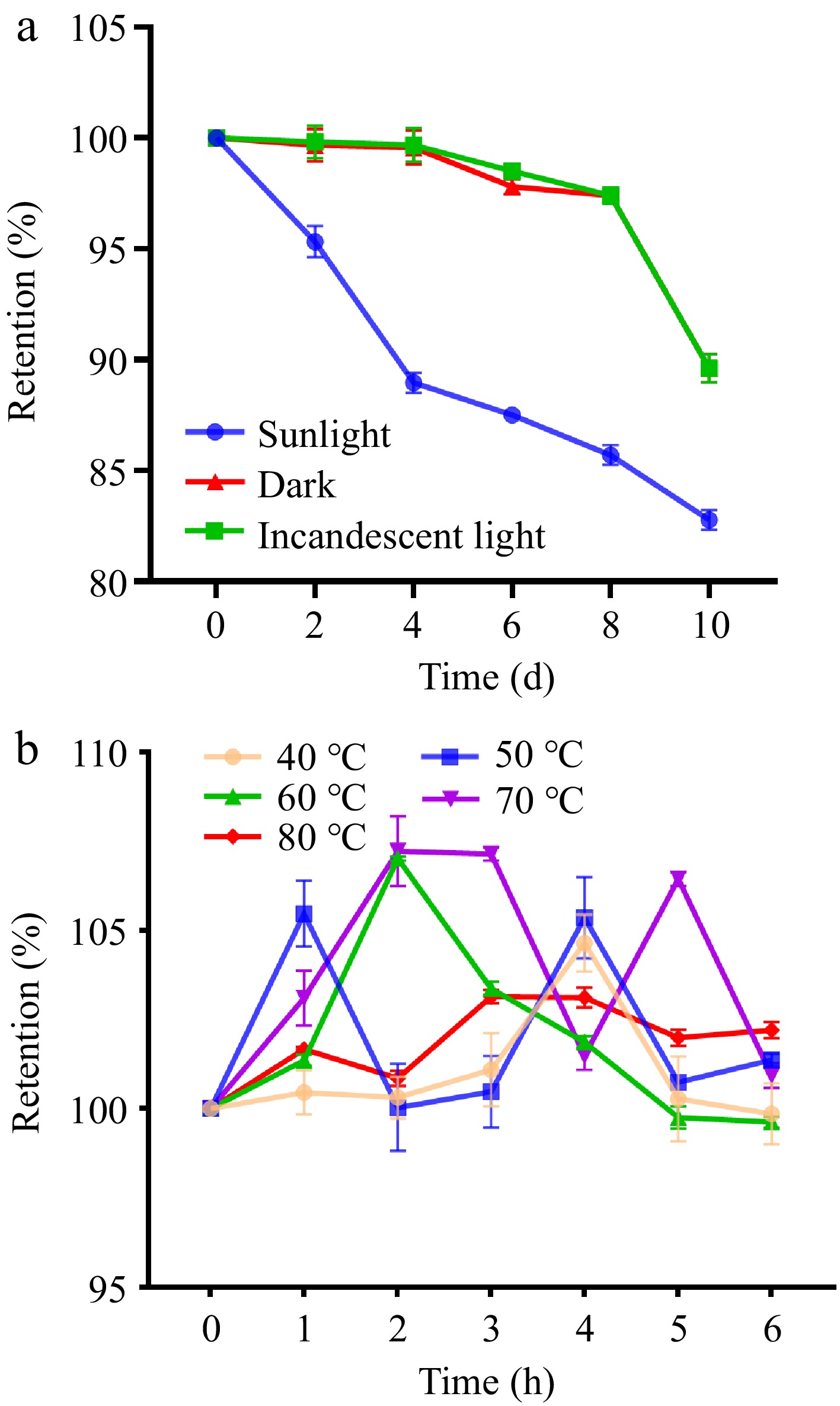
Figure 5.
Stability results of purified UF3 under (a) different light conditions, and (b) different temperatures.
Changes in antioxidant activities of purified UF3 in in vitro digestion
-
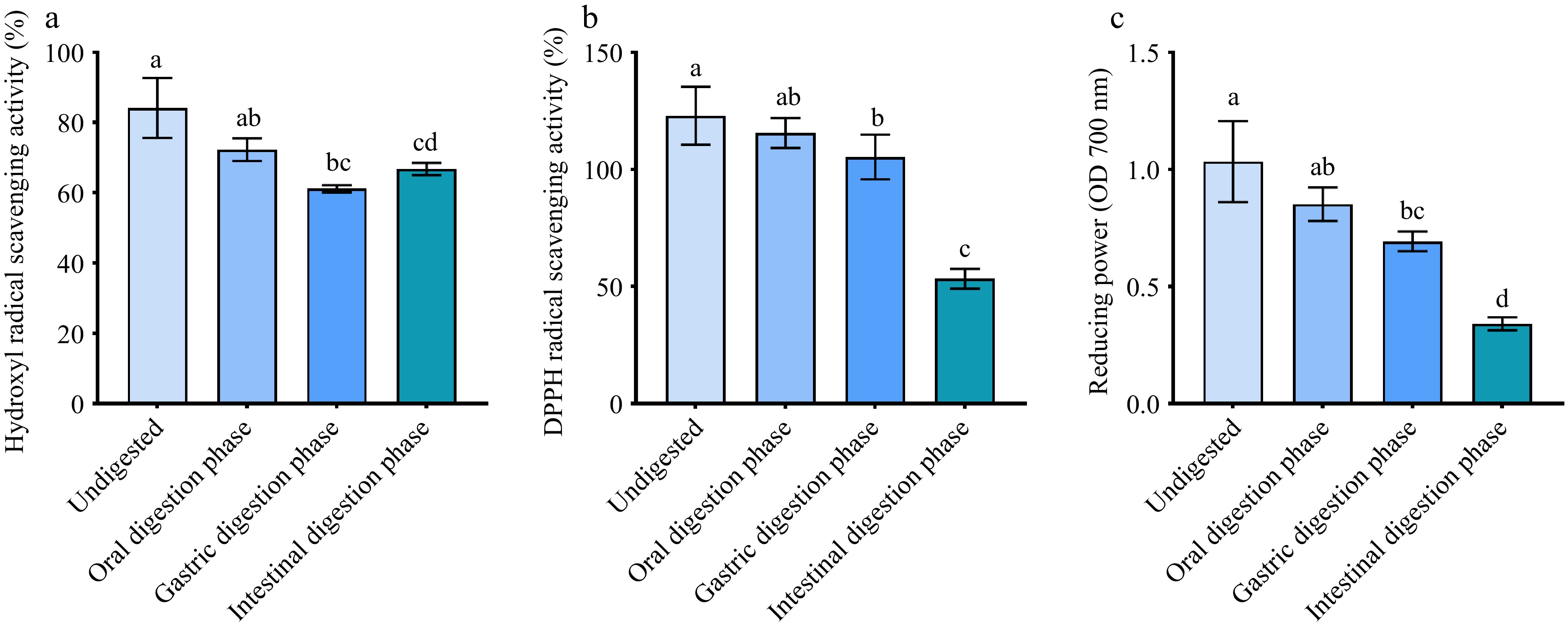
To assess the effect of in vitro enzymatic digestion on the antioxidant activity of purified UF3, the change of hydroxyl radical scavenging activity, DPPH scavenging activity, and reducing power of purified UF3 during the in vitro digestion process were investigated. As presented in Fig. 6a−c, the in vitro enzymatic digestion weakened the antioxidant activity of purified UF3. The antioxidant activities of melanoidins may be directly related to its structure, and in vitro digestion could result in a change in the structure of purified UF3. There are two reasons for this: firstly, the generation of new non-covalently bound melanoidin compounds in the presence of digestive enzymes, and secondly, the free radical scavenging activity of food melanoidins attributed to the presence of phenolic groups in their structure[32,36]. Although the antioxidant activity of digested samples was lower than that of undigested samples, all digested samples still exhibited a good antioxidant activity. After in vitro intestinal digestion, the hydroxyl radical scavenging activity, DPPH scavenging activity, and reducing power of digested UF3 was 65%, 50%, and 0.33, respectively. It could be seen that the purified UF3 extracted from BLB has potential antioxidant application value in vivo.
-
This study demonstrated that melanoidin fractions with LMW (3 kDa < MWC < 10 kDa) were dominant fractions in low thermal induced BLB, and the dominant fractions exhibited excellent antioxidant activities. The dominant melanoidin fractions (UF3) were purified, preliminarily characterized, and its antioxidant activities checked by in vitro digestion. The results of FTIR spectroscopy revealed that purified UF3 showed a typical structure of melanoidins. The optimum purification conditions proved to be efficient for the purification of UF3 from BLB. The purified UF3 was stable in the dark and under light conditions without ultraviolet rays. However, the purified UF3 with LMW was unstable under heat treatment. The antioxidant activities of purified UF3 decreased after in vitro intestinal digestion, yet it exhibited a certain level of antioxidant activity. These results suggest that the UF3 extracted from low thermally induced BLB could be considered as a potential antioxidant ingredient and as a potential antioxidant component for the development of orally consumable functional foods.
-
The authors confirm contribution to the paper as follows: study conception and design: Chen J; experimental processing: Wang J, Liu Y; data collection and validation: Wang J, Li H; analysis and interpretation of results: Li H, Wang W; draft manuscript preparation: Chen J, Pan Y; language editing, manuscript revision: Hu Y. All authors reviewed the results and approved the final version of the manuscript.
-
Data are available from the corresponding author on reasonable request.
This study was partly supported by the Innovative Team of Modern Agricultural Industry Technology System in Tianjin (ITTHRS2021000) and Key R & D Project of Xinjiang Uygur Autonomous Region (2022B02030).
-
The authors declare that they have no conflict of interest.
-
Authors contributed equally: Junran Chen, Jie Wang
- Supplemental File 1 Screening of macroporous adsorption resin for purification of melanoidins from low thermal induced black Lycium barbarum L.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Chen J, Wang J, Liu Y, Li H, Wang W, et al. 2024. Study on physicochemical properties and antioxidant activities of melanoidins extracted from low thermal induced black Lycium barbarum L. Food Innovation and Advances 3(3): 288−294 doi: 10.48130/fia-0024-0027
Study on physicochemical properties and antioxidant activities of melanoidins extracted from low thermal induced black Lycium barbarum L.
- Received: 30 March 2024
- Revised: 11 August 2024
- Accepted: 12 August 2024
- Published online: 30 August 2024
Abstract: In this study, static and dynamic desorption methods, infrared spectroscopy and, in vitro antioxidant modeling were used to isolate, purify, and investigate the bioactivity of melanoidins extracted from hypoheat-induced Lycium barbarum L. The results showed that melanoidin fractions with molecular weight in the range of 3−10 kDa were the dominant and most valuable fractions. In the purification phase, the optimal purification conditions were: a loading concentration of 4 mg·mL−1, elution volume of 6 BV, and an elution flow rate of 1 mL·min−1. Purified dominant melanoidin fractions (UF3) exhibited typical Maillard reaction (MR) characteristics in FTIR. The storage stability showed that sunlight and heat treatment exacerbated the instability of the purified UF3. At the same time it was relatively stable under dark conditions and incandescent light, with a retention rate of about 90%. After in vitro digestion, the purified UF3 still exhibited good antioxidant activity, and the DPPH scavenging activity and hydroxyl free radical scavenging ability reached more than 60%.
-
Key words:
- Melanoidins /
- Low thermal induced /
- Purification /
- Antioxidant activity /
- In vitro digestion