-
Polysaccharides are heavily used in foods, cosmetics and preventive medicines due to their various bioactivities and low side effects[1]. To purify polysaccharides with high yield, a high number of strategies have been reported[2,3], including size exclusion chromatography, ultrafiltration, dialysis, water extraction combined with ethanol precipitation, machine-assisted extraction, and enzymolysis approaches, etc. In spite of this, time-consuming separation and impurity removal steps are often essential for these methods. Simple and efficient strategies for polysaccharide extraction are still scarce, but highly desired.
Magnetic solid phase extraction (MSPE) has been proven to be a powerful tool for separation and purification related applications[4−6], in which the magnetic nanoparticles are applied as sorbents, then the extraction and elution processes can be easily completed by external magnetic field while, without extra instrument-assisted separation operations such as centrifugation and ultrafiltration, and high pressure required in traditional SPE column or cartridge can also be avoided. Large specific surface area of magnetic nanospheres (MNPs) enables MSPE to obtain high extraction capacity, and the vast majority of MNPs can be easily recycled by external magnets. As a result, the operations for separation can be significantly simplified, the extraction efficiency can be improved, and the cost can be also reduced. Admittedly, MSPE provides a promising pathway for polysaccharide purification, but such an idea is still in its infancy.
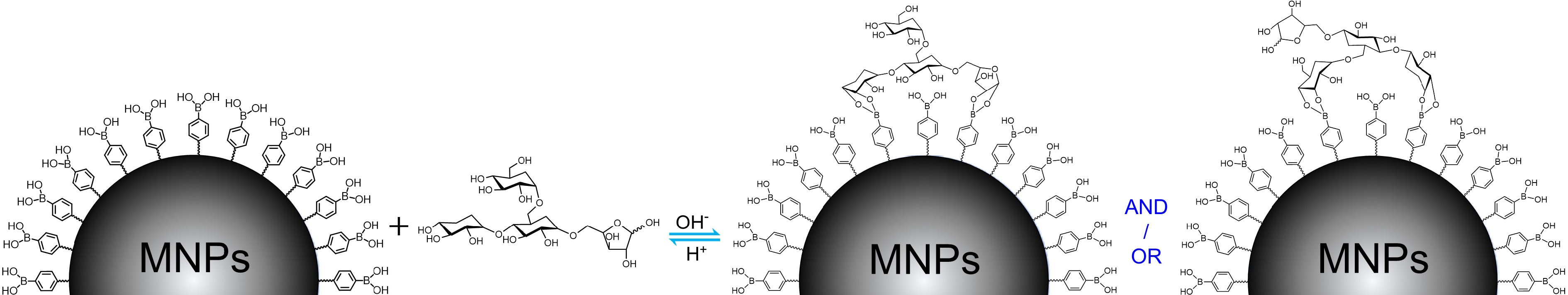
Boronate affinity effect[7−9] is a reversible covalent binding between 1,2- and 1,3-cis-diol containing compounds and boronic acid moieties, of which the covalent five- or six-membered cyclic boronate ester can be formed at a basic pH condition while the bound cis-diol compounds can be reversibly released when the system pH changes to acidic. Thanks to such an unique pH-adjustable binding, boronate affinity effect has been widely used for affinity separation[10] and chemical sensing[11] of cis-diol compounds. Under such a background, a boronate affinity-mediated MSPE (BA-MSPE) strategy was pioneered herein for the easy isolation of polysaccharides. The schematic for this protocol is illustrated in Fig. 1 using the possible structure of tea polysaccharide (TPS) as a representative[12], in which boronic acid-functionalized MNPs were served as sorbents. Because the abundant 1,2- and 1,3-cis-diol structures in backbone and/or branch carbohydrate chains were their universal hallmarks, polysaccharides would be extracted under weak alkaline condition by multisite binding-based positive synergistic boronate affinity effect, and then the extractives could be reversibly released after altering the system pH to acidic. By such a simple process, three polysaccharides, including TPS, Lycium barbarum polysaccharide (LBPS) and soybean polysaccharide (SPS), were effectively isolated from relevant real beverage plants, and their antioxidant and antitumor bioactivities were also demonstrated.
-
Acetic acid (HAc, 99.5%), KH2PO4 (99.5%), NaH2PO4 (99.9%), Na3PO4, H3PO4, NaOH, chloroform (≥ 99%) and FeCl3•6H2O (99%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Ethylene glycol (> 99%), sulfuric acid (96%), 3-methyl-1-phenyl-2-pyrazoline-5-one (PMP, 99%), 4-formylphenylboronic acid (FPBA, 98%), methanol (99.5%), 1,6-hexamethylenediamine (99%) and anhydrous sodium acetate (99.5%) were purchased from Titan Scientific Co., Ltd. (Shanghai, China). 2,2-Diphenyl-1-picrylhydrazyl (DPPH, 98.5%), thiazoyl blue tetrazolium bromide (MTT, > 99%), HPLC-grade acetonitrile (ACN), trifluoroacetic acid (TFA, 99%), anhydrous ethanol and phenol were obtained from Macklin Biochemical Co., Ltd. (Shanghai, China). Pullulan polysaccharide (PPS, 99%), tea polysaccharide (TPS, 50%), soybean polysaccharide (SPS, 70%), Lycium barbarum polysaccharide (LBPS, ≥ 50%), trypan blue (60%) and deoxyguanosine (99%) were from Yuanye Bio-Technology (Shanghai, China). NaBH3CN (95%), guanosine (99%), adenosine (99%), deoxyadenosine (99%) and cytidine (99%) were from Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). Human breast cancer cell line (MCF-7), human lung carcinoma cell line (A549), Roswell Park Memorial Institute 1640 medium (RPMI-1640, containing 2.0 mg/mL D-glucose, 0.3 mg/mL glutamine, 2.0 mg/mL NaHCO3, 80 U/mL penicillin, and 0.08 mg/mL streptomycin), Dulbecco’s Modified Eagle Medium (DMEM, containing 4.5 mg/mL D-glucose, 0.3 mg/mL glutamine, 0.11 mg/mL sodium pyruvate, and penicillin streptomycin), parenzyme cell digestion solution (containing 0.25% trypsin and 0.02% EDTA), and phosphate buffer solution for cell culture (PBS) were purchased from Keygen Biotech (Nanjing, China). Fetal bovine serum (FBS) was purchased from Gibco (Life Technologies, Australia). Cell culture bottles (25 cm2 in growth area) and glass bottom cell culture dishes (Φ 30 mm) used for cell culture and microimaging were obtained from NEST Biotechnology (Wuxi, China). Real-world beverage plants, including Lycium barbarum, tea leaves (green tea) and soybeans, were purchased from a local supermarket. Water used in this study was produced by a Milli-Q system (Millipore, Milford, MA, USA). Except as otherwise noted, the purity grades of all chemicals without specific stipulations were analytically pure and phosphate buffer (PB) solutions used in this work was especially referred to the 0.1 M, pH 8.5 phosphate buffer.
Synthesis of MNPs@B(OH)2
-
MNPs@B(OH)2 were prepared referring to our previous reports[4,5] with slight modifications, in which the preparation of amino group-capped MNPs (bare MNPs) and then their post-modification with FPBA were contained. To synthesize bare MNPs, 480 mL ethylene glycol, 16.0 g FeCl3 and 32.0 g anhydrous sodium acetate were mixed, then the mixture was slowly heated to 55 °C and kept vigorously stirring until the solution became transparent, followed by adding with 104.0 g 1,6-hexamethylenediamine and mixing evenly. After that, the resulting solution was transferred into a polytetra-fluoroethylene autoclave reactor and kept under reaction for 10 h at 198 °C. Finally, the prepared bare MNPs were magnetically separated and washed three times with ethanol and water, then the obtained MNPs were dried in a vacuum oven at 55 °C and stored in an air tight container.
For the boronic acid functionalization, 2.0 g the above prepared MNPs were dispersed into 100 mL absolute ethanol containing 5.0 mg/mL FPBA and 1.0 mg/mL NaBH3CN. After ultrasound for 30 min, the mixed solution was mechanically agitated at 40 °C for 12 h. When the reaction finished, the MNPs@B(OH)2 were magnetically isolated and respectively rinsed three times with ethanol and water, and then harvested after drying at 55 °C and stored under air tight conditions for upcoming assays.
Binding selectivity and adsorption dynamics of BA-MSPE
-
Deoxyguanosine, deoxyadenosine, cytidine, guanosine and adenosine were served as model compounds to investigate the binding selectivity of MNPs@B(OH)2. Briefly, 8 mL aliquots of PB containing 1.0 mg/mL model analytes were supplemented with 35.0 mg MNPs@B(OH)2 or bare MNPs, after fully dispersing by ultrasonic, the resulting mixture solutions were incubated at room temperature for 0.5 h. Then the analyte-extracted MNPs@B(OH)2 or bare MNPs were magnetically separated and redispersed into 1.0 mL desorption solution composed by 0.1 M acetic acid. After desorption for 2 h, the materials were removed by external magnet while the supernatants were collected individually, and their UV-vis absorption spectra were tested on a UV-1800PC spectrophotometer (Mapada Instruments, Shanghai, China). The equilibrium binding capacity of MNPs@B(OH)2 and bare MNPs was figured out with standard curve method, which measured by UV-vis spectrometry and plotted by licensed OriginPro 2016 software. The wavelengths used for plotting standard curves of adenosine, guanosine, cytidine, deoxyadenosine and deoxyguanosine were, respectively, set at 258, 252, 278, 258 and 258 nm.
For the investigation of adsorption dynamics, guanosine, a cis-diol containing compound which can form boronate affinity effect with MNPs@B(OH)2, was selected as a target analyte. The steps were as follows: 35.0 mg MNPs@B(OH)2 was fully dispersed into 5 mL PB containing 0.1 mg/mL guanosine by vortex and ultrasonic. After incubation at room temperature for the appropriate time (0, 5, 10, 15, 20, 30 and 50 min), 200 μL aliquots of this mixture solution were taken out and the supernatants were individually collected after discarding the materials by external magnetic field. Finally, the absorbance of supernatants was measured on a spectrophotometer. The binding dynamics were plotted according to the relationship between the absorbance of supernatants and extraction time.
Influence of pH on BA-MSPE
-
To probe the effect of pH on the extraction performance of MNPs@B(OH)2, the main operations were the same as above except that the consumption of MNPs@B(OH)2 was 10 mg and the solvents used for the preparation of guanosine solutions were replaced by PB with pH ranging from 2.5 to 12.5 with intervals of one pH unit. The absorbance of desorption solutions was tested and the extraction amounts of MNPs@B(OH)2 at binding equilibrium were calculated using the standard curve method. This assay was repeated in triplicate and the data were averaged for plotting. The relationship between equilibrium extraction amounts and system pH was applied to assess the impact of pH on BA-MSPE.
Adsorption isotherms and the comparison of extraction performance between bare MNPs and MNPs@B(OH)2
-
Since the direct spectrometric measurement of polysaccharides was difficult due to their poor UV-vis light absorption properties, color development by phenol-sulfuric acid method was utilized for spectral tests of polysaccharides. With the help of phenol-sulfuric acid chromogenic reaction, standard curves of polysaccharides were tested by UV-vis spectrometry as follows: polysaccharide stock solutions with certain concentration gradients (0.75, 0.5, 0.25, 0.1, 0.05 and 0.005 mg/mL for TPS; 1.0, 0.75, 0.5, 0.25, 0.1, 0.05 and 0.01 mg/mL for PPS; 0.5, 0.4, 0.3, 0.2, 0.1 and 0.075 mg/mL for LBPS; 1.0, 0.75, 0.5, 0.25, 0.1, 0.05 and 0.01 mg/mL for SPS) were prepared at first using 0.1 M HAc aqueous solutions as solvents, then phenol solution (5%, wt%), sulfuric acid and the prepared polysaccharide solutions were mixed in volume ratio of 1:5:1, followed by incubating at 55 °C for 10 min. Thereafter, the digital photos of all solutions were recorded using a smartphone (Vivo X80, Guangdong, China) and their UV-vis spectra were determined on a UV-vis spectrophotometer. Standard curves were plotted by absorbance at 488 nm against polysaccharide concentrations, and the extraction amounts of polysaccharides by MNPs and MNPs@B(OH)2 were inferred from linear regression equations for quantitative comparison. All data were obtained by three tests in parallel for quantifications.
Regarding adsorption isotherms, the details were as follows: A series of TPS stock solutions with a concentration gradient of 0.05, 0.1, 0.5, 1.0, 2.0, 4.0, 6.0, 10.0, 20.0 and 50.0 mg/mL were prepared using PB as solvents, then to each an aliquot of 40.0 mg MNPs@B(OH)2 was added, followed by incubation for 2 h at room temperature. After removing the solvents by magnetic separation, the resulting materials were washed three times with PB, and then supplemented with 1.0 mL aliquots of desorption solution composed of 0.1 M HAc aqueous solutions. After desorption for 2 h, the supernatants were collected via magnetic isolation and their absorbance was measured after color development by phenol-sulfuric acid method. The binding isotherms were obtained through the extraction amounts deduced by the absorbance at 488 nm plotting against TPS concentrations. The Hill equation given below was applied to fit data and estimate the binding properties of MNPs@B(OH)2.
$ y={Q}_{max}\cdot {x}_{n}/({x}_{n}+{K}_{d}^{n}) $ Herein, n was the Hill slope while Qmax and Kd were, respectively, the maximum binding capacity and dissociation constant.
As for the dosage-dependent extraction experiments, the main operations were the same as above except that the dosages of MNPs@B(OH)2 were set as 5, 10, 25, 50, 75, 100 and 150 mg using TPS and LBPS as model analytes both with a concentration of 1.0 mg/mL (dissolved in PB).
Extraction of polysaccharides from real-world beverage plants
-
Three beverage plants, including tea leaves (green tea), soybeans, and Lycium barbarum, were devoted as real samples to extract polysaccharides. Three steps, i.e., the pretreatments of raw materials, the preparation of leaching liquors of crude polysaccharides, and subsequent BA-MSPE, were contained in this experiment.
For the pretreatments of raw materials, the above-mentioned three beverage plants were washed with water several times to remove impurities, followed by drying to a constant weight at 55 °C in an electric oven. Then the powders of raw materials were obtained by quickly crushing in a high speed pulverizer.
To prepare the leaching liquors of crude polysaccharides, the above obtained raw material powders were immersed into PB with a material-to-liquid ratio of 1:50 (1.0 g powder per 50 mL PB). After incubation for 2 h at 60 °C, the leaching liquors and solid matters were centrifugally separated (5000 rpm for 10 min) and individually collected, then the solids were immersed into PB again with the same feed ratio and incubation for another 2 h at 80 °C. After further centrifugal separation, the supernatants were collected and gathered with the filtrates of the previous separation. The total leaching liquors were centrifuged again (10,000 rpm for 5 min) and stored at 4 °C after discarding the precipitation.
BA-MSPE were carried out as follows: 4.0 mL aliquots of freshly prepared leaching liquors were respectively added with 100 mg MNPs or MNPs@B(OH)2, followed by incubating for 0.5 h at room temperature. After magnetic separation, the supernatants were removed and 0.1 M HAc aqueous solutions was supplemented into the polysaccharides-extracted materials with 1.0 mL for each. After desorption for 2 h, the desorption solutions were magnetically collected. After color development with phenol-sulfuric acid method, the UV-vis spectra were measured and their colors were simultaneously recorded. The desorption operations were repeated several times until the color of chromogenic solution was indistinguishable by naked eye. The extraction amounts were figured out and weighted according to standard curve method using the absorbance at 488 nm.
Relative purities of polysaccharide extractives analyzed by UV-vis spectrophotometry
-
In order to survey the relative purities of polysaccharides extracted by MNPs@B(OH)2, the extractives from tea leaves and Lycium barbarum were freeze dried and corresponding standard polysaccharides were devoted as controls in this assay. The main steps for the BA-MSPE of TPS and LBPS were the same as above except that the dosages of leaching liquors and materials were enlarged. In detail, the volume of leaching liquors was set as 400 mL and the usage of MNPs@B(OH)2 was 2.0 g. Concentrations of extracted and standard polysaccharide samples for absorbance tests were set at 1.0 and 0.5 mg/mL, respectively. The relative purities of polysaccharides extracts were calculated by dividing the amount of polysaccharides obtained from extracts into that of standard polysaccharides.
HPLC analysis
-
Freeze-dried extracts of TPS and LBPS are denoted as model polysaccharides herein. Due to their poor light-absorbing properties, PMP-based pre-column derivatizations[13,14] of polysaccharides were essential for HPLC analysis. In detail, 100 μL methanol solution containing 0.5 M PMP was added with equal volume of 0.5 mg/mL polysaccharide solutions, followed by incubation at 70 °C for 2 h. When the labelling reaction was finished, 1.0 mL chloroform was added into the solution, then vortex for 1 min to extract the unreacted PMP reagents. After centrifuging for 1 min at 1000 rpm, the remaining PMP in the bottom layer was removed. Such a PMP separation operation was repeated three times and the resulting PMP-tagged polysaccharides were loaded for HPLC analysis. The standard polysaccharides labelled in the same way were used as benchmarks for qualitative identification and the determinations of HPLC standard curves.
TFA-based hydrolysis was performed to identify the monosaccharide composites of polysaccharides by HPLC, and the details were as follows: polysaccharides were separately dissolved into 2.0 M TFA solution with a final concentration of 10.0 mg/mL, and then reacted in air tight conditions for 6 h at 105 °C. When the reaction completed, the supernatants were centrifugally collected and their pH was adjusted to neutral using 1.0 M NaOH solution. Subsequently, the PMP labelling of hydrolysates and standard monosaccharides was implemented as above. The resulting solutions were finally stored at 4 °C for use.
HPLC conditions were selected referring to an existing method[15]: A Adamas C18-Classic column (SepaChrom S.R.L., 5 μm, 250 mm × 4.6 mm) was equipped for chromatographic separations; The mobile phase was composed by acetonitrile (A) and monopotassium phosphate aqueous solution with pH 6.8 (B) in an isocratic mode with 20% A; Injection volume was set as 20 μL; A pre-equilibration period of 30~60 min was applied between two consecutive separations; The flow rate and column temperature were, respectively, set as 1.0 mL/min and 40 °C, and the wavelength of 248 nm was selected for detection. All buffer and sample solutions were filtrated using a 0.45 μm filter membrane before running.
HPLC standard curves were plotted in terms of the function between peak areas and the concentrations of standard polysaccharides derived by PMP. Briefly, PMP-marked TPS and LBPS stock solutions with concentration gradients of 1.0, 0.5, 0.4, 0.3, 0.2, 0.1, 0.05 and 0.01 mg/mL, and 0.3, 0.2, 0.1, 0.05, 0.01 and 0.005 mg/mL were prepared and their chromatographic retentions were surveyed with the aforementioned conditions. Each sample was tested in triplicate and the weighted peak area was used for plotting standard curves, then the concentrations of polysaccharides in extracts were deduced by linear regression equations.
Reusability tests of MNPs@B(OH)2
-
The recyclability of MNPs@B(OH)2 was investigated by consecutive extraction and desorption 10 times. In detail, 80.0 mg MNPs@B(OH)2 was dispersed into 20 mL PB containing 3.0 mg/mL TPS, then incubation for 1 h at room temperature. After that, the materials were magnetically collected and washed with 5 mL PB for 5 min, followed by desorption for 0.5 h with 1 mL HAc aqueous solution (0.1 M). Finally, the supernatants were separately collected after magnetic isolation. After color development by phenyl-sulfuric acid method, their UV-vis spectra were tested and digital photos were recorded. Such an extraction-elution process was repeated for 10 cycles. A pre-equilibrium period of 1 h by PB (10 mL) was adopted for MNPs@B(OH)2 between two extractions.
Antioxidant capacity assessed by DPPH radical scavenging assay
-
Two aliquots of 1.0 mL ethanol solution containing 0.06 mg/mL DPPH were, respectively, mixed with 0.3 mL TPS and LBPS solution (dissolved in ethanol, and both with a concentration of 0.5 mg/mL). The DPPH solution combined with an equal volume of ethanol was used as the control group. After incubation in the dark for appropriate periods (0, 1, 3, 5, 10, 20, 30, 45 and 60 min), the digital photos were recorded using a smartphone and the UV-vis spectra were measured on a spectrophotometer. The radical scavenging activities of TPS and LBPS were evaluated by the following expression:
$ Radical\;scavenging\;acitivity\;\left({\text{%}}\right)\;=\;\left(1-\frac{{A}_{T}}{{A}_{C}}\right)\;\times\; 100 $ In which, AT and AC were, respectively, on behalf of the absorbance of polysaccharides-treated group and control group (without additives).
Cell culture and antitumor activity evaluation
-
MCF-7 cell was cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum at 37 °C in a humidified chamber containing 5% CO2. A549 and DU145 cells were cultured in DMEM medium with 10% fetal bovine serum at 37 °C in a humidified chamber containing 5% CO2. All the cell experiments were implemented when the confluence reached ~80%. MTT assays and trypan blue staining experiments were adopted to estimate cell viability and apoptosis, respectively.
Details for MTT assay: The cells cultured in 96-well microplate were washed with 1 × PBS three times, followed by supplementing with relevant culture medium containing polysaccharide extracts with different concentrations ranging from 0 (control), 0.25, 1.0, 1.5, 2.5 to 5.0 mg/mL (200 μL per well). After culturing for another 24 h, the culture medium was removed and the remaining cells in microplate were cultured with PBS containing 5.0 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 50 μL per well), and then cultured for 4 h. The remaining MTT reagent was discarded and 100 μL DMSO was supplemented into each well, and then slowly shaken for 10 min to dissolve the formazan in cells. Finally, the absorbance of the DMSO solutions was determined at 492 nm on a Synergy H1M microplate reader (BioTek, Winooski, VT, USA). The formula given below was utilized to calculate the cell viability (%):
$ Cell\;viability=\frac{{{A}_{T}-A}_{b}}{{{A}_{C}-A}_{b}}\times 100{\text{%}} $ Herein, AT, AC and Ab represented the absorbance value of treatment group, control group (cells without additives) and 96-well microplate substrate, respectively.
Trypan blue staining trials were performed as follows: The preculture with polysaccharide extract-added medium was the same as above, but only the cells cultured by the medium containing 0 and 5.0 mg/mL polysaccharide extractions were selected for staining and comparison. The pretreated cells were subsequently stained by 0.4% (wt%) trypan blue solution for 3 min, then microscopic images of the resulting cells were recorded on an inverted fluorescence microscope (Sunny Optical XD-FRL, 10 × objectives).
-
Two steps were concluded for the preparation of MNPs@B(OH)2, namely the synthesis of amino group-capped MNPs (bare MNPs) and boronic acid functionalization of bare MNPs. The synthetic route was illustrated in Supplemental Fig. S1, in which bare MNPs were fabricated via the one-step solvothermal method[4,5], and then the boronic acid modification was carried out in terms of Schiff-base reaction between amino groups onto bare MNPs and the formyl groups of FPBA[16,17]. Transmission electron microscopy (TEM) characterization indicated that both bare MNPs and MNPs@B(OH)2 were of fine water dispersibility and regular morphology, and the particle size of bare MNPs and MNPs@B(OH)2 was respectively estimated to be ca. 70~120 nm and 80~140 nm from TEM and DLS analysis (Fig. 2a − c). Magnetic separation of MNPs@B(OH)2 could be easily achieved by external magnetic field (inset in Fig. 2b), which facilitated the operations of SPE. The slight increase in particle size of MNPs@B(OH)2 as compared with bare MNPs might be caused by the repeated magnetic separation during the post modification of boronic acid (FPBA).
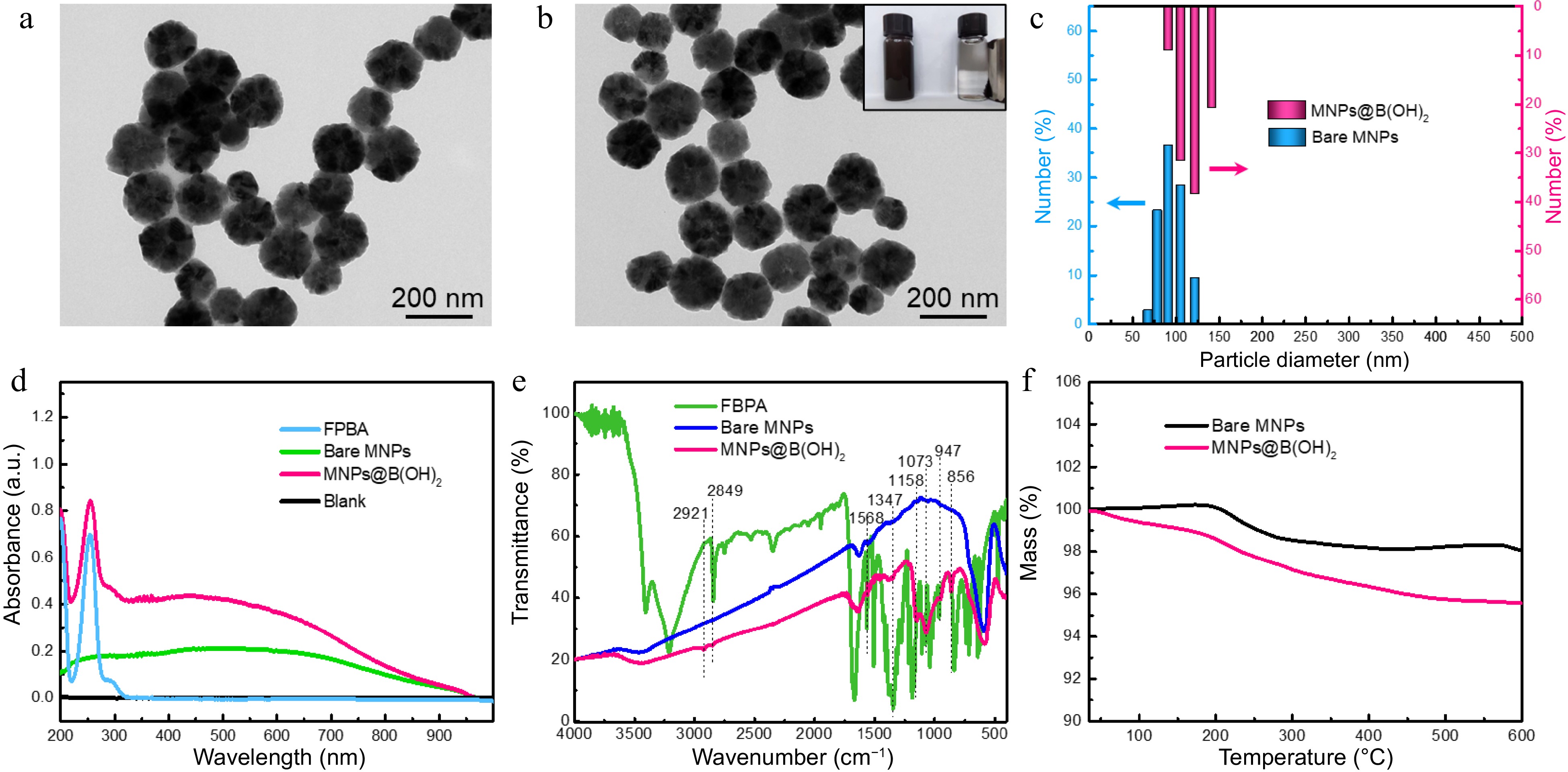
Figure 2.
TEM characterization of (a) bare MNPs and (b) boronic acid-grafted MNPs, along with their particle size distributions analyzed by (c) DLS; (d) UV-vis spectra, (e) FT-IR spectra, and (f) TGA tests of MNPs materials with or without modification by FPBA. Inset in (b) was the digital photo showing the magnetic separation behavior of MNPs@B(OH)2 by an external magnet.
The boronic acid functionalization was firstly evaluated by UV-vis spectrometry, and the characteristic absorption at 260 nm (produced by aromatic ring) of MNPs@B(OH)2 suggested that FPBA was successfully modified onto MNPs (Fig. 2d). FT-IR was further applied to confirm this claim. As seen in Fig. 2e, wide absorption band at ~3,413 cm−1 should be allotted to the stretching vibration of -OH or -NH2; the bands at 2,921 and 2,849 cm−1 should be assigned to the stretching vibration of C-H in methylene groups; the peak at 1,568 cm−1 should be attributed to the in-plane bending vibration of N-H of secondary amine while the peaks at 1,347 cm−1 was associated with the C-B vibrations; 1,158 and 1,073 cm−1 indicated the existence of C-N; the bands at 947 and 856 cm−1 could be, respectively, identified by the out-of-plane deformation vibration of hydroxyl group in boronic acid group and the p-disubstitution of the aromatic ring. These results were in agreement with previous literature reports[5,18] and affirmed that the post-modification of FPBA was workable. Additionally, the greater mass loss of MNPs@B(OH)2 than that of bare MNPs (4.5% vs 1.1%) in TGA tests (Fig. 2f) also validated the reasonability of this claim.
Boronate affinity-mediated MSPE
-
Boranate affinity has been well proved to be of excellent class recognition selectivity toward cis-diol containing compounds over non-cis-diol compounds[10,19−21]. Thus, the binding selectivity of MNPs@B(OH)2 was firstly examined using cytidine, guanosine, adenosine, deoxyguanosine and deoxyadenosine as model analytes, among which cytidine, guanosine and adenosine were cis-diol compounds. As exhibited in Fig. 3a & b, only limited extraction capacity of bare MNPs could be observed toward model analytes, as contrasts, MNPs@B(OH)2 displayed high binding ability toward cytidine, guanosine and adenosine, giving an equilibrium binding capacity (Qe) of 81.44 vs 1.87 μg, 230.41 vs −3.21 μg, and 240.92 vs 57.35 μg, respectively, which suggested that MNPs@B(OH)2 were of good recognition selectivity toward cis-diol compounds against non-cis-diol compounds. The influence of pH on the binding capacity of MNPs@B(OH)2 was given in Fig. 3c & d, and the results indicated that optimal binding performance could be achieved at pH 7.5~8.5, which could be rationalized by the fact that boronate affinity effect would be active at such a pH around pKa of FPBA[22,23]. Such a weak alkaline working pH is beneficial to maintain the structural stability of polysaccharide. Binding dynamics assay declared that the binding equilibrium could be reached within 10 min (Fig. 3e & f), which is consistent with the rapid binding feature of boronate affinity[10], and also provided a reference for selection of extraction time during polysaccharide MSPE. These findings revealed that the binding between MNPs@B(OH)2 and cis-diol compounds was dominated by boronate affinity effect, laying the foundation for upcoming BA-MSPE of polysaccharides.
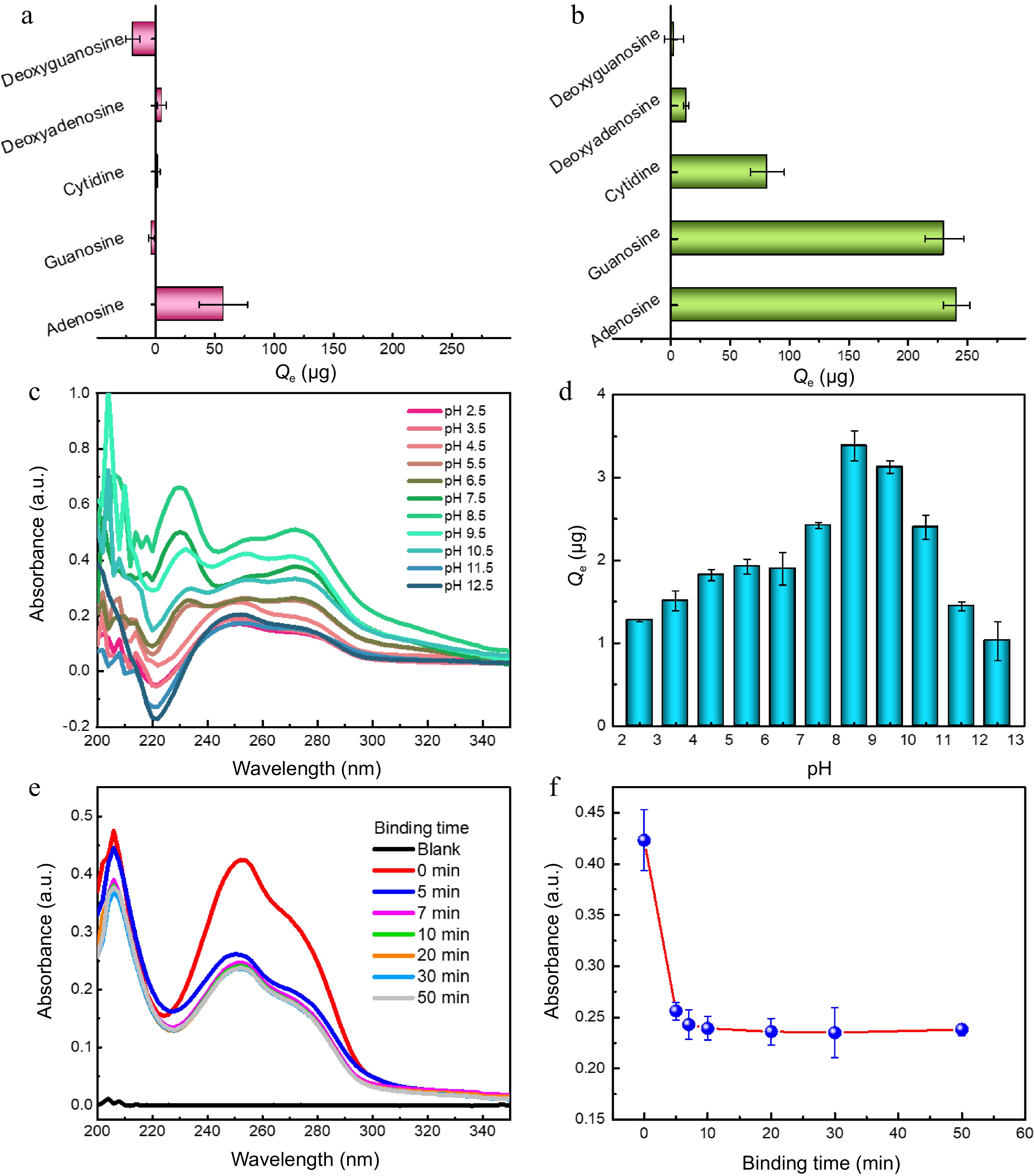
Figure 3.
Binding selectivity of (a) bare MNPs and (b0 MNPs@B(OH)2 toward five model compounds; Typical UV-vis spectra of guanosine extracted by MNPs@B(OH)2 at (c) different pH and their (d) quantitative comparison; (e) UV-vis spectra of guanosine in supernatants after extraction by MNPs@B(OH)2 at different extraction time, and (f) the absorbance of guanosine in supernatants as a function of extraction time. All tests were carried out at least three times in parallel.
The extraction performance of bare MNPs and MNPs@B(OH)2 was compared using SPS, PPS, LBPS and TPS as model polysaccharides, and the results were provided in Fig. 4a & b. Obviously, the equilibrium binding capacity (Qe) of MNPs@B(OH)2 was higher than that of bare MNPs in all cases, and a deeper color also appeared after color development by the phenol-sulfuric acid method, which demonstrated that boronate affinity was activated and essential for MSPE of polysaccharides. Zeta (ζ) potential tests disclosed that MNPs@B(OH)2 were negatively charged at the given conditions (phosphate buffer solution with pH 8.5) while the ζ potentials of PPS-/TPS-extracted MNPs@B(OH)2 were slightly enhanced (Supplemental Fig. S2), offering a value of −41.6 ± 2.68 mV, −38.3 ± 3.12 mV, and −38.7 ± 2.26 mV, respectively. The negative charge of MNPs@B(OH)2 ought to ascribe to the formation of boronic anions with the boron in its tetragonal hybridization[24] at pH 8.5 while the increased surface potential after extraction of polysaccharides could be clarified by the electroneutrality and relatively large molecular weight of polysaccharides, which affect the surface charge of MNPs@B(OH)2. These results proved that MNPs@B(OH)2-mediated MSPE of polysaccharides was workable, and boronic acid played a vital role in this process.
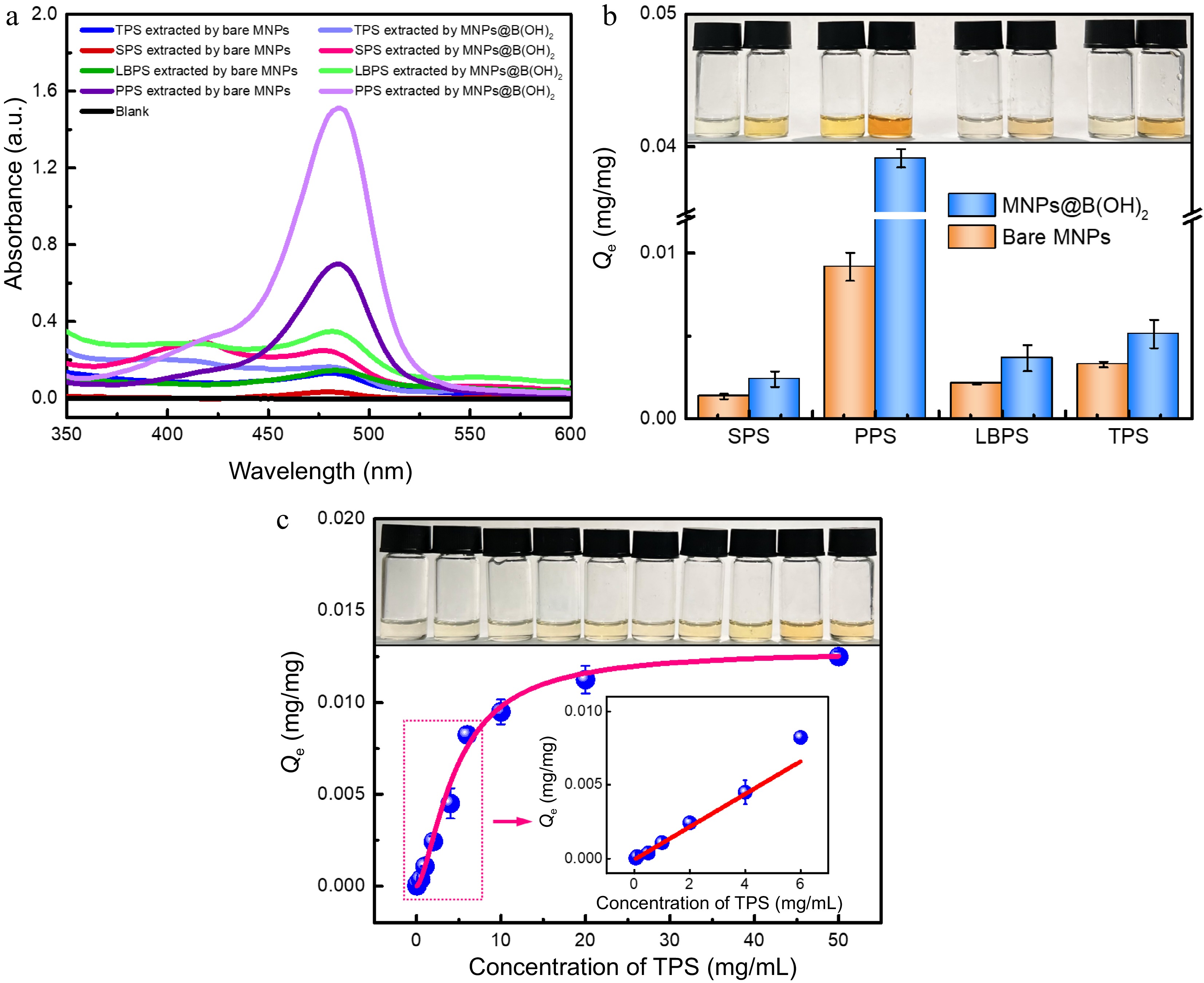
Figure 4.
UV-vis spectra of four polysaccharide extractives by different MNPs after (a) color development by the phenol-sulfuric acid method, and the (b) equilibrium binding capacity of bare MNPs and MNPs@B(OH)2 toward four model polysaccharides, all with a concentration of 1.0 mg/mL); (c) Adsorption isotherms for TPS binding to MNPs@B(OH)2. Insets in (b) and (c): digital photos of polysaccharide extractives (derived by phenol-sulfuric acid chromogenic reaction) with the same order as relevant horizontal axis. Diagrams inserted in (c): linear fitting in the working range of 0.05~6.0 mg/mL (y = 0.0445x − 0.0029, R2 = 0.9579). All measurements were repeated in triplicate at least for quantitative calculations.
Adsorption isotherm was further used to assess the binding properties of MNPs@B(OH)2 using tea polysaccharide (TPS) as a model analyte, in which the standard curve method presented in Supplemental Fig. S3 and Supplemental Table S1 was utilized for quantification, and the Hill equation[25−27] was selected to nonlinearly fit the data. The theoretical maximum binding capacity (Qmax) of MNPs@B(OH)2 toward TPS was deduced to be 12.93 μg/mg (Fig. 4c), accompanying a dissociation constant (Kd) of 4.72 mg/mL or 4.63 × 10−3~4.63 × 10−6 M (R2 = 0.9896, the molecular weight of TPS from tea leaves was ca. 1.02~1020 kDa according to literature reports[28]). Such a Kd level was lower than that of boronic acid and small molecular cis-diol compounds (Kd was usually on the order of magnitudes of 10−1~10−4 M[22−24]), indicating higher binding force between boronic acid and polysaccharides as compared with the binding between boronic acid and small molecular cis-diol compounds. This outcome would be explained by the plenty of 1,2- and 1,3-cis-diol structures of polysaccharide and consequently formed positive synergetic effects by the multi-sites binding between boronic acid and polysaccharide. It also implied the correctness of the possible binding mechanism proposed in Fig. 1. Moreover, a fine linear correlation (y = 0.0445x − 0.0029, R2 = 0.9579) between the Qe of MNPs@B(OH)2 and TPS concentrations could be found in the concentration range of 0.05~6.0 mg/mL, and a good color evolution of polysaccharide extractives after color development by phenol-sulfuric acid method also emerged as polysaccharide concentrations increased, paving the way for quantification of BA-MPSE and giving a guideline for the consumption of materials in real applications.
Extraction of polysaccharides from real-world beverage plants
-
The above-mentioned results encouraged us to explore the feasibility of polysaccharides BA-MSPE in real beverage plants. Lycium barbarum, tea leaves (green tea) and soybeans were devoted as real plant samples, and the extraction of polysaccharides from these plants by bare MNPs and MNPs@B(OH)2 was compared in Fig. 5. After chromogenesis by phenol-sulfuric acid method, strong absorption of leaching liquors at 488 nm demonstrated that polysaccharides were successfully released from plant samples. Higher absorbance of extractives by MNPs@B(OH)2 than those by bare MNPs declared better MSPE performance of MNPs@B(OH)2 (Fig. 5a~c), giving the binding capacity 4.6 times as high in MNPs@B(OH)2 as in bare MNPs (LBPS, Fig. 5d). The possible reason for the variation in the relative binding capacity of MNPs and MNPs@B(OH)2 in Figs 4b & 5d would be attributed to the fact that the initial concentrations of standard polysaccharides and the polysaccharides in leaching liquors of real beverage plants were different. The digital photos of freeze-dried polysaccharide extractives and standard polysaccharide samples were showed in Supplemental Fig. S4, and the similar appearance and colors between them also implied that it was practicable to extract polysaccharides in real beverage plants by MNPs@B(OH)2.
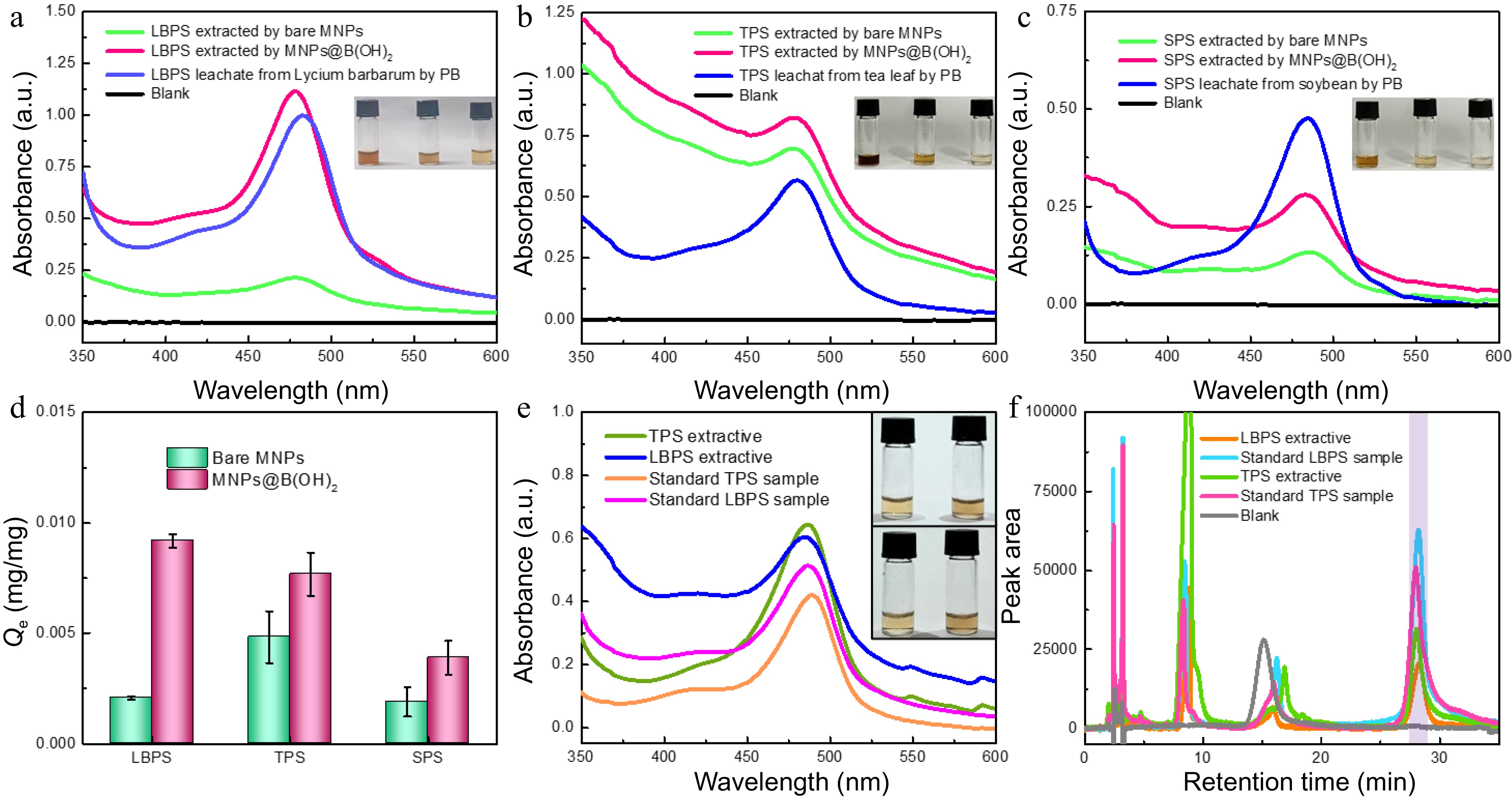
Figure 5.
UV-vis spectra of polysaccharide leaching liquors and extracts from (a) Lycium barbarum, (b) tea leaves and (c) soybeans by different materials. The comparison in extraction performance of bare MNPs and MNP@B(OH)2 (d, n = 3); (e) UV-vis spectra of TPS and LBPS extractives (1.0 mg/mL) and standard polysaccharide solutions (0.5 mg/mL); (f) HPLC chromatograms of TPS and LBPS extractives (1.0 mg/mL for TPS, and 0.5 mg/mL for LBPS) and standard polysaccharide solutions (0.5 mg/mL for TPS, and 0.3 mg/mL for LBPS) after labelling with PMP , in which light violet zone indicated the peak position of polysaccharides. Insets in (a)−(c) were, respectively, the digital photos of relevant polysaccharides obtained in different pathways after color development with phenol-sulfuric acid chromogenic method, and the order was leachates and the extractives by MNPs@B(OH)2 and bare MNPs from left to right. The inset in (e) was the photos of polysaccharide extractives (top) and standard polysaccharide samples (bottom) of TPS (left) and LBPS (right) with the same concentrations as UV-vis spectra tests. All UV-vis spectrograms were obtained after phenol-sulfuric acid chromogenic reaction.
The relative purities of TPB and LBPS extractives were roughly estimated by UV-vis spectrometry using standard polysaccharides as benchmarks and standard curve method for quantification. As displayed in Fig. 5e, the same characteristic bands at ~488 nm and the similar colors between polysaccharide extractives and standard polysaccharide samples after color development by phenol-sulfuric acid chromogenic reaction demonstrated that polysaccharides were efficiently extracted by MNPs@B(OH)2, showing a deduced relative purity of (56.8 ± 0.9)% and (69.1 ± 1.9)% for LBPS and TPS (Supplemental Table S1), respectively. HPLC, a powerful tool for quantification of polysaccharides[29,30], was applied to further confirm these outcomes using PMP labeling reaction for the pre-column derivatization of polysaccharides. Standard curve method showed in Supplemental Fig. S5 and Supplemental Table S2 were employed to infer the content of polysaccharides in extracts. As shown in Fig. 5f, the similar chromatographic retention behaviors could be found between polysaccharide extractives and standard polysaccharides, and the peaks at retention time near 28 min (light violet area in Fig. 5f) could be allotted to the chromatographic curves of polysaccharides by virtue of the mapping between peak area and concentrations, which further verified that polysaccharides were effectively extracted by MNPs@B(OH)2. The relative purities were, respectively, (43.1 ± 1.3)% and (59.2 ± 5.3)% for LBPS and TPS. Higher purities produced by UV-vis spectrometry than those from HPLC might be attributed to the existence of unknown impurities, while such a positive error could be avoided thanks to the separation effect of HPLC. Although the purity level was not so high, it was acceptable considering that only once extraction was implemented in such a facile way of BA-MSPE.
FT-IR spectrometry was used for the structure identification of extracted polysaccharides. As seen in Supplemental Fig. S6, the absorption bands at 3429/3413 cm−1 and 2931/2923 cm−1 were assigned to the stretching vibration of -OH groups and C-H (in -CH2- groups); the band at 1650 cm−1 was due to the stretching vibration of aldehydic carbonyl; the band at 1405/1409 cm−1 was on count of the deformation vibration of -CH2- groups; the peaks at 1248/1251 cm−1 and 1023/1054 cm−1 were attributed to the stretching vibration of C-OH side groups or C-O-C glycosidic bond vibrations, suggesting the possible structure of pyranose ring in sugar residues; the absorption bands at 937/944 cm−1 and 849/850 cm−1 were, respectively, caused by β- and α-glycosidic bonds. These results are in agreement with the literature reports[15, 31], and also double-checked the validity of BA-MSPE for polysaccharides. The main absorption bands were basically the same for TPS and LBPS, indicating their primary chemical structures were similar. Meanwhile, the similarity in characteristic peaks between polysaccharide extracts and standard polysaccharide samples suggested that the structural integrities of extracted polysaccharides were well maintained, which would benefit from the mild extraction conditions during the process of BA-MSPE.
HPLC analysis was applied to roughly estimate the monosaccharide composites of extracted LBPS and TPS. The polysaccharides were first hydrolyzed by the trifluoroacetic acid method and subsequently labeled by PMP prior to sample loading. As presented in Supplemental Fig. S7, six chromatographic peaks could be found from the chromatographic curves of TPS and LBPS, and the principal compositions of xylose (Xyl), arabinose (Ara), glucose (Glc), and galactose (Gal) could be identified. Detailly, peaks 1~6 would be respectively assigned to Xyl, Ara, Xyl, Ara, Glc and Gal for LBPS, and Xyl, Xyl, Ara, Glc, Ara and Gal for TPS as compared with the retention of monosaccharides, giving peak area percentages of 1.5%, 1.5%, 14.8%, 14.2%, 22.1% and 45.9% for LBPS, and 14.4%, 42.4%, 20.1%, 6.3%, 5.0% and 11.8% for TPS. Since no UV absorption signal could be produced by monosaccharides themselves, it was rational to roughly estimate the relative content of monosaccharides by peak area percentages. It was clear that the predominant monosaccharides were Glc and Gal in LBPS, and Xyl and Ara in TPS, which is basically consistent with the results reported in the literature[28, 32, 33]. The content of Xyl in TPS was somewhat different from that reported in the literature[28], and the reason might be related to the difference of tea categories, origin, as well as the hydrolysis conditions.
The relationship between extraction capacity and the dosage of MNPs@B(OH)2 used for MSPE was furtherly probed using TPS and LBPS as model polysaccharides, and the consequences were presented in Supplemental Fig. S8. Although the relative binding capacity of MNPs@B(OH)2 was not so high (Fig. 5d) due to their large specific gravity (chemical compositions of MNPs were mainly Fe3O4 and/or Fe2O3[34]), the extracted amounts of two polysaccharides were linearly enhanced as the consumption of MNPs@B(OH)2 increased from 10 to 150 mg (Supplemental Fig. S8a−c), giving a linear regression equation of y = 0.0056x + 0.4535 (R2 = 0.9392) for TPS, and y = 0.0073x + 0.2953 (R2 = 0.9294) for LBPS, respectively. Correspondingly, the colors of polysaccharide extractives were gradually deepened as the increase of MNPs@B(OH)2 loading amounts (Supplemental Fig. S8d). Clearly, to some extent, such a dosage-dependent extraction capacity remedied the foible of relatively low binding capacity of MNPs@B(OH)2, and provided a guidance for their dosage selection in real applications.
The reusability of MNPs@B(OH)2 for BA-MSPE was further explored using TPS as a model polysaccharide, and the results are showed in Supplemental Fig. S9. The fluctuation in binding capacity of MNPs@B(OH)2 after continuous extraction and desorption ten times was less than 13%, demonstrating an acceptable recyclability of MNPs@B(OH)2 for polysaccharide extraction. Likewise, after color development with phenyl-sulfuric acid method, the colors of TPS extractives obtained by ten consecutive extractions were very close, which confirms the above-stated claim.
Antioxidant and antitumor bioactivities of extracted polysaccharides
-
Antioxidant activity of the obtained TPS and LBPS extractives by MNPs@B(OH)2 was investigated by DPPH radical scavenging assays[35−37]. As shown in Fig. 6, no obvious change could be observed in the absorbance of DPPH after monitoring continuously for 1 h in the absence of polysaccharide (∆A < 1.9%, Fig. 6a & d), by contrast, the absorbance of DPPH was significantly decreased in the presence of TPS and LBPS even their concentration as low as 0.2 mg/mL (Fig. 6b−d), showing a free radical scavenging rate of 31.4% and 18.8%, respectively. Correspondingly, the color of DPPH solution without the addition of polysaccharides was stable while the color variations of polysaccharide-added DPPH were eye-catching enough. These findings certified that the polysaccharides extracted by MNPs@B(OH)2 were of fine antioxidant activity. The possible reasons for stronger antioxidant capability of TPS than that of LBPS could ascribe to its higher relative purity, broader distribution in molecular weight[28] and abundant active functional groups[38].
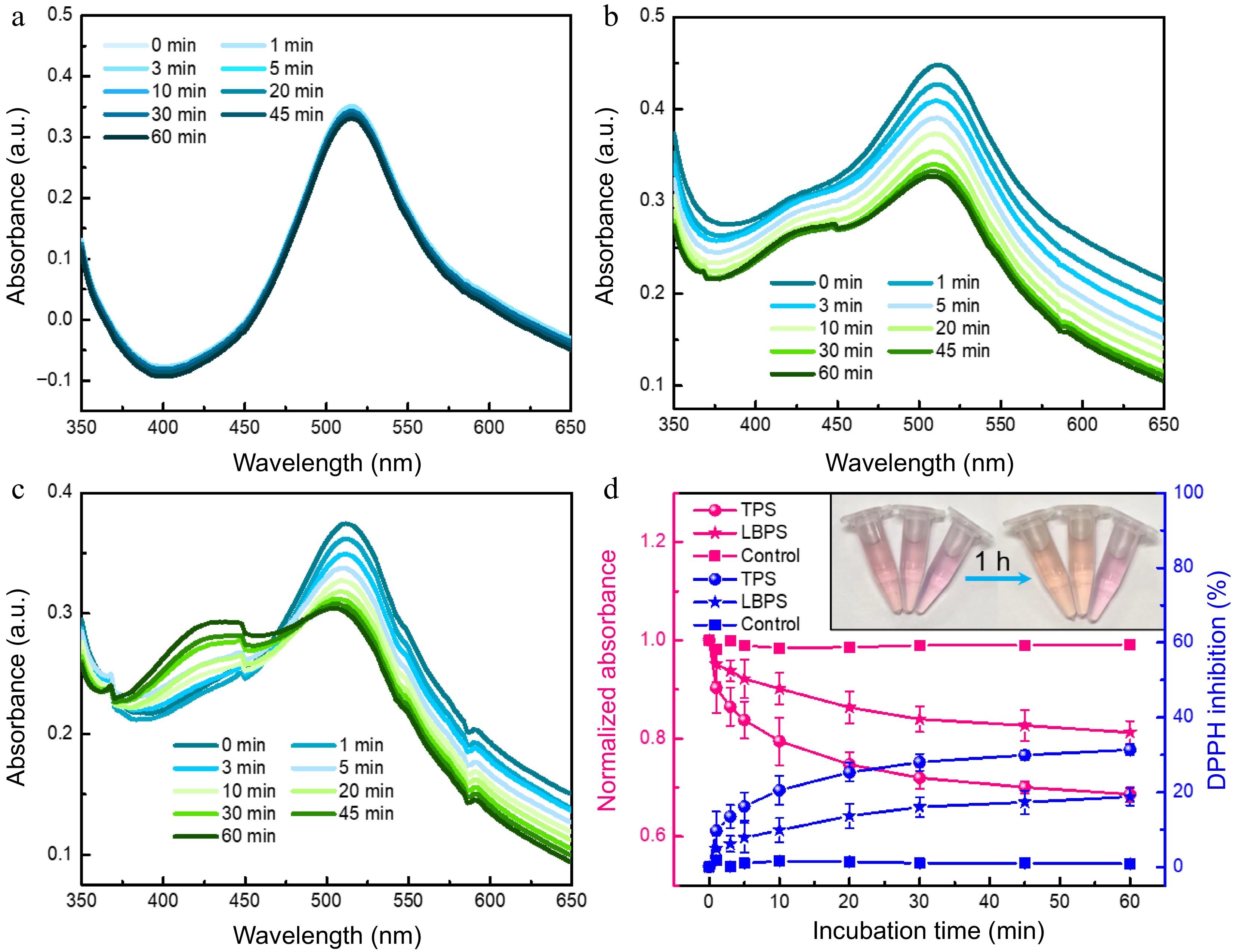
Figure 6.
UV-vis spectra of (a) DPPH, (b) TPS- and (c) LBPS- added DPPH obtained at incubation time ranging from 0 to 60 min. Antioxidant ability of TPS and LBPS assessed by DPPH scavenging activity assay (d, n = 3). The final concentration of both TPS and LBPS added in DPPH was 0.2 mg/mL.
The antitumor activities of LBPS and TPS were subsequently probed by microscopic imaging, MTT trials, along with trypan blue staining assays[39] using A549 and MCF-7 as representative carcinoma cell lines, and the results were presented in Supplemental Figs 7 & S10. Microscopic imaging implied that the cells morphology changed significantly, focusing on cell shrinkage, deteriorated adhesion ability, and the abnormal distribution of intracellular contents. Irreversible vacuolation emerged widely in both A549 and MCF-7 cells under the culture condition in the presence of LBPS (Fig. 7c, d, Supplemental Fig. S10c, d), while cell disruption was more distinct after culturing with TPS (Fig. 7e, f, Supplemental Fig. S10e, f), and a large number of cell fragments could be found in this case, suggesting cells were dying and cell functional statuses changed. These phenomena might be closely related with different apoptosis pathways induced by TPS and LBPS. TPS have been proved to be of lysosomes targetability and induced apoptosis by a lysosomal-mitochondrial pathway mediated caspase cascade[40], while the inhibitions in proliferation, migration and survival of tumor cells by LBPS were testified to be related with Pi3K/AKT signaling pathway[41]. Furthermore, all cells were well stained by trypan blue after administrating by LBPS and TPS (Figs 7d, f, Supplemental Fig. S10d, f), as controls, only a few cells were stained in control groups, which further confirmed that polysaccharide extracts were of antitumor activity and caused cell death. The relative cell viabilities were profiled by MTT assays and the results were given in Fig. 7g, h. An obvious dosage-dependent cytotoxicity could be observed for both LBPS and TPS extractives, showing a relative cell survival rate less than 60.0% and 22.8% for A549 cells, and 43.4% and 8.2% for MCF-7 cells after respectively treating by LBPS and TPS with an equal concentration of 5.0 mg/mL. The higher cell mortality rate induced by TPS as compared with LBPS might be thanks to its relatively higher purity obtained during BA-MSPE. These findings were consistent with the results of literature reports[40−43], and confirmed the reliability of antitumor activities of polysaccharide extractives.
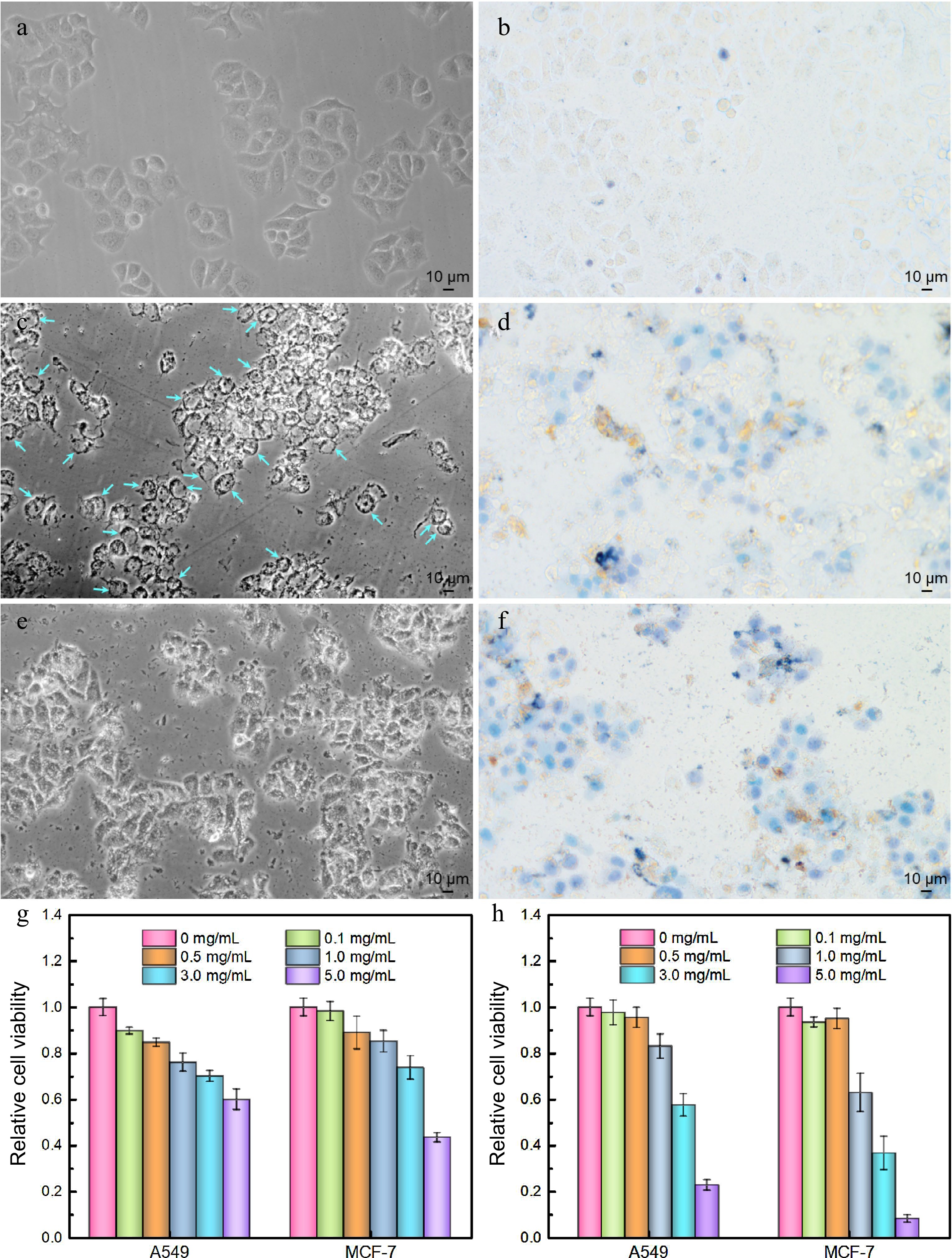
Figure 7.
Antitumor activities of polysaccharide extracts assessed by optical microscopic imaging (a) − (f) using MCF-7 cells as a model cell line and (g), (h) MTT assays. (a) Cell imaging of control group, (b) trypan blue stained control group, (c) LBPS-treated group, (d) trypan blue stained LBPS-treated group, (e) TPS-treated group, and (f) trypan blue stained TPS-treated group. The dosage of TPS and LBPS in (c) − (f) was set as 5.0 mg/mL. Relative cell viabilities of A549 and MCF-7 cells after administrating by (g) LBPS and (h) TPS by MTT assays. Blue arrows in (c) on behalf of the typical vacuolated cells.
-
Boronic acid-functionalized magnetic nanoparticles (MNPs@B(OH)2) have been prepared and used as sorbents for boronate affinity-mediated MSPE of polysaccharides in this work. Boronate affinity effect and its work parameters were investigated, the mechanism for the binding of polysaccharides by MNPs@B(OH)2 was discussed, and the extraction conditions were also optimized. Three polysaccharides, including TPS, LBPS and SPS, were successfully extracted from relevant real-world beverage plants, and the main active ingredients in extracts were identified by several instrumental analysis techniques, such as UV-vis/FT-IR spectrometry and HPLC. In the end, the extracted TPS and LBPS were experimentally proven to be of fine antioxidant and antitumor bioactivities in terms of DPPH radical scavenging experiments, trypan blue staining, as well as MTT assays. Since the operations of BA-MSPE were straightforward and do not necessitate the use of organic solvents or other intricate impurities elimination steps during BA-MSPE, coupled with the fine recyclability of MNPs@B(OH)2, this approach may have more potential for the simple separation and purification of cis-diol containing compounds in the fields of food and agricultural product processing.
This work was funded by the National Natural Science Foundation of China (Grant No. 21904003), the University Scientific Research Project of Anhui Province (2022AH050296), the Natural Science Foundation of Anhui Province (Grant No. 2108085QB85), the Open Project of Anhui Engineering Technology Research Center of Biochemical Pharmaceutical (Bengbu Medical College) (Grant No. 2022SYKFZ02), and the Student Research Training Program of Anhui University of Technology (Grant No. 202210360031, 202210360038).
-
The authors declare that they have no conflict of interest.
-
# These authors contributed equally: Yuwen Ding, Haiyang Li
- Supplemental Fig. S1 Synthetic route of amino group-capped MNPs (a) and boronic acid-modified MNPs (b).
- Supplemental Fig. S2 Representative ζ potential distribution curves of MNPs@B(OH)2 (a), PPS-extracted MNPs@B(OH)2 (b), and TPS-bound MNPs@B(OH)2 (c); The quantitative comparison of their ζ potentials (d).
- Supplemental Fig. S3 UV-vis spectra of polysaccharides with different concentrations (a, c, e) measured after phenol-sulfuric acid chromogenesis, and the standard curves fitted by the relationship between absorbance and polysaccharides concentrations (b, d, f). The details for linear regression equation and related working parameters were listed in Table S1.
- Supplemental Fig. S4 Digital photos of standard polysaccharide samples (1, 2) and freeze-dried extractives (3, 4) of TPS (1, 3) and LBPS (2, 4) by MNPs@B(OH)2.
- Supplemental Fig. S5 HPLC chromatograms (a, c) of polysaccharides with different concentrations, and the standard curves (b, d) obtained by the relationship between peak area and polysaccharides concentrations. (a, b): LBPS; (c, d): TPS. Linear equations, their working parameters along with the tested purities of polysaccharides were given in Table S2.
- Supplemental Fig. S6 FT-IR spectra of polysaccharide extractives (blue) and standard polysaccharide samples (red). (a) TPS; (b) LBPS.
- Supplemental Fig. S7 Chromatograms of several monosaccharides and the hydrolysates of LBPS and TPS after labelling by PMP. (b) Partial enlargement of chromatographic curves for peak 2 in (a). Peaks identification for LBPS and TPS: (1, 3) xylose (Xyl); (2, 6) arabinose (Ara); (4) mannose (Man) and Ara; (5) glucose (Glc); (7) galactose (Gal).
- Supplemental Fig. S8 UV-vis spectra of TPS (a) and LBPS (b) extractives from tea leaves and Lycium barbarum by MNPs@B(OH)2 with different doages; The extraction capacity of TPS and LBPS as a function of MNPs@B(OH)2 usage (c); Digital photos of TPS (top) and LBPS (bottom) extractives recorded after color development by phenol-sulfuric acid method with a decreased dosage of MNPs@B(OH)2 sorbents from left to right (d), which corresponding to the amounts used in (a-c). Linear equations in (c) were, respectively, y=0.0056x+0.4535, R2= 0.9392 (TPS) and y=0.0073x+0.2953, R2= 0.9294 (LBPS), both with a dosage gradient of MNPs@B(OH)2 ranged from 10 to 150 mg.
- Supplemental Fig. S9 Reusability of MNPs@B(OH)2 for polysaccharide extraction. UV-vis spectra of TPS extractives obtained by continuous extraction for ten times (a) and the fluctuation of relative binding capacity of MNPs@B(OH)2 after ten consecutive extractions (b). Inset in (b) was the chromogenic photos of TPS extractives obtained by ten extractions. UV-vis spectra and digital photos were produced with the help of color development by phenyl-sulfuric acid method.
- Supplemental Fig. S10 Optical microscopic imaging of A549 cells before and after treating with polysaccharide extractives. Treatments in (a-f): control group (a), trypan blue stained control group (b), LBPS treated group (c), trypan blue stained LBPS treated group (d), TPS treated group (e), and trypan blue stained TPS treated group (f). The concentration of polysaccharide extractives for cell treatment was set as 5.0 mg/mL.
- Supplemental Table S1 Linear regression equations, relevant running parameters along with the tested relative purities (the purities of standard polysaccharides were used as references) of polysaccharides by UV-vis spectrometry.
- Supplemental Table S2 Linear regression equations, their working parameters and the measured relative purities (the purities of standard polysaccharides were used as references) of polysaccharides by HPLC.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Ding Y, Li H, Liu T, Liu Y, Yan M, et al. 2023. Boronate affinity-mediated magnetic solid phase extraction and bioactivities of polysaccharides from beverage plants. Beverage Plant Research 3:14 doi: 10.48130/BPR-2023-0014
Boronate affinity-mediated magnetic solid phase extraction and bioactivities of polysaccharides from beverage plants
- Received: 19 April 2023
- Revised: 07 May 2023
- Accepted: 11 May 2023
- Published online: 14 June 2023
Abstract: Polysaccharides are of great significance in food production, but their isolation highly relies on multi-staged liquid-liquid extraction. In this study, a boronate affinity-mediated magnetic solid phase extraction (BA-MSPE) method was initiated for the effortless and efficient extraction of polysaccharides using boronic acid-grafted magnetic nanospheres (MNPs@B(OH)2) as extractants. MNPs@B(OH)2 showed fine class selectivity toward cis-diol containing compounds at weak alkaline condition (pH 7.5~8.5) and higher binding capacity than that of MNPs without boronic acid functionalization. Fast binding dynamics with a binding equilibrium within 10 min, stronger affinity toward polysaccharides (Kd as low as 10−3~10−6 M level) than that of small molecular cis-diol compounds (Kd in the range of 10−1~10−4 M level), and good recyclability (the binding capacity decreased less than 13% after ten times consecutive extraction) could also be observed for MNPs@B(OH)2. Finally, the BA-MSPE of polysaccharides was performed with three beverage plants as real samples, including tea leaves, soybeans, and Lycium barbarum. Antioxidant activity of polysaccharide extractives was verified by DPPH radical scavenging assays, giving a radical scavenging rate of 31.4% and 18.8% for crude extractives of TPS (tea polysaccharide) and LBPS (Lycium barbarum polysaccharide), respectively. Microscopic imaging combining with MTT and trypan blue staining trials uncovered that the extractives were of dosage-dependent antitumor bioactivities, giving the cell mortality rates over 91.8% and 77.2% for MCF-7 and A549 cells in the presence of 5.0 mg/mL TPS, and 56.6% and 40.0% with the equal dosage of LBPS, respectively. As the BA-MSPE strategy is simple and eco-friendly, there will be more potential for the application of cis-diol compound purification.
-
Key words:
- Boronate affinity /
- Solid phase extraction /
- Polysaccharide /
- Bioactivity /
- Beverage plant