-
Kidney stones (KS) are a common disease and their prevalence has been increasing over the last decades[1]. Environmental factors, especially dietary factors, may influence the occurrence of KS[2]. The prevalence varies from country to country, with rates of 7%−13% in North America, 5%−9% in Europe, and 1%−5% in Asia[3]. Notably, the relapse rate of renal stones is high, with about half of the patients having recurring KS within 10 years[4]Khalili et al. investigated the risk factors for kidney stone prevalence among the adult population in Rafsanjan, southeastern Iran, and statistically found that gender, hypertension, obesity, diabetes and personal habits were important predisposing factors for kidney stones[5]. In addition, in Taiwan, men had a higher prevalence and recurrence rate than women[6]. Moreover, the socioeconomic burden of the cost of KS diagnosis and treatment was not negligible[7]. Upwards of ten billion dollars a year in the USA has been spent on KS disease[8].
KS are usually composed of calcium oxalate, calcium phosphate (apatite and brushite) or less commonly, uric acid[9]. It has been demonstrated that diet affected the content of urine, and earlier research has linked dietary patterns closely to the incidence of KS[10], and people who consume more animal protein and less water were more prone to develop KS[11]. A substantial number of experimental studies and large cohort studies have produced enough proof that dietary management could prevent KS[12]. For example, in a prospective cohort research including 469 patients with symptomatic stones and 387 controls using a food frequency questionnaire, Chewcharat et al. found that intake of calcium and potassium might contribute to prevent the development of recurrent symptomatic KS[13]. In addition, experimental investigations and cohort studies have shown that the excessive intake of calcium or oxalate could increase the formation of KS[14,15]. Nevertheless, abundant intake of calcium and oxalate could be only part of the causes for forming KS, and the other key factors causing KS were not well explored to date, which should account for the low efficiency for prevention and control of KS in the last decades.
It is presumed that all the stones begin with crystallization, and crystals must be from some kind of solute supersaturations[16]. It is however largely unknown how the supersaturations may occur in specific renal locales[17].
The benefits of fresh fruits to human health have been extensively explored, and known as the powerful sources of dietary antioxidants, particularly polyphenols, such as various flavonoids, phenolic acids and tannins[18]. Despite the fact that the digestive toxicity of some kinds of tannins on livestock being known, it has not been well understood whether fruit tannins may be harmful to humans in some aspects.
Cholesterol is well documented as a major component of gallstones, and epidemiologic investigations had shown that gallstones are highly associated with total fat intake[19]. So far, little is known about the dietary fat or cholesterol involvement in the formation of KS. Characterization of the mechanisms of KS formation underlying dietary intake should help in the design of strategies to prevent KS.
Interactions of cholesterol and fruit tannins were investigated in a mouse model of urinary stones. We found that the synergistic action of cholesterol with tannins plays a key role in formation of various urinary stones.
-
Tannins were extracted from mature green apples (Malusdomestica Borkh. cv. Red Fuji) and further purified by size exclusion chromatograph (Sephadex G-25 Medium, Sigma-Aldrich). Polyclonal antibody of rabbits against the apple tannins was raised as described by Xi et al.[20].
Animal experiments
-
The study was approved by the Animal Ethics Review Committee of the Supervision, Inspection, and Testing Center of Genetically Modified Organisms (Beijing, China). Male CD-1 mice (8 weeks old, 20−22 g) were allowed free access to sterilized lab chow and water for one week before experiments.
The animal experiments in our research were carried out in accordance with the Guide for the Animal Experimental Welfare and Ethics in the Food Science and Nutritional Engineering College of China Agricultural University and were approved by the Animal Experimental Welfare and Ethical Inspection Committee in China Agricultural University. All efforts were made during the animal experiments to minimize suffering.
Twelve mice were placed in each of the experimental groups at random. EG = 20 mg ethylene glycol per 1 mL water, according to Okada et al.[21]. CH = 10 mg cholesterol per 1 mL corn oil; AT = 2 mg apple tannins per 1 mL water.
Each mouse was administrated as in the following schedule:
(1) Control: 0.8 mL saline, 1 h interval, 0.6 mL saline;
(2) CH: 0.8 mL CH, 1 h interval, 0.6 mL saline;
(3) AT: 0.8 mL saline, 1 h interval, 0.6 mL AT;
(4) CH + AT (CT): 0.8 mL CH, 1 h interval, 0.6 mL AT;
(5) EG: 0.8 mL EG, 2 h interval, 0.8 mL saline;
(6) EG + CH (EC): 0.8 mL EG, 2 h interval, 0.8 mL 8 mg CH;
(7) EG + AT (ET): 0.8 mL EG, 1 h interval, 0.6 mL AT;
(8) EG + CH + AT (ECT): 0.8 mL EG, 1 h interval, 0.8 mL CH, 1 h interval, 0.6 mL AT.
All the gavages for the each group were repeated for three times in 3 d. Food and water were freely available. Just after the last gavage, the mice were kept in standard mouse cages with no food and water for 4 h; then samples of urine were collected. Thereafter, the mice were housed for an additional 3 weeks.
The cervical dislocation method was used to kill the mice. The left kidney of each mouse was cut longitudinally, and one half part was fresh frozen in OCT (Optimum cutting temperature compound), and the other half of the kidney was fixed in 10% formalin.
Crystal number count in urine and observation of the crystallization in kidney tissue
-
Dripping 10 μl urine sample on a counting plate, the crystal number (length > 10 nm) was counted under a polarizing microscope (Cossim-PLJ-135A, Beijing, China).
The sample section of frozen kidney was put on an adhesive microscope slide and observed under a polarizing microscope.
Hematoxylin-eosin staining
-
Hematoxylin/eosin staining was carried out using the previously reported method of Paragas et al.[22] .
Confocal microscopy observation of localization of tannins and cholesterol in sections of kidney
-
The kidney frozen sections (20 μm) were prepared and were incubated with 1% PBS-BSA at 37 °C for 30 min, then were incubated with antibodies (rabbit anti-tannins, 1:200) at 4 °C overnight. After being washed twice, sections were then treated for 45 min at room temperature with the secondary antibody (Beyotime, Shanghai, China) that was coupled to FITC-phalloidin (fluorescein isothiocyanate-conjugated phalloidin)[23]. After 10 min of staining with DAPI (4′,6-diamidino-2-phenylindole, Sigma-Aldrich), the kidney segment was rinsed with PBS. Finally, anti-fadefluorescence mounting solution was used to mount the produced slices so they could be examined using a confocal microscope. The FITC-phalloidin and DAPI nuclear dyes were excited with argon lasers at 488 and 405 nm, respectively. The crystals were seen to reflect the red light of the Kr-laser at a wavelength of about 633 nm[24]. Cholesterol in samples were stained by Filipin kit (Genmed, Shanghai, China) and the dyes were excited by a 430 nm argon laser[25].
Statistical analysis
-
The statistical analysis was performed using IBM SPSS statistics software Version 19 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) and Duncan's test were used to assess the data.
-
In this study we found the particles sizes of urinary sediment in CT-mice or ECT mice were much bigger than those mice gavaged with AT, CH, EG, EG + AT or EG + CH (Fig. 1a & b). Crystals with diameters greater than 20 μm could be observed in urine of mice gavaged with CT or ECT, but not observed in urine of other groups. Comparing with the CH group, crystal numbers increased 37% in urine of CT-mice.
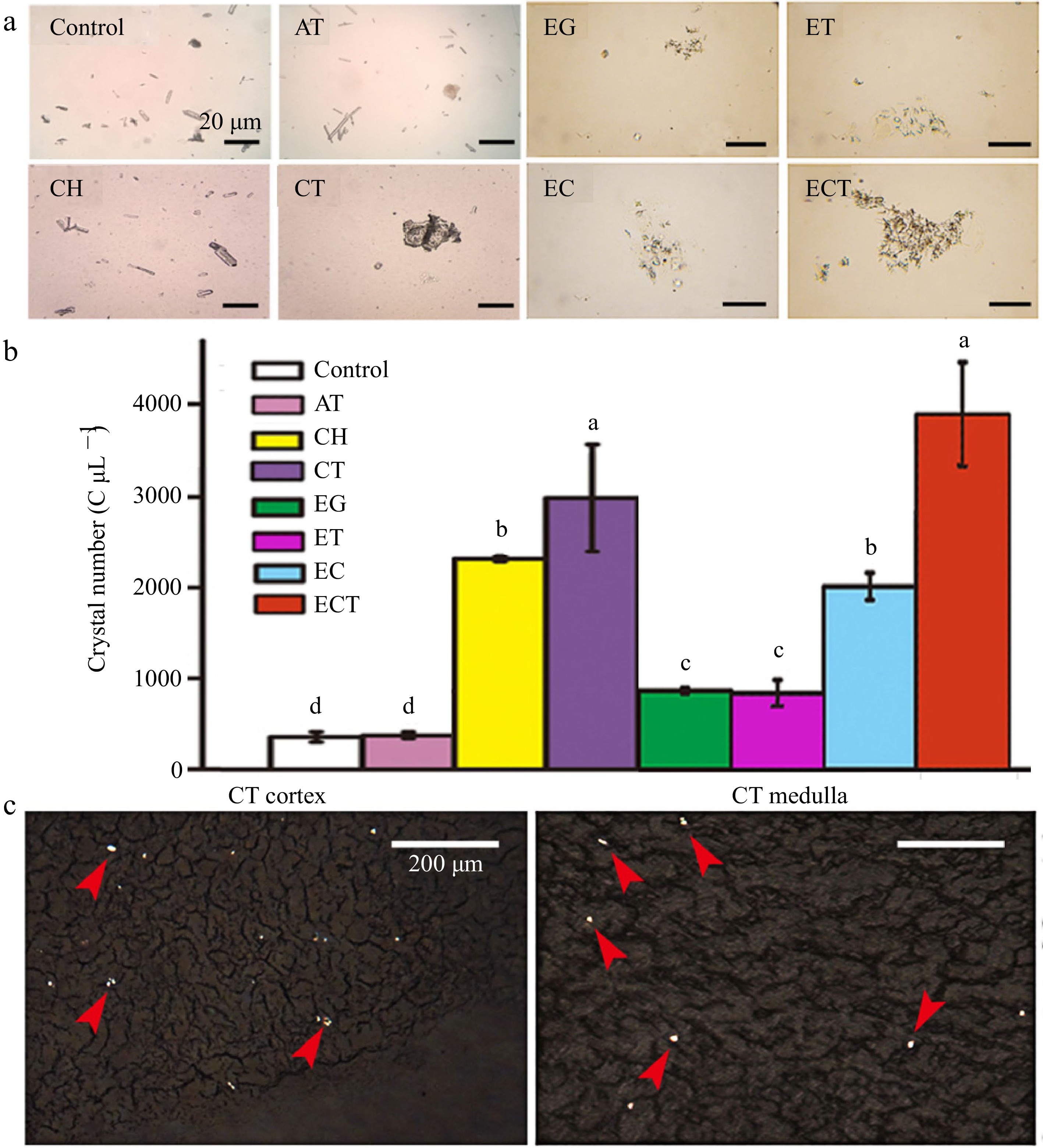
Figure 1.
(a) Microphotography, (b) crystal numbers of urine and the (c) frozen kidney sections. Urine was collected after the mice gavaged with AT, CH, CT, EG, ET, EC or ECT for 3 d. Frozen kidney sections of the mice gavaged with CT were observed under polarized microscope, the red arrows indicate the crystals induced by the treatment of CT. The crystal numbers in different groups were about 432, 456, 2,336, 2,984, 952, 904, 2,080, 3,928 C μL−1, respectively. Vertical bars on data in the columnar graph represented standard deviation; values with different letters are significantly different at p < 0.05.
It is known that ethylene glycol (EG) can promote oxalate excretion in kidney, inducing the urinary stones formation.
Crystalline analysis in kidney tissues
-
Crystals could be observed under polarizing microscope in unstained frozen sections of kidney tissues of CT-mice (Fig. 1c) and numerous crystalline substances were observed clearly in sections of kidney tissues of ECT-mice (Fig. 2).
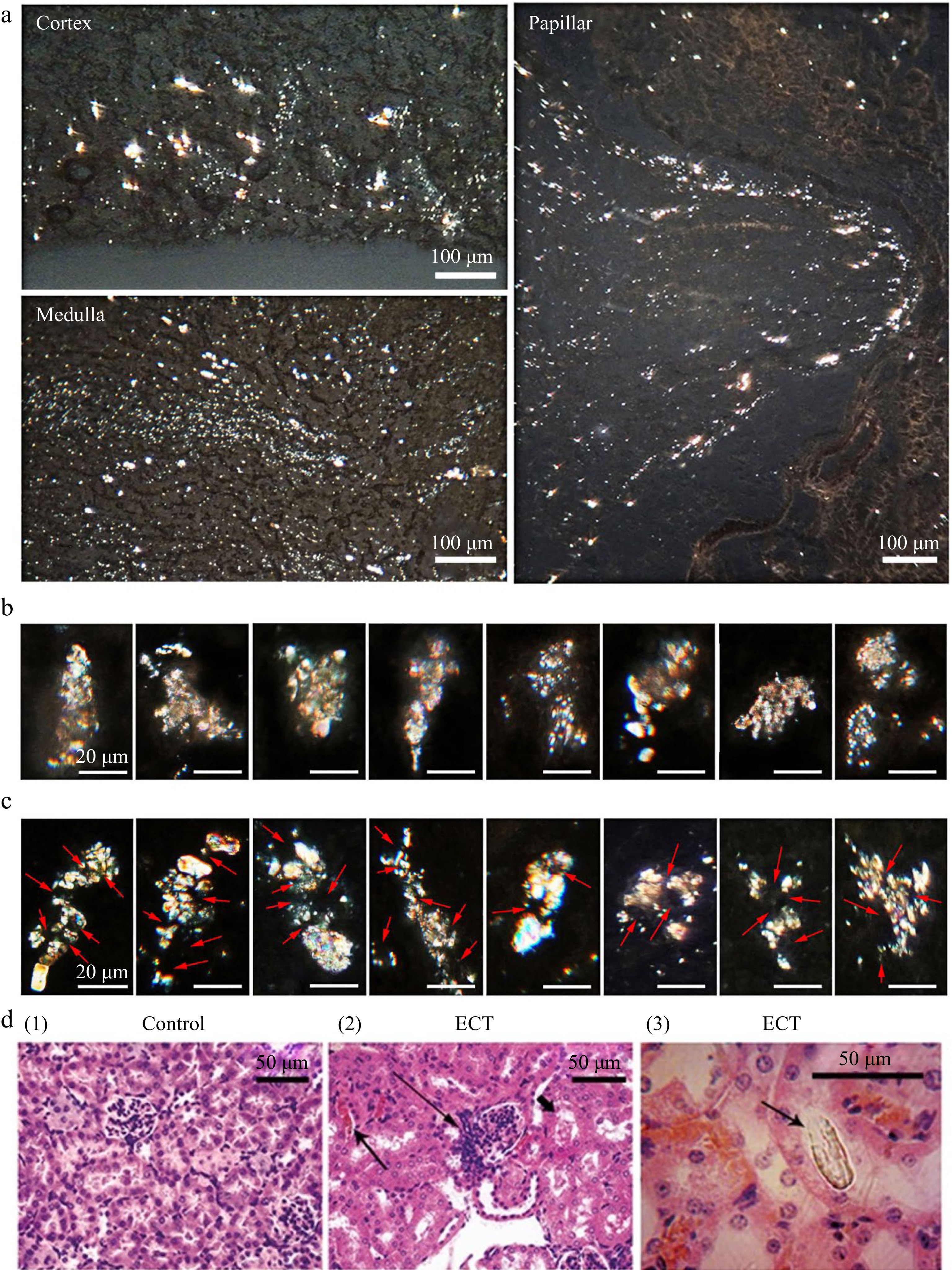
Figure 2.
Frozen kidney sections of ECT-mice observed under polarized microscope and HE section of kidney. (a) Crystallization in kidney observed under polarized microscope after the mice gavaged with ECT for 3 d. (b) Filter cake shaped crystal grains (microstones) in kidneys of ECT-mice. (c) Multiple nuclei (arrow) crystal grains (microstones) in kidneys of ECT-mice. Each of the groups contained 12 mice. (d) HE stained kidney sections of (1) the control and (2) ECT-mice observed under optical microscope; prominent hyperplasia of the juxtaglomerular apparatus (long arrow), eosinophilic casts (arrow) and marked vascular stasis within the interstitial capillary lumina (short arrow); (3) the HE section observed under polarized microscope, arrow indicates a distinct crystal.
In ECT-mice, silt stream like crystalline deposits were observed in the cortex, medulla and papilla (Fig. 2a). Crystalline grains (usually 20−50 μm wide and 50−150 μm long) consisting of adhesion crystalline particles were seen at various sites of the kidney, especially at the renal papilla. Generally there were two types of crystal grains, namely, the filter cake shaped grain (small crystals being held in filter meshes, Fig. 2b), and the multiple nuclei grain (small crystals being adhered on some amorphous nuclei, Fig. 2c).
Paraffin embedding and HE staining
-
The HE staining kidney sections of the ECT-mice showed prominent hyperplasia of the juxtaglomerular apparatus (long arrows), eosinophilic casts (arrows) and obvious vascular stasis within the interstitial capillary lumina (short arrows). Inflammation is seen around the tubules with crystalline deposits in distal tubule or collecting ducts in kidneys of mice gavaged with ECT as shown in Fig. 2d (2).
Compared to the numerous crystalline deposits observed in frozen kidney sections of ECT-mice, only a few big crystals in the HE staining sections could be seen under polarizing microscope shown in Fig. 2d (3). The small crystals and crystalline grains could be lost entirely during HE staining.
Micro-stones observed in the kidney
-
Some micro-stones (wide > 100 μm, long > 250 μm) were clearly seen in kidney of ECT-mice under polarizing microscope, which were opaque under transmitted light, and were white under reflected light in a standard optical microscope (Fig. 3a).
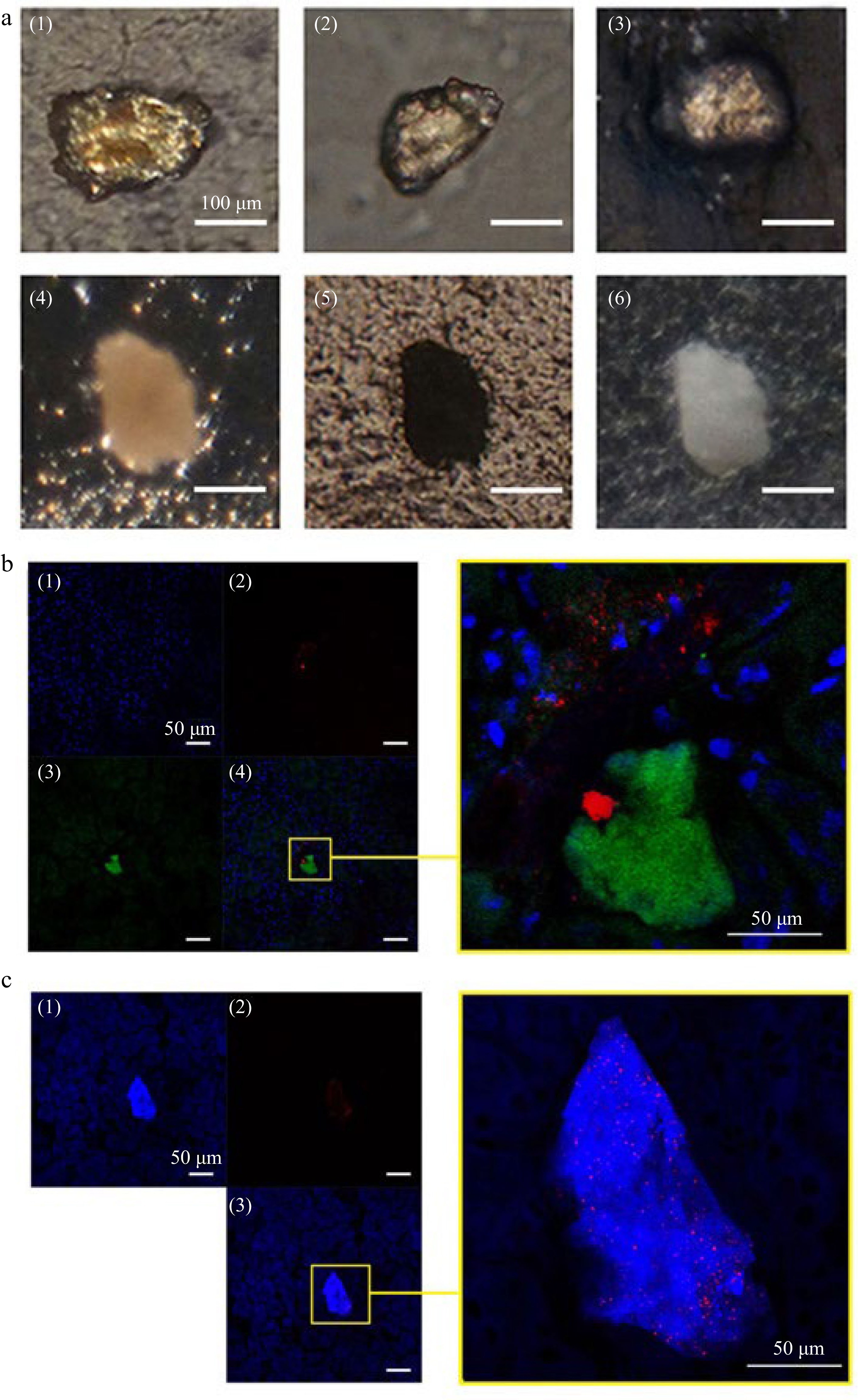
Figure 3.
Stone like grains and CLSM observation on AT or CH deposits and crystal distribution in kidney of mice gavaged with ECT. (a) Stone like crystalline grains in kidney of mice gavaged with ECT 3 times (1)−(4) under polarized microscope; (5) under transmitted light microscope; (6) under reflected light microscope. (b) (1) Cell nuclei (blue) were stained with DAPI and (2) AT (green) was immunolocalized with antibody against the tannins and visualized with FITC-phalloidin; (3) crystals (red) was visualized by reflecting light at λ633 nm of Kr–laser; the right, a local part magnification in (4). (c) (1) Total cholesterol (blue) were stained with Filipin and (2) crystals (red) were visualized by reflecting the light at λ633 nm of Kr–laser; the right, a local part magnification in (3).
Tannins and CH deposits in kidney
-
By detection of immunofluorescence with antibodies against apple tannins, remarkable tannin deposits could be observed as green fluorescence in kidney of ECT-mice (Fig. 3b). CH precipitations in kidney were visualized by the Filipin-fluorescence (blue) detection. As shown in Fig. 3c, remarkable cholesterol deposits were observed in kidney sections of ECT-mice.
By using the Kr-laser's red light at a wavelength of 633 nm to reflect off certain crystals, the locations of crystal deposits in the kidney could be visualized (Fig. 3b & c). We found the crystals were either adhered to tannin deposits (Fig. 3b) or co-deposited with CH (Fig. 3c). These results were consistent with the observation under a polarized microscope.
The results coming from the mouse model strongly suggest that the synergistic action of cholesterol with tannins is a crucial factor in the cause of urinary stones.
-
Unlike other hereditary or well-defined acquired diseases, diet plays a critical role in the formation of KS. The development of urinary stones may be influenced by food, according to various epidemiologic and metabolic research[26−28]. In general, the development of KS and its recurrence may be prevented by consuming less animal protein, lots of fluids, fruits, and green leafy vegetables with low oxalate concentration[12]. In addition, the best beneficial diet for KS patients appeared to be a well-balanced vegetarian diet that included dairy products[29]. Notably, there was still controversy about whether vitamin D consumption increased the occurrence of KS[30]. The majority of previous studies have focused on characteristics of various renal stones and the macro components involved, and almost ignored the role of those trace chemicals in the stones. Indeed, cholesterol is well documented as a major component of gallstones[19]. In this study, it was initially found that cholesterol was one of the key factors related to KS formation in mice (Fig. 1a & b). This finding further supports the view that obesity and being overweight were unfavorable factors for KS formation[31], and many researchers have demonstrated that obesity is positively related to over intake of fat[32].
Based on molecular structure, fruit tannins are classified as condensed tannins or hydrolyzable tannins[33]. We found that gavage with cholesterol plus apple condensed tannins could cause distinct crystal formation in mouse kidneys (Fig. 1c), which could be owing to the synergistic action of cholesterol with tannins (SACT). This view was proved by immunocytochemical localization of the tannin deposition in kidney (Fig. 3b), as well as chemical and enzyme-chemical localizations of cholesterol deposition in urinary tract (Fig. 3c).
The SACT can be critical once there is one or more other risk factors of stones, just as the extreme effects of SACT on renal stone formation observed in mice gavaged with ECT as shown in Figs 2 & 3.
The formation of KS involves complex interactions between multiple factors, including genetic predisposition, dietary habits, and physiological metabolism. Cholesterol and tannins are two substances that have been implicated in the development of KS. Exploring the processes involving cholesterol and tannins from both chemical and physiological perspectives contributes to an overall understanding of KS formation. In terms of chemistry, oversaturation of substances including calcium, oxalate, and phosphate in the urine can cause crystals to form and develop, which can start the process of KS formation. Cholesterol can indirectly promote the KS formation through adding the concentration of bile acids, which can increase the solubility of calcium oxalate crystals. Tannins, a group of polyphenolic compounds found in various plant-based foods, can interact with proteins and other macromolecules, leading to the formation of tannin-protein complexes. These complexes can potentially act as nucleation sites for crystal growth, thus contributing to KS formation. From a physiological standpoint, KS formation involves alterations in protein and mRNA expression in the kidneys. Elevated levels of certain proteins (osteopontin and matrix Gla protein) are linked to an increased risk of KS, as they can promote crystal retention and aggregation. Furthermore, expression changes of specific genes related to calcium and oxalate homeostasis can also contribute to the development of KS. In summary, the link between cholesterol, tannins and KS formation may be understood by examining the chemical processes of stone formation and changes in protein and mRNA expression in the kidney. A more detailed understanding of these mechanisms may help to identify potential therapeutic targets and preventive strategies for KS formation. Mechanisms implicated in the SACT inducing KS may have several routes (Fig. 4). Firstly, the SACT-deposits may block the renal tubules to a certain extent, which then can reduce the urine flow and enhance water reabsorption, whereas those solutes with low solubility, such as oxalate, calcium phosphate, cysteine, urine acid, etc. will be concentrated and supersaturated, as shown in Fig. 4b. Secondly, the SACT-deposits may act as 'a filter cake' and aggregate with the filter residues, such as crystalline and other deposits (Fig. 2b, Fig. 4c). Thirdly, the SACT-deposits may also act as nuclei of heterogeneous nucleation with some other crystalline (Fig. 2c, Fig. 4d).
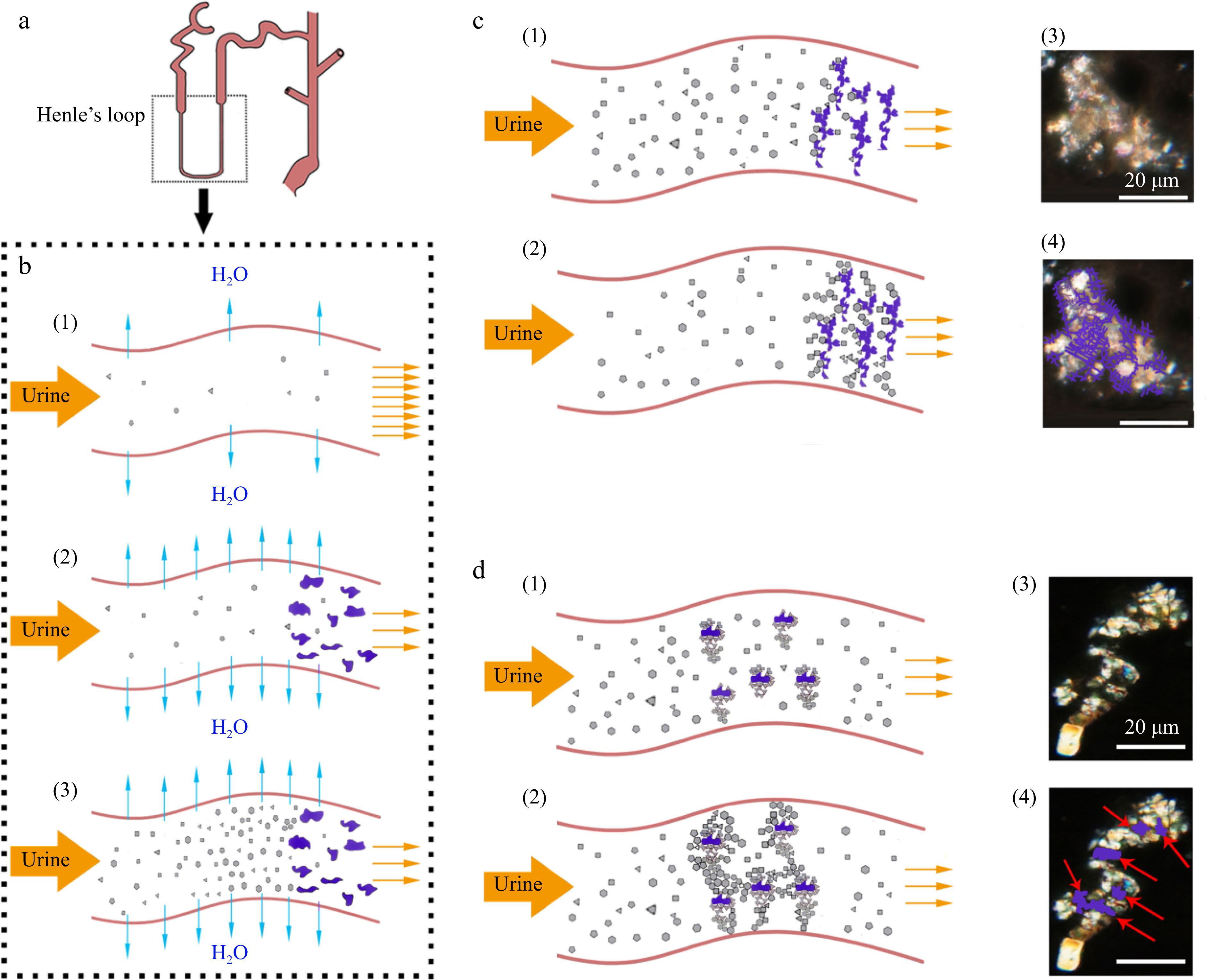
Figure 4.
Proposed model of synergistic action of cholesterol with tannins (SACT) causing formation of renal stones. (a) Diagrammatic structure of the renal tube. (b) (1) Urine normal flow in the renal tube; (2) cholesterol with tannins co-precipitating in the renal tube; (3) supersaturation of urine solutes caused by SACT. (c) (1), (2) SACT caused formation of filter cake shaped crystal grains (microstone) in the renal tube; (3), (4) similar case observed in kidney sections of ECT-mice under polarized microscope, purple lines indicating the amorphous deposits. (d) (1), (2) SACT caused formation of multiple nuclei crystal grains in the renal tube; (3), (4) multiple nuclei crystal grains observed in kidney sections of ECT-mice under polarized microscope, red arrows indicating the amorphous nuclei (the purple loci).
It is believed that the high incidence of renal stones during summer is usually attributed to dehydration then may cause concentration of salts in the kidneys. A complementary view supported by our study was that the high consumption of fruits and fat could add to the risk of KS, which was consistent with the fact that KS dramatically rose in the summer and fall[34], because most of the fruits rich in condensed tannins were usually consumed in these seasons. Meanwhile, the increase in the incidence of KS was also in parallel with the increase in consumption of fresh fruits with a year-round supply by residents both in developing and developed countries.
It has been reported that intake of fruit could assist in lowering the chance of developing KS as most fruits are abundant in citric acid[35], which is not contradictory with our notion that the high risk of KS is specified to intake of fruit rich in condensed tannins, such as not fully ripened apple and banana, along with fatty meat simultaneously.
A high cholesterol diet is also associated with high protein and high salt intake, which are also the contributing factors for KS formation[36]. Cholesterol plus fruit tannins also showed other aspects of nephrotoxicity in mice, such as remarkable increases in urine proteins as well as atrophic glomeruli and eosinophilic casts (Fig. 3d), even without forming the stones, which may give a clue to the studies on other renal diseases.
The clinic significance of this study, based our findings and our primary study on the intervention of KS, is that the prevalence of KS can be greatly reduced by diet management to avoid simultaneous intakes of fat with food rich in condensed tannins.
In conclusion, experiments with mice showed that cholesterol and tannins may co-precipitate in renal tubules, solute supersaturation in kidney urine in renal tubules, formations of crystals/stones in cortex renal pyramid, and renal pelvis. These demonstrated that interaction of cholesterol and tannins was vital for the formation of various KS. The renewed theory of KS formation should help to design a new strategy for more effective prevention of various urine stones.
Compliance with ethical standards
-
Ethical approval: The study was approved by the Animal Ethics Review Committee of the Supervision, Inspection, and Testing Center of Genetically Modified Organisms (Beijing, China).
Informed consent: Due to retrospective nature of study, waiver of informed consent was obtained by the Animal Ethics Review Committee.
This work was supported by the National Natural Science Foundation of China (No.32172270).
-
The authors declare that they have no conflict of interest.
-
These authors contributed equally: Yu Xi, Xiangquan Zeng, Yijing Pu
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Xi Y, Zeng X, Pu Y, Li X, Che H, et al. 2023. The synergistic effect of dietary cholesterol with fruit tannins in forming kidney stones. Food Innovation and Advances 2(2):163−170 doi: 10.48130/FIA-2023-0019
The synergistic effect of dietary cholesterol with fruit tannins in forming kidney stones
- Received: 14 March 2023
- Accepted: 22 May 2023
- Published online: 20 June 2023
Abstract: Prevalence of kidney stones has increased continously over several decades worldwide, the major causes of which are largely unknown. To explore the dietary causes of kidney stones, and reveal mechanisms underlying dietary risk factors inducing kidney stones, animal experiments using mice as the disease model were performed. Eight-week old male CD-1 mice were treated by ethylene glycol, cholesterol or/and apple tannins for 3 d, respectively. In the present study, the crystalline analysis in urine and kidney tissues, HE staining kidney sections as well as observation of micro-stones, tannins and cholesterol deposition in kidneys of mice in different groups were conducted. We found that gavage with ethylene glycol, cholesterol and tannins resulted in mice urine solute supersaturation in renal tubules and forming kidney stones. Significant cholesterol and tannin deposits in mouse kidney were observed by laser confocal microscopy and crystals were shown either adhered with or co-deposited with cholesterol and tannin deposits. The primary crystals were found in renal cortex, medullar, especially papilla in the kidney sections under polarized microscope. These findings demonstrate that interaction of cholesterol and tannins in kidney plays a critical role in the formation of kidney stones.
-
Key words:
- Polyphenol /
- Condensed tannins /
- Cholesterol /
- Kidney stones /
- Synergistic effect.












