-
The utilization rate of nitrogen (N) by plants is one of the important limiting factors for plant yield. Glutamine, which is primarily produced from assimilated nitrogen and plays a central role in the nitrogen cycle, can be transformed into asparagine (Asn) by aspargine synthase (AS) or into glutamate under GOGAT action[1,2]. Glutamine synthase (GS) is the rate-limiting enzyme in nitrogen assimilation, as it is the first enzyme in the assimilation of inorganic nitrogen to organic nitrogen[3,4].
Tea [Camellia sinensis (L.) O. Kuntze], an important commercial crop, with young shoots and leaves as the main picked parts. In the case of N deficiency, the chlorophyll content in new shoots reduce and photosynthesis weakens, resulting in a decrease of tea yield; in the case of sufficient N, plants grow vigorously and buds germinate faster, resulting in the number of tip pickings and tea production increase. Therefore, the effects of temperature, different nitrogen sources, and shading methods on nitrogen utilization in tea plants were studied[5−9]. For GS, apart from the fundamental role in N assimilation of tea plants, the products come from the GS-GOGAT pathway can also serve as precursors for some N-containing organic molecules, like free amino acids, pigments and so on. Therefore, GS is essential for primary metabolism, secondary metabolism and quality in tea plant[10,11].
MicroRNA (miRNA) is a non-coding small RNA molecule of about 20−24 bases synthesized by the organism itself. In recent years, miRNAs have been shown to act as key regulators of development, growth, and stress tolerance in plants. MiRNAs, such as miR156s, miR159s, miR160s, miR162s, miR166s, miR167s, miR169s, miR171s, miR172s, miR319s, miR393s, miR394s, miR396s, miR397s, miR398s, miR399s and miR408s have been reported to regulate plant growth under N deficiency[12,13]. MiR396 was one of the most conserved miRNA families in plants[14]. Studies have shown that miR396 could control grain size and yield by modulating the development of auxiliary branches and spikelets or brassinosteroid-induced genes[15−17], miR396-OsGRFs module could offer resistance to Magnaporthe oryzae and brown planthopper[18,19]. However, research on functions of miR396 in tea plant are limited and not yet clear[20].
In our previous study, CsmiR396d showed lower expression in young tissues and higher expression in mature tissues, which was opposite to the trend of ʟ-theanine content. Degradome data analysis indicated CsGS2 as the target gene of CsmiR396d[21], suggesting that CsmiR396d may play a role in the metabolism of tea plant glutamine. Therefore, this study aimed to verify the targeting relationship between them by using 5'RLM-RACE and tobacco transient expression. Then the role of CsmiR396d and CsGS2 in glutamine metabolism were explored through Arabidopsis thaliana stable overexpression and the transient expression system of tea leaves. This study may provide reference for N utilization and provide a theoretical basis for quality improvement of tea plant.
-
In October 2018, from the tea plantation of Huazhong Agricultural University (Wuhan, China), we collected seven-year-old tea cultivar 'Fuding Dabaicha' (FDDBC) including bud (B), first leaf (FL), mature leaf (ML), old leaf (OL), lateral root (LR), flower (F), and seed (S). Nicotiana benthamiana and Arabidopsis thaliana were cultured in a 3:1 ratio of soil to vermiculite. Tea branches used for the transient transformation system were cultured in Hoagland's liquid nutrient solution. All materials were collected with three biological replicates, frozen immediately in liquid nitrogen, and stored in -80°C freezers before further use. Glutamine and L-theanine standard were purchased from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) for miR396d and CsGS2
-
The high-throughput sequencing data were validated by using qRT-PCR to quantify the expression levels of CsmiR396d and CsGS2 in seven tissues. The total RNA of each sample was isolated by using EASYspin plant RNA kit as directed of the manufacturer (Aidlab, Beijing, China). After the quality and purity of RNA were checked, the reverse transcription reactions of the CsGS2 and CsmiR396d were carried out using the First-strand cDNA synthesis kit(Simgen, Hangzhou, China). The specific stem-loop RT primers of CsmiR396d was designed base on the mature sequence, U6 and GAPDH were reference genes for CsmiR396d and CsGS2, respectively (Supplemental Table S1). PCR procedure followed a previous article[22].
5'RLM-RACE
-
To validate the cleavage sites of CsmiR396d in CsGS2, which was identified by the degradome data, a modified 5′RLM-RACE was performed according to a previous study[22]. Briefly, T4 RNA ligase to ligate the 5′RACE-adapter and 1,000 ng total RNA then reverse-transcribed to cDNA. The cleavage sites of CsGS2 were verified by two rounds of PCR with primers CsGS2-out and CsGS2-in, respectively (Supplemental Table S1). Next, the purified PCR products were cloned into the pTOPO-TA vector (Aidlab, Beijing, China), followed by transforming these vectors into DH5α competent cells (AngYuBio Shanghai, China) and sequencing. Finally, based on the sequencing results, the cleavage sites were mapped.
MiRNA cleavage assay by transient expression in tobacco leaves
-
The cleavage effect of CsmiR396d on CsGS2 was verified by the tobacco transient expression system[23,24]. Briefly, PCR was used to amplify the sequence containing the CsmiR396d precursor (101 bp) from the DNA of 'FDDBC' to obtain the MIR gene, and then this gene was homologously recombined into the pBI121 vector which was double digested by XbaI and SacI. Meanwhile, two complementary target sites (21 nt) of CsGS2 were synthesized, and after annealing the two oligonucleotide DNAs, the double-stranded DNA was inserted into the pMS4 vector which was double digested by XhoI and XbaI. In order to ensure the experimental rigor and exclude other interferences, a CsmiRN91 over-expression vector unable to complement with CsGS2 and a pMS4-mimic target site (21 nt) unable to complement with CsmiR396d were constructed as negative controls.
Transient transformation in tea plant leaves
-
A previously constructed transient transformation system of tea leaves was used for CsmiR396d silencing, CsmiR396d over-expression, CsGS2 silencing, and CsGS2 over-expression[22]. The roles of CsmiR396d and CsGS2 in tea plant glutamine metabolism were analyzed by measuring the expression levels of CsmiR396d, CsGS2, CsTSI (CSS0049154.1), CsGS1.1 (CSS0037306.1), CsGS1.2 (CSS0015313.1), and CsMYB73 (CSS0029616.1) in these treatment groups (primers are shown in Supplemental Table S1).
Stable transformation in Arabidopsis
-
The sequence containing the CsmiR396d precursor (101bp) and the open reading frame (ORF) of CsGS2 were obtained from the DNA and cDNA of 'FDDBC', respectively. Then they were inserted into the pBinRed3 vector which was digested with XbaI and HindIII. Agrobacterium (GV3101, AngYuBio) harboring pBinRed3-CsmiR396d and pBinRed3-CsGS2 were used for genetic transformation of Arabidopsis Col-0 using the floral dip method[25,26].
The positive transformants of CsmiR396d and CsGS2 were identified by fluorescence observation and PCR. Briefly, under an ultraviolet lamp (LUYOR-3410GR), the transgenic seeds with red fluorescence were screened out, then sown in nutrient soil with the standard of six plants every four lines. Two weeks later, the leaves were harvested, and DNA was isolated as instructed in the Simgen's Plant DNA Kit (3201050). The forward primer used for PCR identification was a segment of 5'-CGTCTTCAAAGCAAGTGGATT-3' on the 35S promoter, and the reverse primers of CsmiR396d and CsGS2 were the reverse primer of CsMIR396d gene cloning primer and CsGS2 cloning primer, respectively.
In order to analyze the roles of CsmiR396d and CsGS2 in the glutamine metabolism of Arabidopsis over-expression lines, we used TAIR database to obtain five glutamine metabolism-related genes: AtGS2 (AT5G35630.1), AtGLN1;1 (AT5G37600.1), AtGLN1;3 (AT3G17820.1), AtGLN1;4 (AT5G16570.1), and AtGLN1;5 (AT1G48470.1). Finally, the expression levels of these five genes in CsmiR396d and CsGS2 Arabidopsis over-expression lines were analyzed.
Determination of glutamine and L-theanine contents
-
The extraction of glutamine and L-theanine from Arabidopsis thaliana leaves and tea leaves followed a previous article[21]. The isolation and determination were performed as previously reported with slight modifications on a C18 reversed-phase column (Agilent 250 mm × 4.6 mm × 5 μm) using a triple quadrupole LC/MS QUANTIS (Thermo Fisher, TSQ Altis/TSQ Quantis)[27]. Mobile phases A and B were pure water and acetonitrile, respectively, with an injection volume of 10 μL, and a flow rate of 0.30 mL/min. The mass spectrometer worked in a positive ion mode and at 210 nm detection wavelength. The elution program was: phase B increased linearly from 1% to 10% between 0 and 5 min, linearly up to 50% in 7 min, up to 80% in 1 min, held at 80% in 8-13.5 min, down to 1% in 14 min and held for 6 min. The glutamine and ʟ-theanine contents in the samples were qualitatively determined according to the retention time and ion characteristics of the standard. The relative quantification of the samples was carried out according to the peak area and the sample data in each treatment.
Statistical analysis
-
Difference among means within different treatments were analyzed using the software of SPSS 25.0 (SPSS. Inc, USA). The data were checked for normality first and then analyzed of variance (ANOVAs). GraphPad Prism 8.0.2 were used for column chart figures, and Photoshop CS6 was used for other figures.
-
In our previous degradome sequencing data, CsGS2 was one of the target genes of CsmiR396d. We used qRT-PCR to examine the expression levels of CsmiR396d and CsGS2 in seven tissues of tea plant. CsmiR396d was seen to vary greatly in its expression in the seven tissues, expression level increased with the maturity of leaves, highest in old leaves and flowers, significantly decreased in lateral roots, and almost no expression in seed. This expression trend is consistent with sRNA sequencing data (BioProject: PRJNA680379). Meanwhile, the expression of CsGS2 followed an opposite trend, consistent with the expression signature of miRNAs and target genes (Fig. 1a).
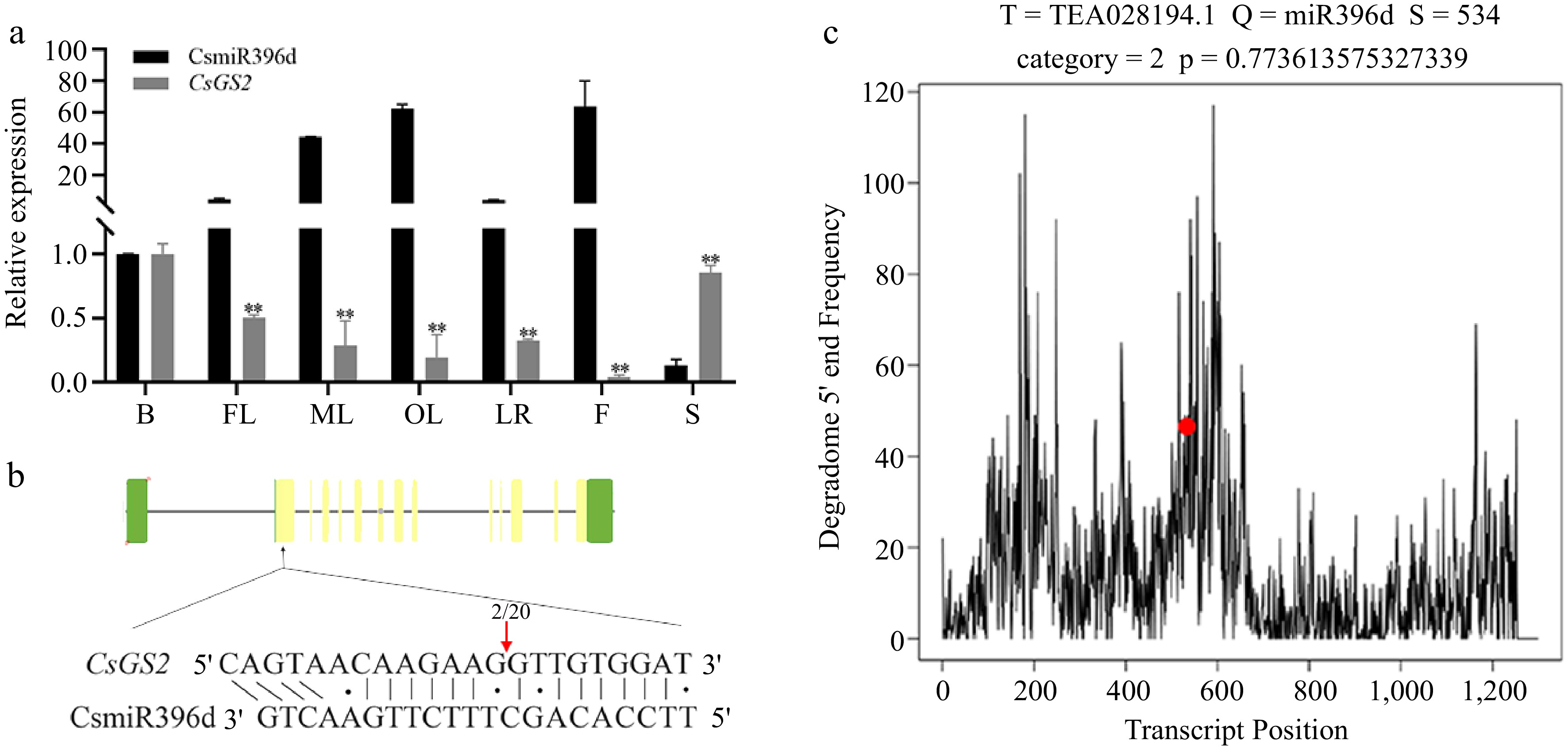
Figure 1.
Expression and relationship of CsmiR396d and CsGS2 of tea plant. (a) Expression of CsmiR396d and CsGS2 in seven tissues. B, bud; FL, first leaf; ML, mature leaf; OL, old leaf; LR, lateral root; F, flower; S, seed. (b) 5'RLM-RACE experiment. The frequency of clone corresponding to the cleavage site (arrow) is shown as a fraction, with the number of clones matching the target message in the denominator. (c) T-plot of degradome sequencing.
Verification of interaction mode between CsmiR396d and CsGS2 in tea plant
-
The splicing of CsmiR396d on CsGS2 in tea plant was verified by 5′RLM-RACE. Compared with the (Coding sequence) CDS of CsGS2, the degradation fragment obtained by two rounds of PCR amplification using specific primers showed that CsmiR396d cleaved CsGS2 from the 13th position of the 5 'end (Fig. 1b, c).
Verification of interaction mode between CsmiR396d and CsGS2 in tobacco leaves
-
To validate the cleavage of CsGS2 at the target sites by CsmiR396d in vitro, the 21 nt target sites sequences were inserted before a green fluorescent protein (GFP) reporter in pMS4 vector, recombined the precursor sequence of CsmiR396d with the pBI121 vector, followed by co-transferring them into tobacco leaves and observing the fluorescence signal changes in GFP (Fig. 2a, b). In the transient experiment on tobacco leaves, when mixing the Agrobacterium solution of CsmiR396d and CsGS2, the green fluorescence signal on tobacco leaves was weakening. By contrast, no matter in CsGS2 and CsmiRN91 or in mimic and CsmiR396d bacterial solution, the green fluorescence signal on tobacco leaves was stronger. These results showed that CsmiR396d can degrade CsGS2 by binding to its target site, thereby inhibiting the expression of GFP gene (Fig. 2c, d).
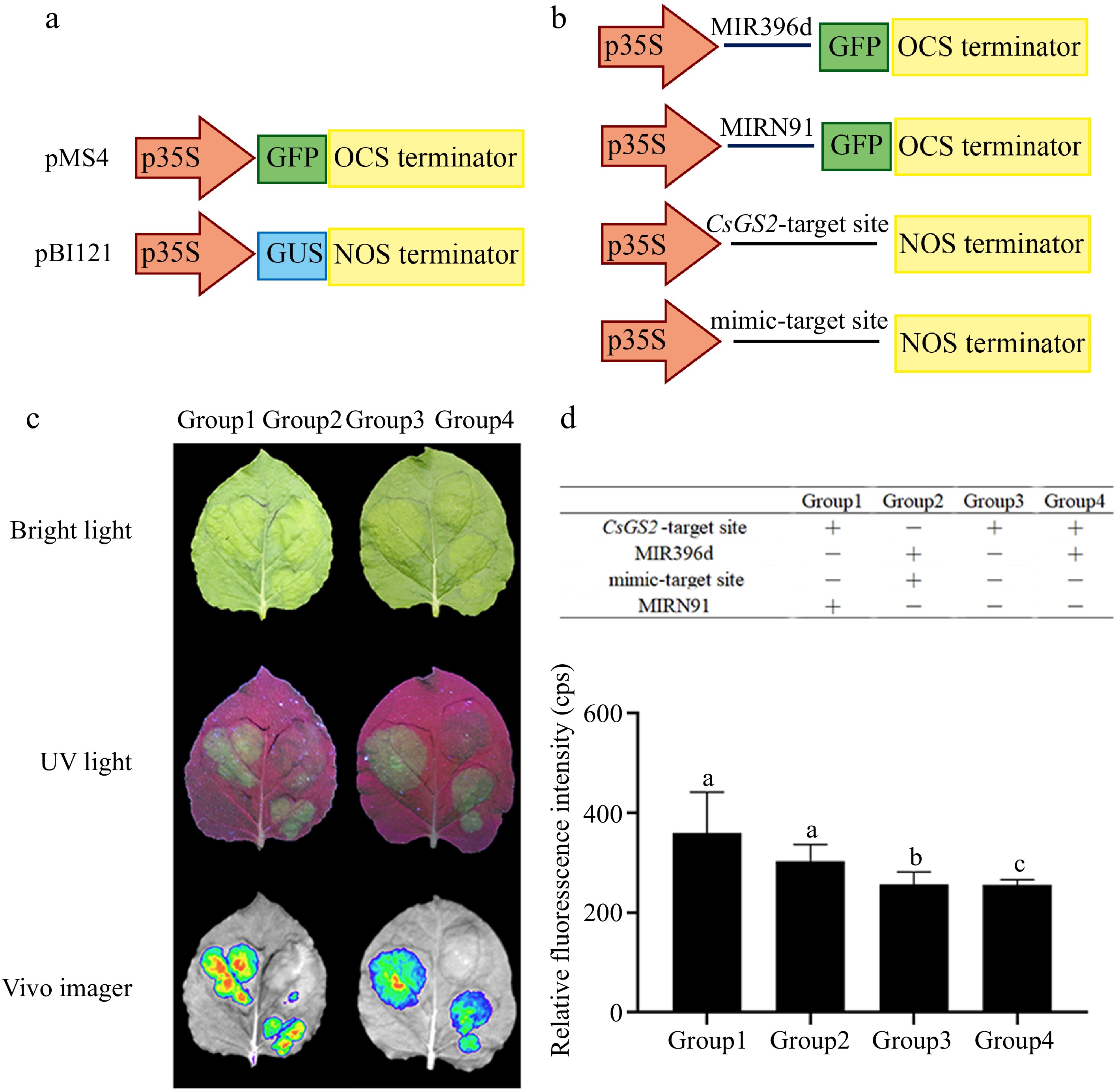
Figure 2.
Interaction between CsGS2 and CsmiR396d verified by tobacco transient system. (a), (b) Schematic of co-transformation vector construction. (c) The GFP fluorescence signals of different combinations under bright light, UV and vivo imager. (d) Relative fluorescence intensities in different combinations.
Determination of gene expression pattern and glutamine content in tea plant leaves
-
In order to understand the roles of CsmiR396d and CsGS2 in glutamine metabolism in tea plants, CsmiR396d and CsGS2 were silenced and over-expressed in tea leaves by asODNs injection and Agrobacterium infection, respectively.
In CsmiR396 gene-silenced tea leaves, compared with the control, CsGS2 showed a significant increase (p < 0.01), coupled with an increase in glutamine content. But there were no significant difference (p > 0.05) between CsGS1.1 and CsGS1.2. In the CsmiR396 over-expressed leaves, when compared with the control, CsGS2 was significantly down-regulated in expression, CsGS1.1 showed no significant difference in expression, and CsGS1.2 was significantly higher in expression (p < 0.05). Whether in CsmiR396d gene silenced or over-expressed leaves, the expression of CsMYB73 showed an obvious increase (p < 0.05), in contrast to a decrease in the expression level of CsTSI (p < 0.05) relative to the control. Meanwhile, the content of ʟ-theanine is consistent with the expression of CsmiR396d (Fig. 3).
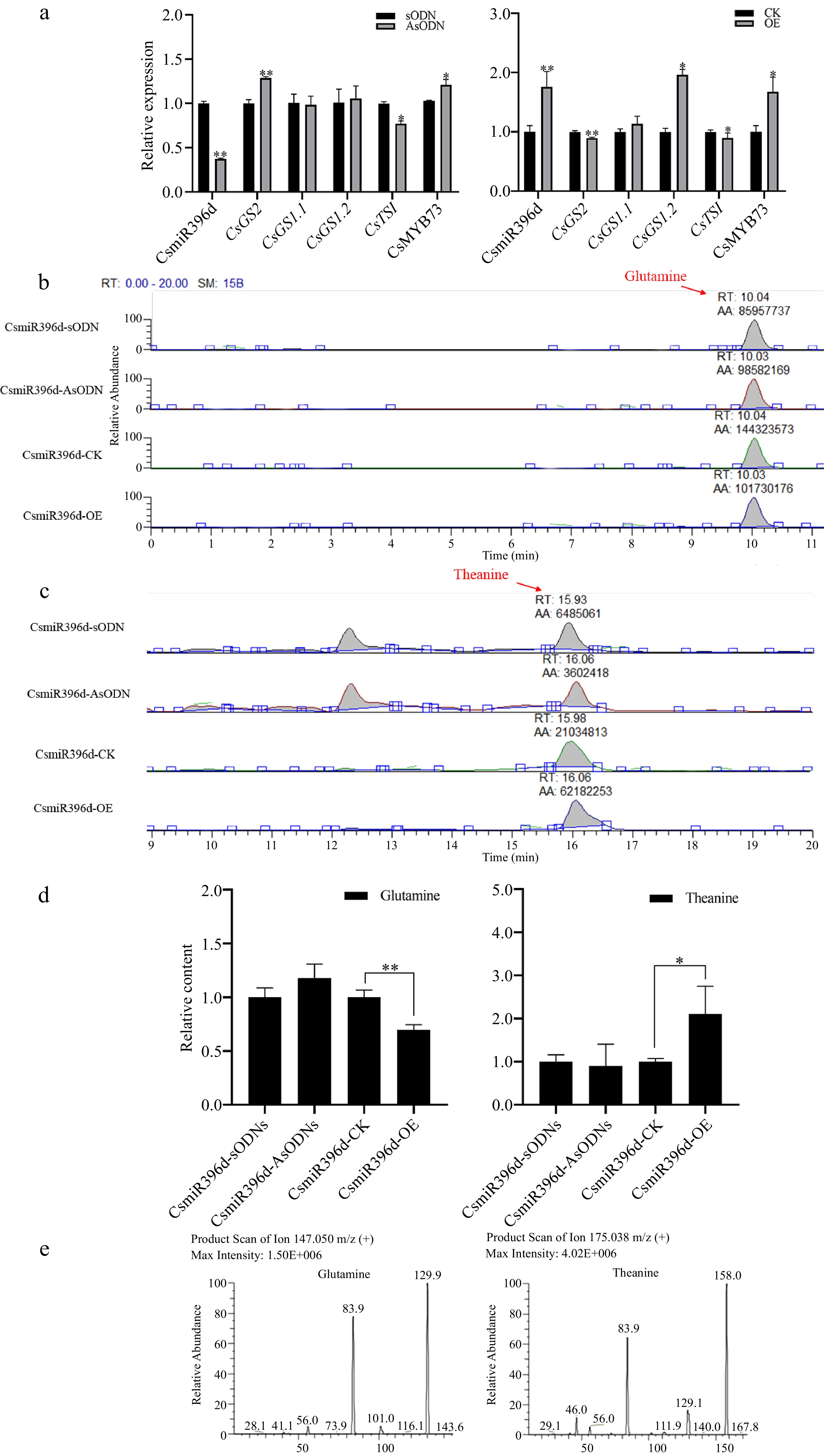
Figure 3.
The role of CsmiR396d in glutamine metabolism in tea plant. (a) Changes of glutamine metabolism-related genes in CsmiR396d silenced and over-expressed leaves. (b)−(d) HPLC analysis of glutamine and ʟ-theanine content in CsmiR396d silencing and over-expression leaves. (e) Mass spectra of glutamine (MS/MS of m/z 147) and theanine (MS/MS of m/z 175). *, ** indicate significant difference at p < 0.05 and p < 0.01, respectively.
Compared with the control, in CsGS2 gene-silenced tea leaves, the expression of CsmiR396d was seen to increase significantly (p < 0.01), CsGS1.1, showed a slight but not significant increase, and the expressions of CsGS1.2 (p < 0.05), CsTSI (p < 0.01) and CSMYB73 (p < 0.05) were significantly down-regulated. In CsGS2over-expressed leaves, CsmiR396d showed no significant change in the expression level (p > 0.05), but the glutamine content in leaves increased significantly (p < 0.01). CsTSI showed a downtrend inexpression, while ʟ-theanine content increased in both CsGS2-silenced and over-expressed leaves (p < 0.05) (Fig. 4).
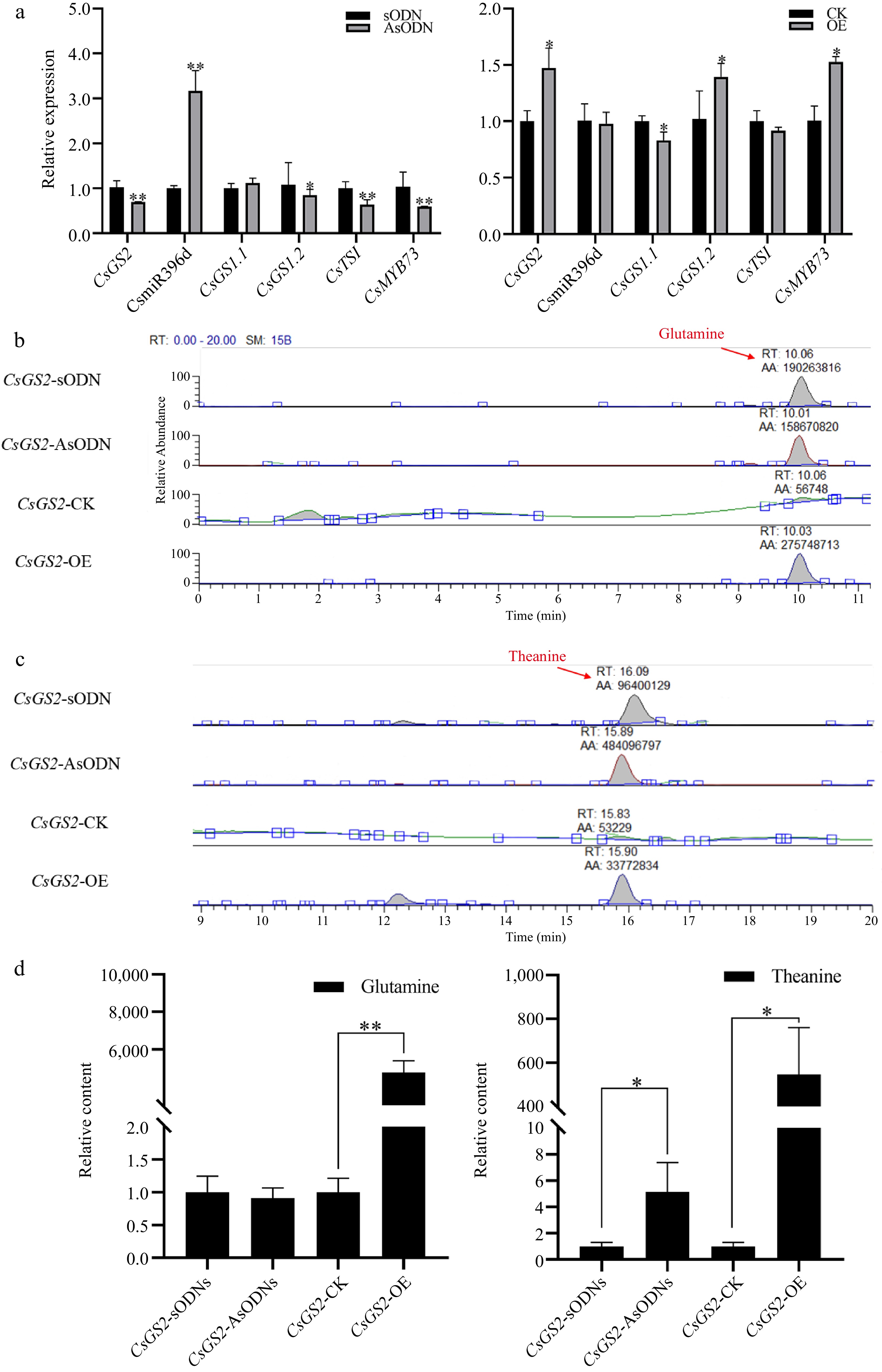
Figure 4.
The role of CsGS2 in glutamine metabolism in tea plant. (a) Changes of glutamine metabolism-related genes in CsGS2 silenced and over-expressed leaves. (b)−(d) HPLC analysis of glutamine and ʟ-theanine content in CsGS2 silenced and over-expressed leaves. *, ** indicate significant difference at p < 0.05 and p < 0.01, respectively.
Determination of gene expression pattern and glutamine content in Arabidopsis Csmi396d and CsGS2 over-expressed lines
-
Compared with WT Arabidopsis, the expression of GS2 in CsmiR396d over-expressed plants were significantly decreased (p < 0.01), the transcript levels of AtGS2, AtGLN1;1, AtGLN1;3 and glutamine content were remarkably decreased in transgenic Arabidopsis lines harboring the CsmiR396d gene (Fig. 5).
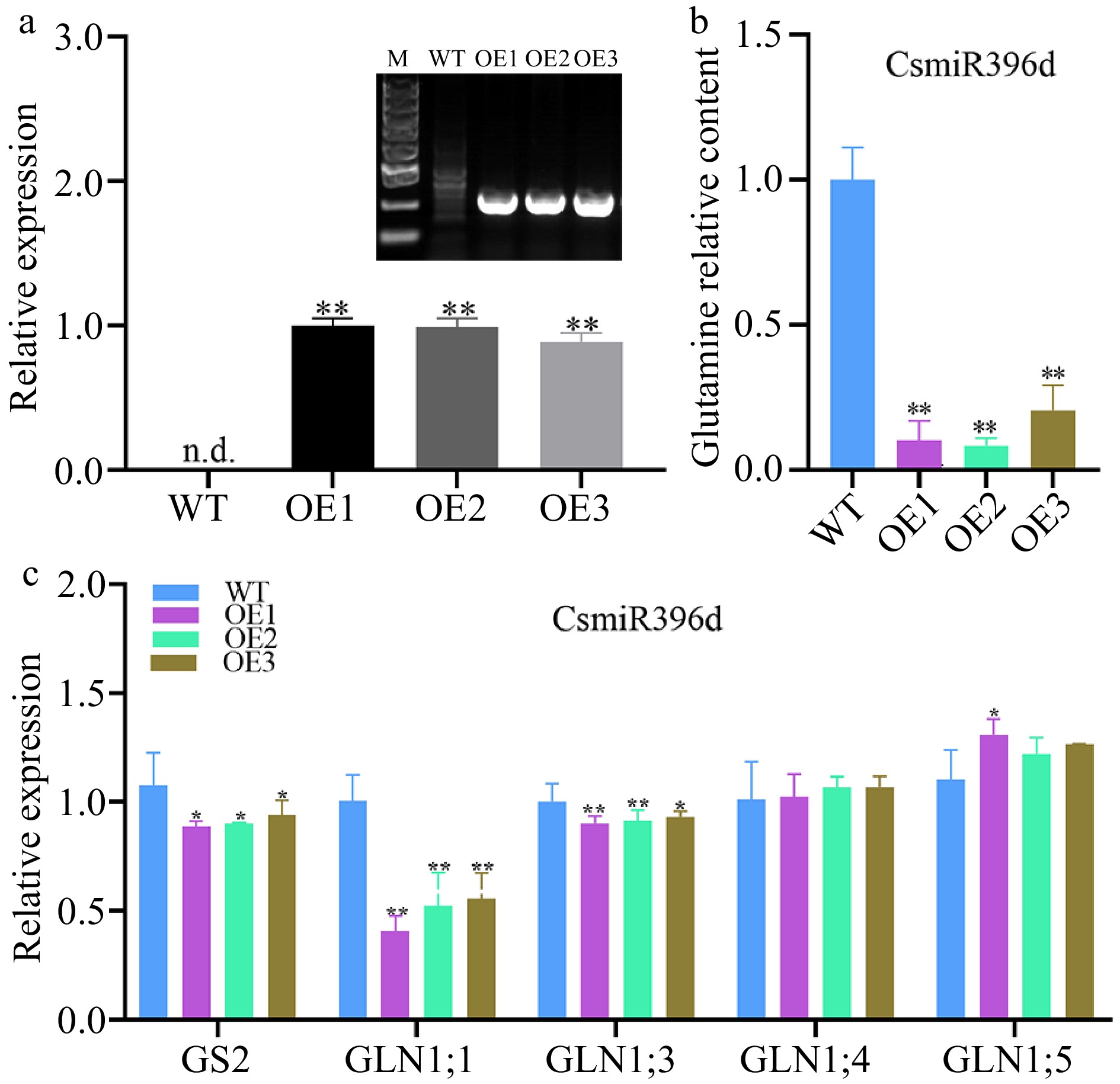
Figure 5.
The role of CsmiR396d in glutamine metabolism in Arabidopsis. (a) Positive identification in WT and CsmiR396d over-expressed lines. (b) Glutamine content in WT and CsmiR396d over-expressed lines. (c) Expression levels of glutamine metabolism related genes in WT and CsmiR396d over-expressed lines. *, ** indicate significant difference at p < 0.05 and p < 0.01, respectively.
Different from the CsmiR396d over expression line, the transcript levels of AtGS2, AtGLN1;4 were remarkably increased in transgenic Arabidopsis lines harboring the CsGS2 gene. Compared with WT Arabidopsis, the expression of GS2 and the content of glutamine in CsGS2 over-expressed plants were significantly increased (p < 0.01) (Fig. 6).
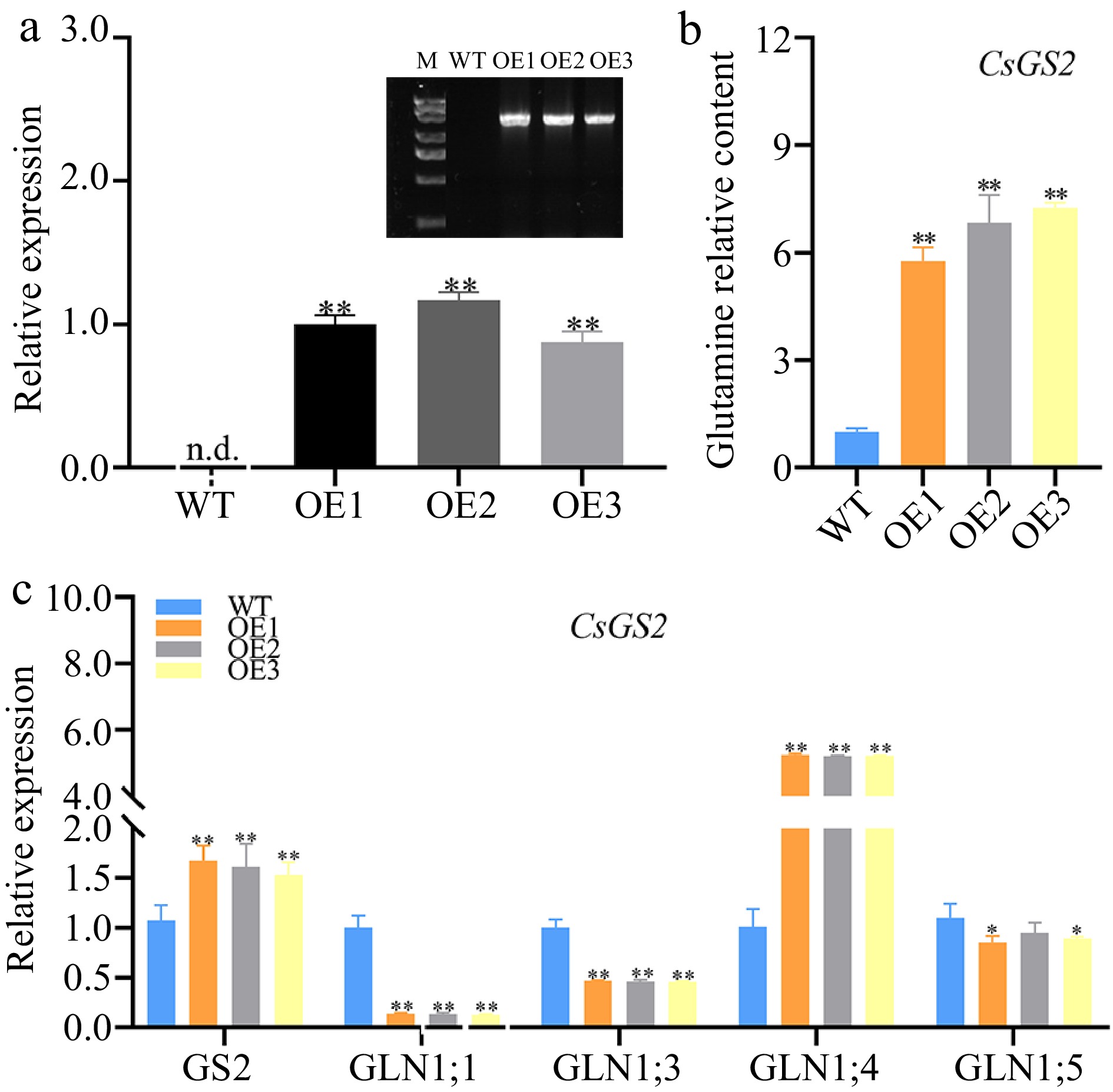
Figure 6.
The role of CsGS2 in glutamine metabolism in Arabidopsis. (a) Positive identification in WT and CsGS2 over-expressed lines. (b) Glutamine content in WT and CsGS2 over-expressed lines. (c) Expression levels of glutamine metabolism related genes in WT and CsGS2 over-expressed lines. *, ** indicate significant difference at p < 0.05 and p < 0.01, respectively.
-
MiRNAs has wide impacts on many aspects of the plant development, and the most of them are highly conserved. MiR396 is relatively conserved and its conserved targets belong to the growth regulating factor (GRF) family. Studies have shown that miR396s-GRF module plays an important effect in various stages of plant growth and development[28]. For example, OsGRF binds the promoters of OsJMJ706 and OsCR4, OsGRF7 through binds the ACRGDA motif in the promoter of aP450 gene and ARF12, playing a role in floret development and plant architecture, respectively[29,30]. In addition to GRFs, miR396s also regulates other targets. In Brassicaceae and Cleomaceae families, miR396s play a role in leaf margin and vein patterning by controlling the transcription factor bHLH74[31]. In Helianthus annuus, miR396s and HaWRKY6 are involved in high-temperature protection[32]. These results suggest that miR396s might acquire some new target genes in the evolution. In plant studies, most of the target genes of miR396 are transcription factors, and to our knowledge, no report is available about structural genes directly regulated by miR396 and affecting glutamine content in tea plant.
In this study, we combined qRT-PCR and tobacco transient expression with 5′RLM-RACE to confirm the interaction between CsmiR396d and CsGS2. In most plants, GS2 activity was higher in leaves, which was basically consistent with the expression levels of CsGS2 we measured in seven tissues. CsDOF has been reported to directly bind AAAG motif in the CsGS2 promoter, promote its activity, and increase glutamine accumulation in tea plant[33]. This is consistent with our analysis of the CsGS2 over-expressed line, but opposite to the case in the CsmiR396d over-expressed line, indicating that the glutamine content is related to the regulation of CsGS2 by CsmiR396d in tea plant. In CsGS2 over-expressed leaves, the expression level of CsmiR396d showed no significant change, in contrast to a significant increase in the glutamine content of leaves. This suggests that under normal conditions, the expression levels of CsmiR396d and CsGS2 are relatively fixed in tea leaves. When the expression of CsGS2 decreases as a target gene, the consumption of CsmiR396d in leaves also decreases, leading to an increase in its expression level; when the expression of CsGS2 increases, the total amount of CsmiR396d in leaves remains unchanged, and its expression level is not significantly different from the control.
The expression level of CsGS1.1 and CsGS2 in tea plant leaves showed an opposite trend, in contrast to a consistent expression pattern of CsGS1.2 and CsGS2 in tea leaves. A possible explanation is that CsGS2 is located in the chloroplast, and CsGS1.1, CsGS1.2 and CsTS1 are located in the cytoplasm[34]. When the CsGS2 gene is silenced, the expression of CsGS1 increases to compensate for the decrease of CsGS2 and maintain the N balance in plants. Previous studies have found that CsMYB73 binds the promoter regions of CsGS1 and CsGS2 through the MYB recognition sequence, repressing the transcription of both genes[35]. Therefore, in CsmiR396d gene-silenced tea leaves, due to the increased expression of CsGS2, the plant maintained a normal growth state by up-regulating the expression of CsMYB73. However, when the expression of CsGS2 in leaves was below the normal level, the expression of CsMYB73 showed an upward trend relative to the control. In CsGS2 silenced and over-expressed tea leaves, CsMYB73 was consistent with CsGS1.2 and CsGS2 in their expression trend, but opposite to the trend of CsGS1.1, indicating that CsMYB73 may bind the promoter of CsGS1.1 and inhibit its transcription.
Theanine resembles glutamine in both structure and properties[36]. Recent studies have found that the glutamine synthase gene is highly homologous to the theanine synthase gene. CsGS can not only catalyze glutamic acid and NH3 to generate glutamine amide, but also can catalyze the synthesis of theanine[27,34]. In CsmiR396 silenced and over-expressed tea leaves, the expression of CsTSI also showed a decrease, while the L-theanine content was consistent with the expression of the CsmiR396 gene. This can be attributed to the change of CsGS2 gene, a feedback regulation of tea plant on N metabolism balance in leaves. The theanine content increased in CsmiR396d and CsGS2 silenced and over-expressed leaves. When CsGS2 was silenced and the glutamine content decreased, the tea plant maintained its own N supply by increasing L-theanine content to keep element balance. When CsGS2 was over-expressed, the L-theanine content in leaves increased sharply, possibly due to the function of theanine synthase. These results indicate that the content of L-theanine in tea leaves can also be affected by CsGS2 and regulation of CsmiR396d.
-
In this study, we conducted a vivo and vitro analysis to validation cutting of CsGS2 by miR396d. And their role in glutamine metabolism was demonstrated by silencing or overexpressing CsmiR396d and CsGS2 in tea plant leaves and Arabidopsis. Our study revealed the possible role of miRNA in regulating the important gene GS2 for nitrogen assimilation, which will provides a theoretical basis and reference for regulating nitrogen utilization in plants at the post transcriptional level.
-
The authors confirm contribution to the paper as follows: investigation: Li H, He H, Yan M, Lin Q, Qin F, Li W; conceptualization: Li H, Wang M, Wang P, Zhao H, Wang Y, Ni D, Guo F; data analysis, software, methodology, Writing - original draft: Li H; writing - review and editing: Wang M, Wang P, Zhao H, Wang Y, Ni D, Guo F; funding acquisition, project administration, resources, supervision: Guo F. All authors have read and agreed to the published version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are not publicly available due to management requests, but are available from the corresponding author on reasonable request.
This work was support by National Key Research and Development Program of China (2022YFD1200505), National Natural Science Foundation of China (32272766), and the department of Science and Technology of Hubei Province, the Program of Horticultural Crop Germplasm Resources in Hubei Province (2021DFE016). We thank Professor Feng Li (Huazhong Agricultural University) for providing the pMS4 vector used in this study.
-
The authors declare that they have no conflict of interest.
- Supplemental Table S1 Sequence information of all primers used in this experiment.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Li H, He H, Yan M, Lin Q, Qin F, et al. 2024. CsmiR396d targeting of CsGS2 plays an important role in glutamine metabolism of tea plant (Camellia sinensis). Beverage Plant Research 4: e005 doi: 10.48130/bpr-0023-0038
CsmiR396d targeting of CsGS2 plays an important role in glutamine metabolism of tea plant (Camellia sinensis)
- Received: 24 September 2023
- Revised: 06 November 2023
- Accepted: 15 November 2023
- Published online: 01 February 2024
Abstract: Nitrogen (N) is a critical element to improve tea production and quality. However, the role of miRNA in nitrogen nutrition of tea plants is still unclear. Glutamine, produced from assimilated nitrogen, plays a central role in the nitrogen cycle. In this study, 5'RNA ligase-mediated rapid-amplification of cDNA ends (5'RLM-RACE) and transient tobacco transformation experiments confirmed glutamine synthetase (CsGS2) was cleaved by CsmiR396d. Gene silencing and over-expression experiments of tea plant leaves show when CsmiR396d was over-expressed, the expression level of CsGS2 and the content of glutamine were decreased; when miR396d was silenced, the expression level of CsGS2 and the content of glutamine were increased. The results of the over-expression experiment in Arabidopsis were consistent with those in tea leaves. These results revealed the regulatory role of CsmiR396d in nitrogen nutrition of tea plant, which will provide further information and theoretical basis for tea plant utilization and quality improvement.














