-
Cocoa (Theobroma cacao L.), the raw material of chocolate, is one of the important estate commodities for many people in several producing countries in Africa, Central America, South America, and Asia, including Indonesia. In Indonesia, the majority of cocoa growers have small-sized cocoa farms, and cocoa trees are cultivated by mostly smallholder farmers. However, area cultivation gradually decreased in the last ten years, 1,421,009 Ha as of 2022, which decreased from 1,740,612 Ha as of 2013[1]. Sulawesi Island is the largest producer of cocoa beans in Indonesia, with nearly 60% production per year. Like other perennial crops, many cacao trees are damaged by old and new fungal diseases, leading to significant yield losses[2−4].
Lasiodiplodia theobromae, a member of the family Botryosphaeriaceae, is a diverse fungus and often resides in plant systems without producing disease symptoms[5]. In the plant tissue, the fungus can colonize the plant tissue as a latent pathogen following endophytic infections[6−8]. On the other hand, L. theobromae causes disease in many plants[9−18]. On cocoa, L. theobromae is a unique pathogen and is considered an important pathogen because the pathogen causes several diseases, including diebacks, stem cankers, leaf blight, and pod rot[12,19−23]. Also, the pathogen has been isolated from numerous tissues and conditions, including tissues showing symptoms of vascular streak dieback (VSD) disease[21,24]. In Indonesia, the pathogen is considered a newly emerging threat to cocoa production in Sulawesi[22]. Currently, the disease associated with the pathogen appears to be alarming. Monitoring of the Lasiodiplodia cocoa dieback disease was conducted on a cocoa farm in East Luwu, South Sulawesi, in August 2019, and the incidence of the disease was as high as 30%. In 2022 and 2023, the disease occurrences were observed in other cocoa areas in South Sulawesi, including Pinrang, Soppeng, Enrekang and Luwu Regency. Also, the disease was detected in two cocoa regencies in Southeast Sulawesi. L. theobromae is known to kill tissue on the vital organs, stems, pods, and even trunks of cocoa, causing substantial yield losses and tree mortality may occur[18−20,23]. Similar aggressivity of L. theobromae to Phytophthora palmivora on stems of cocoa was reported[25].
The impact of the disease caused by L. theobromae appears to be increasing, perhaps in association with a pressure of abiotic and biotic stresses[26]. Abiotic factors like temperature and drought are known to influence the interactions between plant hosts and L. theobromae[27−29]. Drought incidence may result from poor irrigation, high or low temperature, or unbalanced soil application of mineral salts and fertilizers[30−32]. In addition, the incidence of drought in cacao cultivation areas is related to global warming. So, climate change issues on cocoa and the tropics, in general, are becoming an increasing concern[33−40].
Drought stress could make plants more susceptible to infection by pathogens through predisposition changes in plants[5,41−43]. The family Botryosphaeriaceae members are recognized opportunistic pathogens that cause severe diseases in drought-stressed plants[43,44]. Drought stress may influence the interactions between L. theobromae and their plant hosts[5], including the interaction between L. theobromae and cocoa. In a previous study, water stress was applied to 6-month-old cocoa seedlings inoculated by L. theobromae presumably to increase the susceptibility of cocoa, but no comparison was made between watering treatment and water stress treatment[19]. Little is known about the interaction between the disease caused by Lasiodiplodia and cocoa under drought stress conditions, particularly in Sulawesi, where cocoa dieback disease caused by Lasiodiplodia has occurred. Sulawesi, the largest plantation area of cocoa in Indonesia has experienced a prolonged drought and is estimated to face a long drought session. Considering the increasing frequency of drought conditions forecasted in Indonesia[45−47] and the potential economic damage of L. theobromae as an emerging threat to cocoa sustainability in Sulawesi, this study aimed to evaluate the effect of drought stress on cocoa clone MCC 02 interaction with L. theobromae.
-
Masamba Cocoa Clone (MCC) 02 cocoa clone, also known as '45', was selected by local farmer selections. The clone originated from the North Luwu Regency of South Sulawesi Province, Indonesia, and it was invented in 2006 by two local farmers M. Nasir and H. Andi Mulyadi. In 1987, a farmer M. Nasir identified one superior cocoa tree on his farm, and then H. Andi Mulyadi propagated the cocoa tree through clonal propagation using a side grafting technique. MCC 02 is considered a high-yield clone (> 3 tons ha−1 per year), resistant to VSD and pod rot diseases, and cocoa pod borer (CPB)[48]. Also, the clone has been planted widely by farmers in Sulawesi and Indonesia. In addition, the clone has been certified and recommended by the Indonesian government to be planted[49]. Currently, MCC 02 is a favorite clone and comparatively tolerant to cocoa dieback caused by L. theobromae. The clone has been distributed to almost all provinces in Indonesia, and its population is higher than other cocoa clones.
Preparations of rootstock, scion, and grafting
-
Seeds of the clonal trees of MCC 02 were harvested for rootstock production. After the seeds were selected, the seeds were washed, removed from their placenta, soaked overnight, and treated with 1% Dithane M-45 fungicide (a.i. mancozeb 80%). Then, the seeds were placed in the germination sack. The germinated seeds were planted in poly-ethylene (PE) bags (15 cm × 22 cm) containing soil. Seedlings were placed in a nursery shade house with ultraviolet (UV) plastic as a roof and maintained with good irrigation. The temperature inside the nursery ranged from 27 to 33 °C during the daytime, and relative humidity ranged from 76% to 90%.
Five-month-old seedling rootstocks were selected for grafting. Grafting was performed in a nursery shade house. The nursery shade house is surrounded by cocoa, durian, and rambutan trees and located in the Village of Tarengge, Wotu District, East Luwu Regency, in South Sulawesi (2°33'28.3" S, 120°47'53.8" E). Meanwhile, healthy scions of MCC 02 that measured a length of ± 9 cm and a diameter of 5−9 mm and contained green-brownish to brownish buds were taken from plagiotropic branches. Also, the healthy scions were taken from mature and productive trees on the same farm. The grafting process was conducted based on the procedure of Asman et al.[50].
Preparation of the L. theobromae culture
-
The isolate of L. theobromae (CAS0321) used in this study was isolated from cocoa that was associated with dieback symptoms in South Sulawesi, Indonesia. Among four Lasiodiplodia that were obtained, the L. theobromae isolate CAS0321 was more virulent based on bioassay and pathogenicity tests in a previous study. The fungal inoculum was maintained as pure cultures in-vitro on Potato Dextrose Agar (PDA; Merck) medium at 25−28 °C in the dark.
The identity of L. theobromae isolate (CAS0321) and three other Lasiodiplodia isolates were confirmed by morphological identification and performed sequencing of the internal transcribed spacer (ITS) and the elongation factor 1-alpha (EF1α) regions after PCR amplification. PCR amplification of the ITS region and elongation factor 1-alpha (EF1α) of the template DNA of Lasiodiplodia was performed using the primers pairs ITS1-ITS4[51] and primer pairs EF1-688F and EF1-1251R described by Alves et al.[52], respectively. For polymerase chain reaction (PCR) amplification of ITS and EF1α were performed using (2×) MyTaq HS Red Mix (Bioline, BIO-25048) with the following conditions: Initial denaturation at 95 °C for 3 min (ITS) / 2 min (EF1α), then 35 cycles (ITS) / 35 cycles (EF1α) of denaturation at 95 °C for 30 s (ITS) / 30 s (EF1α), annealing at 55 °C for 15 s (ITS) / 30 s (EF1α), extension at 72 °C for 30 s (ITS) / 45 s (EF1α), and final extension at 72 °C for 4 min (ITS)/5 min (EF1α).
The PCR products were electrophoresed in a 1% TBE agarose gel. The size of the amplified PCR products was determined using a 100 bp DNA ladder. DNA sequencing through Bi-directional sequencing was conducted by 1st Base, Apical Scientific Company, Selangor, Malaysia.
Plant inoculation of L. theobromae
-
The stem inoculation was performed on each scion of the clone MCC 02 grafted on MCC 02 seedling rootstock when the grafted scion was four months old. For inoculation, the surface of the stem bark was disinfected with 70% ethanol and left to dry. A 9-mm square cut was made into the wood between two nodes. An 8-mm diameter mycelial PDA round plug was removed from the edge of actively growing cultures and placed onto the stem wounds, with the mycelium facing the cambium. The inoculated wound was wrapped with Parafilm M (Bemis) to prevent desiccation and contamination. The Parafilm was removed at the end of the experiment. Control plants were inoculated with sterile PDA agar plugs.
The air temperature was recorded daily in the greenhouse and varied between 29.8 to 33.9 °C, and relative humidity ranged from 88.8% to 99% during the daytime. After inoculation, the temperature varied from 30.2−35 °C, and relative humidity ranged from 69% to 99% from 10:00 am to 4:00 pm (daytime).
Watering treatments
-
Water content was determined by a gravimetric method of three representative samples of poly-bags with soil. Each poly-bag was weighed to obtain the poly-bag wet weight after flooding the soil and then drained overnight through gravitational drainage. Then, the soil was allowed to dry in an oven at 100 °C for 24 h and then weighed to obtain the poly-bag's dry weight. Soil water content was determined using the following formula;
$ Soil\; water\; content\; \left(\text{%}\right)=\dfrac{(WW-DW)}{DW}\times100 $ where, WW, wet soil weight; DW, dry soil weight
During the experiment, the sets composed of poly-bag, plant and soil were weighed daily using digital scales to monitor the required weight. Correction in weight difference on subsequent days was conducted by adding water to maintain until reaching the desired field capacity (FC). The period of water stress was 30 d.
Drought treatments
-
Graft-propagated cocoa plants were maintained under greenhouse conditions before drought imposition. The greenhouse was located in the area of the Faculty of Agriculture, Hasanuddin University, city of Makassar, South Sulawesi (5°07'53.3" S, 119°29'05.8" E). There are two levels of water treatments, namely, water-stressed (WS), corresponding to 25% field capacity (FC), and well-watered (WW), corresponding to 80% FC. 25% FC was selected for water stress treatment because the level was considered moderate water stress, while below 25% FC was considered too dry and severe, which may impair the plants and thus influence the study. Also, there are two kinds of inoculum treatments (fungus and control). Then, there are two different experiments (timings of inoculation), namely:
1. Inoculation of L. theobromae was applied to plants simultaneously with the initiation of water stress. The experiment was designed with the following treatments:
a. Water-stressed (WS) - 25% FC + L. theobromae
b. Well-watered (WW) - 80% FC + L. theobromae
c. Water-stressed (WS) - 25% FC + Control
d. Well-watered (WW) - 80% FC + Control
2. Inoculation of L. theobromae was applied to plants seven days after water stress commenced. The experiment was designed with the following treatments:
a. Water-stressed (WS) - 25% FC + L. theobromae
b. Well-watered (WW) - 80% FC + L. theobromae
c. Water-stressed (WS) - 25% FC + Control
d. Well-watered (WW) - 80% FC + Control
The trial was arranged as a completely randomized design, and the experiment combinations were repeated once. Each treatment was repeated with four replications per treatment combination. Also, each treatment consisted of six plants per replication, providing a total of 192 plants for all treatment combinations.
Evaluation of infection
-
The disease development was evaluated by its severity weekly. In addition, the scion was cross-sectioned at the end of the experiment (2 months after inoculation). The dark brown to black vascular streaking area was measured with a digital calliper. The disease severity was measured every week by developing severity scores:
The severity of the disease is determined by scoring symptoms in individual seedlings as follows: 0 (nil) = No visible symptoms; 1 (low) = percentage of chlorotic/necrotic: below 50%, branch remain alive; 2 (moderate) = percentage of chlorotic/necrotic: above 50%, branch remain alive; 3 (moderately-high) = percentage of branch dieback: below 50%; 4 (very high) = percentage of branch dieback: above 50%−80%; 5 (very high) = percentage of branch dieback: 80%−100%.
Mean disease severity was calculated using the formula[53]:
$ \mathit{I}=\dfrac{\sum_{ }^{ }\left(\mathit{n}\times\mathit{v}\right)}{\mathit{Z}\times\mathit{N}}\times100 $ where n represents the number of infected plants on each score; v is a score on each infestation category; Z is the highest score; and N represents the total number of plants observed.
The disease progression was evaluated by the area under the disease progress curve (AUDPC) that was generally calculated from the initial scoring to the last as the total area under the graph of disease severity against time. Specifically, in this study, the AUDPC value was determined according to the dieback severity estimates corresponding to the disease ratings[54,55]:
$ AUDPC={\sum _{i=1}^{n-1}}\dfrac{{y}_{i}+{y}_{i+1}}{2}\times ({t}_{i+1}-{t}_{i}) $ where, yi is an assessment of a disease percentage at the i-th observation, ti is time at the i-th observation, and n is the total number of observations.
Re-isolation
-
Lasiodiplodia was reisolated from four plants per treatment at the end of the experiment. Lasiodiplodia from symptomatic inoculated stems were re-identified using morphological colony characteristics. Re-isolation was conducted from treated and untreated plants onto a PDA medium. Seedling stems were surface sterilized with NaOCl solution (5%) for 3 min and rinsed in sterilized water three times. Approximately 3–5 mm diameter pieces of stems were placed on a PDA medium supplemented with chloramphenicol and incubated at room temperature in the dark.
Data statistical analysis
-
Analysis of differences in dieback severity, scion survival, and vascular streaking progression on the two types of experiment was conducted by one-way analysis of variance (ANOVA). Factorial or two-way ANOVA was used to determine the effects of every single factor (water treatment and inoculum type) and their interaction. When significant differences were detected, means were separated by Tukey's test at the level of significance (p < 0.05) or 5% probability level. The normality data was checked by the skewness and kurtosis test.
-
The plants inoculated with L. theobromae under two distinct watering regime treatments as summarized in Figs 1−3 and Tables 1−3, respectively, showed a marked difference in disease severity between well-watered and water-stressed treatments. During the whole period of the evaluation (seven to 56 d after inoculation), the mean disease incidence under the water stress treatment was significantly different. The external symptoms, such as dieback, chlorotic, and necrotic, were visible 7 d after inoculation (Fig. 5a−g) and then increased gradually. After that, no other symptoms appeared until the end of the observation in both experiments (Fig. 6a−g). Meanwhile, the plants treated with a PDA agar plug (healthy control) in both watering regime treatments remain symptomless regardless of water stress (Fig. 5h & i).
In the experiment where the plants were inoculated with L. theobromae simultaneously to water stress imposition (Fig. 1), inoculation treatment with water stress induced the highest incidence of dieback (40.8%), followed by inoculation treatment with well-watered (15.8%) by the time of the first observation (7 d after inoculation). These two treatments are significantly different. Also, the inoculation treatment under water-stressed condition leads to significantly higher disease incidents than that in the control (no inoculation), either under water-stressed condition (0%) or under well-watered condition (0%). However, there was no significant difference between the inoculation treatment and the control (no inoculation) under well-watered condition. Meanwhile, at the second evaluation (14 d after inoculation), the mean percentage of dieback increased (42.5%) on the plot with inoculation treatment accompanied by water stress, and there was no more increase in dieback incidence until the end of the evaluation. In contrast, the dieback incidence on the plot with inoculation treatment with well-watering increased at the fourth evaluation (28 d after inoculation) (19.2%). Then, the dieback severity was constant until the end of the evaluation. For the controls, the plants remained healthy until the end of the experiment. Similarly, the AUDPC values indicate that inoculation treatment under water stress caused the highest degree of dieback. Moreover, the effect of the water stress imposition (factor 1) was not significant on dieback severity from the beginning of the evaluation (1 week after inoculation) until the end of the evaluation. Meanwhile, the effect of the inoculum type (factor 2) was significant in any evaluation event. On the other hand, the interaction (water stress imposition x inoculum type) effect was not significant, in either the percentage of dieback severity or AUDPC disease severity (Table 1).
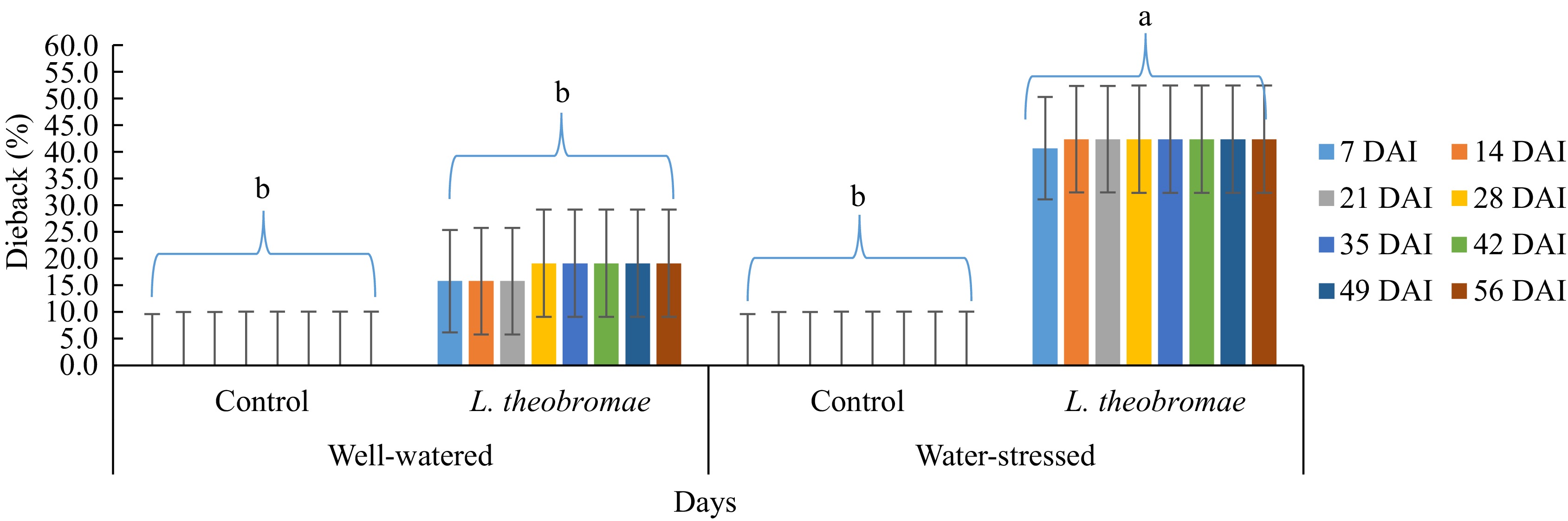
Figure 1.
Dieback severity in cocoa MCC 02 clone inoculated with L. theobromae simultaneous to water-stress imposition and with PDA as a control inoculum for 56 d. Bars with the same letter indicate no significant difference according to the Tukey's test at p ≤ 0.05. DAI: Days after inoculation.
Table 1. Two-way ANOVA of dieback severity in two different watering regimes and two different inoculum types. Cocoa MCC 02 clone inoculated with L. theobromae simultaneous to water stress imposition and with PDA as a control inoculum for 56 d and evaluated 1, 2, 3, 4, and 5 weeks after inoculation in South Sulawesi (from January 2023 to March 2023). In all treatments, dieback symptoms were first observed one week after inoculation.
Watering regime/
inoculum typeDieback severity
1 week after inoculationDieback severity
2 weeks after inoculationDieback severity
3 weeks after inoculationDieback severity
4 weeks after inoculationDieback severity
5 weeks after inoculationMean AUDPC disease severity
for 8 weeksAverages for each watering regime Well-watered 7.92% 7.92% 7.92% 9.58% 9.58% 62.92% Water-stressed 20.42% 20.42% 20.42% 21.25% 21.25% 148.33% Tukey's test 15.08% (NS) 15.08% (NS) 15.08% (NS) 13.73% (NS) 13.73% (NS) 98.85% (NS) Averages for each inoculation time Control 0.00 0.00 0.00 0.00 0.00 0.00 L. theobromae 28.33% 28.33% 28.33% 30.83% 30.83% 211.25% Tukey's test 15.08% (**) 15.08% (**) 15.08% (**) 13.73% (**) 13.73% (**) 98.85% (**) Analysis of variance (p-value) Watering regime (W) NS NS NS NS NS NS Inoculum type (I) ** ** ** ** ** ** W × I NS NS NS NS NS NS ** and NS indicate statistical significance at p < 0.01, 0.05, and not significant by Tukey's test analysis (p < 0.05), respectively. Mean disease severity as described on Materials and methods section. In the experiment where the plants were inoculated with L. theobromae seven days after water stress imposition (Fig. 2), at the beginning of the evaluation, the highest severity of dieback was recorded at the inoculation treatment under water stress (35.0%). The value was significantly higher than those in the plot of the inoculation treatment with well-watering (5.0%) and both controls (water-stressed or well-watered) (0%). Similar to the experiment where the plants were inoculated simultaneously to water stress imposition, the mean percentage of dieback on the plot with inoculation treatment with water stress increased (38.3%) in the second observation. In addition, the severity increased on the plot with inoculation treatment with well-watering as well (6.7%). However, they were significantly different. The dieback severity under both water-stressed and well-watered conditions remained steady after the second evaluation until the end of the experiment. In addition, the plants in the control groups, both well-watered and water-stressed, remained symptomless (0%) until the end of the evaluation. Likewise, the AUDPC value of the plot with inoculation treatment with water stress was significantly high. Furthermore, there is an effect of the water stress imposition (factor 1) on dieback severity where the influence was highly significant from the beginning of the evaluation (1 week after inoculation) until the end of the evaluation. Similarly, the inoculum type (factor 2) and interaction (water-stress imposition × inoculation time) effects were highly significant in any evaluation event, either a percentage of dieback severity or AUDPC disease severity (Table 2).
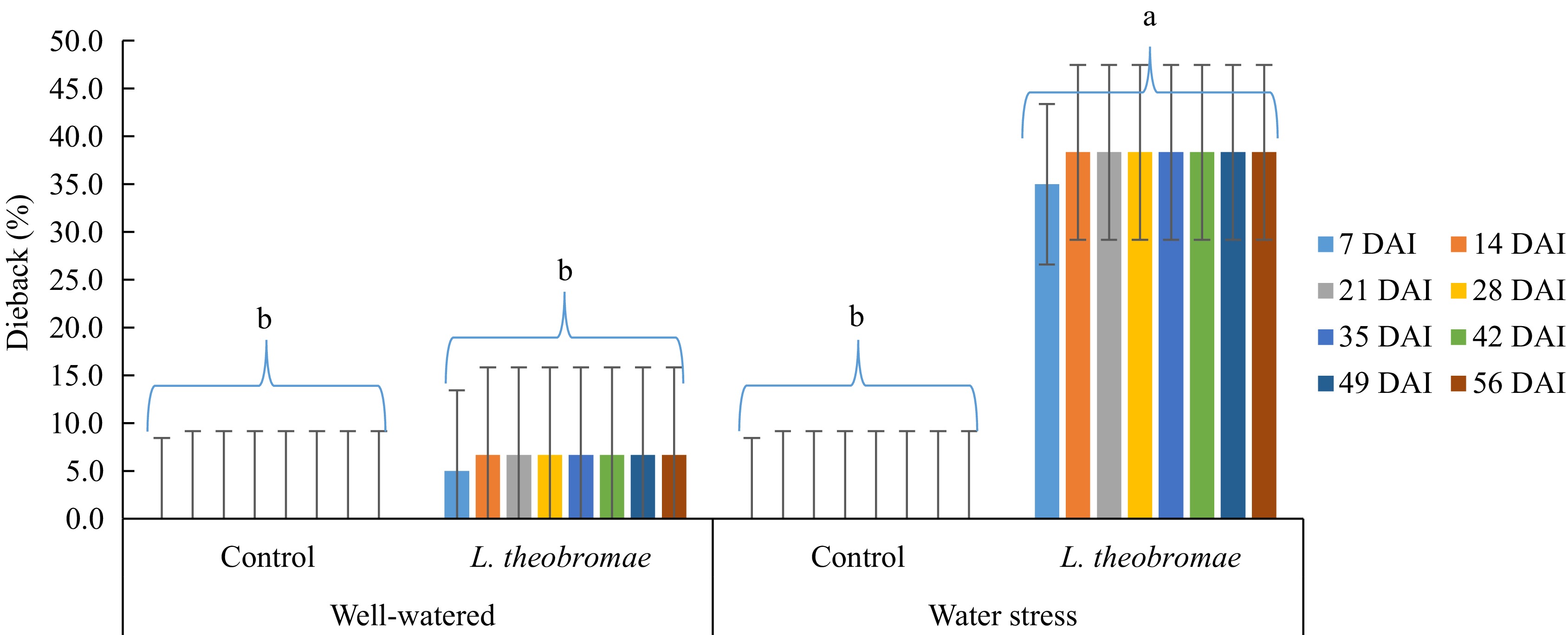
Figure 2.
Dieback severity in MCC 02 cocoa clone inoculated with L. theobromae 7 d after the initiation of water-stress imposition and with PDA as a control inoculum for 56 d. Bars with the same letter indicate no significant difference according to the Tukey's test at p ≤ 0.05. DAI: Days after inoculation.
Table 2. Two-way ANOVA of dieback severity in two different watering regimes and two different inoculum types. Cocoa MCC 02 clone inoculated with L. theobromae 7 d after the initiation of water-stress imposition and with PDA as a control inoculum for 56 d and evaluated 1, 2, 3, 4, and 5 weeks after inoculation in South Sulawesi (from January 2023 to March 2023). In all treatments, dieback symptoms were first observed one week after inoculation.
Watering regime/
inoculum typeDieback severity
1 week after inoculationDieback severity
2 weeks after inoculationDieback severity
3 weeks after inoculationDieback severity
4 weeks after inoculationDieback severity
5 weeks after inoculationMean AUDPC disease severity for 8 weeks Averages for each watering regime Well-watered 2.50% 3.33% 3.33% 3.33% 3.33% 22.92% Water-stressed 17.50% 19.17% 19.17% 19.17% 19.17% 133.33% Tukey's test 8.29% (**) 7.52% (**) 7.52% (**) 7.52% (**) 7.52% (**) 52.62% (**) Averages for each inoculation time Control 0.00 0.00 0.00 0.00 0.00 0.00 L. theobromae 20.00% 22.50% 22.50% 22.50% 22.50% 156.25% Tukey's test 8.29% (**) 7.52% (**) 7.52% (**) 7.52% (**) 7.52% (**) 52.62% (**) Analysis of variance (p-value) Watering regime (W) ** ** ** ** ** ** Inoculum type (I) ** ** ** ** ** ** W × I ** ** ** ** ** ** ** indicate statistical significance at p < 0.01, 0.05 by Tukey's test analysis (p < 0.05). Mean disease severity as described on materials and methods. When AUDPC values of dieback severity were compared in two different watering regimes and two different times of inoculation of L. theobromae, the AUDPC values with the simultaneous and delayed inoculation treatments under water-stressed condition were significantly higher than those with the simultaneous and delayed inoculation treatments under well-watered condition as well as those in the controls (Fig. 3). In addition, the AUDPC value with the delayed inoculation treatment under well-watered condition was not significantly different from the control (Fig. 3). The combination of water stress imposition and inoculation time showed a variety of dieback severity. There is an effect of the water stress imposition (factor 1) on dieback severity where the influence was highly significant from the beginning of the evaluation (1 week after inoculation) until the end of the evaluation. Meanwhile, inoculation time (factor 2) and interaction (water-stress imposition × inoculation time) effects were not significant in any evaluation event, either percentage of dieback severity or AUDPC disease severity (Table 3).
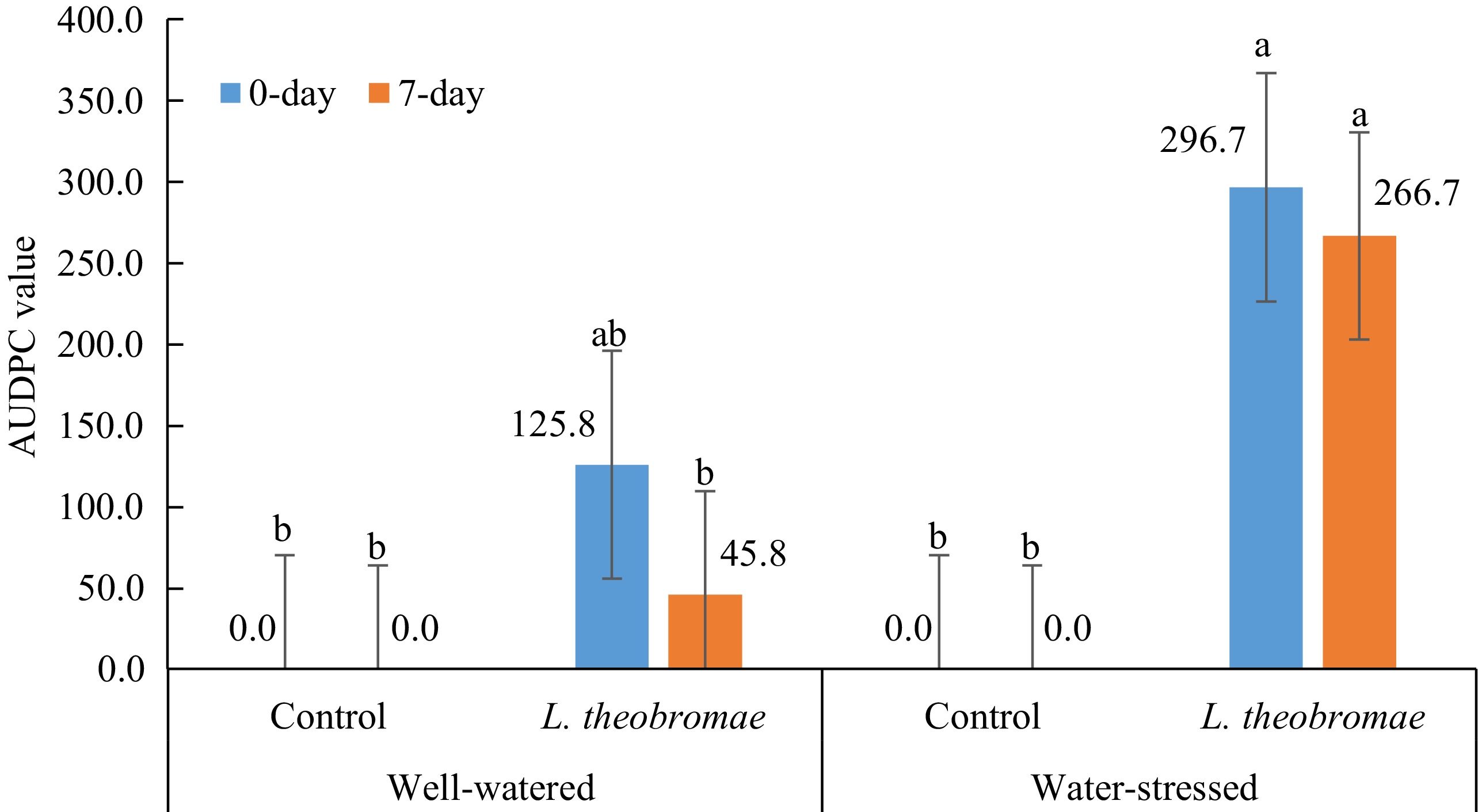
Figure 3.
Mean AUDPC values of dieback severity in two different watering regimes and two different times of inoculation of L. theobromae evaluated 1, 2, 3, 4, and 5 weeks after inoculation in South Sulawesi (from January 2023 to March 2023). In all treatments, dieback symptoms were first observed one week after inoculation. Bars with the same letter do not differ significantly according to the Tukey's test analysis (p < 0.05).
Table 3. Two-way ANOVA of dieback severity in four different watering regimes and two different times of inoculation of L. theobromae evaluated 1, 2, 3, 4, and 5 weeks after inoculation in South Sulawesi (from January 2023 to March 2023). In all treatments, dieback symptoms were first observed one week after inoculation.
Watering regime/
inoculation timeDieback severity
1 week after inoculationDieback severity
2 weeks after inoculationDieback severity
3 weeks after inoculationDieback severity
4 weeks after inoculationDieback severity
5 weeks after inoculationMean AUDPC disease severity for 8 weeks Averages for each watering regime Well-watered LT 5.21%b 5.63%b 5.63%b 6.46%b 6.46%b 42.92%b Water-stressed LT 18.96%a 20.21%a 20.21%a 20.21%a 20.21%a 140.83%a Well-watered CO 0.00b 0.00b 0.00b 0.00b 0.00b 0.00b Water-stressed CO 0.00b 0.00b 0.00b 0.00b 0.00b 0.00b Tukey's test 13.11% (**) 12.56% (**) 12.56% (**) 11.77% (**) 11.77% (**) 83.99% (**) Averages for each inoculation time 0-day 28.33% 29.17% 29.17% 30.83% 30.83% 211.25% 7-day 20.00% 22.50% 22.50% 22.50% 22.50% 156.25% Tukey's test NS NS NS NS NS NS Analysis of variance (p-value) Watering regime (W) ** ** ** ** ** ** Inoculation time (I) NS NS NS NS NS NS W × I NS NS NS NS NS NS Numbers in the same column followed by the same letter are not significantly different by Tukey's test analysis (p < 0.05). ** and NS indicate statistical significance at p < 0.01, 0.05, and not significant, respectively. Mean disease severity as described in the Materials and methods. LT: L. theobromae; CO: Control, a PDA plug. Inoculation of L. theobromae with water-stress imposition consistently resulted in higher dieback severity, regardless of the inoculation time, than inoculation of L. theobromae under well-watered condition (Figs 1−3 & Tables 1−3). In addition, black conidiomata were apparent after the branch or stem was colonized thoroughly by L. theobromae (Fig. 6h).
Survival of the scions and vascular streaking progression
-
The treatments of water stress showed significantly different rates of survival of scions after inoculation of L. theobromae and without inoculation (controls). The lowest survival rates of scions were observed by the water-stressed treatments, with simultaneous inoculation and inoculation on the seventh day after water-stress imposition at 50% and 66.7%, respectively (Fig. 4). Meanwhile, the largest survival rates of scions after inoculation were perceived by well-watering treatments with simultaneous inoculation and inoculation on the seventh day after initiation of well-watering, 95.8% and 91.7%, respectively (Fig. 4). Controls on both water stress and well-watered treatments remained healthy (100% survival rate) until the end of the experiment.
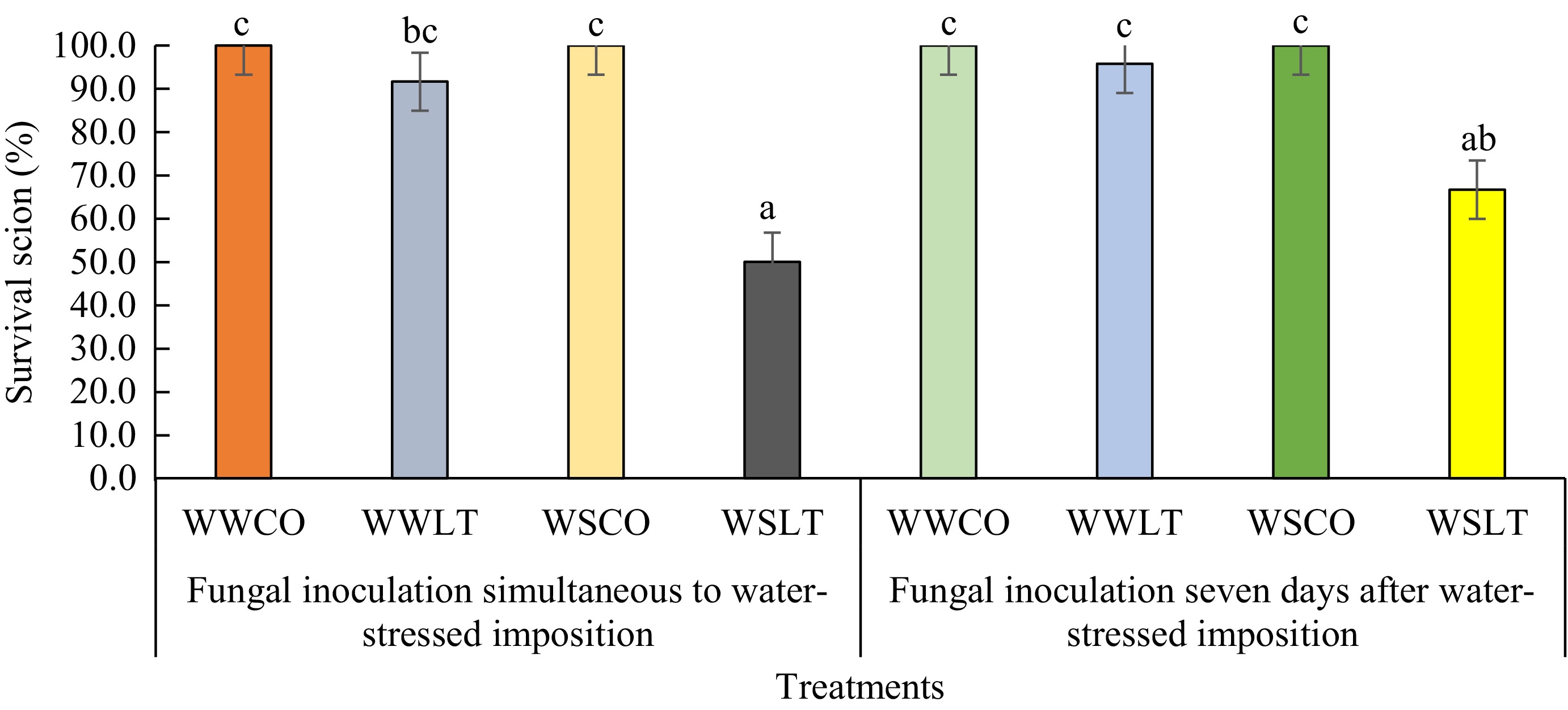
Figure 4.
Survival rates of scions inoculated by L. theobromae in two different experiments: Fungal inoculation simultaneous to water stress imposition (Experiment 1) and 7 d after the initiation of water stress imposition (Experiment 2), with PDA as a control inoculum for 64 d. Differences in letters above the bar on each treatment indicate statistically significant differences by Tukey's test analysis (p < 0.05). WWCO: Well-watered treated with a PDA plug (control); WWLT: Well-watered with L. theobromae inoculation; WSCO: Water-stressed treated with a PDA plug (control); WSLT: Water-stressed with L. theobromae inoculation.
On the surviving scions, vascular streaking was visible in the vertical sections of all the fungal-inoculated scions (Table 4, Fig. 6i−o). The vascular streaking length showed no significant difference in all fungal inoculated treatments, regardless of water stress and timing of inoculation. However, vascular streaking spread faster on the scions with water-stress treatment than on those with well-watering. Also, the percentage of colonization of the pathogen on the scions with the water-stress treatments was higher than that on the scions that were well-watered. In addition, the pathogen moved upward and downward from the inoculation site. Controls on both water stress and well-watering remained healthy in the vascular until the end of the experiment (Fig. 6p−r).
Table 4. Vascular streaking length (mm) in the stem of MCC 02 cocoa clone inoculated with Lasiodiplodia theobromae simultaneous to water-stress imposition (Experiment 1) and 7 d after the initiation of water-stressed imposition (Experiment 2) with PDA as a control inoculum for 64 d.
No. Experiment Treatment Vascular streaking (mm) Vascular streaking length compared
to scion lengthDistance of from inoculation site to edge of lesion (mm) Upward Downward 1 L. theobromae inoculation simultaneous to water-stressed imposition Well-watered Control 0.0b 0.0c 0.0b 0.0b L. theobromae 71.3a 72.6%ab 34.5a 36.8a Water-stressed Control 0.0b 0.0c 0.0b 0.0b L. theobromae 80.2a 83.7%a 40.0a 40.2a 2 L. theobromae inoculation seven days after water-stressed imposition Well-watered Control 0.0b 0.0c 0.0b 0.0b L. theobromae 71.2a 65.1%b 38.6a 32.7a Water-stressed Control 0.0b 0.0c 0.0b 0.0b L. theobromae 82.6a 87.3%a 42.4a 40.2a Tukey's test at α = 0.05 16.84 (**) 15.13% (**) 8.67 (**) 10.41 (**) Columns with the same letter do not differ significantly according to Tukey's test at α = 0.05. 
Figure 5.
Various initial symptoms on leaves of cocoa scions inoculated by L. theobromae (simultaneously or 7 d after water imposition) under well-watered and water-stressed conditions. (a), (b) dieback; (c)−(e) chlorotic and mixed of chlorotic and necrotic; (f), (g) chlorotic; (h), (i) control (a PDA plug).

Figure 6.
Various advanced symptoms on cocoa scions inoculated with L. theobromae simultaneously with water imposition and 7 d after the initiation of water imposition. (a)−(c) dieback on all branches, three, two, and one branch(es), respectively; (d)−(g) dieback on one side of the branch; (g)browning leaf at lower leaf; (h) presence of black conidiomata (red circle) on the fungal inoculated stem; (i)−(o) vertical section of fungal inoculated scion stems showed vascular streaking; (p)−(r) vertical section of control showed symptomless/no vascular streaking.
-
The current study explored the influence and the interaction of drought stress and inoculation of L. theobromae to dieback disease on T. cocoa clone MCC 02. For these purposes, we determined such responses as dieback, leaf chlorotic and necrotic, and vascular streaking of cocoa to L. theobromae infection under drought treatments through artificial inoculation on potted plants prepared from a typical cocoa clone in Sulawesi. The results indicated that water deprivation promoted disease development, regardless of the timing of inoculation during drought stress. Generally, drought situations influence plants by inducing damage to water relations and making plants more predisposed to pathogen onset and other biotic attacks. Also, the severity of the disease probably elevates with drought stress[56−58]. In addition, drought stress may exacerbate the development of the disease in the trees[43,58−62].
Although drought stress is the most abiotic factor studied on tree susceptibility to pathogens[43,44,63,64], the role of drought on cocoa susceptibility to L. theobromae in Sulawesi had remained poorly understood. To the authors' knowledge, this research is the first attempt to evaluate the interaction of drought stress and disease caused by L. theobromae in Indonesia. The present results showed the apparent destructive effect of the water stress under infection of the pathogen. Similarly, it has been known that water stress increases the susceptibility of perennial plants to L. theobromae and other Botryosphaeriaceae species[43,44,65].
Dieback and vascular streaking symptoms were clearly expressed on each inoculated plant. The results found here corroborate the pathogenicity of L. theobromae to cocoa clone MCC 02 and are in accordance with the previous study that indicates L. theobromae is one of the most aggressive and destructive phytopathogenic fungi responsible for causing such broad disease symptoms as dieback, canker, chlorotic, root and collar root and leaf blight in many plants[9−15,18,23,66−70]. In addition, the increase in disease severity in a plant exposed to water stress may predisposed by host physiology status where during water stress, accumulation and production of the certain compounds that induce disease defense were altered, including amino acids and reactive oxygen species (ROS)[65,71]. Also, the production of biochemical defense may decrease because of water stress[56,72].
Relatively few studies have directly addressed the mechanism of interaction and how L. theobromae became more severe under water stress on cocoa when most pathogens strive in opposite conditions. However, drought could make trees more susceptible to pathogens because drought decreases the availability of plant resources for defenses against plant pathogens. How drought affects pathogen survival is not clear. However, some fungal pathogens are very adaptable, and the design of fungal reproductive systems is diverse to manage fluctuating environmental conditions[73]. Moreover, a framework estimates that necrotrophs fungal pathogens, which mostly relies on nutrients from dead tree cells, accelerate drought-induced tree mortality by colonizing sapwood and damaging plant transport systems[59].
Stress duration plays an important role in host-pathogen interaction. A study conducted on stems of European white birch revealed that Botryosphaeria dothidea causes canker after exposure to water stress for a minimum of 3 d at the acceptable level[74]. Also, host-pathogen interaction was influenced by the timing of the different stresses. A study on dogwood revealed that water imposition before the inoculation of L. theobromae resulted in a greater effect on canker development than post-inoculation water stress[5].
L. theobromae can grow in wide different environmental conditions and a wide range of temperatures[75−77]. The average temperature during the experiment was relatively in the high range (29.8–33.9 °C). However, with such a temperature range, L. theobromae can grow optimally and colonize the plant aggressively[75−77]. In addition, such a temperature range seemed to increase the virulence of Botryosphaeriaceae spp.[27,77]. However, at this point, more studies are needed to evaluate the effect of temperature on the development of disease severity in cocoa.
Although this research is only tested on potted plants in a greenhouse using artificial inoculation, only performed on a single clone and cannot represent directly mature trees that are established in the field, the results of this study showed obvious pictures of the importance of drought × L. theobromae interaction-inducing disease.
The interaction of drought and L. theobromae reported here suggested that water limitation before and after fungal inoculation makes plants more susceptible to fungal pathogens and weakens plant defense against pathogen infection, indicating that water deprivation supports the effectivity of pathogen infection (predisposition). Also, this study emphasized that the influence of water deficit on plant physiological status was more prominent compared to the fungal infection.
More integrated studies are needed to follow up on the findings here and to tackle the severity of dieback diseases in cocoa under drought conditions in which more frequent and longer are predicted, the evaluation of different cocoa clones will be necessary to gain more understanding of the effect of drought stress on dieback disease development under L. theobromae infection, and evaluation of other abiotic and biotic stress factors on dieback disease development under L. theobromae infection will help comprehend the dynamics of the disease in the field.
-
This study has shown that drought stress increases the severity of the dieback disease caused by L. theobromae on cocoa clone MCC 02. It was also observed that drought stress × L. theobromae interaction increased the length of vascular streaking in the stems of cocoa. In addition, we observed external disease symptoms and vascular streaking at the interaction between well-watered × L. theobromae. This research extends our knowledge of the impact of drought on plant-pathogen interaction in cocoa and water stress should be avoided in cocoa plantations due to its detrimental impact on severity of dieback diseases.
-
The authors confirm contribution to the paper as follows: study conception and design: Asman A, Rosmana A, Iwanami T; material preparation and data collection: Asman A, Rosmana A; analysis and interpretation of results: Asman A, Iwanami T, Rosmana A; draft manuscript preparation: Asman A; review and editing: Iwanami T, Asman A, Rosmana A. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
-
The first author would like to thank Mr. Anwar (Ayye) for his assistance in providing cocoa seedlings, and Nurmala Rasyda, S.P., Mr Kamaruddin, Ardan, Ahmad, S.P., M.Si, Husnul Chatimah, S.P., Inayah Maghfirah Ramadhani, S.P., Fadia Ersya Matterru, S.P., Dian Anugrah, S.P., and Asri for the technical assistance.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Asman A, Iwanami T, Rosmana A. 2024. Effect of drought stress on dieback disease development under Lasiodiplodia theobromae infection in cocoa clone 'MCC 02'. Beverage Plant Research 4: e034 doi: 10.48130/bpr-0024-0023
Effect of drought stress on dieback disease development under Lasiodiplodia theobromae infection in cocoa clone 'MCC 02'
- Received: 04 May 2024
- Revised: 22 May 2024
- Accepted: 04 June 2024
- Published online: 10 September 2024
Abstract: Cocoa is spread over tropical countries, being extensively cultivated by mostly smallholders and processed by industries as the raw material of chocolate. Pathogens and drought are one of the biotic and abiotic factors limiting the productivity of cocoa. The main objective of this study was to evaluate the pathogenicity of Lasiodiplodia theobromae in drought-stressed and well-watered cocoa clone MCC 02. MCC 02, a popular cocoa clone in Sulawesi (Indonesia), was evaluated in the greenhouse for infection of L. theobromae under water-stressed and well-watered conditions. Dieback, leaf chlorotic and necrotic, scion survival, and vascular streaking were determined. The results indicated that the treated cocoa seedlings exposed to water stress corresponding to 25% field capacity during both inoculation of the pathogen simultaneously with the initiation of water stress or seven days after water stress commenced were more susceptible to L. theobromae than well-watered ones. However, this effect was mainly relevant when the pathogen was inoculated through a wound on the stem. Moreover, the severity of the disease on inoculation of L. theobromae simultaneously with the initiation of water stress was higher than that of the disease on inoculation seven days after water stress commenced but not significantly different. This study demonstrates the potential threat of drought stress to cocoa plants under the infestation of L. theobromae and emphasizes the significant effect of water stress in interaction with L. theobromae that should be considered in plant management, especially under the climate change scenario in Sulawesi, in which the drought will increase and last longer.
-
Key words:
- Abiotic and biotic stress /
- Cocoa /
- Lasiodiplodia /
- Sulawesi /
- Water stress.













