-
Microalgae are chlorophyll-containing, photosynthetic aquatic organisms with basic growth requirements that allow them to sustainably yield valuable bioactive compounds, such as lipids, proteins, and carbohydrates[1]. The large quantities of macronutrients present in microalgae make it a valuable potential for food and pharmaceutical applications. In addition, the chemical composition of microalgae displays the availability of a broad array of functional properties for various food applications[2]. Currently, the main microalgal species considered as potentially promising in the food industry due to the high presence of bioactive compounds includes Chlorella, Arthrospira platensis, Isochrysis galbana, Dunaliella salina, Porphyridium sp. and Haemotococcus[3].
While market opportunities for microalgae are still not comparable to traditional food commodities, the microalgae-based industry is showing consistent and remarkable expansion[4]. This is evidenced by the substantial number of studies characterising microalgae, which have established the pronounced variation in the biochemical composition due to different factors, such as species, seasonal changes, cultivation conditions, and developmental stage during harvest[5]. Given the diversity of microalgal species, they must undergo a comprehensive physicochemical characterisation[6]. Such investigations are particularly important since the direct incorporation of microalgal biomass into food formulation results in nutritional supplementation and modifications in the structural properties of products[7]. Bernaerts et al. have reviewed the functionality of microalgae and their polymers for their structuring and texturing potential, which pointed towards rheological improvement when microalgae is incorporated into food[8]. In addition, algae have different volatile flavour properties that increase their potential as ingredients in various food products[9]. Nevertheless, while the nutritional aspects are substantially studied, there is a need for a comprehensive study that combines chemical composition, rheological properties, and volatile flavour attributes of microalgae in a more harmonised way. The lack of a more integrative strategy in previous studies have made it difficult to make conclusive remarks regarding the suitability of microalgae biomass as functional ingredients for specific food applications.
The objective of this research work was to comprehensively characterise the chemical composition, microstructural and rheological properties, and volatile-flavour related attributes of four microalgal species, namely Arthrospira, Isochrysis, Nannochloropsis, and Tetraselmis species. These microalgal biomass are commercially available and could easily be obtained as functional ingredients that may be directly incorporated into food. Among these, the blue-green cyanobacteria Arthrospira sp. is the most largely cultivated, produced, and marketed species. It is known to be rich in proteins, vitamins, antioxidants, and fatty acids and has been popularly utilised for human and animal health[10]. Arthrospira sp. has been added directly to different food products, such as baked goods, pasta, and meat products, to improve their nutritional properties. Isochrysis sp. is a rich source of vitamin A and provitamin as well as carotenoids that have antioxidative and inflammatory activities[11]. Unlike Arthrospira sp., food incorporated with Isochrysis sp. is still very limited to mostly baked goods, albeit with promising nutritional benefits[12]. Nannochloropsis sp. has the promising benefits for commercialisation as a source of eicosapentaenoic acid (EPA), which has been utilised as a dietary supplement of omega-3 fatty acid to manage cholesterol levels in the treatment of cardiovascular diseases[13]. Incorporation of Nannochloropsis sp. biomass to dough has had considerable impact on the colour, although not so much on texture properties[12]. Tetraselmis sp. is known to have high polyunsaturated fatty acids (PUFA), chlorophyll content, α-tocopherol, and vitamin A while having proteins and polysaccharides that show favourable emulsifying and foaming properties[14]. Incorporation of Tetraselmis sp. has been mostly explored in dough and other baked goods. Nevertheless, while Arthrospira sp. has long been a part of human diet, Isochrysis sp., Nannochloropsis sp., and Tetraselmis sp. are typically only used in aquaculture feed[7].
Due to the great taxonomic diversity of microalgae, even within species, evaluation of relevant properties is still necessary prior to utilization in the food sector. Understanding the microalgal biodiversity and the corresponding heterogeneity variation in biochemical, morphological, and structural properties is crucial in choosing the microalgal species for food applications. The strength of this research lies in the multi-platform analytical approach, which integrates untargeted fingerprinting and targeted profiling with chemometrics data analysis. Advanced chemometrics were employed to integrate proximate composition, microstructural, and rheological properties with the volatile flavour-related attributes. This allowed for the determination of unique and discriminant markers that reflect the distinct characteristics of each microalgal species, indicating their potential as functional ingredients.
-
Lyophilised biomass of Arthrospira was supplied by BioBalance, New Zealand (NZ). Wet pastes of Isochrysis, Nannochloropsis, and Tetraselmis were bought from Reed Mariculture (USA). Reed Mariculture grew the microalgal biomass in sealed photobioreactors with saltwater media and harvesting was done by flowthrough centrifugation. This was followed by resuspension in buffer salt solution before being immediately transferred in plastic containers. Nannochloropsis sp. and Tetraselmis sp. were kept frozen at −20 °C while Isochrysis sp. was maintained under refrigerated conditions (4 °C) during storage and transport. The samples were kept at refrigerated conditions and freeze-dried within 48 h of arrival in the laboratory of the Department of Food Science, Otago University, New Zealand. After being freeze-dried, the powders were vacuum-packed and kept at −20 °C until they were analysed.
Proximate analysis was performed on lyophilised samples. All other analyses were conducted on microalgal suspensions. To prepare the microalgal suspension, lyophilised biomass was dispersed in distilled water at 8% (w/v) concentration, stirred overnight (10 °C), homogenised using a homogeniser (ULTRA-TURRAX®, Krackeler Scientific, NY, USA) for a homogenous suspension, and quick-frozen with liquid nitrogen[15]. Samples for particle size distribution were stored at –20 °C until analysis. All experiments were conducted in triplicate using independently prepared suspensions.
Chemical composition
Proximate composition
-
Moisture and ash contents were determined by drying samples in a vacuum oven (65 °C) until constant weight loss and decomposition in a muffle furnace (550 °C), respectively[16]. Average moisture values were used in calculating percentages of chemical composition as % dry basis (DB). Lipid was extracted using the modified Bligh and Dyer method[17]. Nitrogen value was determined using the Kjeldahl method[18]. Protein content was calculated by converting the measured nitrogen using specific conversion factors for Arthrospira (5.95), Nannochloropsis (4.95), Isochrysis (4.59), and Tetraselmis (4.8)[19,20]. Total carbohydrate was determined by subtracting the sum of the moisture, crude fat, crude protein, and ash values from 100.
Pigment content
-
The pigment content of all microalgal samples was determined based on a modified methanol extraction method, wherein microalgal suspension was mixed in methanol, vortexed, incubated, and supernatant was collected after centrifugation[21]. Supernatant absorbance was measured at a spectrum of 350 to 850 nm, wherein maximum absorbance of chlorophyll a is 666 nm, chlorophyll b is at 653 nm, and total carotene at 470 nm. Chlorophyll a, chlorophyll b, and carotenoids were determined as µg·mL−1 using Eqns (1), (2), and (3)[22] and converted to mg/g dry weight of microalgae:
$ {Chlorophylla}\;\left({C}_{a}\right)=15.65\;{{A}}_{666}-7.34\;{{A}}_{653} $ (1) $ Chlorophyllb \;(C_b)=27.05\;A_{653}-11.21\;A_{666} $ (2) $ Carotenoids=\dfrac{1000\;A_{470}-2.86\;C_a-129.2\;C_b}{221}$ (3) Total phenolic content
-
Total phenolic content was estimated using a modified Folin-Ciocalteau method[23] . Absorbance was measured at 765 nm against methanol as blank. Total phenolic content of samples was quantified with reference to the gallic acid standard curve (0–500 mg·L−1) and reported as gallic acid equivalent (GAE)/g dry weight of microalgae.
Microstructural and rheological properties
Morphological characteristics
-
An optical microscope (Ceti Magnum, Medline Scientific, UK) equipped with a video camera and interfaced with the software ToupView (ToupTek Photonics, China) was used. Images were taken by 40 × dry objective lens.
Particle size distribution
-
Sample particle size distribution (PSD) was evaluated by a laser diffraction particle size distribution analyser (Partica LA-950V2, Retsch Technology GmbH, Germany) with distilled water as dispersant[24]. Particle size parameters of the volume distribution were determined using the LA-950 software.
Rheological properties
-
Rheological analysis was perfomed using a controlled-stress rheometer (20 °C) (HAAKE Rheostress 1, Germany) with a double gap cylinder (DG 41-bob and DG 43-cup, 5.1 mm gap)[15]. Steady-state measurements were performed to determine the flow behaviour of suspensions at a shear rate of 0.1 to 100 s−1).
Volatile flavour-related attributes
Fatty acid profile using GC-FID
-
Fatty acid methyl esters (FAME) were prepared from extracted lipid with a combination of diethyl ether as organic solvent, boron trifluoride as derivatisation agent, and pentadecaenoic acid (C15:0) as internal standard[25]. FAME was analysed using a GC system coupled with a flame ionisation detector (GC-FID) (689A; Agilent Technologies, USA) equipped with an autosampler (7683 series injector, Agilent Technologies, USA) and fitted with a BPX70 capillary column (SGE, Australia). Sample injection was carried out in split mode with hydrogen as carrier gas. The GC oven temperature regime involved a starting temperature of 120 °C that increased to 225 °C at 3 °C/min, followed by a 10 °C/min ramp up to 245 °C, and kept at 245 ° for 2 min. The detector temperature was set at 250 °C.
Obtained chromatograms were analysed with GC ChemStation (Build 4.01, Agilent Technologies, USA). FAME peaks were manually identified by matching retention time with commercial standards (FAMQ-005, AccuStandards, USA). After manual peak alignment and removal of interfering background was done, the proportion of signal abundance of each fatty acid was calculated in % abundance of total signal abundance.
Volatile profile using HS-SPME GC-MS
-
Volatile analysis was performed using headspace solid-phase microextraction technique coupled with gas chromatography-mass spectroscopy (HS-SPME GC-MS) technique. HS-SPME was carried out on vialed samples (1:2:4 sample : ultrapure water : saturated NaCl solution). Volatile extraction was done with a pre-conditioned HS-SPME fibre coated with 30/50 µm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) (Supelco, USA) that was exposed to the vial headspace and desorbed into the GC injection port. Chromatographic separation was carried out in a ZB-Wax column (60 m × 0.32 mm × 0.5 µm) (Phenomenex) with helium as carrier gas. GC oven temperature was: 50 °C for 5 min, increased to 210 °C at 5 °C/min, ramped to 240 °C at 10 °C/min for 5 min, and then cooled to 50 °C. Mass spectra were obtained by electronic ionisation at 70 eV and scanned from 35 to 400 m/z. MS ion source and MS quad temperatures were 230 and 150 °C, respectively[26]. Six replicates were performed for each sample.
Resulting chromatograms were processed through a series of preprocessing steps using the Automated Mass Spectral Deconvolution and Identification System (AMDIS) software (Version 2.72, National Institute of Standards and Technology (NIST), USA) and Mass Profiler Professional (MPP) software (Version 14.9.1, Agilent Technologies, USA). Compound identification was made using the NIST mass spectral library (NIST14, Version 2.20, NIST, USA) and validated.
Univariate statistical analysis
-
All experiments were conducted in triplicates except for the volatile analysis. Experimental error was determined for the triplicate assays and expressed as standard deviation. Statistical analyses (ANOVA and Tukey tests) at p < 0.05 were carried out using Minitab 18 software (Minitab Inc., USA).
Multivariate data analysis (MVDA)
-
MVDA was employed on data matrix containing the chemical composition, microstructural and rheological parameters, and volatile flavour-related attributes. Initially, all data were mean centred and variables were given equal variance. MVDA was performed in two stages using Solo (Version 8.2.1, Eigenvector Research, USA): principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA).
Selection of discriminant variables was performed with variable identification (VID) coefficient calculation. Only variables with an absolute VID higher than 0.80 were deemed important by confirming significant testing.
-
The chemical composition of the selected microalgal biomass (Table 1) have been corrected for the moisture contents of each dried biomass. Each biomass composition was distinct but generally consistent with reported literature. The wide differences among microalgal species are in accordance with their different taxonomic position.
Table 1. Chemical composition and physical properties of the microalgal biomass used in this study.
Parameters Arthrospira sp. Isochrysis sp. Nannochloropsis sp. Tetraselmis sp. Proximate composition Crude lipid (%, DB) 15.60 ± 1.12a 12.01 ± 0.24b 17.62 ± 0.84a 8.80 ± 0.22c Crude protein (%, DB) 57.92 ± 0.52a 10.11 ± 0.12d 25.44 ± 0.79b 15.54 ± 0.49c Total ash (%, DB) 5.81 ± 0.02d 38.55 ± 0.08a 25.82 ± 0.15c 33.50 ± 0.39b Carbohydrate (%, DB) 14.06 ± 0.40c 18.75 ± 0.05b 19.68 ± 0.04b 25.42 ± 0.65a Pigments Chlorophyll a (mg/g DM) 0.051 ± 0.003b 0.070 ± 0.001a 0.008 ± 0.001c 0.046 ± 0.001b Carotenoids (mg/g DM) 0.023 ± 0.001b 0.137 ± 0.003a 0.005 ± 0.000d 0.014 ± 0.000c Total phenolic content (mg GAE/100 g DM) 245.64 ± 7.92a 242.80 ± 2.92a 195.63 ± 8.55c 221.50 ± 9.24b Particle size distribution d (0.1) (µm) 5.65 ± 0.10 2.69 ± 0.02 1.40 ± 0.00 5.80 ± 0.06 d (0.5) (µm) 9.08 ± 0.13 4.21 ± 0.03 2.35 ± 0.01 8.82 ± 0.03 d (0.9) (µm) 14.48 ± 0.16 6.44 ± 0.08 4.27 ± 0.04 13.13 ± 0.05 Rheological properties Consistency coefficient, K (Pa·sn) 0.013 ± 0.000b 0.020 ± 0.001a 0.008 ± 0.000c 0.004 ± 0.000d Flow behaviour index, n (−) 0.900 ± 0.002a 0.774 ± 0.002c 0.802 ± 0.006b 0.907 ± 0.013a Yield stress, σ0 (Pa) 0.003 ± 0.001a 0.004 ± 0.002a 0.002 ± 0.000a 0.001 ± 0.000a Values are mean ± standard deviation from independent replicates (n = 3). Means with different superscripts in the same row indicate a significant difference (p < 0.05). % DB refers to % dry basis. Lipid content of the selected microalgae ranged from approximately 10% and 20% of the biomass. Lipid content of Arthrospira (15.60%) is considerably higher than cited in the literature[6]. Lipid content of Isochrysis (12.01%), Nannochloropsis (17.62%), and Tetraselmis (8.80%) were generally comparable to reported ranges[27].
Arthrospira had the highest protein content (57.92%) and was comparable to the literature[19,28]. The relatively high protein content of Arthrospira could be attributed to the abundance of water-soluble phycobiliproteins, which are known to have a direct and significant relationship with the amount of protein in algal biomass[29]. The protein content of Nannochloropsis (25.44%) was similar to the literature[30]. In contrast, protein content of Isochrysis (10.11%) and Tetraselmis (15.54%) were both lower than previously reported[6,31].
Ash content of Isochrysis (38.55%) and Tetraselmis (33.50%) were relatively higher[28]. This may result from the varying salinities in cultivation mediums used by other authors from the conditions utilised for the microalgal biomass in this study. Additionally, harvested biomass was resuspended in buffer salt solution prior to packing for delivery as a wet paste, a step not included in sample preparation by others[28]. Low ash content of freshwater algae Arthrospira (5.81%) was comparable to that reported in the literature[30]. Available carbohydrate of Tetraselmis was highest (25.42%) while the other species were in the same range relatively at 14.06%−19.68% and were comparable to the literature[28].
The considerable diversity of the chemical compositions among the microalgal species is attributable to differences in cultivation practices, seasonal and geographical influences, and genetic modifications[5]. It should be noted that the use of freeze-dried microalgal biomass was reported to have minimal impact on the chemical composition, particularly protein and lipid. Hence, it is widely applicable to microalgal-based analytical and extraction procedures[32].
Pigments
-
Pigment content of samples (Table 1) calculated from the spectra of the methanol extracts included chlorophyll a, chlorophyll b, and carotenoids. Chlorophyll b was present at significantly lower amounts than chlorophyll a and carotenoids for all species except Tetraselmis. Unlike chlorophyll a, which is ubiquitous in all algal classes, chlorophyll b is exclusive to some algal classes only, specifically Chlorophyceae and Cryptophyceae[33]. As such, only chlorophyll a was included in Table 1
Isochrysis had highest chlorophyll a (0.070 ± 0.001 mg/g DM) and carotenoids (0.137 ± 0.003 mg/g DM) values. These pigments have been previously reported in this microalgae[34]. Isochrysis biomass and suspensions had a distinct yellow-brownish colour that could be attributed to this species being characteristically rich in carotenoids, which mainly comprised of fucoxanthin[35].
Arthrospira was high in green-coloured chlorophyll a (0.051 ± 0.003 mg/g DM) and had carotenoids (0.023 ± 0.001 mg/g DM). The blue-green colour that was observable in the samples exhibited the presence of phycobiliproteins, which has been reported in aqueous extracts of this species[36]. The relatively low amount of carotenoids of Arthrospira was comparable to a previous report[37].
Tetraselmis suspensions had intense green colour and contained comparable amounts of chlorophyll a (0.046 ± 0.001 mg/g DM) and carotenoids (0.014 ± 0.000 mg/g DM) to Arthrospira. Chlorophyll b was present in likewise significant amount (0.042 ± 0.002 mg/g DM, not shown in Table 1) in Tetraselmis. The presence of chlorophyll a has been reported on Tetraselmis along with chlorophyll derivatives like chlorophyll b[38]. The same research group reported that Tetraselmis have high levels of a wide variety of carotenoids, with the presence of both α- and β-carotenes and their derivatives due to the carotenoid biosynthesis pathway.
While Nannochloropsis displayed intense green colour, this species had the lowest chlorophyll a (0.008 ± 0.001 mg/g DM) and carotenoid (0.005 ± 0.000 mg/g DM) content among the samples. The low amounts of measured pigment for Nannochloropsis may be attributed to their rigid algal cells, which inhibited release of pigments to the soluble fraction. Nannochloropsis is known to have chlorophyll a as the dominant pigment[30] while having limited carotenoid content[35]. The application of pre-treatment, such as ultrasound treatment and drying, on Nannochloropsis were observed to increase recovery of pigments and other valuable compounds[39].
Total phenolic content
-
Although all the samples are rich in phenolic compounds (Table 1), both Arthrospira and Isochrysis have the highest amounts of total phenolics. Phenolic content of Arthrospira (245.64 mg GAE/g DM) is lower than previously reported[40]. Similarly, phenolic content of Isochrysis (242.80 mg GAE/g DM) is lower than the total content reported by others (308 mg GAE/g DM)[41]. The same research group also determined that the total phenolic content of Isochrysis is significantly (p < 0.05) higher than that of Tetraselmis. The variability of the results compared to the reported values can be attributed to different cultivation practices of the microalgal biomass[5].
The abundant presence of phenolic compounds in Arthrospira conforms with the literature, which indicated these compounds have antioxidant properties and functionalities that can improve the immune system[40]. On the contrary, the phenolic contents of other cyanobacteria and freshwater green algae were deemed not major contributors to the antioxidant properties of these microalgae[23]. The release of valuable health-relevant compounds, including pigments and phenolic compounds, from microalgae may not be readily available because of the rigid microalgal cells. Therefore, it may be beneficial to enhance the accessibility of these compounds by utilising a mechanical cell disruption technique, such as high-pressure homogenisation, bead milling, pulsed electric field, and ultrasonic processing[42].
Microstructural and rheological properties
Morphological characteristics
-
The optical microscopic images (Fig. 1) highlight the diverse morphological features of the microalgal biomass. The cyanobacterium Arthrospira is observed with multicellular cylindrical trichomes, albeit considerable detachment from the spiral structure has occurred. The individual cylindrical trichomes had diameters that were within the reported range of 2.5 to 6 µm and have thin and fragile peptidoglycan cell walls, which allow partial disruption during sample preparation[43]. The small ellipsoidal shape of Isochrysis (Haptophyta) cells was observed with the distinct flagellar root system. Isochrysis cells were determined to be 3.5 to 6 µm in size and had no distinct cell walls but consisted of plasma membrane covering only[44]. The tiny cells of the Nannochloropsis (Eustigmatophyceae) cells were visibly subspherical in shape. Planktonic Nannochloropsis cells, which could be either subspherical at 2 to 4 µm or cylindrical at 3–4 × 1.5 μm, have a rigid cell wall that has bilayered trilaminar sheath outer layer[45]. Tetraselmis (Chlorophyta) cells had elliptical shape with observable flagella appendage. Tetraselmis cells are typically highly motile when alive due to four flagella and can have a cell size of 9 to 15 µm[46].
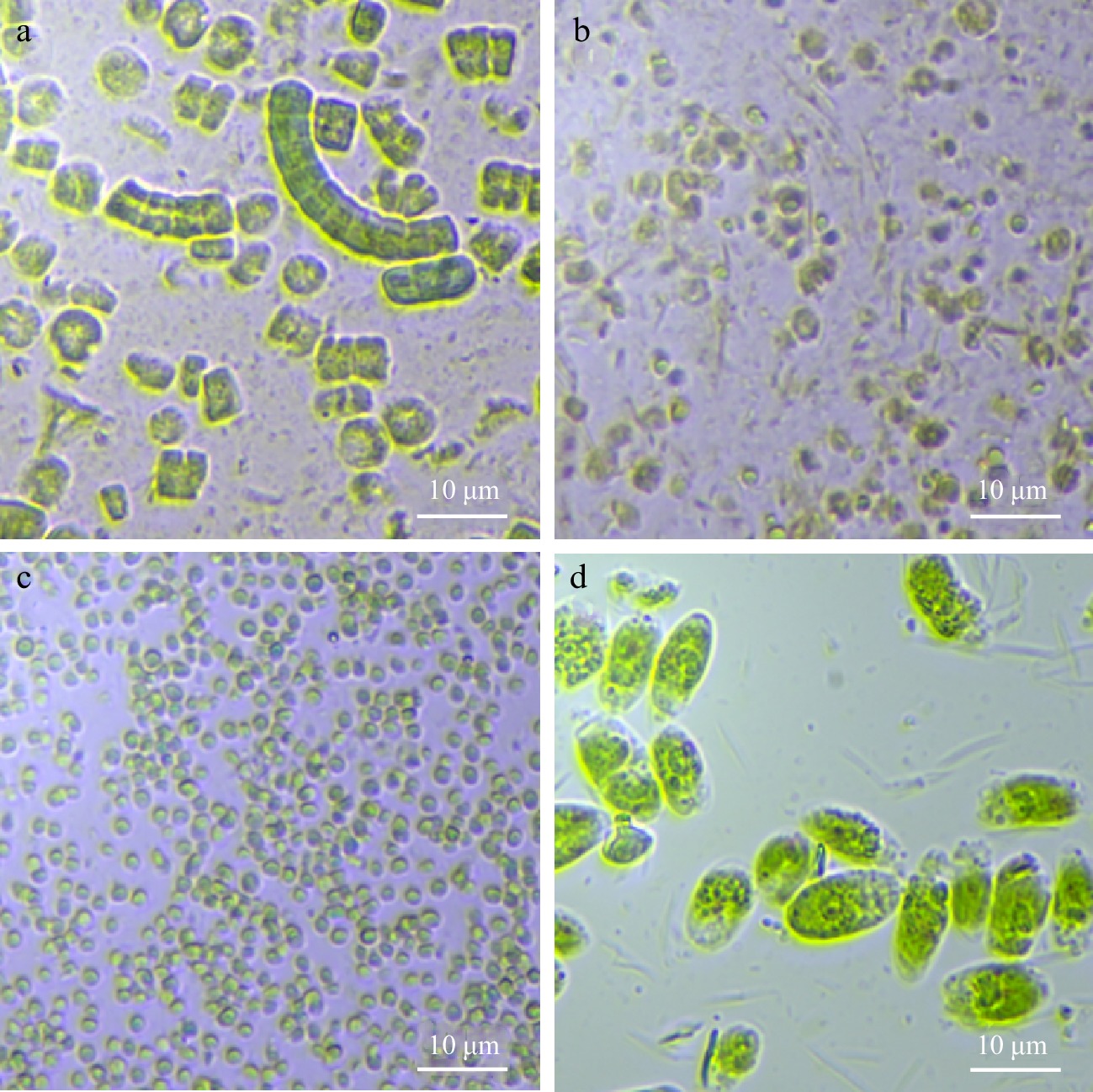
Figure 1.
Microscopic images of microalgal suspensions at 40 × magnification: (a) Arthrospira sp., (b) Isochrysis sp., (c) Nannochloropsis sp., and (d) Tetraselmis sp.
Particle size distribution
-
Particle size distribution of all the samples (Table 1) displayed a unimodal characteristic with a singular modal peak. The particle size, which refers to sizes of a single algal cell and/or complex of cells (i.e., Arthrospira), ranged depending on the microalgal species. Larger cells of Arthrospira and Tetraselmis were similar in size, followed by Isochrysis cells, while Nannochloropsis cells were the smallest among the microalgae. The results correspond to the microstructure images (Fig. 1). The distribution of Arthrospira, Nannochloropsis, and Tetraselmis in this study was similar to that obtained by previous researchers[24]. However, a comparison for Isochrysis was not performed due to the unavailability of literature for this species.
Rheological properties
-
Flow curve of the samples (Fig. 2) generally depict shear-thinning flow behaviour, as exhibited by a decrease in viscosity with increasing shear rate. This non-Newtonian behaviour could be attributed to the composition of the microalgal suspensions, which are composed of the liquid phase with extracellular polymeric substances, algae cells, and cell debris[24].
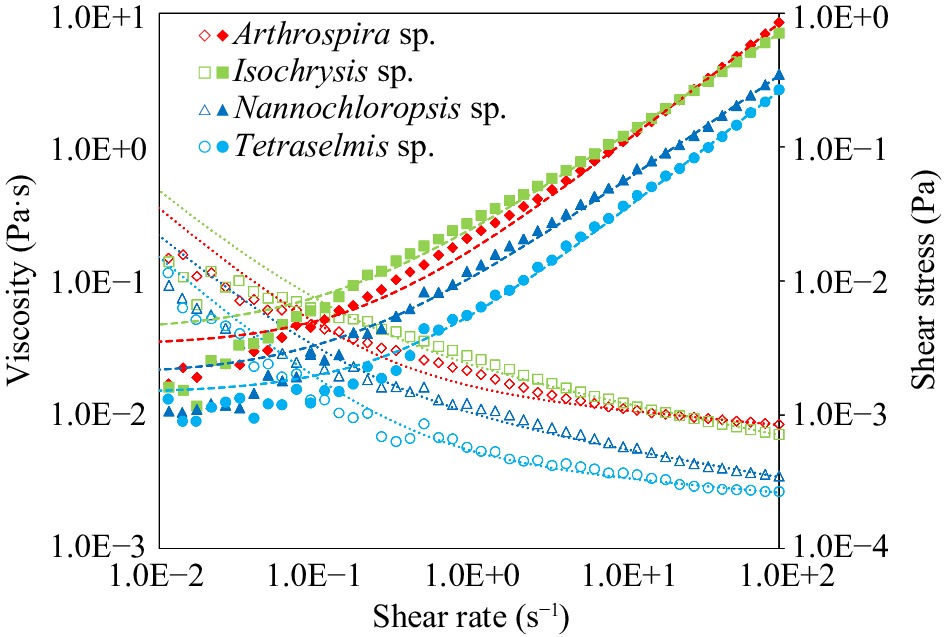
Figure 2.
Viscosity (Pa·s) and shear stress (Pa) vs shear rate (s−1) of untreated microalgal suspensions of Arthrospira sp., Isochrysis sp., Nannochloropsis sp., and Tetraselmis sp. Data points are means based on three replicates. Lines represent the Herschel-Bulkley fit.
The shear-thinning behaviour of microalgal suspensions with concentrations higher than 5 vol. % had been discussed previously by other research groups[24]. The shear stress-shear strain data were fitted into the Herschel-Bulkley mathematical model, which had a low chi-square value indicating the precision was adequate and the rheological measurements collected were reliable. The model is expressed as Eqn 4:
$ {\sigma }={{\sigma }}_{0}+{k}\cdot {\dot{{\gamma }}}^{{n}} $ (4) where
$ \sigma $ $ {\sigma }_{0} $ $ k $ $ \dot{\gamma } $ $ n $ $ {\sigma }_{0} $ The highest K among the microalgal suspensions was observed in Isochrysis (0.020 Pa·sn), which also had high viscosity. This trend exhibited by Isochrysis could be attributed to high ash (38.55%) and considerable carbohydrate (18.75%) contents of the biomass. Carbohydrates are an essential fraction of the microalgal biomass and are structural biopolymers that could exhibit texturising properties[8]. Arthrospira had the second-highest K (0.013 Pa·sn), indicating high viscosity as well. This behaviour could be attributed to the high protein content (57%) of Arthrospira since higher protein-protein crosslinking interactions allow for better network formation. The gelling property could also be due to exopolysaccharides in Arthrospira biomass that enables the formation of a weak gel[31]. Conversely, the K of Tetraselmis (0.004 Pa·sn) of was five-fold lower than that of Isochrysis and indicative of a weak potential for microstructural development. In terms of n, all the microalgal suspensions had values < 1, indicating shear-thinning behaviour. Arthrospira and Tetraselmis were at the higher range of n (0.900−0.907), while Isochrysis and Nannochloropsis were at the lower range (0.774−0.802). This implies that the latter two species had a greater propensity to behave as shear-thinning fluids. All samples had very low values of σ0 that were not significantly (p > 0.05) different from each other and suggested that a small amount of force is needed to initiate their flow.
Volatile flavour-related attributes
Fatty acid profiles
-
In the relative fatty acid profiles of the samples (Table 2), 16 fatty acids were identified, with each microalga having distinct profile. Different lipid compositions may be due to species variation and differences in cultivation method and production conditions[5].
Table 2. The selected microalgae's relative fatty acid abundance as fatty acid methyl esters (FAME) by gas chromatography coupled with a flame ionisation detector (GC-FID).
Fatty acids Arthrospira
sp.Isochrysis
sp.Nannochloropsis
sp.Tetraselmis
sp.C12:0 ND ND ND 5.72 ± 0.48 C14:0 2.52 ± 0.42b 12.18 ± 1.75a 1.84 ± 0.33b 2.22 ± 0.02b C14:1 ND 7.83 ± 1.18 ND ND C16:0 45.30 ± 0.95a 9.86 ± 1.71c 21.53 ± 0.16b 22.37 ± 0.41b C16:1n7 3.34 ± 0.11c 5.40 ± 0.36b 26.87 ± 0.95a 1.33 ± 0.15d C18:0 1.81 ± 0.80b ND 0.81 ± 0.42b 15.44 ± 0.85a C18:1n9t ND ND 0.60 ± 0.01b 1.86 ± 0.07a C18:1n9c 2.89 ± 0.18b 10.16 ± 1.14a 4.46 ± 0.03b 10.21 ± 0.16a C18:2n6c 22.58 ± 1.08a 6.03 ± 0.42b 4.01 ± 0.04c 7.38 ± 0.24b C18:3n3 ND 5.20 ± 0.17b ND 12.71 ± 0.41a C18:3n6 19.61 ± 0.46a ND 0.99 ± 0.01c 2.39 ± 0.10b C20:0 ND 25.01 ± 2.97a ND 8.47 ± 0.26b C20:3n3 ND ND ND ND C20:4n6 ND ND 4.48 ± 0.12a 1.39 ± 0.13b C20:5n3 ND ND 32.76 ± 0.10a 5.59 ± 0.11b C22:6n3 ND 15.99 ± 1.57 ND ND Total SFA 49.63 ± 1.14b 47.67 ± 0.96b 24.18 ± 0.58c 53.47 ± 00.09a Total MUFA 6.23 ± 0.05d 23.73 ± 2.76b 32.14 ± 0.84a 16.52 ± 0.53c Total PUFA 42.20 ± 1.25a 27.61 ± 1.17b 42.34 ± 0.10a 29.00 ± 0.26b Values are expressed as mean ± standard deviation (n = 3). Means with a different superscript in the same row indicate a significant difference (p < 0.05). ND means not detected. Arthrospira lipid was rich in unsaturated fatty acids, with the major fatty acids being palmitic acid (C16:0, 45.30% ± 0.95%), linoleic acid (C18:2n6c, 22.58% ± 0.6%) and γ-linolenic acid (C18:3n6, 19.61% ± 0.3%). Similar major fatty acids have been reported in Arthrospira at varying concentrations[37]. For Isochrysis, the most abundant fatty acid was arachidonic acid (C20:0, 25.01% ± 2.97%), while the other significant composition was DHA (C22:6n3, 15.99% ± 1.57%). Isochrysis is also identified as a rich source of PUFA, primarily EPA and DHA[27], although EPA was not identified in the current research. Other fatty acids present in moderate amounts, such as myristic acid, palmitic acid, and linoleic acid, and the abundant occurrence of DHA have previously been reported[48]. For Nannochloropsis, the most abundant fatty acid was EPA (C20:5n3, 32.76% ± 0.10%) followed by palmitoleic (C16:1n7, 26.87% ± 0.95%) and palmitic acid (C16:0, 21.53% ± 0.16%). These three fatty acids being the dominant in Nannochloropsis is consistent with other reports[49]. Nannochloropsis is likewise reportedly abundant in PUFA, with major relevant fatty acids such as EPA and DHA[27]. For Tetraselmis, the most abundant fatty acid was palmitic acid (22.37% ± 0.41%), followed by stearic (C18:0, 15.44% ± 0.85%) and α-linolenic acid (C18:3n3, 12.71% ± 0.41%). The abundance of palmitic acid in Tetraselmis has been reported previously[41]. In general, the abundant presence of PUFA in all samples is desirable as they can be effective in disease treatment and prevention of cardiovascular and inflammatory diseases[3,13,41].
Volatile profiles
-
The volatile compounds identified through an untargeted HS-SPME GC-MS technique were approximately 136 across the four microalgal species (Supplemental Table S1) with each species having unique volatile profiles. The identified compounds belonged to varied chemical classes in the present work, including aldehydes, hydrocarbons, ketones, alcohols, furans, pyrazines, esters, and terpenes.
The identified volatiles occur based on the chemical composition of the microalgal biomass, and some of the compounds have been reported in Arthrospira, Nannochloropsis, and Tetraselmis by other authors[9,50]. This is the first report on the volatile profile of Isochrysis, leading to a very limited comparison. Aldehydes, ketones, alcohols, and hydrocarbons are volatile compounds characteristically associated with lipid oxidation. These are reaction products formed when several decomposition reactions co-occur during lipid oxidation[51]. Aldehydes have a low odour threshold and are considered significant headspace volatile compounds[9]. While hydrocarbons are present as a major group of volatiles in microalgae, hydrocarbons typically have a high odour threshold, hence are not considered relevant from an aroma point of view but have biological and ecological significance as volatile markers[52]. Alcohols are present in microalgae samples in appreciable amounts, and they contribute to a strong, pungent odour of microalgae[53]. Furans can also be present and formed in foods in small amounts, with one of the most studied furan formation pathways being lipid oxidation[54].
Investigating the inter-relationship of different attributes using MVDA
-
The multiplatform analytical approach followed by chemometrics could help better understand the inter-relationship between various attributes and the different microalgal species. Hence, data from the chemical, rheological, fatty acid, and volatile assays were collated into a single data matrix and examined with MVDA. The integrated data matrix was first analysed with PCA (not shown) as an exploratory technique to detect outliers and distinguish trends or groupings. This was followed by supervised PLS-DA modelling to investigate the classification of the four microalgal species. For the model, the different attributes were considered X-variables and the microalgal species as categorical Y-variables. The first three latent variables (LVs) explained 98.30% of the cumulative variance, which denotes the presence of classification among the samples. Cross-validation was used to select the optimum LVs that can maximally explain the variance with the root mean squared error of the cross-validation (RMSECV) kept to a minimum.
A PLS-DA biplot was constructed with the first two highest LVs to visualize the classification/groupings. Level of classification can be interpreted based on the distance between the classes while the significance of compounds for classification can be explained based on their distribution on the plot. Variables projected far away from the centre of the coordinate and close to a particular species have high contribution to the classification. The X- and Y-variances explained by each LV are indicated in the respective axes. The PLS-DA biplot (Fig. 3) shows a clear separation among the species, especially with Arthrospira and Nannochloropsis being the most distant from each other and the other two species. Isochrysis and Tetraselmis showed distinct groupings but their relative closeness could indicate similarities in certain attributes.
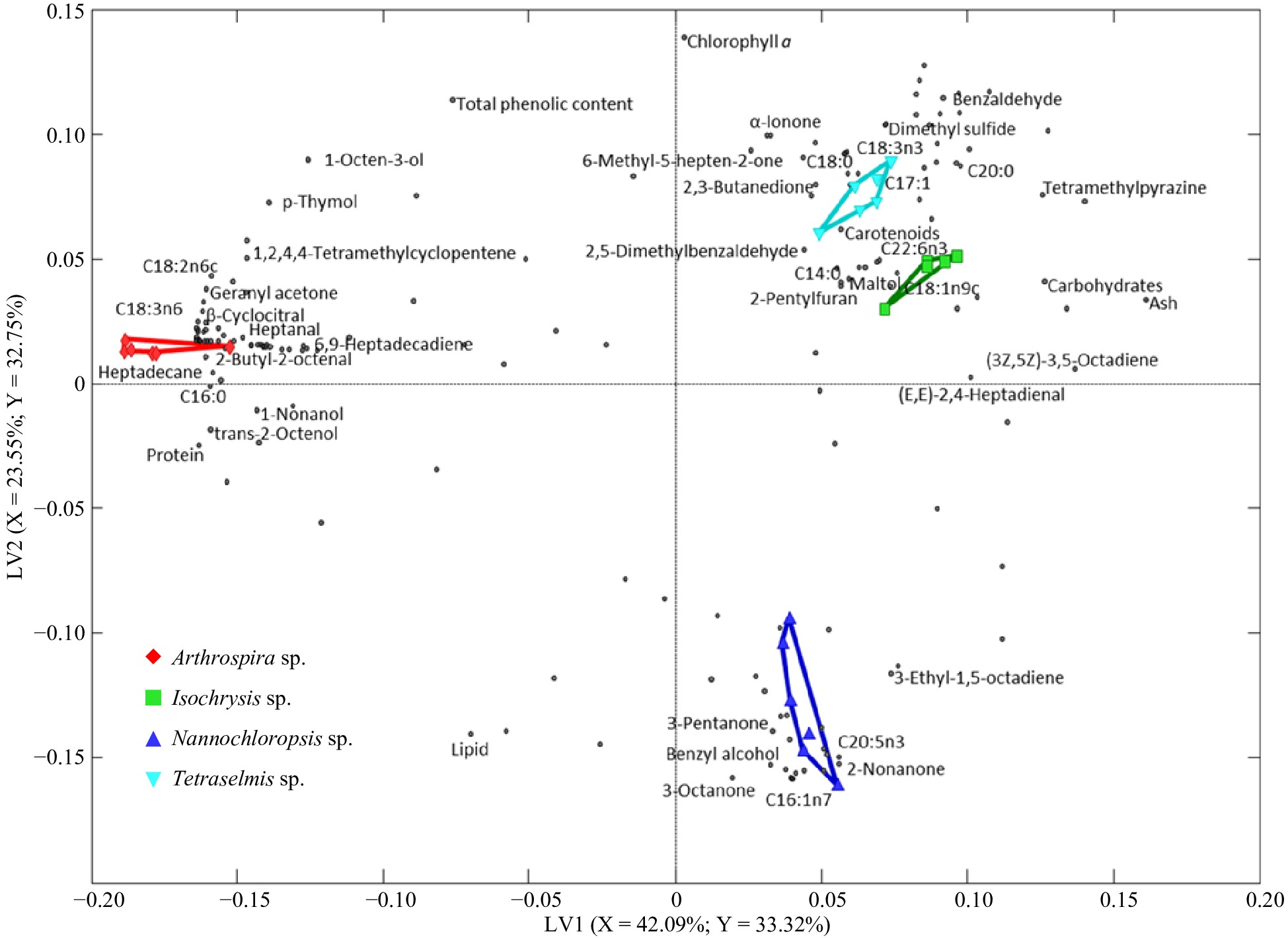
Figure 3.
PLS-DA biplots describe the variation among the selected microalgae. Differently shaped symbols represent the different microalgae: Arthrospira sp., Isochrysis sp., Nannochloropsis sp., and Tetraselmis sp. The dots represent components.
VID technique enabled selection of discriminant compounds (Table 3), where positive VID coefficient represents higher amounts detected in the related species than the others and vice-versa. The discriminant markers associated with Arthrospira, Isochrysis, Nannochloropsis, and Tetraselmis were 45, 13, 22, and 16, respectively. To show differences among the microalgal species, selected representative markers are presented (Fig. 4).
Table 3. Discriminant compounds and attributes selected per microalgal species based on the VID method confirmed with significant testing, listed in decreasing order of VID coefficient. Retention indices (RI) for the individual volatile compounds were calculated, and references were obtained from the National Institute Standards and Technology Standard Reference Database[60]. Individual fatty acids were identified by matching retention time with commercial standards.
VID Identity RI calculated RI reference Chemical class VID Identity RI calculated RI reference Chemical class Arthrospira sp. 0.982 2-Nonanone 1381 1390 Ketone 0.996 3-Ethyl-2,5-dimethylpyrazine 1438 1443 Pyrazine 0.98 (Z)-2-Pentenol 1304 1318 Alcohol 0.996 Safranal 1650 1616 Aldehyde 0.976 1-Penten-3-one 1016 1019 Ketone 0.995 1-Decene 1032 1050 Hydrocarbon 0.974 C16:1n7 (Palmitoleic acid) 0.994 1R-α-Pinene 1019 1013 Terpene 0.972 C20:5n3 (Eicosapentaenoic acid, EPA) 0.993 2,2,6-Trimethylcyclohexanone 1317 1319 Ketone 0.962 1-Heptanol 1440 1453 Alcohol 0.993 C18:3n6 (γ-Linolenic acid, GLA) 0.946 3-Octanone 1247 1253 Ketone 0.992 2-Butyl-2-octenal 1664 1656 Aldehyde 0.936 Benzyl alcohol 1867 1870 Alcohol 0.992 2,4-Dimethylbenzaldehyde 1737 1728 Aldehyde 0.888 3-Pentanone 973 980 Ketone 0.982 α-Cyclocitral 1442 1425 Terpene 0.885 1-Penten-3-ol 1147 1159 Alcohol 0.98 β-Cyclocitral 1626 1611 Terpene 0.832 2,7-Octadienol 1666 − Alcohol 0.979 β-Ionone epoxide 1997 1962 Ketone 0.815 3-Ethyl-1,5-octadiene 1019 1015 Hydrocarbon 0.975 C18:2n6c (Linoleic acid) 0.812 (3E,5E)-3,5-Octadien-2-one 1566 1570 Ketone 0.97 trans-β-Ionone 1942 1940 Terpene −0.838 Chlorophyll a 0.964 Protein −0.839 Total phenolic content 0.957 Heptadecane 1690 1700 Hydrocarbon Tetraselmis sp. 0.953 Heptanal 1177 1184 Aldehyde 0.976 C18:3n3 (α-Linolenic acid, ALA) 0.947 trans-2-Octenol 1598 1614 Alcohol 0.974 Dimethyl sulphide 743 754 Sulphur compound 0.942 C16:0 (Palmitic acid) 0.969 C12:0 (Lauric acid) 0.942 Nonanal 1387 1391 Aldehyde 0.957 2-Ethyl-3,5,6-trimethylpyrazine 1505 1506 Pyrazine 0.937 Pentadecane 1487 1500 Hydrocarbon 0.955 (Z)-4-Heptenal 1234 1240 Aldehyde 0.936 Hexadecane 1587 1600 Hydrocarbon 0.947 C18:0 (Stearic acid) 0.929 Geranyl acetone 1846 1859 Ketone 0.925 α-Ionone 1853 1840 Terpene 0.925 Octanal 1281 1289 Aldehyde 0.918 C18:1n9c (Oleic acid) 0.893 Isophorone 1404 1591 Ketone 0.894 Carbohydrates 0.887 1,2,4,4-Tetramethylcyclopentene 932 − Hydrocarbon 0.857 Benzaldehyde 1526 1520 Aldehyde 0.869 2,2,4,6,6-Pentamethylheptane 944 949 Hydrocarbon 0.853 6-Methyl-5-hepten-2-one 1329 1338 Ketone 0.863 β-Pinene 1091 1112 Terpene 0.844 2,3-Butanedione 970 979 Ketone 0.858 D-Limonene 1187 − Terpene −0.87 Lipid 0.856 1-Nonanol 1643 1660 Alcohol Isochrysis sp. 0.851 m-Xylene 1131 1143 Hydrocarbon 0.96 C22:6n3 (Docosahexaenoic acid, DHA) 0.851 α-Ionene 1697 1565 Hydrocarbon 0.96 C14:1 (Myristoleic acid) 0.843 1-Dodecene 1227 1243 Hydrocarbon 0.951 C20:0 (Arachidonic acid) 0.818 Hexyl acetate 1261 1272 Ester 0.948 Carotenoids 0.801 1-Octen-3-ol 1431 1450 Alcohol 0.936 3-Methyl-1,4-heptadiene 914 − Hydrocarbon −0.8 (3Z,5Z)-3,5-Octadiene 925 − Hydrocarbon 0.933 C14:0 (Myristic acid) −0.945 Ash 0.916 3-Methyl-2-(3,7,11-trimethyldodecyl) furan 2097 − Furan Nannochloropsis sp. 0.906 2,5-Dimethylbenzaldehyde 1746 1683 Aldehyde 0.991 2-Undecanone 1591 1598 Ketone 0.886 Maltol 1960 1969 Ketone 0.986 (E)-2-Pentenal 1125 1127 Aldehyde 0.865 (E,E)-2,4-Heptadienal 1460 1495 Aldehyde 0.985 1,3-Pentadiene 97 624 Hydrocarbon 0.835 2-pentylfuran 1221 1231 Furan Retention indices (RI) for the individual volatile compounds were calculated and reference obtained from the National Institute Standards and Technology Standard Reference Database (National Institute of Standards and Technology n.d.). Individual fatty acids were identified by matching retention time with commercial standards. 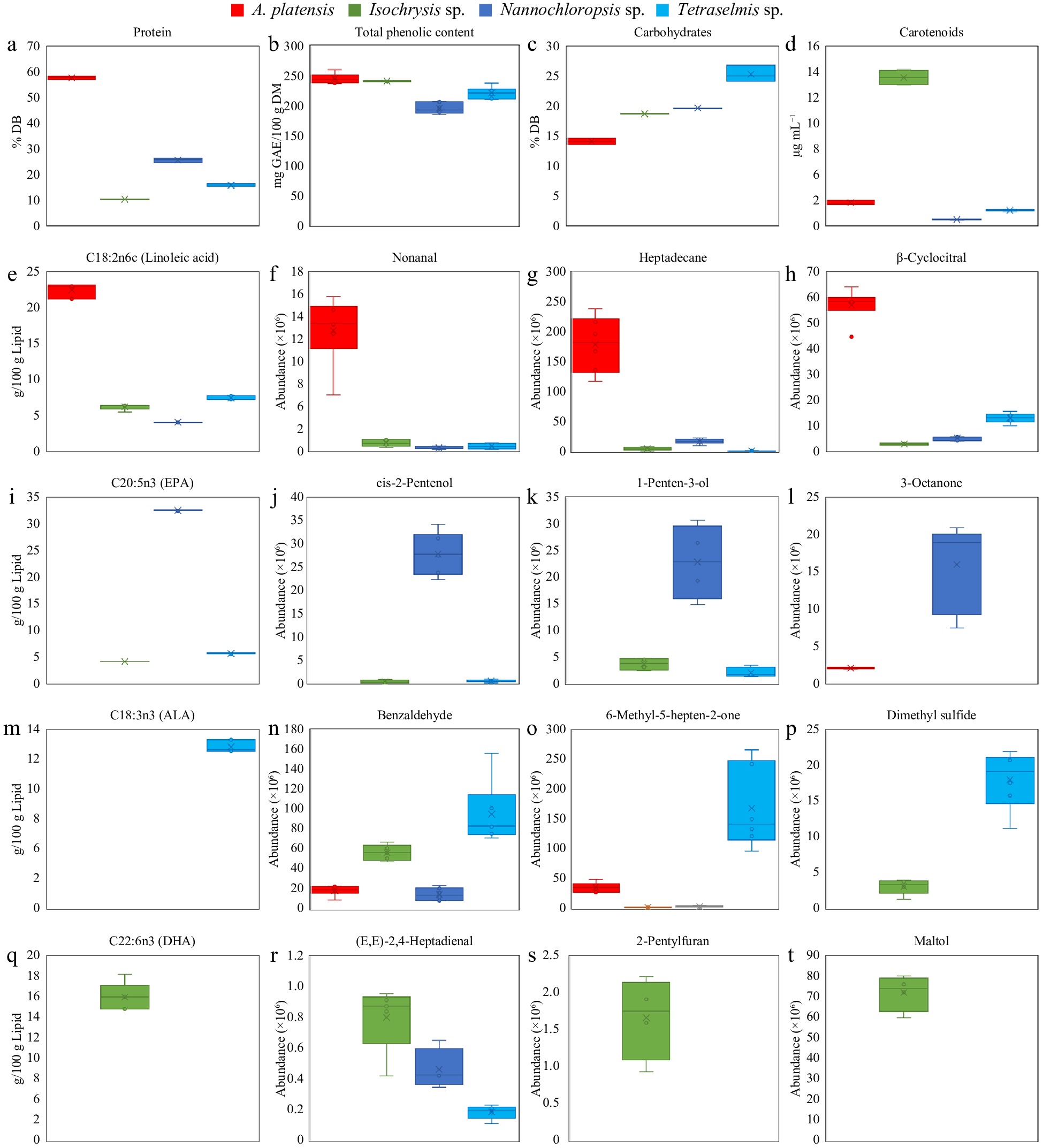
Figure 4.
Individual plots of some representative discriminant compounds show variation among A. platensis, Isochrysis sp., Nannochloropsis sp., and Tetraselmis sp. Values are mean ± standard error (n = 3).
Arthrospira
-
Arthrospira contains a high amount of protein, palmitic, linoleic, and γ-linolenic acid and is dominated by hydrocarbons, aldehydes, and terpenes. The prominent presence of protein (Fig. 4a) and fatty acids in this species corresponds to the literature[28,40]. Presence of these PUFA in Arthrospira could account for most of the dominant discriminant volatile compounds identified in the biomass through the HS-SPME GC-MS technique (Fig. 4e−h).
Between the three major chemical groups, aldehydes have lower odour threshold values and are deemed to be highly relevant headspace volatiles. Aldehydes selected in Arthrospira included safranal, 2-butyl-2-octenal, 2,4-dimethylbenzaldehyde, heptanal, nonanal, and octanal. Aldehydes detected in microalgal biomass have been mostly connected to the oxidation of PUFA by enzymatic reactions[55]. The high concentration of PUFA has been attributed to a greater number of linear aldehydes with the occurrence of chemical lipid oxidation and aromatic aldehydes associated with enzymatic lipid and protein oxidation[9]. This corresponds to the high amount of PUFA in Arthrospira as observed with linoleic acid (Fig. 4e). Among aldehydes, nonanal (Fig. 4f) was identified as a volatile biomarker to indicate changes in different growth phases[55].
Among hydrocarbons, heptadecane (Fig. 4g) was the major compound identified, followed by smaller amounts of hexadecane and pentadecane, which is in accordance with other studies[50]. Hydrocarbons are associated with neutral lipids obtained from the lipid fraction of microalgal biomass[51]. With the abundance of PUFA in Arthrospira, the findings corroborate the reported link of hydrocarbons to lipid peroxidation in microalgae[52]. Significant presence of terpenes is represented by β-cyclocitral (Fig. 4h), among others. While this is the first time that β-cyclocitral is reported in Arthrospira, this carotenoid-degradation product has been reported in other microalgae[9].
Nannochloropsis
-
Nannochloropsis can be characterised as an abundant source of lipid rich in PUFA and having a volatile profile that dominantly consists of ketones and alcohols (Fig. 4i−l).
Discriminant fatty acids in Nannochloropsis include EPA (Fig. 4i) and palmitoleic acid. Substantial amount of EPA in Nannochloropsis has been consistently reported in the literature and is desirable because of the numerous health benefits of this PUFA[56]. Nannochloropsis-derived EPA was found to control cholesterol levels and is beneficial to the cardiovascular health of the general population[13]. The abundance of palmitoleic acid has also been reported in Nannochloropsis[49].
Compared to other species, Nannochloropsis had the greatest number of alcohols identified to be discriminant. (Z)-2-Pentenol (Fig. 4j ) and 1-penten-3-ol (Fig. 4k) were the most abundant that have also been identified by other researchers in Nannochloropsis[9,55]. Zhou et al. reported that the relative contents of alcohols in Nannochloropsis were stable at different growth phases, whereas other microalgal species had decreasing trend[55]. While it was unclear what caused the observed trend, this could explain the predominance of alcohol volatile compounds in Nannochloropsis. Other studies reported short-chain alcohols, ketones, and aldehydes as the most representative volatile compounds for Nannochloropsis using PCA analysis[9]. Among ketones, 3-pentatone (Fig. 4k) and 1-penten-3-one has been identified previously[9]. In contrast, the generally lower values of chlorophyll a and total phenolic content (Fig. 4b) of Nannochloropsis compared to the other species are represented by these attributes' negative VID coefficient.
Tetraselmis
-
Tetraselmis can be described as rich in carbohydrates and α-linolenic acid with mainly ketones and aldehydes as volatile compounds (Fig. 4m−p). Superior amounts of carbohydrates (Fig. 4c) in Tetraselmis could be attributed to cell walls being rich in intracellular starch and complex polysaccharides[57]. The negative VID of lipid emphasizes the lower range of lipid content in Tetraselmis compared to other species, in accordance to other reports[57]. While α-linolenic acid (Fig. 4m) was previously found in other microalgal species[28], current work observed that this PUFA was absent in other species but richly present in Tetraselmis.
Aldehydes and ketones were the major discriminant volatiles in Tetraselmis. (Z)-4-heptenal and benzaldehyde could be attributed to oxidation reactions of PUFA that are relatively abundant in the biomass. High proportions of benzaldehyde (Fig. 4n) are analogous to other reports and can be accredited to phenylalanine's enzymatic and chemical degradation via amino acid biosynthetic pathway[9]. A significant ketone identified is 6-methyl-5-hepten-2-one (Fig. 4o) that others have consistently reported[9,53]. Tetraselmis had the unique presence of sulphur-containing compounds, specifically dimethyl sulphide, as discriminant. Dimethyl sulphide (Fig. 4p), the only sulphur-containing compound detected, was also found in Isochrysis but present at much higher concentrations in Tetraselmis. This compound has only been previously reported in Tetraselmis and Rhodomonas and attributed to the enzymatic and chemical degradation of dimethylsulfoniopropionate[9].
Isochrysis
-
Isochrysis is rich in carotenoids, DHA, arachidonic acid, myristic acid, and contained mostly aldehydes and furans. The abundance of identified fatty acids is directly related to the presence of discriminant volatiles (Fig. 4q−t). The substantially high carotenoid content (Fig. 4d) warrants this attribute as an appropriate discriminant marker for this species. Carotenoid-rich Isochrysis are in accordance with other studies[48,57]. Among the fatty acids, DHA, arachidonic acid, and myristic acid have sharply greater amounts in Isochrysis than in the other species, a feature consistently reported in the literature for this species[28,57]. Among other fatty acids, DHA (Fig. 4q) had one of the highest VID among the discriminant variables and reflects Isochrysis having the highest DHA level. Significantly high concentration of DHA in Isochrysis is desirable as they attenuate risk factors of cardiovascular and other chronic diseases[58].
Discriminant aldehydes in Isochrysis were 2,5-dimethylbenzaldehyde, and (E,E)-2,4-heptadienal, with the latter having been detected in Rhodomonas and Botryococcus species[9]. Additionally, an interesting compound selected to be discriminant in Isochrysis is 2-pentylfuran (Fig. 4s), likewise reported in Botryococcus and Chlorella[9]. Other cyanobacteria that had a detectable presence of 2-pentylfuran, an important lipid degradation product, are Arthrospira, Anabaena, and Nostoc genera[50]. Moreover, previous studies have identified linolenic and linoleic acids, beta-carotene, ascorbic acid, amino acids, and carbohydrates, which are considerably present in Isochrysis, as precursors in furan formation[54]. The ketone maltol (Fig. 4t) contributed substantially to the volatile profile of Isochrysis. This is the first time in this study on Isochrysis that maltol is identified in microalgae, although it has been reported in certain seaweeds[59]. Ketones are generally considered lipid oxidation or degradation products[51].
For all the microalgal species, it is notable that the discriminant markers were mostly health-relevant compounds that are considered to have food and pharmaceutical applications. The high protein content and richness in valuable fatty acids of Arthrospira verifies its distinction as a foremost microalgal biomass for commercialization since it has numerous applications as health supplement or supplementary food ingredient. Additionally, Arthrospira have been associated with desirable bioactive properties indicating favorable contribution to nutraceutical industry[40]. For Nannochloropsis, its differentiation as an EPA-rich biomass builds up to the body of evidence that this species is ideal as a functional ingredient, with reported positive effect on cardiovascular health among other health benefits[13]. Tetraselmis is distinguished as having PUFA-rich lipid, in the form of γ-linolenic acid, and confirms the viability of this microalgal biomass as PUFA supplement for human nutrition[13]. Meanwhile, Isochrysis has been differentiated for the high levels of carotenoids as well as DHA, which is a very relevant PUFA for human health. This shows its potential as a novel functional ingredient, along with the other microalgal species.
-
There is an apparent variation in the physicochemical properties of the four microalgal species. Chemometrics analysis to the multivariate data revealed the distinctness of each microalgal species based on integrated microalgal properties. The major discriminant volatile markers, indicative of each species' distinct volatile profiles were aldehydes, terpene, and hydrocarbon for Arthrospira, ketones and alcohols for Nannochloropsis, aldehydes, ketones, and sulphur-containing compounds for Tetraselmis, and furans and aldehydes for Isochrysis. The main discriminant fatty acids included γ-linolenic acid for Arthrospira, DHA for Isochrysis, EPA for Nannochloropsis, and α-linolenic acid for Tetraselmis.
As presented in this study, the rich abundance of proteins, carbohydrates, lipids, and other bioactive compounds in microalgae enables a complex association that could potentially result in microalgae-enriched biomass with promising rheological, volatile, and nutritional characteristics. The diverse profiles of microalgae allow a varied and expanded application in the food industry and the pharmaceuticals and nutraceutical sectors. Findings suggest that desirable compounds, like pigments, total phenolic contents, and other macronutrients, may be appreciated with or without the intended rheological/textural and volatile/aroma impact, depending on the type of microalgae utilised. In the case of Arthrospira, while it has and can further be used for protein supplementation in health drink juices, it can also be used as a structuring/thickening agent in different food products. Isochrysis, Nannochloropsis, and Tetraselmis species have great potential as ingredients in the development of functional foods. They can be utilised in various suitable food matrices (e.g., pasta, baked products, and processed meat products) that complements their unique volatile flavour-related attributes for greater consumer acceptance.
While the present work clearly shows the effectivity of chemometrics approach in identifying the distinguishing qualities of different microalgal species, it would be worthwhile to further characterize microalgal biomass as affected by growth conditions and developmental stage. Additionally, integrating other data such as amino acid and sugar profiles could elucidate other notable unique microalgal characteristics.
The HS-SPME GC-MS fingerprinting approach employed in this research provides insights into the volatile composition of microalgae. However, it has limitations when directly correlating with the (off) flavor profile of the product. While the identified volatile compounds can be linked to reaction pathways or specific food characteristics, caution was exercised in attributing selected compounds to undesirable odor notes commonly found in microalgae-based products. (Off) flavor attributes are best assessed using descriptive sensory analysis, a facet not covered in this research. Future investigations have the potential to correlate instrumental attributes with sensory data, identifying compounds contributing to the off-flavor profile of microalgae.
-
The authors confirm contribution to the paper as follows: conceptualisation: Magpusao J, Kebede B; Investigation: Magpusao J; Methodology: Magpusao J, Oey I, Kebede B; data analysis and visualization: Magpusao J, Kebede B; supervision: Kebede B, Oey I; writing - original draft & editing: Magpusao J; writing - review & editing: Oey I, Kebede B. All authors reviewed the results and approved the final version of the manuscript.
-
The study's supporting data will be provided by the corresponding author upon reasonable request.
-
Johannes Magpusao acknowledges the New Zealand Development Scholarship funded through the Ministry of Foreign Affairs and Trade for her doctoral scholarship.
-
The authors declare that they have no conflict of interest. Indrawati Oey is the Editorial Board member of Food Innovation and Advances who was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer-review handled independently of this Editorial Board member and the research groups.
- Supplemental Table S1 List of volatile compounds found in microalgal aqueous suspensions determined by headspace- solid-phase microextraction gas chromatography-mass spectrometry (HS- SPME GC-MS).
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Magpusao J, Oey I, Kebede B. 2024. Chemical, rheological, and volatile profiling of microalgae Arthrospira, Isochrysis, Nannochloropsis, and Tetraselmis species. Food Innovation and Advances 3(2): 75−87 doi: 10.48130/fia-0024-0007
Chemical, rheological, and volatile profiling of microalgae Arthrospira, Isochrysis, Nannochloropsis, and Tetraselmis species
- Received: 13 December 2023
- Revised: 19 March 2024
- Accepted: 19 March 2024
- Published online: 22 April 2024
Abstract: Microalgae are increasingly regarded as a sustainable source of novel food and functional products due to their nutritional composition. This study aimed to conduct an in-depth analysis of the chemical, microstructural and rheological, and volatile-flavour related properties of Arthrospira, Isochrysis, Nannochloropsis, and Tetraselmis species. Chemometric data analysis was employed to integrate the multivariate data, investigate the classification among the four species, and identify discriminating and distinct features. Arthrospira is high in protein content, and Nannochloropsis is lipid-rich with dominantly polyunsaturated fatty acids. Isochrysis is rich in carotenoids and total phenolics, while Tetraselmis is high in carbohydrates. Key discriminant volatile markers encompass aldehydes, terpenes, and hydrocarbons for Arthrospira; ketones and alcohols for Nannochloropsis; aldehydes, ketones, and sulfur-containing compounds for Tetraselmis; and furans and aldehydes for Isochrysis. Moreover, Arthrospira and Isochrysis demonstrate elevated viscosity and notable thickening potential. In summary, the different microalgal biomass studied in this study showcase unique compositional, rheological, and volatile properties, highlighting their potential as functional ingredients for diverse applications in the food and pharmaceutical industries.
-
Key words:
- Microalgae /
- Chemical composition /
- Rheology /
- Volatile /
- Fatty acid /
- Chemometrics













