-
Wolfberry (Lycium chinense Miller) is a deciduous shrub in the Solanaceae family, found in China and other parts of Asia. Its fruit, bright orange-red oval berries measuring 1−2 cm in length, is traditionally used as an herb and functional food in Asian countries. Polysaccharides, flavonoids, and polyphenols are recognized as the primary bioactive constituents of wolfberry, showcasing a range of effects including anti-aging[1], neuroprotection, hypoglycemic, and hypolipidemic properties[2], immune modulation, anti-tumor activity[3], and cellular protection[4]. As a result, the consumption of wolfberry is anticipated to mitigate the risks associated with certain diseases and conditions linked to oxidative stress, while simultaneously enhancing antioxidant defense mechanisms. To optimize the absorption efficiency of polyphenols and other beneficial substances in wolfberries within the human body and fully harness their antioxidant functions, the adoption of innovative technologies becomes imperative[5]. Lactic acid fermentation emerges as a widely employed technique in the processing of functional foods, renowned for its capacity to significantly enhance various nutritional components. This method holds promise for enhancing the bioavailability and beneficial effects of wolfberry constituents, thereby potentially maximizing their health-promoting properties.
Fermentation by lactic acid bacteria (LAB) has the potential to enhance a variety of bioactive compounds within the substrate[6], making it a promising alternative for the processing of wolfberry. The organic acids, esters, and other substances produced during fermentation can boost the antioxidant capacity of wolfberry pulp[5], providing beneficial effects on human health post-consumption. Prebiotic compounds, identified as 'selective fermentation agents facilitating specific changes in the composition and/or functioning of gut microbiota, beneficial for the well-being and health of the host', has been observed in research to stimulate probiotic proliferation in fermentation products, thereby improving fermentation efficiency and overall effectiveness. Numerous studies have highlighted the advantages of employing Lactobacillus plantarum in the biological processing of fruits and vegetables. For instance, in addition to improving sensory perceptions, fermentation can significantly augment aroma components in apple juice[7], kiwi fruit[8], and wolfberry juice[9]. This enhances health benefits, as the hydrolase of L. plantarum releases antioxidant compounds from conjugated phytochemicals in fruits and vegetables[10,11]. To the best of our knowledge, the current research on wolfberry mainly focuses on the fermentation of its plant components or extracts, and the fermentation of fruit pulp has not been reflected in the literature. The fermentation process of wolfberry pulp is complex and influenced by various factors such as the amount of added prebiotics, the inoculum size of lactic acid bacteria, and the fermentation time. Therefore, developing an accurate kinetic model is not only advantageous for comprehending and forecasting the dynamic alterations of several crucial parameters throughout the fermentation process, but it also aids in optimizing fermentation conditions. This, in turn, aims to achieve increased efficiency and energy savings through model optimization.
Following the above discussion, this study was conducted in three distinct parts. Firstly, the changes of substrates, products and bacterial number parameters in the process of fermentation of wolfberry pulp by Lactobacillus plantarum were recorded, and the kinetic model equation of lactic acid fermentation of wolfberry pulp was emphatically explored. In addition, the changes in phenolic compounds and antioxidant activity in unfermented goji berry pulp and fermented goji berry pulp were analyzed. In the third part, the volatile aroma components of wolfberry were detected by GC-IMS to clarify the effect of fermentation on the aroma change of wolfberry pulp.
-
Dried wolfberry sourced from Ningxia Runxinyuan Wolfberry Development Co., Ltd. (Zhongning, China). Lactobacillus plantarum CCFM1050 obtained from the Fruit and Vegetable Storage and Processing Laboratory of the School of Food Science and Engineering, Northwest A&F University (Yangling, China). DPPH (D4313), ABTS(IA0010), Caffeic acid (SC8010), Gallic acid (SG8040), Rutin (SR8250), and other phenolic standard products were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China).
Preparation of wolfberry pulp
-
Dried wolfberry was thoroughly rinsed with tap water. Spoiled berries were removed, and the remaining berries were soaked in tap water at a ratio of 1:5 (w/v). After pulp beating, the mixture was homogenized under a colloid mill for 10 min. The resulting wolfberry pulp was then sterilized at 100 °C for 5 min cooled and set aside for use.
Preparation of bacterial cultures
-
Lactobacillus plantarum CCFM1050, stored in a glycerol solution at −80 °C was first thawed at room temperature and then activated in 20 mL MRS broth at 37 °C for 12 h. One hundred μL bacterial culture was activated twice, washed with 20 mL of physiological saline, and utilized as an inoculum for wolfberry pulp.
Prebiotic screening single factor experiment
-
The activated bacterial culture's viability was determined using the coating plate method. A 1.0 × 108 CFU/100 g concentration served as the 1% inoculum. Firstly, inulin, lactulose, and oligosaccharides were individually added to 50 g of wolfberry pulp at 1%, 3%, 5%, 7%, and 9% concentrations. Then after 24 h of fermentation, measurements were taken for total acid content and viable bacterial count.
Construction of fermentation dynamics model
-
Leveraging the insights gleaned from prior response surface optimization experiments, Lactobacillus plantarum CCFM1050 was employed as the fermentation strain. Primarily, each 100 g of wolfberry pulp received supplementation of 1 × 108 bacterial solution, coupled with the addition of 0.06 g of oligosaccharides for the fermentation process. Next, sampling intervals of 6 h was maintained throughout the fermentation trajectory to scrutinize the dynamics of viable bacteria, reducing sugars, and total acid content. The model's parameters were methodically computed based on the empirical data.
Dynamic model of bacterial growth
-
The growth of bacterial cells typically adheres to an 'S' curve, a pattern well-suited for simulation by the logistic equation[12]. This equation effectively captures the changes in bacterial growth and the distinct expression of bacterial growth metabolism rates, as observed in the wolfberry pulp fermentation process.
The logistic equation is expressed as follows:
$ \dfrac{\text{1}}{\text{x}}\dfrac{\text{dx}}{\text{dt}}=\text{μ}\text{m}\left(\text{1}-\dfrac{\text{X}}{{\text{X}}_{\text{m}}}\right) $ (1) At the commencement of fermentation (t = 0) and with the initial microbial population denoted as X = Xm, the integral deformation equation is substituted, yielding the following expression:
$ \text{X}=\dfrac{{\text{X}}_{\text{0}}{\text{e}}^{{\text{μ}}_{\text{m}}\text{t}}}{\text{1}-\dfrac{{\text{X}}_{\text{0}}}{{\text{X}}_{\text{m}}}\text{(}\text{1}-{\text{e}}^{{\text{μ}}_{\text{m}}\text{t}}\text{)}} $ (2) In the formula, X0 is the initial number of live lactic acid bacteria during growth / (108 CFU/mL); Xm represents the highest viable count of lactic acid bacteria during the growth period/(108 CFU/mL); μm is the maximum specific growth rate/h−1, t denotes time in hours.
Upon converting Eqn (2) into a deformed form, simultaneous logarithmic transformation of both sides results in:
$ \text{ln}\dfrac{\text{X}}{{\text{X}}_{\text{m}}-\text{X}}={\text{μ}}_{\text{m}}\text{t}-\ln\left(\dfrac{{\text{X}}_{\text{m}}}{\text{X}}-\text{1}\right) $ (3) Product generation kinetics model
-
In bacterial fermentation, the relationship between cell growth rate and product generation rate can manifest in three ways: coupled with bacterial growth, partially coupled, and non-coupled[13]. The product generation rate does not perfectly align with any single model, introducing a degree of error. Consequently, the Leudeking-Piret equation is employed:
$ \dfrac{\text{dP}}{\text{dt}}=\text{α}\dfrac{\text{dX}}{\text{dt}}+\text{βX} $ (4) In the formula, P represents the total acid concentration after fermentation, (g/L); X denotes the number of live bacteria, (108 CFU/mL); T is the fermentation time, h; α is the product synthesis constant associated with bacterial growth; β is the product synthesis constant associated with bacterial volume.
In the context of lactic acid bacteria fermenting wolfberry pulp to produce acid, the process conforms to a partially coupled type, specifically α ≠ 0 and β ≠ 0. At this stage, the bacterial body is in a stable phase: dX/dt = 0, X = Xm, so that:
$ \text{β}=\dfrac{\text{dP}}{\text{dt}\times {\text{X}}_{\text{m}}} $ (5) When taking t = 0 and P = 0 as the initial conditions, the integral results in:
$ \begin{split}&\rm P={\text α}A(t)+{\text β}B(t) \\&\text{A}\left(\text{t}\right)={\text{X}}_{\text{0}}\left[\dfrac{{\text{e}}^{{\text{μ}}_{\text{m}}\text{t}}}{\text{1}-\dfrac{{\text{X}}_{\text{0}}}{{\text{X}}_{\text{m}}}\left(\text{1}-{\text{e}}^{{\text{μ}}_{\text{m}}\text{t}}\right)}-\text{1}\right] \\& \text{B}\left(\text{t}\right)=\dfrac{{\text{X}}_{\text{m}}}{{\text{μ}}_{\text{m}}}\ln\left[\text{1}-\dfrac{{\text{X}}_{\text{0}}}{{\text{X}}_{\text{m}}}\left(\text{1}-{\text{e}}^{{\text{μ}}_{\text{m}}\text{t}}\right)\right] \end{split}$ (6) Substrate consumption dynamics model
-
Throughout the fermentation process of lactic acid bacteria, the sustenance of normal physiological activities in cells necessitates specific substrates. The energy generated through substrate consumption serves both microbial growth and the production of new metabolites. The dynamics of substrate consumption can be effectively described using the Luedeking equation:
$ -\dfrac{\text{dS}}{\text{dt}}=\text{γ}\dfrac{\text{dX}}{\text{dt}}+\text{δX} $ (7) In the formula, S represents the substrate concentration/(g/100 mL), X denotes the number of live bacteria/(108 CFU/mL), T is time/h, γ and δ are substrate consumption parameters.
When the bacterial body is in a stable phase with dX/dt = 0 and X = Xm, the following can be derived from Eqn (7):
$ \text{δ}=-\dfrac{\text{dS}}{\text{dt}\;\text{×}\;{\text{X}}_{\text{m}}} $ (8) After integration:
$\begin{split}& S=S_0-\gamma (t)-\delta D(t)\\&\text{C}\left(\text{t}\right)={\text{X}}_{\text{0}}\left[\dfrac{{\text{e}}^{{\text{μ}}_{\text{m}}\text{t}}}{\text{1}-\dfrac{{\text{X}}_{\text{0}}}{{\text{X}}_{\text{m}}}\left(\text{1}-{\text{e}}^{{\text{μ}}_{\text{m}}\text{t}}\right)}-\text{1}\right] \\& \text{D}\left(\text{t}\right)=\dfrac{{\text{X}}_{\text{m}}}{{\text{μ}}_{\text{m}}}\ln\left[\text{1}-\dfrac{{\text{X}}_{\text{0}}}{{\text{X}}_{\text{m}}}\left(\text{1}-{\text{e}}^{{\text{μ}}_{\text{m}}\text{t}}\right)\right] \end{split}$ (9) Determination of chemical substances and antioxidant activity during fermentation
Determination of total phenolic and total flavonoids content
-
The estimation of total phenolic content was performed by slightly modifying the Folin-Ciocalteu procedure[14]. To put it briefly, 1 mL of the sample, once diluted, was amalgamated with 2 mL of the Folin-Ciocalteu reagent (1:9, v/v). This was followed by the introduction of 2 mL of Na2CO3 solution (75 g/L). The blend was then left undisturbed in a dark environment at room temperature for 30 min. Post this incubation, the absorbance of the concoction was gauged at 760 nm.
The total flavonoid content (TFC) was evaluated using the AlCl3 colorimetric method, as described by Wu et al.[15]. Initially, 2 mL of the diluted phenolic extract was incubated with 0.25 mL of NaNO2 solution at a concentration of 50 g/L for 5 min. Then, 0.5 mL of AlCl3 solution, prepared at a concentration of 100 g/L, was added, followed by a further 5 min incubation. Subsequently, 1 mL of NaOH solution, with a concentration of 2 mol/L, was introduced and allowed to react for 10 min. The absorbance of the resulting solution was measured at 510 nm.
DPPH and ABTS radical scavenging rate
-
The DPPH radical scavenging activity was assessed using a modified protocol based on prior methods[15]. A total of 0.4 mL of the appropriately diluted sample was combined with 9.6 mL of a methanolic DPPH solution (at a concentration of 20 mg/mL). The resulting solution was subsequently incubated at a consistent temperature of 37 °C in a light-restricted environment for exactly 30 min. Following this incubation period, the absorbance of the mixture was carefully measured at a target wavelength of 517 nm.
The ABTS radical scavenging activity was determined with slight modifications to previously described methods[16]. The process commenced with the incubation of 2.45 mmol/L K2S2O8 and 7 mmol/L ABTS in a 1:1 (v/v) ratio, maintained in a dark environment for 16 h to synthesize the ABTS radical cationic reagents. The subsequent step involved diluting the reaction mixture with 80% ethanol to obtain an absorbance of 0.70 ± 0.02 at a wavelength of 734 nm. Thereafter, a 0.6 mL sample was mixed with 5.4 mL of the ABTS radical cation solution, allowing for a 6 min reaction period. The absorbance of the mixture was then precisely measured at 734 nm. The outcome of this assay was expressed as the percentage of ABTS free radical inhibition.
Phenolic profiles
-
The samples were extracted three times by ethyl acetate, combined with organic phases, evaporated by a rotary evaporator, and then reconstituted with methanol for testing.
High-Performance Liquid Chromatography (HPLC) was utilized to determine the phenolic profiles, adhering to the methodology delineated in a prior study[15]. The flow rate was set at 1 mL/min, and the detection was facilitated by a UV–visible spectrophotometer, monitoring at a wavelength of 280 nm. The quantification of the phenolic compounds was achieved by correlating the obtained peak areas with those of established external standards.
Phenolic profiles were quantified by HPLC according to previously reported method[15]. The mobile phase, consisting of a 1% formic acid solution (referred to as solvent A) and acetonitrile (designated as solvent B), were combined according to a specific gradient elution scheme. This program was as follows: from the start to 5 min, the proportion of solvent B was maintained at 5%; from 5 to 25 min, it increased to 12%; from 25 to 40 min, the concentration rose to 30%; between 40 and 50 min, solvent B constituted 45% of the mixture; and from 50 to 60 min, the program returned to an initial 5% concentration of B. The column oven temperature was set at 30 °C and 10 μL of samples were injected into the sampler. Separation was carried out on a Symmetry C18 column (4.6 mm × 250 mm, 5 μm, Waters). The flow rate was set at 1 mL/min, and the detection was facilitated by a UV–visible spectrophotometer, monitoring at a wavelength of 280 nm. The quantification of the phenolic compounds was achieved by correlating the obtained peak areas with those of established external standards.
GC-IMS detection
-
The analysis of Volatile Organic Compounds (VOCs) was conducted using the FlavourSpec® analytical system (G.A.S., Dortmund, Germany)[17]. The initial step involved the preparation of wolfberry pulp samples, where 5 g were precisely measured and deposited into a 20 mL headspace vial. This vial was then subjected to a thermal treatment at a temperature of 60 °C for 30 min to facilitate the release of VOCs. Following the incubation period, the samples were injected into the gas chromatography system using an injection needle heated to 85 °C, with a specified air volume of 500 μL at the top. The Gas Chromatography-Ion Mobility Spectrometry (GC-IMS) technique was subsequently employed to resolve the volatile components within the sample. The separation process occurred in a capillary column (MXT5, 15 m × 0.53 mm inner diameter). Nitrogen gas, with a purity level of no less than 99.999%, was chosen as the carrier gas. The flow rate of the carrier gas was programmed to initiate at 2 mL/min, which was sustained for the first 2 min. This rate was then incremented to 10 mL/min over 8 min, further increased to 100 mL/min within the subsequent 10 min, and ultimately raised to 150 mL/min in another 10 min. The ions resulting from the analytical process were directed into a drift tube, which was maintained at a temperature of 45 °C. The drift gas, set at a flow rate of 150 mL/min, facilitated the ion mobility within the drift tube, thereby completing the VOC assessment procedure.
Statistical analysis
-
Sample preparation and analysis were conducted in triplicate to ensure consistency. Data were displayed as Mean ± SEM and processed using Origin 2022 software (Origin Lab, China). Statistical analysis was carried out via one-way ANOVA, with a p-value less than 0.05 indicating statistical significance. HS-GC-IMS data were extracted through the GC-IMS library retrieval method, and a bi-dimensional cross-qualitative approach was employed for qualitative analysis.
-
Some studies have reported that lactulose and inulin promote the growth of Lactobacillus bulgaricus and Lactobacillus casei[18], to improve the fermentation efficiency of wolfberry pulp, we explored the effects of three prebiotics (inulin, lactulose, and galacto-oligosaccharides) on the fermentation of wolfberry. Figure 1a illustrates the impact of adding three prebiotics on total acidity during fermentation. It is evident from Fig. 1a that among the five prebiotics tested, wolfberry pulp supplemented with galactooligosaccharide produced higher total acidity after fermentation compared to pulp supplemented with inulin or lactulose. Different dosages of lactulose did not significantly influence the change in total acidity during fermentation (p > 0.05). Inulin, at varying dosages, had a certain impact on total acid formation, exhibiting a trend similar to that of galactooligosaccharides. The highest total acid content was observed in wolfberry pulp supplemented with 7% galactooligosaccharide, reaching 33.84 mg/mL. Figure 1b illustrates the quantification of viable bacteria after 48 h of fermentation with the three prebiotics at varying concentrations. The supplementation of prebiotics significantly enhanced the population of viable bacteria in fermented wolfberry pulp. The addition of lactulose maximizes the preservation of lactic acid bacteria activity (1.30 × 108 CFU/mL), aligning with findings from Ricardo's study[19]. As the dosage of prebiotics increases, the count of lactic acid bacteria also rises. When the dosage reaches 5%, the number of viable bacteria tends to stabilize. In the experimental group supplemented with galactooligosaccharides, at a 7% addition rate, the population of live bacteria peaks (1.18 × 108 CFU/mL), and as the addition rate continues to increase to 9%, the count of live bacteria decreases (1.17 × 108 CFU/mL). This decline may be attributed to the excessive addition of galactooligosaccharides hindering the growth of lactic acid bacteria. This observation is in harmony with the observed change in total acid depicted in Fig. 1a. Based on the combined data from Fig. 1a & b, galactooligosaccharides were ultimately selected as the prebiotics for augmenting the fermentation of wolfberry pulp.
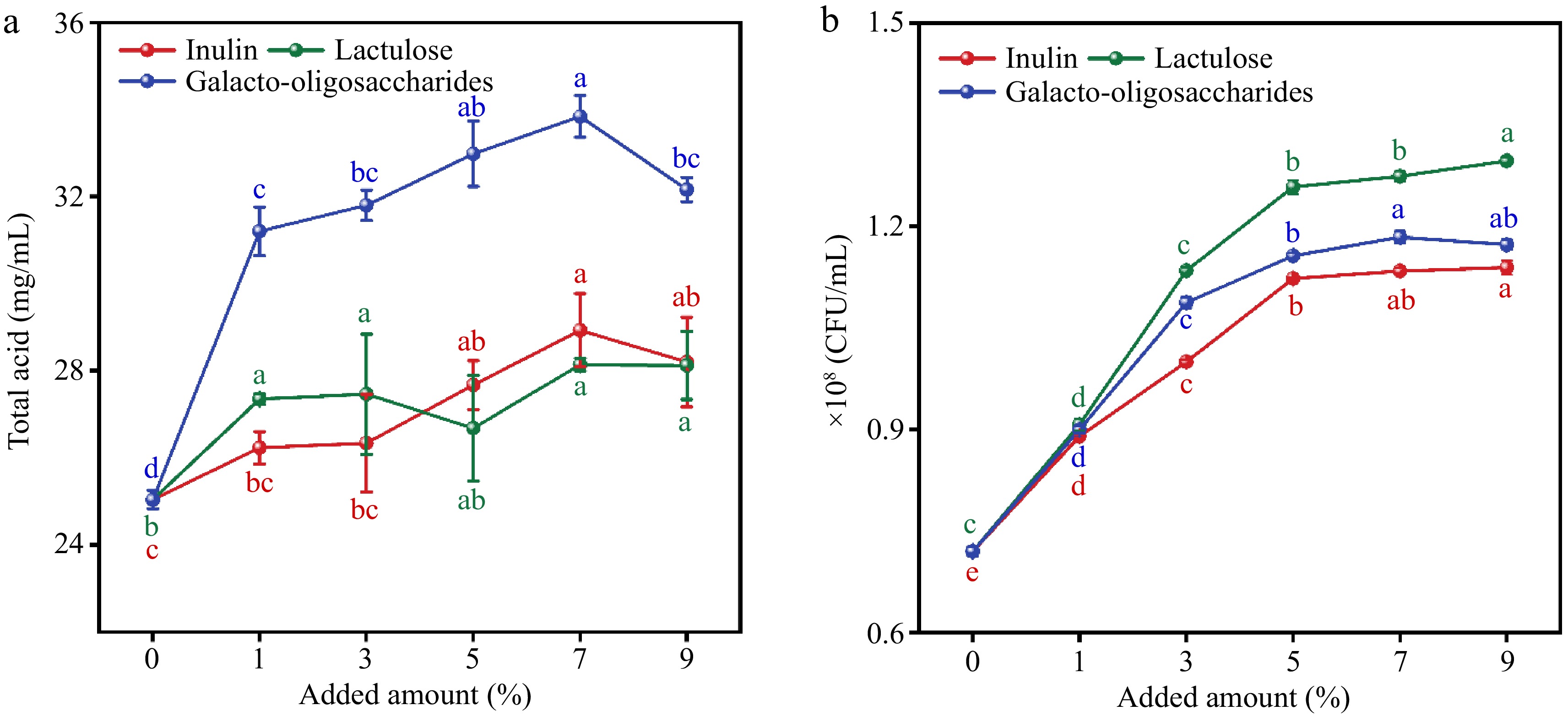
Figure 1.
Effects of adding three prebiotics (inulin, lactulose, and oligosaccharides) on (a) total acid and (b) viable bacterial count during fermentation process. The different lowercase letters marking in the figure represent different levels of significance (p < 0.05).
Establishment of fermentation dynamics model equations
Dynamic model of bacterial growth
-
Figure 2a showed the overall changes in the number of viable bacteria, total acids, and total sugars over 72 h of fermentation. Throughout the 72-h fermentation period, lactic acid bacteria underwent distinct phases, including an adjustment period, logarithmic period, stable period, and apoptosis period. Notably, the construction of fermentation kinetics models typically focuses on the changes in microbial numbers before entering the apoptosis period.
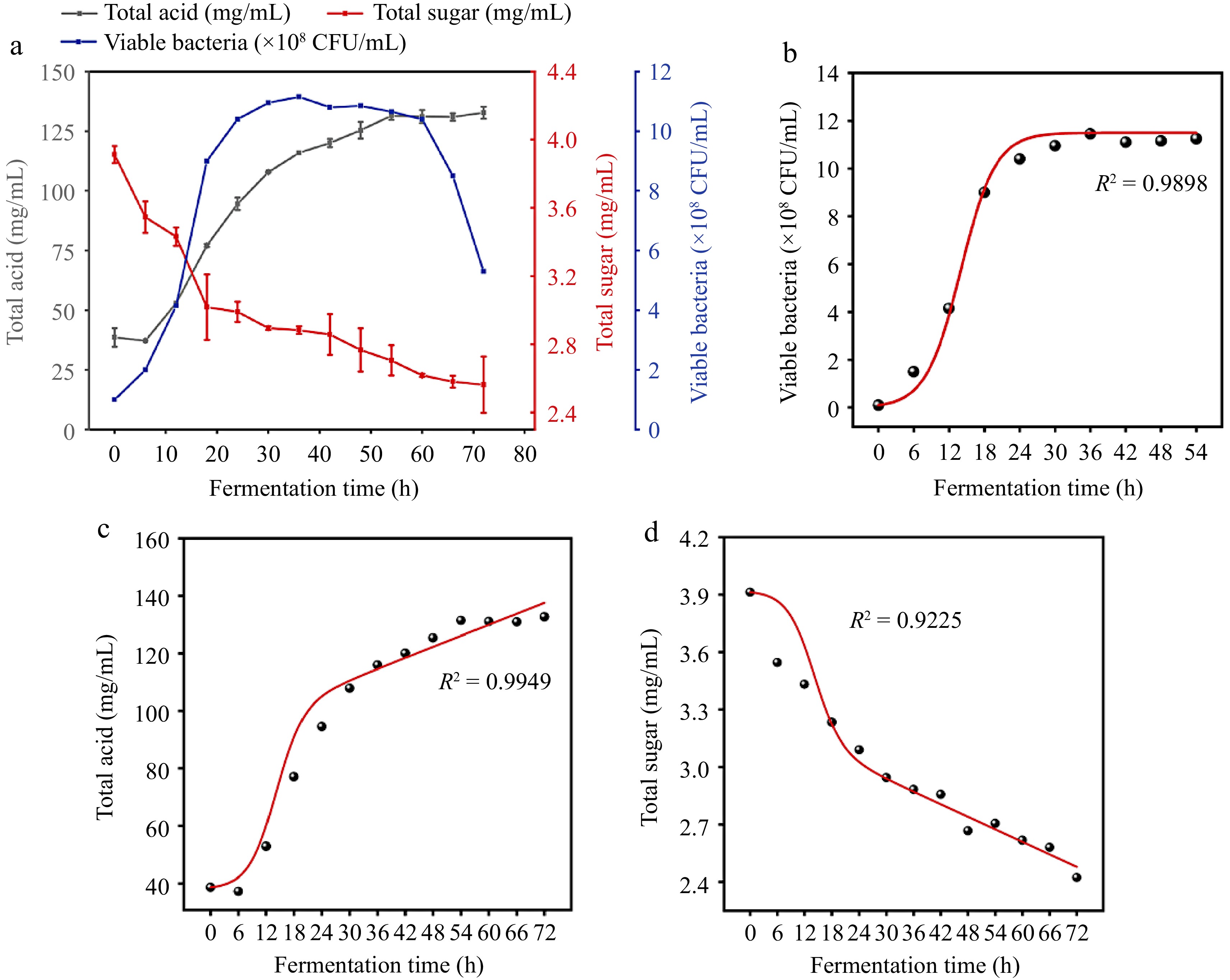
Figure 2.
(a) Changes in total sugar, total acid, and bacterial count within 72 h of fermentation. (b) Fitting curve of the kinetic equation for bacterial growth. (c) Fitting curve of the kinetic equation for total acid production. (d) Fitting curve of the kinetic equation for substrate consumption.
In Fig. 2b, it is observed that after 54 h of fermenting wolfberry pulp, plant lactobacilli entered the apoptosis period. Consequently, our study concentrated on developing kinetics models specifically within the initial 54 h of fermentation.
Figure 2a, combined with Eqn (3), yields a plot of
$ \text{ln}\dfrac{\text{X}}{{\text{X}}_{\text{m}}-\text{X}} $ $\rm \text{ln}\dfrac{\text{X}}{{\text{X}}_{\text{m}}-\text{X}} =0.3393t-4.2829,\quad R^{2}=0.9960 $ From this, it can be deduced that μm = 0.3393. Given X0 = 0.1 × 108 CFU/mL and Xm = 11.45 × 108 CFU/mL, substituting into Eqn (3), the kinetic equation for bacterial growth during wolfberry pulp fermentation is determined as:
$\rm X= \dfrac{\text{0.1}{\text{e}}^{\text{0.3393t}}}{\text{0.9913+0.0087}{\text{e}}^{\text{0.3393t}}} $ (10) Upon fitting the equation with experimental data points, an R² value of 0.9898 was obtained. This high R² value signifies that the equation effectively represents the changes in bacterial growth over time. The robust fit of the equation to the experimental data highlights its reliability and accuracy in capturing the dynamics of bacterial growth during the fermentation process of wolfberry pulp.
Product generation kinetics model
-
Based on the total acid production curve in Fig. 2a, it is known that Lactobacillus plantarum is in the stable growth phase after 24 h, where
$ \dfrac{\text{dX}}{\text{dt}} $ From Eqn (5), it is derived that: β =
$ \dfrac{\text{dP}}{\text{dt}} $ $ \dfrac{\text{1.9374}}{\text{11.45}} $ Substituting μm, X0, Xm, β, and α into Eqns (6), the product generation kinetic model is obtained. After calculation, the product generation kinetics equation for wolfberry pulp fermentation process is:
$ \begin{split}\rm X =\;&0.4457 \times \left( \dfrac{{\text{e}}^{\text{0.3393t}}}{\text{0.9913+0.0087}{\text{e}}^{\text{0.3393t}}} -1\right)+\\&2.6223 \times \ln(0.9913+0.0087 {\text{e}}^{\text{0.3393t}} ) \end{split}$ (11) Following the fitting of the equation with experimental data points, an R² value of 0.9949 was achieved. This high R² value indicates that the equation aptly represents the process of total acid generation during the fermentation of wolfberry pulp. The close alignment between the model and experimental data underscores the accuracy and reliability of the equation in capturing the dynamics of total acid production (Fig. 2c).
Substrate consumption dynamics model
-
Based on the substrate consumption data from the change in Fig. 2a, taking the data during the stable growth phase of bacterial cells, the relationship between total sugar content S and time t is determined as: S = −0.01789t + 0.6359, with a correlation coefficient R2 = 0.9179. From Eqn (8), it is found that: δ = 0.0194.
Substituting parameters X0, Xm, and δ into Eqn (9) to plot S0 − S − δD(t) against C(t), the relationship between them is: S0 − S − δD(t) = 0.1524C(t), with a correlation coefficient R2 = 0.9114. Thus, γ is determined to be 0.1524. After calculation, the kinetic equation for substrate consumption is:
$\begin{split}\rm X=\;&3.9126-0.007 \times \left( \dfrac{{\text{e}}^{\text{0.3393t}}}{\text{0.9913+0.0087}{\text{e}}^{\text{0.3393t}}} -1\right)-\\&0.0322 \times \ln (0.9913+0.0087 {\text{e}}^{\text{0.3393t}} ) \end{split}$ (12) Upon fitting the equation with experimental data points, an R² value of 0.9225 was attained. This R² value indicates that the equation effectively represents the process of total sugar consumption during the fermentation of wolfberry pulp. While slightly lower than perfect correlation, the substantial R² value demonstrates the reliability of the equation in capturing the dynamics of total sugar utilization (Fig. 2d).
Effects of fermentation on phytochemicals and antioxidant activity of wolfberry pulp
Total phenolic and total flavonoids content and antioxidant activity
-
Phenolic compounds contribute to antioxidant and anti-inflammatory health benefits. It has been pointed out that lactic acid bacteria can enhance the bioavailability of phenolic compounds by converting them into smaller, more absorbable molecules. They also release conjugated phenolics from fermentable materials which further improve its utilization rate and health benefits to the human body[20]. Figure 3 illustrates the alterations in total phenols and flavonoids before and after fermentation, along with the shifts in DPPH radical and ABTS radical scavenging rates. Upon comparing the total phenolic and flavonoid content in wolfberry pulp pre- and post-fermentation, the findings revealed a significant increase after fermentation (p < 0.05). The post-fermentation content of total phenols and flavonoids was 1.15 and 1.16 times higher than their respective levels before fermentation. Typically, phenolic content undergoes augmentation through enzymatic reactions during fermentation, wherein β-Glucosidase catalyzes the hydrolysis of glycosidic bonds, leading to the release of phenolic glycosides[15]. DPPH radical scavenging rate and ABTS radical scavenging rate serve as common metrics for assessing food antioxidant activity. Following fermentation, the DPPH radical scavenging rate in wolfberry pulp significantly increased, reaching 67.16% ± 6.70% (p < 0.05) after 48 h, and the ABTS radical scavenging rate increased from 26.50% ± 1.20% to 32.10% ± 0.90%. The alteration in free radical scavenging rate demonstrated a positive correlation with the heightened content of total phenols and flavonoids. Phenols are recognized as pivotal contributors that profoundly influence the antioxidant capacity of the food system[21]. Concurrently, the metabolic activity of lactic acid bacteria produces various phenolic compounds, which help improve the overall antioxidant capacity of food[22]. To investigate how the fermentation of Lactobacillus plantarum changes the content of phenolic compounds in wolfberry pulp, HPLC was used to determine the content of monomeric phenols in wolfberry pulp.
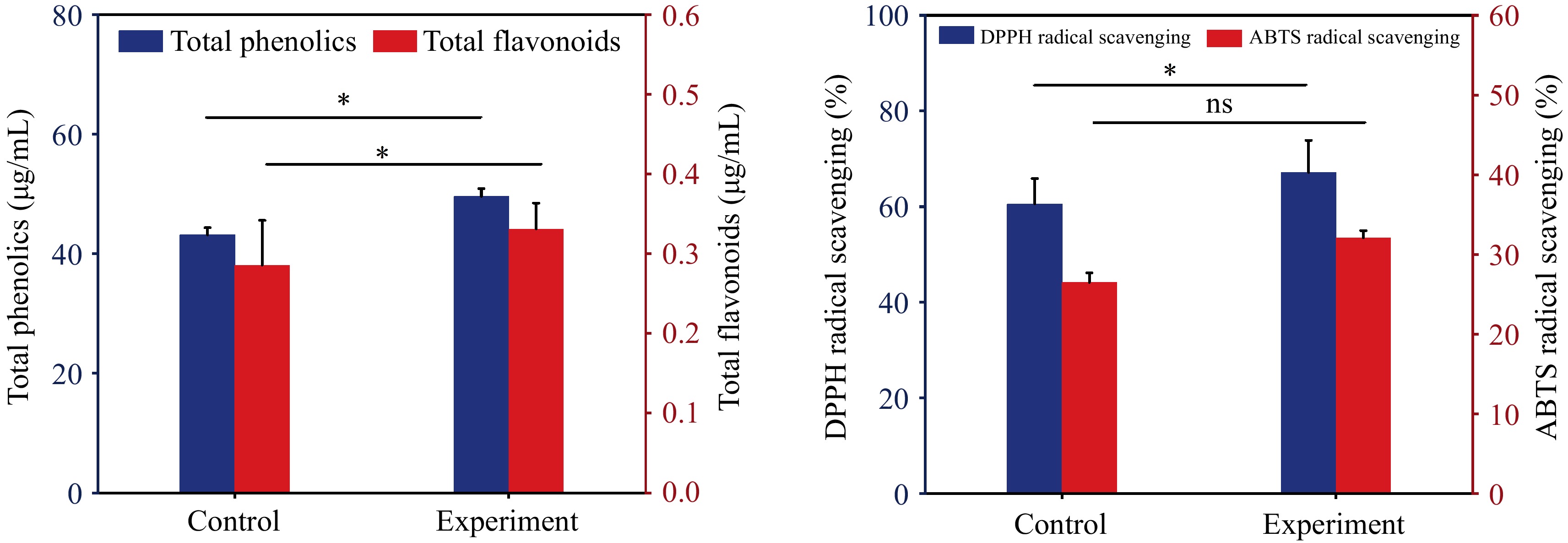
Figure 3.
(a) Effect of fermentation on the content of total phenols and flavonoids in wolfberry pulp. (b) Effect of DPPH and ABTS radical scavenging rate. * represents different levels of significance (p < 0.05), while ns represents non significance (p > 0.05).
Phenolic profiles
-
A blank control group (1) was set up along with four experimental groups, namely: after enzymolysis (2); add probiotics (3); cultivation without added prebiotics (4); adding prebiotic inference (5). The detection of five sets of samples provided a detailed explanation of the changes in composition during the production of fermented wolfberry pulp. Eight phenolic compounds, comprising five phenolic acids and three flavonoids in the wolfberry pulp, were qualitatively and quantitatively analyzed using liquid chromatography (Table 1). Chlorogenic acid, the predominant phenolic acid in wolfberry, significantly increased through fermentation (p < 0.05). In wolfberry pulp fermented with oligosaccharides, the chlorogenic acid content was 1.16 times higher than that in the blank control group, aligning with the changes in total phenols. Other phenolic acids, including gallic acid, caffeic acid, and ferulic acid, exhibited varying increases in wolfberry after enzymatic hydrolysis and fermentation. A distinctive pattern was observed in p-Coumaric acid, which increased to more than four times (23.28 ± 0.81 μg/mL) its original value (5.32 ± 0.46 μg/mL) after enzymatic hydrolysis. However, after fermentation, the p-Coumaric acid content decreased again, matching that of the blank group (5.37 ± 0.13 μg/mL). This might be attributed to the poor stability of coumaric acid in low-pH environments. Three flavonoids, catechin, rutin, and quercetin, all showed significant increases after fermentation (p < 0.05). Rutin, a common dietary flavone, possesses pharmacological properties, including antibacterial, anti-inflammatory, anti-cancer, anti-diabetic effects[23]. Among the eight monomeric phenols detected rutin exhibited the highest content, attributable to the inherent richness of rutin in wolfberry itself. The alterations in monomeric phenolic substances in wolfberry pulp before and after fermentation further indicate that fermentation can stimulate the generation of phenolic substances in wolfberry pulp, thereby enhancing its antioxidant activity.
Table 1. Phytochemical profiles in wolfberry pulp before and after LAB fermentation (μg/mL).
Phytochemicals 1 2 3 4 5 Gallic acid 3.49 ± 0.74c 4.74 ± 0.38b 4.87 ± 0.30b 7.76 ± 0.02a 7.92 ± 0.28a Catechin 33.79 ± 4.83b 37.70 ± 2.76a 39.95 ± 3.87a 42.36 ± 2.01a 42.31 ± 2.78a p-Coumaric acid 5.32 ± 0.46b 23.28 ± 0.81a 21.09 ± 1.06a 5.27 ± 0.55b 5.37 ± 0.13b Chlorogenic acid 36.63 ± 10.11c 109.29 ± 3.87b 107.56 ± 3.95b 165.56 ± 4.08a 159.04 ± 8.58a Caffeic acid 2.68 ± 0.68ab 1.39 ± 0.81b 4.30 ± 0.79a 4.57 ± 0.99a 3.11 ± 0.54ab Ferulic acid 5.97 ± 0.25c 8.12 ± 0.80ab 8.37 ± 0.82a 7.45 ± 0.41abc 6.55 ± 0.26bc Rutin 208.09 ± 19.27b 311.27 ± 41.04ab 263.13 ± 16.56ab 361.47 ± 23.15a 265.70 ± 60.17ab Quercetin 41.60 ± 1.09c 39.93 ± 1.76c 38.88 ± 0.38c 58.21 ± 1.88a 53.29 ± 1.79b Values with different superscripts in the same row have significant differences (p < 0.05). Among them: without any processing (control) (1); after enzymatic hydrolysis (2); add prebiotics (3); fermentation without added prebiotics (4); adding prebiotic fermentation (5). Identification and analysis of volatile components in wolfberry samples
-
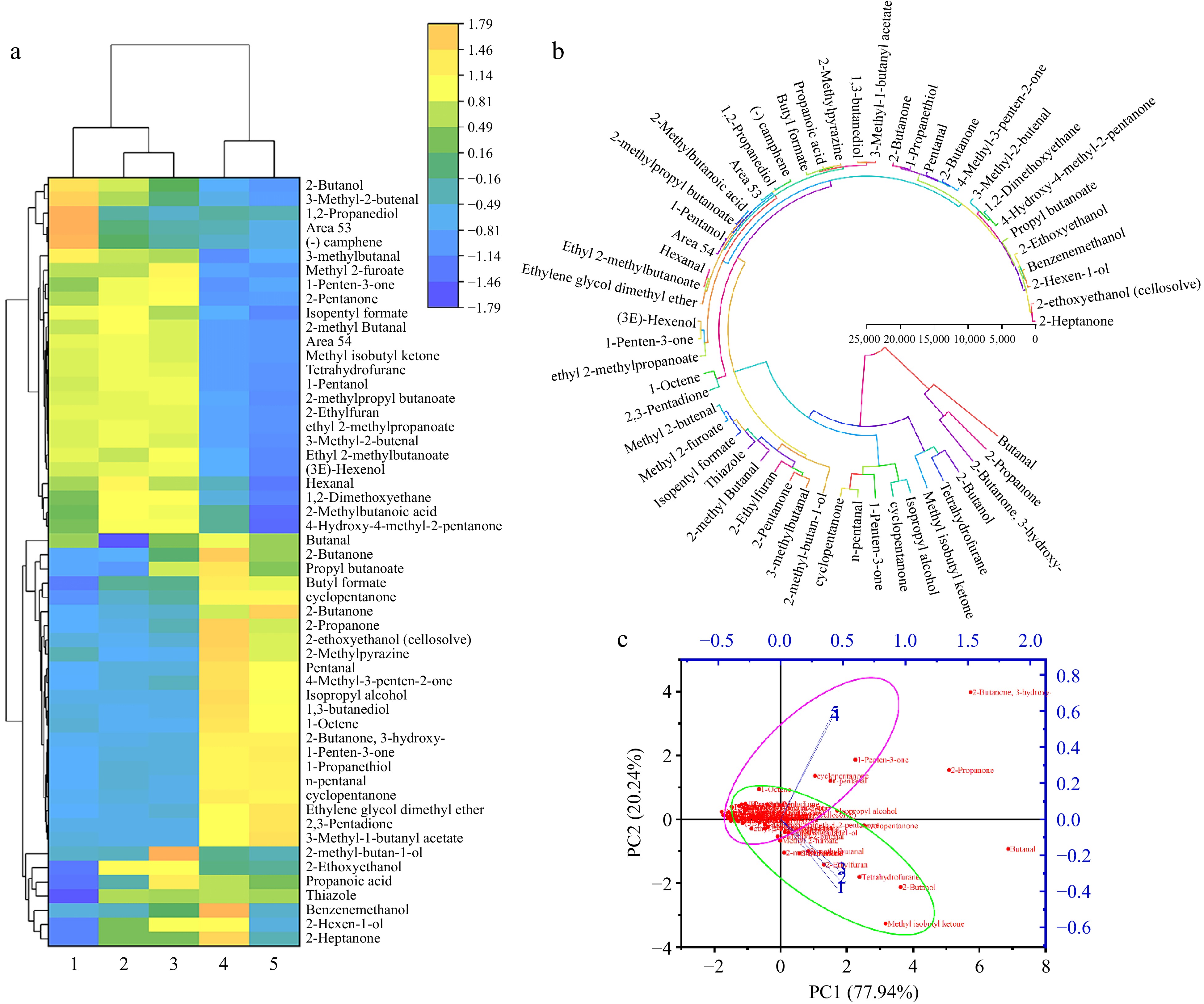
Five sets of samples underwent analysis using HS-GC-IMS (Fig. 4). A total of 51 primary volatile aroma components were identified, comprising 12 alcohols, seven aldehydes, two acids, eight esters, 12 ketones, and 10 other compounds. Two among them lacked matching names in the database, suggesting they might constitute unique aroma components of wolfberry. In comparison to the original pulp, enzymatic hydrolysis resulted in an increase in hexanal, 4-methyl-4-hydroxy-2-pentanone, 2-methyl-butan-1-ol, and other substances. However, during subsequent fermentation, these substances were utilized and consumed by microorganisms as substrates (Fig. 5). Fermentation introduced more flavor components to the wolfberry pulp, such as 2-butanone, butyl formate, and 4-methyl-3-penten-2-one. Ketones can exhibit distinct flavor characteristics at low concentrations[24]. HS-GC-IMS detection results indicated that fermentation could augment the content of various ketones in wolfberry pulp. A trace number of harmful components to human health were detected in the samples before fermentation, such as tetrahydrofuran and 2-ethylfuran, which may be characteristic flavor components of wolfberry. The content of furan compounds also significantly decreased after fermentation. This suggested that fermentation could degrade some residual harmful components while enhancing the presence of beneficial aroma components. Principal component analysis demonstrated a significant distinction between the blank control group and the two groups without fermentation treatment, as well as the two groups with fermentation treatment. PC1 and PC2 contributed 77.94% and 20.24%, respectively, resulting in a total contribution rate of 98.18%.
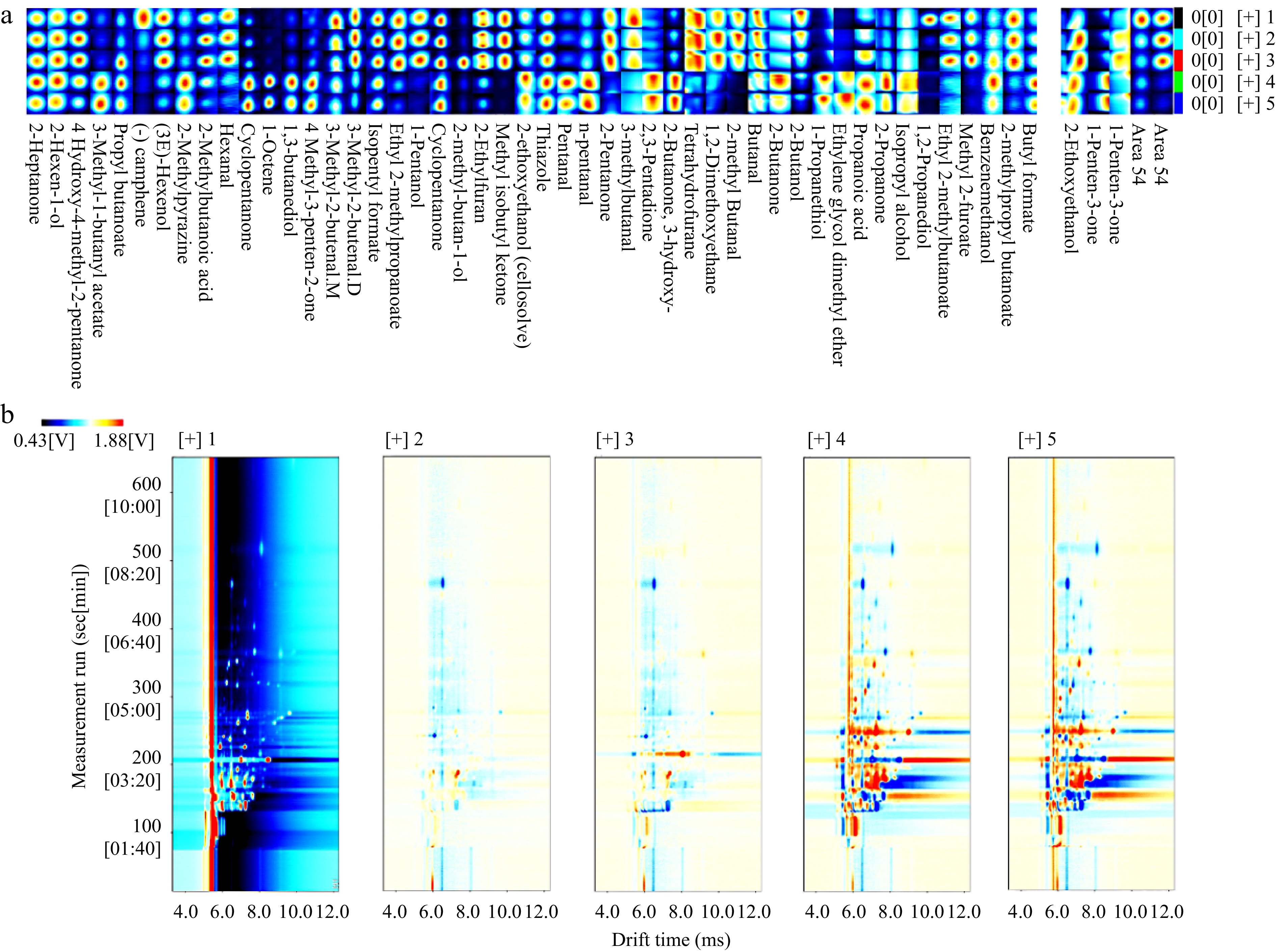
Figure 4.
(a) Fingerprint of volatile aroma components in five samples of wolfberry pulp. (b) Subtraction plot of different wolfberry pulp samples. Among them: without any processing (control) (1); after enzymatic hydrolysis (2); add prebiotics (3); fermentation without added prebiotics (4); adding prebiotic fermentation (5).
-
This study demonstrated the substantial impact of lactic acid bacteria fermentation on the flavor, chemical characteristics, and antioxidant activity of wolfberry pulp. Lab fermentation significantly increased the content of total phenols and flavonoids in wolfberry pulp (p < 0.05), subsequently enhancing its antioxidant activity with a positive correlation. The alterations in volatile aroma components in wolfberry pulp before and after fermentation were scrutinized using HS-GC-IMS. The findings revealed that lab fermentation augmented various aldehydes, ketones, and esters, introducing novel flavors like sourness and fruitiness to the fermented wolfberry pulp. Concerning fermentation efficiency, the addition of galactooligosaccharides expedited the fermentation process of wolfberry pulp, elevating the production of total acids and yielding a more favorable fermentation outcome. In summary, this study unveiled the fermentation kinetic model of L. plantarum CCFM1050 on wolfberry pulp with galactooligosaccharides. It concluded that lactic acid fermentation could enhance the flavor and functional properties of wolfberry pulp, offering insights for its advanced processing and potential guidance for the future development of the wolfberry industry.
-
The authors confirm contribution to the paper as follows: conceptualization, funding acquisition: Peng Q; investigation: Liu H, Wang Y, Chen T; methodology: Liu H, Li N; data curation, formal analysis, draft manuscript preparation: Liu H; validation, software: Li N; resources, supervision, manuscript revision & editing: Yang H, Peng Q. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This work was supported by Beijing Engineering and Technology Research Center of Food Additives, Beijing Technology & Business University (BTBU); Science and Technology Project of Xining, Grant/Award Number: 2022-Y-12; Open Foundation of the Key Laboratory of Seaweed Fertilizers, Ministry of Agriculture and Rural Affairs, Grant/Award Number: KLSF-2023-010. The authors would like to thank the shared instrument platform of the College of Food Science and Engineering of Northwest A&F University, Mrs. Ma (Instrument shared platform of College of Food Science & Engineering, Northwest A&F University) for the assistance with food grade laboratory, Mrs. Wang (Instrument shared platform of College of Food Science & Engineering, Northwest A&F University) for the assistance with HPLC (LC-2030 PLUS, Shimadzu), Mrs. Cao (Scientific Research Center of College of Horticulture, Northwest A&F University) for the assistance with HS-GC-IMS (FlavourSpec®, G.A.S., Dortmund, Germany).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Liu H, Li N, Wang Y, Cheng T, Yang H, et al. 2024. Study on fermentation kinetics, antioxidant activity and flavor characteristics of Lactobacillus plantarum CCFM1050 fermented wolfberry pulp. Food Innovation and Advances 3(2): 126−134 doi: 10.48130/fia-0024-0012
Study on fermentation kinetics, antioxidant activity and flavor characteristics of Lactobacillus plantarum CCFM1050 fermented wolfberry pulp
- Received: 20 March 2024
- Revised: 07 May 2024
- Accepted: 08 May 2024
- Published online: 27 May 2024
Abstract: As a superfruit, wolfberry has extremely high nutritional value, and how to enhance the accessibility of its nutrients is the core of current research. This study focused on exploring the kinetic model of Lactobacillus plantarum CCFM1050 fermentation of wolfberry and the potential alterations of antioxidant activity and volatile flavor compounds induced by lactic acid fermentation. we monitored cell counts, product formation, and substrate changes over a 72-h period of wolfberry fermentation. A kinetic model was developed to illustrate cell growth, substrate consumption, and product accumulation during wolfberry pulp fermentation. Phenolic substance analysis revealed a significant increase in total phenol and flavonoid content in wolfberry pulp during fermentation, reaching 1.16 and 1.15 times, respectively, compared to pre-fermentation levels. The elevated levels of phenolic substances led to a substantial increase in DPPH and ABTS free radical scavenging rates in fermented wolfberry pulp, reaching 67.16% and 32.10%, respectively. Volatile components of samples were analyzed using the HS-GC-IMS method, and fingerprints of wolfberry pulp before and after fermentation were established. A total of 51 compounds were identified, including 12 alcohols, seven aldehydes, two acids, eight esters, and 12 ketones, contributing to an enhanced flavor profile in the fermented wolfberry pulp. This study is helpful for understanding the kinetic changes in the lactic acid fermentation of wolfberry, the changes of antioxidant active substances and VOCs, and provides guidance for the industrial processing of wolfberry.
-
Key words:
- Wolfberry /
- Lactobacillus plantarum /
- Kinetic model /
- Antioxidant activity /
- Aroma components