-
Fungal infections pose a major challenge within the food supply, leading to substantial postharvest losses. The Food and Agriculture Organization (FAO) data estimated that the amount of food wasted worldwide on farms, in transportation, storage, wholesale, and processing, reached 13.2% in 2021. This is comparable to earlier estimations from the time reporting started, which were 13.3% in 2020 and 13% in 2016[1]. Factors such as rough handling, postharvest metabolism and disease, inadequate transportation, improper storage, and distribution are reported to be responsible for this waste[2].
It is worth noting that fungal pathogens are one of the most severe causes of the deterioration and considerable postharvest losses in fruits and vegetables. In addition, certain fungi may cause harm by releasing mycotoxin[3]. Mycotoxins produced by specific fungal genera and species are low molecular weight compounds that affect nearly 25% of food and feed crop output worldwide[4], and are extremely harmful to humans and animals[5]. For example, freshly picked fruits and their derivatives are contaminated with a highly toxic mycotoxin such as patulin (PAT), mostly produced by pathogens including Aspergillus spp., Penicillium spp., and Byssochlamys spp. When the fruits are infected by the pathogen, decay occurs together with the production of PAT[6]. Furthermore, other mycotoxins such as Aflatoxin (AF) and fumonisin (FUM) are among the priority mycotoxins to control in foods. These toxins contaminated breast milk and complementary foods in Nigeria, increasing infant mycotoxin exposure. AFs are classified as class 1 human liver carcinogens, while the prevalence of birth abnormalities and esophageal cancer have been linked to FUM consumption[7].
Black spot caused by Alternaria alternata is among postharvest diseases that occur naturally in fruits, vegetables, and cereals, which has gained global attention due to its high occurrence in fruits and their derived products. Furthermore, Alternaria spp. produce mycotoxins widely known as Alternaria toxins. These toxins were detected in tomatoes and their products[8], cherries[9], apples[10], and jujube juice[11]. Alternaria toxins have been considered one of the most serious risk factors for human health as they may cause esophageal cancer[12]. These phenomena deserve global attention to minimize and eliminate the potential dietary risks as well as ensure public health[13]. Apart from A. alternata, Botrytis cinerea is also globally recognized as a necrotrophic fungus responsible for gray mold disease in hundreds of host plants, which can produce toxins and reactive oxygen species (ROS) to kill the host cells[14]. In addition, it has a wide host range, several attack mechanisms, high genetic diversity, and adaptable phases to withstand harsh environments, making it difficult to control[15].
Control of fruit postharvest disease was generally performed by the treatment of fungicides such as 2-amino-pyrimidine, benzimidazoles, carboxanilides, phosphorothiolates, morpholines, dicarboximides, phenylamides, and sterol demethylation inhibitors (DMIs)[16]. Synthetic and organic fungicides together hold a majority share in the European Union market for pesticides, making up over 60% of total sales[17]. However, their application is vital in determining their environmental effects[18]. For instance, the overall soil microbial activity decreased when difenoconazole was applied at a rate of 500 mg kg−1 DW soil, which negatively impacted soil enzymes, as shown by the respiratory quotient[19]. The predominant and extensively employed group of organic sulfur fungicides, known as Ethylenebisdithiocarbamate (EBDC), has been linked to eye and upper respiratory tract inflammation[20]. Furthermore, there is a widespread prevalence of pathogen resistance to fungicides[16]. The fungus B. cinerea was found to be cross-resistance to dicarboximides[21]. Similarly, resistance to thiabendazole was evident in 70% of Penicillium expansum isolates from grapes, pears, and apples[3]. This resistance emerges as initially, rare mutants survive and spread during treatment with fungicide. It involves various mechanisms, typically by altering the fungicide's primary target in the fungal pathogen[16]. These phenomena have led to the restriction of fungicide utilization in fruit postharvest disease control[22], grown public concern over the sustainable approaches in postharvest disease control, and encouraged more research to develop low-cost and efficient strategies to replace the use of chemical fungicides[3].
Several sustainable strategies in fruit postharvest management have been successfully applied against fungal pathogens as alternatives to chemical fungicides. The utilization of biological control agents (BCAs) is considered a promising alternative to fungicides and has been globally increasing and gaining more attention due to their ability to suppress fruit postharvest diseases caused by pathogenic fungi[23]. Colonization of cherry tomatoes by the yeast Wickerhamomyces anomalus resulted in the inhibition of B. cinerea[24] and A. alternata growth, respectively through the prevention of spore germination, decreased germination tube length, and an increase in defense-related enzyme activities and defense-related genes involved in several metabolisms[25]. Similarly, Pichia fermentans and Lodderomyces elongisporus have been shown to effectively reduce Aspergillus parasiticus growth and AFs contamination in the marinade and its products[26]. The application of (E)-2-hexenal, a plant-volatile organic compound with high bioactivity, significantly suppressed the gray mold incidence in strawberries by consuming glutathione of B. cinerea. When B. cinerea was exposed to higher concentrations of (E)-2-hexenal, the fungal capacity for survival and reproduction was irreversibly lost[27]. The trends in the application of BCAs experienced significant growth from approximately USD 2.1 billion in 2011 to USD 4 billion in 2017[28]. Furthermore, at 50 universities in the United States, biological control education was added to integrated pest management and sustainable agricultural courses and workshops. With this development, biological control has progressively evolved from being essentially an autonomous field to become a more integral component of integrated pest management over the last 24 years[29].
This review aims to summarize the recent developments in the application of biological control and other sustainable approaches in managing fruit postharvest diseases, with an emphasis on A. alternata and B. cinerea, respectively. Furthermore, several action mechanisms, challenges, and prospects of the application of BCAs will also be discussed.
-
The fungus Alternaria is widely distributed and comprises pathogenic, endophytic, and saprobic species that are linked to a broad range of substrates[30]. It is also globally recognized for it's capacity to generate a broad range of secondary metabolites, such as mycotoxins that can contaminate foods and different phytotoxins linked to plant disease, both host- and non-host-specific[31]. This plant pathogen is capable of causing postharvest spoiling of several crops, such as tomatoes, strawberries, apples, melon, pears, and citrus[5], and is reported to infect over 400 host plants[32].
Alternaria alternata is considered one of the most devastating fungi infecting soybean foliar, causing leaf spot and leaf blight diseases[33]. This global fungal genus is also a key player in the grapevine microbiome and has been identified as producing a wide range of secondary metabolites, which are especially important in terms of crop protection and food safety[34]. The fungus A. alternata causes black spot as well as brown spot in citrus mainly through exploiting the surface lesions caused by sunburn, bruising, or fruit cracking[35]. In tomatoes, A. alternata diseases are marked by the appearance of early fruit blight, stem, and canker. In extreme situations, these diseases result in full plant defoliation by reducing the photosynthetic surface of the leaves[36]. Although A. alternata is a necrotrophic pathogen, it can also infect seeds and influence the following generation if the plant sustains significant damage[36]. Furthermore, A. alternata is responsible for latent infections in winter jujubes[37]. Through horizontally transferring a whole pathogenicity chromosome, A. alternata was able to generate the host-specific toxin AAL and infect tomatoes[38]. Plant and animal cells exposed to Alternaria toxins undergo apoptotic morphology as a result of the death process[39]. The toxins produced by A. alternata are responsible for their pathogenicity on tomatoes, inhibiting the sphingolipid biosynthesis in vitro and toxic for certain plant species. A. alternata conidia germinate rapidly in damp environments and start to release toxins before penetrating the tissue[40]. In addition, the toxigenic fungus A. alternata causes brown rot in apples and has been associated with food poisoning since it can produce mycotoxins such as altenuene (ALT), the benzopyrene derivatives alternariol (AOH), the perylene derivative altertoxin (ATX), the tenuazonic acid (TeA), alternariol monomethyl ether (AME), and tentoxin (TEN) during infection[10]. The main Alternaria toxins, including their chemical names, molecular weights, CAS numbers and hazard identifications are compiled in Table 1 as reported in the scientific literature[41,42]. Various techniques have been applied to determine and confirm the identity of Alternaria toxins, including high-performance thin-layer chromatography (HPTLC), thin-layer chromatography (TLC), gas chromatography (GC), and more often liquid chromatography (LC), primarily with ultraviolet (UV) detection, atmospheric pressure chemical ionization (APCI), LC-mass spectroscopy (MS), and LC-MS/MS[41]. Although the Alternaria toxins cause serious issues, none of the Alternaria toxins found in food and feed are subject to specific national or international restrictions[43].
Table 1. The chemical name, molecular formula, molecular weight, CAS number, and hazard identification of major Alternaria toxins.
Chemical structure Mycotoxin Chemical name Molecular formula Molecular
weight (g/mol)Chemical abstracts services (CAS) number Hazard identification Dibenzopyrone derivatives Alternariol (AOH) 3,7,9-trihydroxy-1-methyl-6H-dibenzo[b,d]pyran-6-one C14H10O5 258.226 641-38-3 - AOH, AME, and ALT are less poisonous than other mycotoxins
- At doses of ≥ 1 μM and 25 μM, respectively, AOH and AME markedly enhanced the rate of DNA strand breaks in human colon cancer cellsAlternariol monomethyl ether (AME) 3,7-dihydroxy-9-methoxy-1-methyl-6H-dibenzo[b,d]pyran-6-one C15H12O5 272.253 23452-05-3 Altenuene (ALT) 2α,3α,4aβ-tetrahydro-2,3,7-trihydroxy-9-methoxy-4a-methyl-6H-dibenzo[b,d]pyran-6-one C15H16O6 292.284 29752-43-0 Perylene quinone derivatives Altertoxin I (ATX I) 1,2,7,8,12b-pentahydro-1,4,6b,10-tetrahydroxy-perylene-3,9-dione C20H16O6 352.337 56258-32-3 - Compared to AOH and AME, ATX I, -II, and -III are more powerful mutagens and acute poisons for mice
- ATX-II has high genotoxicity and is the most powerful member of the ATX group with various action mechanismsAltertoxin II (ATX II) [perylo(1,2-b)oxirene-7,11-dione,7a,8a,8b,8c,9,10-hexahydro-1,6,8c-trihydroxy-, (7aR,8aR,8bS,8cR)-] C20H14O6 350.321 56257-59-1 Altertoxin III (ATX III) [perylo(1,2-b:7,8-b’)bisoxirene-5,10-dione, 1a,1b,5a,6a,6b,10a-hexahydro-4,9-dihydroxy-] C20H12O6 348.306 105579-74-6 Tetramic acid derivatives Tenuazonic acid (TeA) 3-acetyl-5-sec-butyl-4-hydroxy-3-pyrrolin-2-one C10H15NO3 197.231 610-88-8 - TeA is more poisonous compared to AOH, AME, and ALT
- By preventing the release of freshly synthesized proteins from the ribosomes, TeA suppresses protein production at the ribosomal level in mammalian cells -
Recent scientific research has identified approximately 35 species within the genus Botrytis, with B. cinerea emerging as the best-known and most thoroughly investigated[44]. Botrytis cinerea is a necrotrophic plant disease with a broad host range that seriously destroys crops worldwide. It has three stages of the infection process: an early stage marked by the development of local necrotic lesions, an intermediate stage when the lesions start to spread more quickly, and a final stage marked by continuous lesion spreading[45]. This fungal pathogen is responsible for gray mold disease and is considered one of the most devastating pathogens in agro-economic crops such as tomatoes, grapes, kiwifruits, strawberries, apples, pears, lettuces, and ornamental crops, and also on hundreds of dicotyledonous plant species[46]. It can produce plant cell wall degrading enzymes, toxins, and an array of cell death-inducing proteins. Furthermore, it also modulates the plant-regulated cell death machinery, leading to local host cell collapse[47]. The fungus could infect plant organs in the fruit, seeds, stems, leaves, and flowers in any period of development[48]. After the establishment of fungal hyphae, B. cinerea may remain inactive for an extended period, making the infected fruits symptomless until the fungus is reactivated by ripening or an appropriate environment[49]. These latent infections go undetected during packaging and transportation until reaching distant markets, where the rots may then be revealed, ultimately leading to significant economic losses[49]. In strawberries, gray mold has a significant latency period to manifest symptoms following infection and remains latent until the fruit reaches ripeness[50]. Flower residue colonization by B. cinerea is thought to be a major infection mechanism in grapes. When the environment is conducive to the development of the disease, the pathogen could persist in the cluster and initiate new infections of the berries[51]. Once B. cinerea inoculum survives on floral residues, it can cause infections on tissue lesions triggered by biotic (powdery mildew infections, fruit flies, and grape moths) or abiotic (hail, wind, or striking amid berries) damage[51]. In the early stages of kiwifruit disease, the fruit does not rapidly decay. Rather, the virus grows again when the fruit matures, either in cold storage or throughout shelf life[52].
-
The reduction of postharvest losses, as well as the need for sustainable disease control has grown as a result of tighter regulations on chemical fungicides. While fungicides are still the primary means of managing postharvest diseases of fruits and vegetables, more studies have been conducted to identify sustainable alternatives due to the negative impacts of fungicides on human health and the environment, as well as the prevalence of pathogen resistance[28]. Sustainable postharvest disease management is provided via biological control, partly due to the emergence of parasitoid and predator resistance. Biological control is the use of natural microorganisms, such as yeast and bacteria, as antagonists in controlling pests, diseases, or weeds through an ecological interaction[53]. This ecosystem service, valued at billions of dollars yearly, has been regarded as a promising alternative because of its cheap cost and long-term effectiveness[54].
Several yeasts have been reported to be successfully applied as BCA against postharvest pathogens[28]. Metschnikowia pulcherrima strain MACH1 effectively inhibited A. alternata and B. cinerea growth in apples via iron depletion[55]. Furthermore, by enhancing the enzymatic system for ROS scavenging in cherry tomatoes, Pichia caribbica was able to greatly reduce the incidence of black spot and sustain the elevated levels of essential antioxidant compounds while simultaneously reducing the generation of O2‾·, H2O2, and malondialdehyde (MDA)[56]. The antagonistic yeast W. anomalus, isolated from the soil in a fruit orchard, significantly controlled postharvest gray mold disease of kiwifruits caused by B. cinerea through preventing fungal pathogen growth in vitro, quickly colonizing kiwifruit wounds and surfaces, adhesion to fungal mycelia's surface, potent biofilm-forming capacity, and production of volatile organic compounds (VOCs) with antifungal properties[57]. Its high-throughput sequencing analysis showed that W. anomalus has a major impact on the fungal community and has a favorable effect on the structure of the kiwifruit epiphytic and endophytic communities[58]. Chitin, extracted from yeast cells, is a successful method for triggering resistance against gray mold decay in tomatoes caused by B. cinerea. Its application also leads to an increase in the accumulation of ROS and deposition of callose[59]. In addition to this evidence, several BCA products such as Candifruit, Aspire, BOTRY-Zen, and BoniProtect have also been developed and commercialized, which thus have the potential to make a significant contribution to the biocontrol of fruit postharvest diseases[60].
Furthermore, to offer a broad range, persistence, and higher levels of yeast concentration against fungal diseases, a variety of natural substances, in conjunction with antagonists, have been recently identified to improve the BCA effects[60]. Several natural compounds and their remarkable effects as enhancers have been applied together with antagonists against postharvest diseases in fruits and vegetables[61]. Generally, these combined treatments serve a sustainable strategy that is highly effective and safe for controlling fruit postharvest diseases, in which disease control at the commercial level (97%–99%) can be achieved through these combined methods[61]. Rhamnolipids, a surfactant produced by Pseudomonas aeruginosa, was more effective in degrading A. alternata disease in cherry tomatoes when combining with Rhodotorula glutinis compared to their single treatment. Furthermore, the activities of phenylalanine ammonialyase (PAL), polyphenoloxidase (PPO), and peroxidase (POD) in cherry tomatoes were significantly stimulated by the combination treatments, which were higher than those of the single treatments[62]. Similarly, the control effects of Cryptococcus laurentii in suppressing gray and blue mold disease in pears were significantly higher after combining with calcium chloride (CaCl2)[63]. Other substances such as ascorbic acid (AA)[64], chitosan[65], methyl jasmonate (MeJa)[66], phytic acid[67], sodium bicarbonate (SBC)[68], and sodium carboxymethyl cellulose (CMC-Na)[69] have also been successfully applied as enhancer to boost the effectiveness of antagonistic yeasts against fungal pathogens (Table 2).
Table 2. Various combination treatments that have been successfully applied in fruit postharvest disease control.
Treatment Target pathogens Target crops Ref. Ascorbic acid + Pichia caribbica Penicillium expansum Apples [64] Chitosan + Pichia anomala Grapes [65] Methyl jasmonate + Meyerozyma guilliermondii Apples [66] Phytic acid + Rhodotorula mucilaginosa Botrytis cinerea Strawberries [67] Sodium bicarbonate + Kloeckera apiculate/Metschnikowia fructicola Cherry fruits [68] Sodium carboxymethyl cellulose + Rhodosporidium paludigenum Alternaria alternata Jujubes [69] Understanding the mechanisms of postharvest BCAs is a foundation for product development and registration. In general, research on biocontrol yeasts mainly includes four major modes of action: (1) antibiotic production; (2) competition for nutrients and space; (3) direct parasitism; and (4) induction of host resistance[70]. The fungal infection mechanism and resistance mechanisms of fruits against fungal pathogens are shown in Fig. 1.
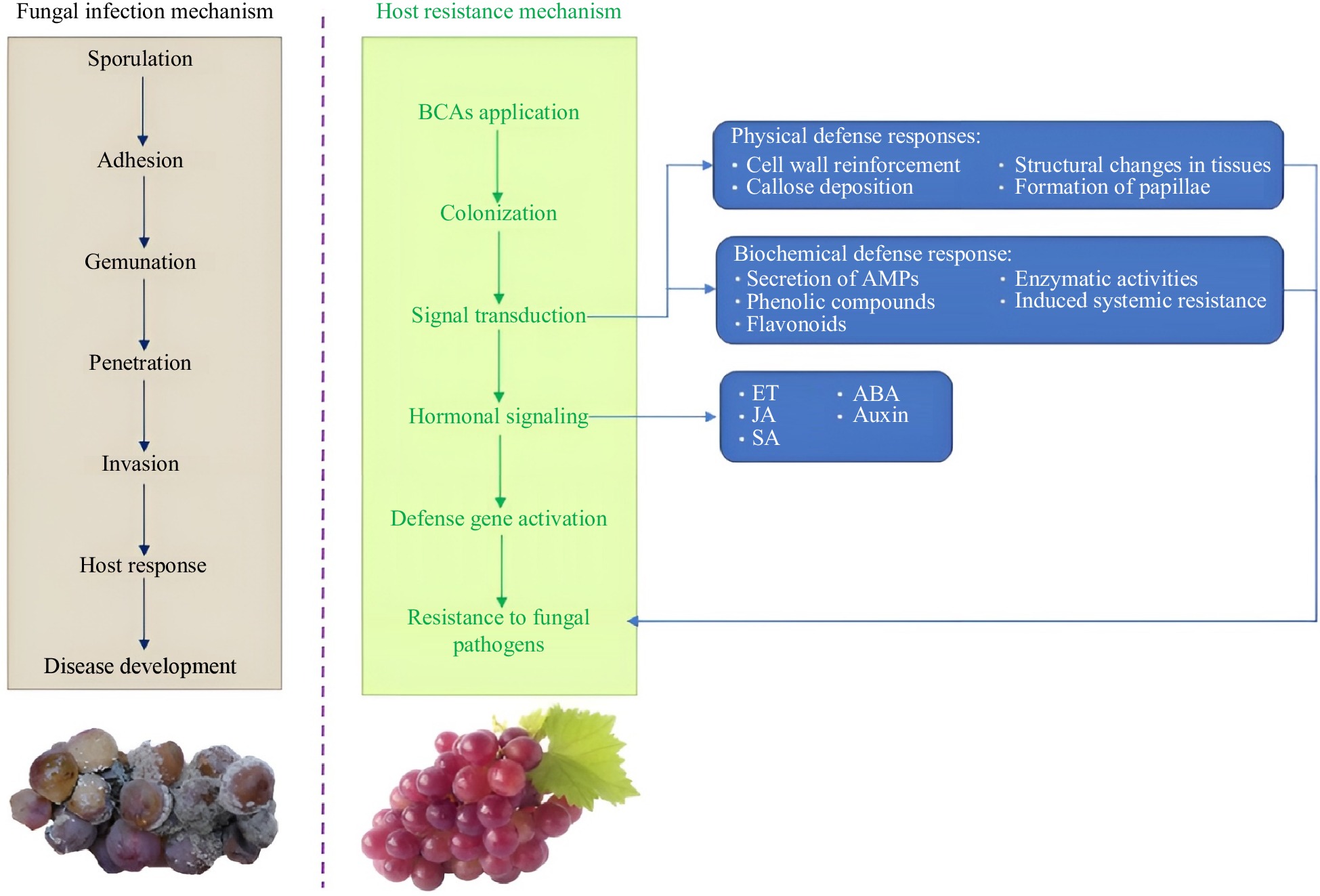
Figure 1.
Fungal infection mechanism and resistance mechanisms of fruits against fungal pathogens after treatment with BCAs.
Antibiotic production
-
Antibiotics are a widely recognized mechanism for the biocontrol activity of microorganisms on fruit wounds, leaf surfaces, and rhizosphere[71]. For example, Pseudomonas aeruginosa could produce the glycolipid antibiotic rhamnolipid B that has the ability to inhibit postharvest disease of fruits and suppress the development of pathogen infection on leaf surfaces[72]. However, although the microorganisms that can produce antibiotics have the potential to be BCAs for the postharvest disease of fruits, due to their high risks and poor safety, the development focused on antagonistic microorganisms that do not produce antibiotics is becoming more necessary[73].
Competition for nutrients and space
-
It is interesting to note that the presence of antagonistic BCAs can have an impact on the postharvest disease of fruits. This is because microorganisms are constantly competing with each other and the host for resources like nutrients and space. The combination treatment of rhamnolipids and R. glutinis inhibited the spore germination of A. alternata in cherry tomatoes through destructing microbial cell membranes, reducing spore movement, and leading to spore collapse[62]. Furthermore, through the use of scanning electron microscopy (SEM) and transmission electron microscopy (TEM), it has been observed that recombinant yeast GS115/CEC possessed the ability to degrade the DNA and RNA of A. alternata fungal cells. This yeast strain induced the expression of pathogenesis-related proteins, thereby effectively reducing postharvest decay in cherry tomatoes[74].
Direct parasitism
-
There is limited information concerning BCAs that directly attack and parasitize fungal pathogens in the postharvest area[71]. However, the yeast cells of Pichia guilliermondii and Debaryomyces hansenii were found to strongly attach to B. cinerea hyphae. When the yeast cells detached the B. cinerea hyphae, the hyphal surface showed signs of deterioration, and the attachment points showed evidence of partial B. cinerea cell wall breakdown. The partial degradation of the cell wall of B. cinerea by P. guilliermondii was related to its persistent attachment to hyphal walls in relation to its production of β-1,3-glucanase[75].
Induction of host resistance
-
The antagonistic yeasts can interact with the host tissue, particularly the wounds, and thereby enhance the process of cicatrization. It is confirmed that applying these antagonists before pathogen infection increases their effectiveness. Furthermore, through elicitors that are either produced or a component of their cell wall, yeast cells may trigger resistance processes in fruit skin[70]. Candida saitoana applied to apple wounds induced the activity of chitinase and structural barriers that develop along the walls of the host cell, like papillae[76]. A similar case was also found in apple wounds, in which Aureobasidium pullulans affected a transient increase in chitinase, β-1,3-glucanase, and peroxidase activities[77].
-
Different secondary metabolites produced by plants, including fruits, leaves, flowers, buds, stems, seeds, barks, and roots have biocidal properties that are suitable for making EOs and can act as fungal inhibitors[78]. EOs are natural antimicrobials extracted from plants that potentially fight a variety of foodborne diseases and spoilage microorganisms[79]. Various EOs, including winter savory, peppermint, oregano, eucalyptus, and wintergreen, as well as their key components such as limonene and carvacrol, have shown promising antimicrobial, antioxidant, insecticidal, and herbicidal properties for the agricultural and food sector[80]. In addition to their biodegradable characteristics, EOs are also able to limit the development of pest resistance and have little effect on non-targeted species[81]. The efficacy of Melaleuca alternifolia EOs against postharvest fungal pathogens has been confirmed in multiple pathogenic systems, including A. alternata in tomatoes, B. cinerea and Rhizopus stolonifera in strawberries, Stemphylium spp. in onions, and Monilinia fructicola in peaches[82]. EOs from Origanum vulgare and their major components (thymol and carvacrol) strongly decreased B. cinerea mycelial growth and spore germination in vitro, as well as postharvest decay in cherry tomatoes[83].
Modified atmosphere packaging (MAP)
-
MAP is reported can extend the shelf life of agricultural goods by preventing anaerobiosis activities[84]. Through package respiration, MAP utilizes materials with a particular gas permeability to regulate changes in oxygen levels. As a result, MAP can sufficiently prevent fresh products from respiring[85]. It has been reported that the application of MAP with medium CO2 levels (nearly 3 kPa) and O2 levels of at least 12 kPa, RH below 90 % could extend the overall quality of red ripe cherry tomatoes and suppress the decay incidence in the fruits caused by A. alternata and B. cinerea, respectively[86].
Edible coating
-
Edible films/coatings are biodegradable polymer thin layers widely used in food packaging that have gained a lot of attention due to their benefits such as being edible, safe, biodegradable, fresh food preservers, preventing spoilage, prolonging the shelf life, and maintaining food qualities, especially during transit and distribution[87]. Gelatin-based edible coatings, including ethanolic propolis (PEE) extract from beehives in the Monte area of Argentina demonstrated exceptional antifungal activity against fungal diseases of A. alternata and B. cinerea in raspberries[88]. Similarly, A. alternata and B. cinerea in vitro colony development was reduced by pectin-based edible coatings encapsulating carvacrol/2-hydroxypropyl-β-cyclodextrin inclusion complex, ultimately proving the application of edible coatings to be a sustainable and environmentally beneficial method of food packaging, as well as a valuable way to extend fruit shelf life[89].
Heat treatments
-
As sessile organisms, plants cannot escape from stress situations, including heat stress, chilling injury, fungal infection, and deterioration. They expend significant energy on adjusting their metabolism to shield themselves from heat-related harm, acclimation activities, or acquired thermotolerance[90]. Heat stress, for example, changes the enzymatic reaction efficiency in the plant cell and impairs the stability of many proteins, as well as membranes, RNA species, and the structure of the cytoskeleton, leading to metabolic imbalance[91]. Heat water treatment, which involves raising the temperature from 40 to 60 °C, is considered the most straightforward and affordable way to protect postharvest quality against fungal pathogens and various postharvest storage issues. This method is possible to use without registration requirements and is entirely safe for both humans and the environment (free of residue and favorable to the environment)[92]. By strengthening their resilience to a variety of environmental stressors, heat treatments can promote host resistance, impede pathogen growth, and improve the effectiveness of BCAs[93].
Cold storage
-
Cold chain logistics for fruit and vegetables is the term for a supply chain system that maintains products at the proper temperature from the point of picking, processing, storing, shipping, and customer sales to guarantee product quality as well as safety and minimize losses. This technique has been globally used to prevent the development of ethylene, preserve the quality of the harvested fruit, and increase the fruit's shelf life[94]. The application of ozone during cold storage successfully delayed and concurrently reduced the postharvest disease in kiwifruits caused by B. cinerea[95].
UV irradiation
-
UV irradiation, classified into three groups including UV-A (long wavelength 320–400 nm); UV-B (medium wavelength 280–320 nm); and UV-C (short wavelength 200–280 nm), is a common method frequently applied for radiation sterilization to lower the bacteria burden in food and avoid unfavorable physicochemical changes[96]. UV-C irradiation displayed a fungicidal potentiality in inhibiting fruit rot disease as well as improving the quality of mangosteen[97] and procrastinating the physiological changes in bell peppers[96]. Furthermore, the physiological basis of this UV successfully reduced the petal specking of B. cinerea in Freesia hybrida L.[98].
-
Growing concerns about the chemical fungicide effects on the environment and human health have led to an increase in the use of alternative methods to control postharvest diseases. Biological control is one type of approach that employs microbes or natural antagonists to manage postharvest diseases and pests. While this approach has demonstrated potential in certain cases, its implementation frequently requires a substantial commitment of resources such as time and cost. One such requirement, the BCAs must be deposited and preserved in a respectable culture collection facility that meets the quality and safety requirements set forth by the Organization for Economic Co-operation and Development (OECD) for biological resource centers (BRCs). This calls for the creation of collection centers in developing countries as well as access to a suitable collection center[28]. Furthermore, biological control dataset analyses usually cover large taxonomy groups of organisms, such as all hymenopteran parasitoids or all insect natural enemies. However, the diversity of ecological situations and interactions taken into consideration rise along with the taxonomic breadth examined to the point that certain patterns could become too intricate to identify[99]. The primary challenge to the release of novel products onto the market has been registration. Despite these challenges and limitations, scientists and researchers are still innovating and creating new alternative techniques. Future research and innovation will probably lead to the development of more efficient and long-lasting techniques. In addition to several BCAs that have been successfully commercialized, due to lower-risk procedures for these materials, EOs have also received approval for use in the US more than in other countries[100]. Ultimately, several variables, such as the type of diseases, the surrounding conditions, and the available resources, will determine the success of alternative approaches. However, with a growing interest in developing and implementing alternative approaches, the prospect for establishing ecologically sustainable pest management strategies in the future will be promising to find sustainable ways to control postharvest diseases of fruit as well as minimize postharvest losses.
-
Alternaria and Botrytis species are among the primary pathogenic fungi in fruits and vegetables that harm humans and create food safety issues. In recent years, various sustainable approaches have been studied for their efficacy and advantages. Due to their characteristics that have been proven safe, eco-friendly, and non-toxic, the application of BCAs is recommended by scientists and global commercial companies to reduce the use of chemical fungicides. Biological control has also gradually changed from being primarily an independent field to a more crucial part of integrated pest management. Furthermore, to offer a broad range, persistence, and higher levels of BCA concentration against fungal diseases, a variety of natural substances, in conjunction with antagonists, have also been recently identified to improve the BCA effects against fungal pathogens. This combination of sustainable methods is expected to achieve disease control at the commercial level. Several BCA products have been developed and commercialized, which thus have the potential to make a significant contribution to the biocontrol of fruit postharvest diseases and can be a promising approach to replace the use of chemical fungicides in controlling postharvest disease of fruits. Ultimately, although the pest control market is still dominated by the use of chemical fungicides, given the increasing interest in creating and utilizing alternative methods, the future is apparent to be favorable for the establishment of ecologically sustainable pest management systems to control the postharvest disease of fruits and minimize postharvest losses.
-
The authors confirm contribution to the paper as follows: writing - original draft: Raynaldo FA; writing - review & editing: Xu Y, Wang Q; resources: Raynaldo FA, Yolandani; supervision, project administration: Wu B, Li D; funding acquisition: Xu Y, Li D. All authors reviewed the results and approved the final version of the manuscript.
-
All data generated or analyzed during this study are included in this published article.
This work was financially supported by the National Key Research and Development Program of China (2023YFD2201300) and the National Natural Science Foundation of China (32202555, 32272776).
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Raynaldo FA, Xu Y, Yolandani, Wang Q, Wu B, et al. 2024. Biological control and other alternatives to chemical fungicides in controlling postharvest disease of fruits caused by Alternaria alternata and Botrytis cinerea. Food Innovation and Advances 3(2): 135−143 doi: 10.48130/fia-0024-0014
Biological control and other alternatives to chemical fungicides in controlling postharvest disease of fruits caused by Alternaria alternata and Botrytis cinerea
- Received: 14 February 2024
- Revised: 16 May 2024
- Accepted: 16 May 2024
- Published online: 27 May 2024
Abstract: Alternaria alternata and Botrytis cinerea are among the primary fungal pathogens of fruits, causing black spot and gray mold disease, respectively. They cause serious losses in yield as well as affect fruit quality. Controlling fruit postharvest diseases largely relies on the use of chemical fungicides. However, the overuse of fungicides makes the produce unsafe due to their residual effects on the environment and human health. Therefore, significant advancements are necessary to investigate and find sustainable ways to prevent postharvest disease of fruits and minimize postharvest losses. This review summarizes the recent developments in the application of biological control and other sustainable approaches in managing fruit postharvest diseases, with an emphasis on A. alternata and B. cinerea, respectively. Furthermore, several action mechanisms, challenges, and prospects for the application of biological control agents (BCAs) are also discussed. Biological control application has been proven to successfully reduce postharvest disease of fruits caused by A. alternata and B. cinerea. In recent years, it has gradually changed from being primarily an independent field to a more crucial part of integrated pest management. Due to their characteristics that are safe, eco-friendly, and non-toxic, several BCAs have also been developed and commercialized. Therefore, biological control has the potential to be a promising approach to replace the use of chemical fungicides in controlling postharvest disease of fruits.
-
Key words:
- Biological control /
- Postharvest disease /
- Alternaria alternata /
- Botrytis cinerea /
- Sustainable methods












