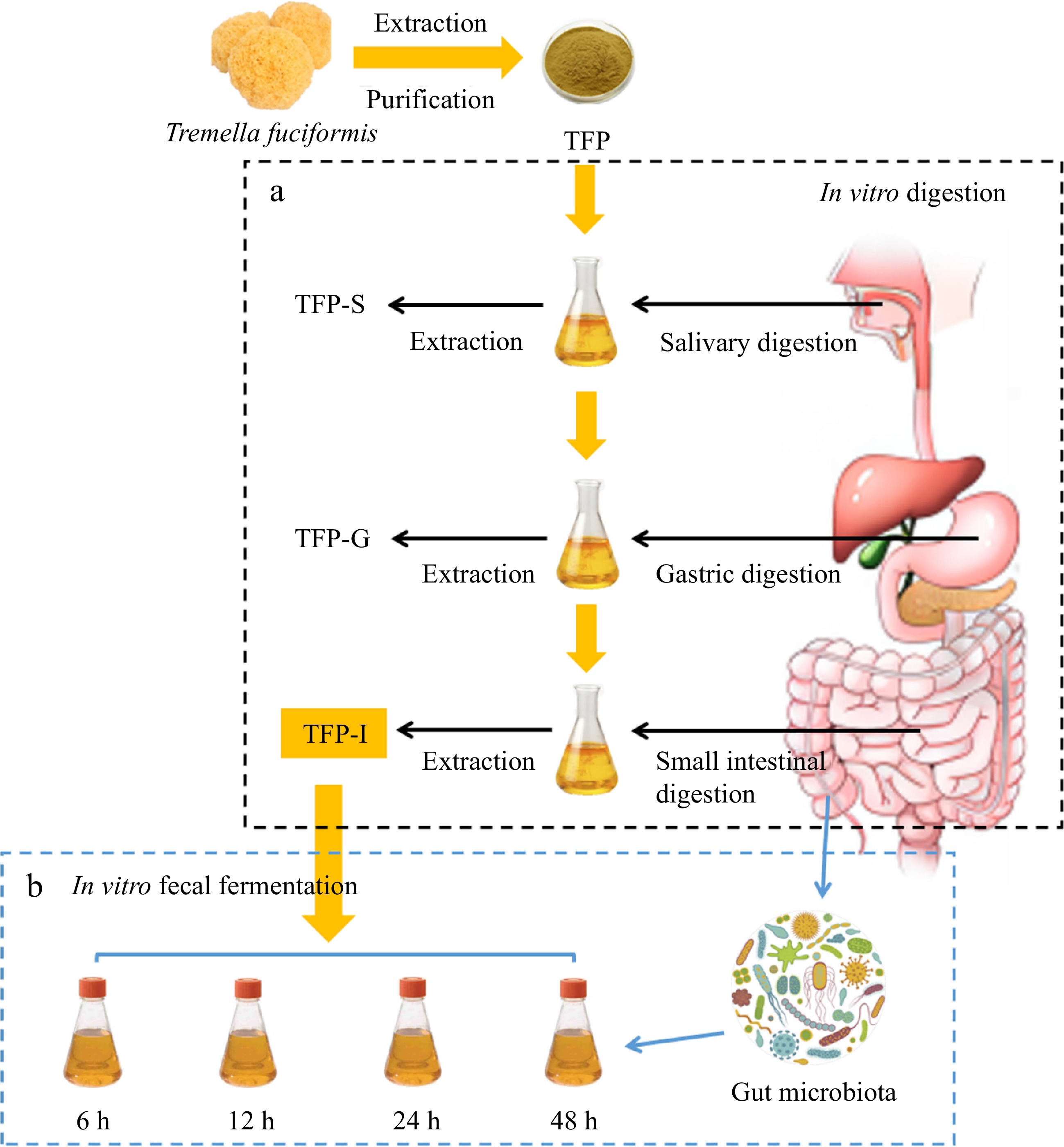
-
Prebiotic digestion and fermentation have drawn more attention in the past several years owing to the beneficial effects of prebiotics on host health[1]. Bioactive polysaccharides extracted from medicinal and edible plants and mushrooms exhibited prebiotic characteristics[2]. As a type of medicinal and edible fungus, Tremella fuciformis is rich in polysaccharides, proteins, dietary fiber, and other bioactive components[3]. In Tremella fuciformis, Tremella fuciformis polysaccharide (TFP) is the major bioactive substance[4] and exhibits various physiological activities such as antioxidant[5], anti-tumor[6], blood sugar control[7], anti-inflammatory[8], and immune-enhancing effects[9]. TFP has been confirmed by Xu et al. to prevent mice from colitis caused by dextran sulfate sodium (DSS), showing a reduction in colonic peroxidase and serum diamine oxidase activity, as well as alleviation of colonic tissue damage[10]. In addition, according to Yui et al., the major chain of TFP was identified as α-D-mannose, with β-D-xylobiose, β-D-gluconic acid, and β-D-xylose attached to the C-2 position of main chain[11]. Due to its excellent physiological activities and structure, the creation of medicinal goods and functional foods have made extensive use of TFP.
It is common knowledge that the bioactivity of polysaccharides is largely associated with their digestion, absorption, and functional properties in the digestive system[12]. Absorption of polysaccharides is a crucial physiological step in the course of digestion and fermentation[13], involving the coordinated actions of various organs in the human digestive system, for instance the small intestine, stomach, and mouth. Eventually, the nutrients can be applied for subsequent fermentation via gut microbiota[14]. Due to technical difficulties and ethical limitations, conducting human experiments to determine the effects of polysaccharides are challenging[15]. Therefore, in vitro models that mimic the human gastrointestinal tract, including the stomach, intestines, and colon, are particularly important for assessing the fermentative and digestive properties of polysaccharides. A study by Wu et al. stated that the TFP was continuously degraded in the process of fermentation, and the total sugar, uronic acid content, molecular weight, and apparent viscosity of TFP decreases significantly with increasing fermentation time[16]. Studies on TFP features related to fermentation and digestion are few. Consequently, it is critical to examine the digestion mechanism of TFP and explore its actual effects in the human body, providing a basis for understanding the bioactivity mechanisms of TFP.
Gut microbiota not only participate in physiological processes such as digestion, absorption, and metabolism of nutrients but also play important roles in immune regulation, biological defense, and maintaining intestinal homeostasis[17]. Intestinal inflammation and other diseases have been intimately linked to an imbalance in the gut microbiota, making the use of natural edible polysaccharides for intervention and regulation of gut diseases, obesity, and type II diabetes a current research focus. Since the human body lacks enzymes that are activated by carbohydrates, the majority of non-starch polysaccharides can only be fermented and utilized by the microbial community in the intestines to maintain microbial balance and diversity[18]. Edible fungal polysaccharides (primarily β-glucans) can reach the distal colon and be degraded by carbohydrate-active enzymes encoded by the gut microbiota, thus raising the number of beneficial bacteria (e.g., Phascolarctobacterium and Bacteroides) to modulate the composition of the intestinal microbiota[19]. As a result, knowledge of the interaction between the gut microbiota and TFP is essential for designing and manufacturing TFP-based functional health foods.
In previous studies, in colitis-affected mice, TFP has been shown to affect the equilibrium of gut microbiota and protect the intestinal barrier. Nevertheless, TFP is a biopolymer that is difficult to absorb and digest, and its exact bioactivity mechanism is yet unknown. In this study, using an in vitro digestion model, the properties of TFP digestion during in vitro digestion were examined, followed by evaluating the interaction between poorly digestible TFP and gut microbiota using an in vitro fecal fermentation model. These findings provide a basis for clarifying the underlying digestive and fermentation mechanisms of TFP and give a theoretical basis for the mechanism of its bioactivity.
-
Tremella fuciformis and Inulin (> 98% purity) were provided by Gutian County, Fujian Province, China and Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China), respectively. The other chemicals were all analytically graded.
Extraction of polysaccharides from Tremella fuciformis
-
Based on previous studies, the hot water extraction of polysaccharides from Tremella fuciformis were performed with slight modifications[20]. Details are supplied in the Supplementary File 1.
In vitro digestion of TFP
-
In accordance with previous methods, with slight changes, in vitro digestion of TFP was performed[21−30]. As shown in Fig. 1a, TFP-I, TFP-G, and TFP-S are the names of TFP samples that were digested under various in vitro digesting circumstances, such as saliva-gastrointestinal, saliva-gastric, and saliva digestion, separately. Details were supplied in Supplementary File 1.
In vitro fecal fermentation of TFP
In vitro fermentation utilizing human fecal inoculum
-
The collection of fresh fecal samples, preparation of fermentation media, and methods for in vitro fermentation can be referenced from previous studies with slight modifications[31]. As shown in Fig. 1b, using TFP-I as the carbon source, it was added to the culture medium and subjected to in vitro fermentation using the collected gut microbiota from healthy individuals, named as TFP group. The group that had no carbon supply was designated as the blank control or BLANK group. To serve as a positive control, inulin (a recognized soluble polysaccharide serving as a prebiotic) was used as a substitute for TFP, named as FOS group. Details are supplied in the Supplementary File 1.
Measurement of CR, pH, carbohydrate content, and gas production during fermentation in vitro
-
Measurement of CR, pH, carbohydrate content, and gas production during fermentation in vitro were conducted in accordance with previous studies[23]. Details are supplied in the Supplementary File 1.
Gut microbiota analysis during in vitro fermentation
-
The fermentation broth was subjected to low-temperature high-speed centrifugation to collect the bacterial pellet in cryovials, which were then stored at −80 °C after liquid nitrogen flash freezing. The gut microbiota investigation was carried out with the methodology outlined in our earlier research[32]. Details are supplied in the Supplementary File 1.
Statistical analysis
-
The data in the results were presented as 'mean ± SD'. Significant differences were indicated by letters (a−e) at p < 0.05.
-
Table 1 reveals that the total polysaccharide content in TFP were 89.62 ± 0.82%, indicating that polysaccharide is the main component of TFP and its content decreased significantly after gastrointestinal digestion[18]. Notably, the Mw of TFP decreased significantly in the digestion stage, and the content of CR increased significantly (Table 2), indicating that the glucoside bond was destroyed, leading to the decrease of Mw of TFP[33,34].
Table 1. Data summarization of TFP, TFP-S, TFP-G and TFP-I.
TFP TFP-S TFP-G TFP-I Total polysaccharides (%) 89.62 ± 0.82a 88.90 ± 0.55ab 87.28 ± 0.78b 86.07 ± 1.03c Total uronic acids (%) 15.35 ± 0.80a 15.83 ± 0.36a 14.74 ± 0.11b 14.15 ± 0.33c Total proteins (%) 2.53 ± 0.05a 1.90 ± 0.01b 0.79 ± 0.01c 0.60 ± 0.02d Molecular weight Mw × 104 (Da) 2.0361 ± 0.0375a 1.9686 ± 0.0412a 1.7864 ± 0.0109b 1.6620 ± 0.0156c Mw/Mn 1.33172 1.2779 1.20094 1.36855 Constituent monosaccharides and molar ratios Man 1.00 1.00 1.00 1.00 GlcA 0.07 0.08 0.08 0.07 Glc 0.86 0.73 0.75 0.58 Xyl 0.42 0.44 0.40 0.41 Fuc 0.19 0.19 0.20 0.20 Table 2. Variations in CR of TFP during in vitro digestion.
Processes Time (h) CR (mg·mL−1) Origin − 0.115 ± 0.001a Saliva digestion stage 0.25 0.113 ± 0.003a 0.5 0.114 ± 0.002a 1 0.116 ± 0.001a Gastric juice digestion stage 0.5 0.312 ± 0.032e 1 0.364 ± 0.002d 2 0.408 ± 0.010c 4 0.583 ± 0.023b 6 0.729 ± 0.016a Small intestinal juice digestion stage 0.5 0.809 ± 0.011c 1 0.836 ± 0.024bc 2 0.880 ± 0.039b 4 0.931 ± 0.022ab 6 0.950 ± 0.005a Changes of CR
-
Table 2 indicates that the content of CR did not vary considerably in the course of salivary digestion[35], but increased significantly after gastric digestion. This is due to the lower pH conditions in the stomach causing the glucoside bond to break, leading to an increase in the reducing end[36,37].
Analysis of monosaccharide composition
-
In Fig. 2a, the monosaccharide composition of TFP includes Fuc, Glc, Man, Gal, Xyl, GlcA, GalA, and Ara. Among them, the major monosaccharides in TFP are Glc and Man. Previous studies have shown that the main chain of TFP consists of (1→3)-α-D-mannopyranosyl residues and the side chains consist of Fucp, β-GlcAp, and β-Xylp residues[38]. The molar ratio of Glc declined after simulated digestion, which could be attributed to the lower pH causing degradation of the polysaccharide side chains[18,37], suggesting that in vitro digestion could affect the monosaccharide composition of polysaccharides.
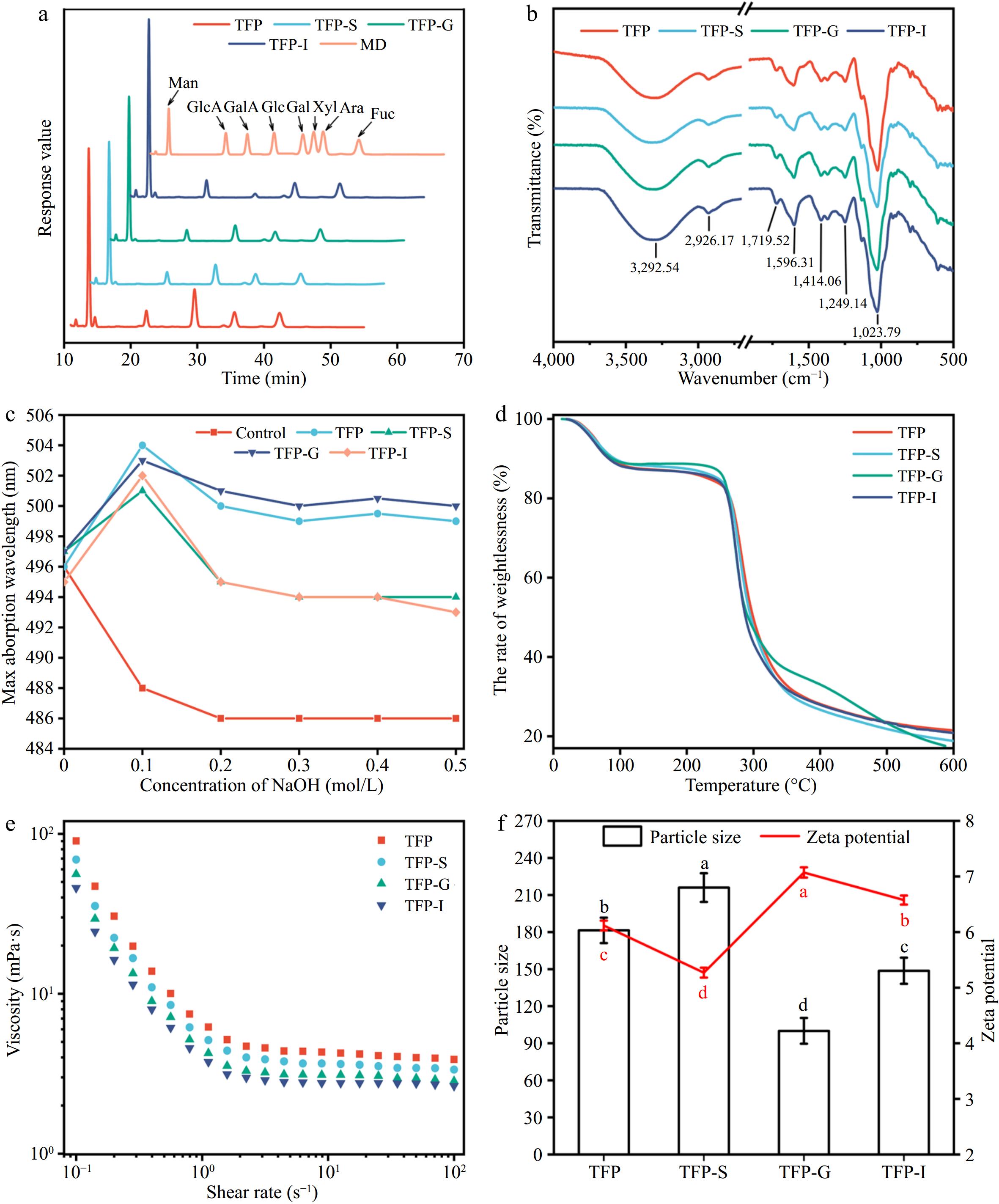
Figure 2.
Variations in structural characterizations of TFP during in vitro digestion. (a) Monosaccharide composition. (b) FT-IR. (c) Congo red staining. (d) Thermogravimetric curve. (e) Rheological properties. (f) Particle size and zeta potential.
FT-IR analysis
-
Figure 2b demonstrated that FT-IR spectra of TFP following the simulated digestion were comparable, suggesting that the RG-I backbone and other structural features of TFP were unaffected by the in vitro simulated digestion process[37]. In particular, the existence of carbohydrates was confirmed by the characteristic peak at 3,600−3,200 cm−1, which correlated with the O-H stretching vibration[39], and the range of 1,400−1,200 cm−1, which correlated with the C-H bending vibration[40,41]. The presence of uronic acids was indicated by the asymmetric stretching vibration of free carboxyl groups, which fell within the range of 1,590−1,644 cm−1[40]. Additionally, at 1,555 cm−1, there was no absorption peak, indicating a very low protein content in the polysaccharide samples. The peak at around 917 cm−1 presented the characteristic vibration of the non-symmetric ring stretching of pyranose[5].
Congo red assay and TG analysis
-
TFP was described as a polysaccharide with a triple helix shape in Fig. 2c, and this structure held unchanged even after in vitro digestion was simulated. The TG curves in Fig. 2d did not reveal any discernible variations between various phases of TFP digestion. At temperatures between 25 and 600 °C, polysaccharides exhibited three stages of thermal degradation[42]. Notably, in the second stage (101−337 °C), there was a sharp decrease in weight, mainly because the anhydrous organic components gradually decompose under high-temperature heating. The rhamnogalacturonan chain was degraded, leading to carbonization and oxidation, causing the volatilization and loss of a large number of volatile small molecules. In the third stage (337−600 °C), aromatic carbon residues undergo combustion[43].
Apparent viscosities, particle size, and zeta potential analysis
-
The apparent viscosities of TFP exhibited a typical Newtonian plateau at high shear rates, as presented in Fig. 2e[29]. Figure 2f exhibits that TFP-G had the lowest particle size, suggesting that TFP dissociates more readily in the acidic environment of the stomach. The highest charge was observed for TFP-G, indicating that the small intestine was able to absorb and utilize TFP-G-digested samples more easily.
Variations in CR, pH, residual carbohydrates and gas production during in vitro fermentation of TFP
-
Enzymes encoded by gut microbiota could break down carbohydrates into fermentable sugars, and the growth metabolism of gut microbiota could influence the content of total carbohydrates in the fermentation medium. As shown in Fig. 3a, the carbohydrate content of all substrates decreased most rapidly during the first 6 h, indicating that the fecal microbiota was in the logarithmic growth phase with the highest carbohydrate consumption[44]. With increasing fermentation time, the total sugar content in BLANK, TFP, and FOS groups all showed a decreasing trend, suggesting varying degrees of carbohydrate utilization and the presence of unfermentable components. Studies have found that the consumption of aloe polysaccharides after 48 h of fermentation was approximately 56%[45], and the total sugar consumption of loquat polysaccharides after fermentation was as high as 85%[46], In this experiment, after 48 h of fermentation, the FOS group consumed approximately 69.90% and the TFP group consumed approximately 66.08%. Therefore, TFP was a good fermentation substrate that could be effectively utilized by microorganisms.

Figure 3.
Variations in CR, pH, residual carbohydrates and gas production during in vitro fermentation of TFP. (a) Total carbohydrates. (b) Reducing sugars. (c) pH value. (d) The amount of gas produced.
As can be seen from Fig. 3b, throughout the entire process of fecal fermentation, the fermentation broth contained very few reducing sugars, ranging from 0.09 ± 0.03 mg/mL to 0.12 ± 0.04 mg/mL, suggesting that the gut microbiota can fully use the reducing sugars generated by TFP-I, with a dynamic balance between enzymatic hydrolysis rate and utilization rate[47].
The pH level is a crucial signal throughout the fermentation process. Figure 3c exhibits that the pH values of the FOS and TFP groups were consistently lower than those of the BLANK group, owing to acidic substances like short-chain fatty acids (SCFAs) were produced throughout the fermentation process through the fermentation of polysaccharides. The development of pathogenic bacteria may be inhibited by the reduction in intestinal pH. Therefore, TFP and inulin could lower the colonic pH and maintain gut health.
The gut microbiota tends to produce gases like CH4, H2 and CO2 while fermenting carbohydrates, which could cause adverse symptoms and were the main reason for the limitation of prebiotic application[48]. In Fig. 3d, the gas production of FOS, TFP, and BLANK groups gradually raised during fermentation. After fermentation for 48 h, the gas volume produced by TFP fermentation (0.53 mL) was significantly lower than that of inulin (1.08 mL), indicating that TFP was a more advantageous prebiotic biomass than inulin in terms of gas production.
Effects of TFP on microbial community compositions
-
Gut microbiota are crucial for the body's ability to absorb and store energy, perform a number of metabolic processes, and control the immune system, which are crucial for human health and disease. Previous studies have found that through altering gut microbiota, TFP reduced colitis caused by DSS in mice. Thus, it was essential to understand the connection between gut bacteria and TFP, as modulating gut microbiota could contribute to disease prevention and promote health.
Figure 4a−c indicated that most bacterial diversity in the samples was covered by the sequencing depth, indicating that the volume of sequencing data was appropriate. The findings in Fig. 4d & e revealed that there were remarkable differences in the gut microbiota composition between the BLANK, FOS, and TFP groups. Figure 4f demonstrated that between the three groups, there were more inter-group differences than intra-group differences, with intra-group differences being very minor. Figure 4g presented a certain distance between the samples in BLANK, FOS and TFP groups, indicating the specificity of bacterial distribution[49]. In summary, the inter-group differences of each experimental group were remarkably different from one another, and these differences outweighed the intragroup differences, suggesting that carbohydrates from different sources had different effects on the microbial community.
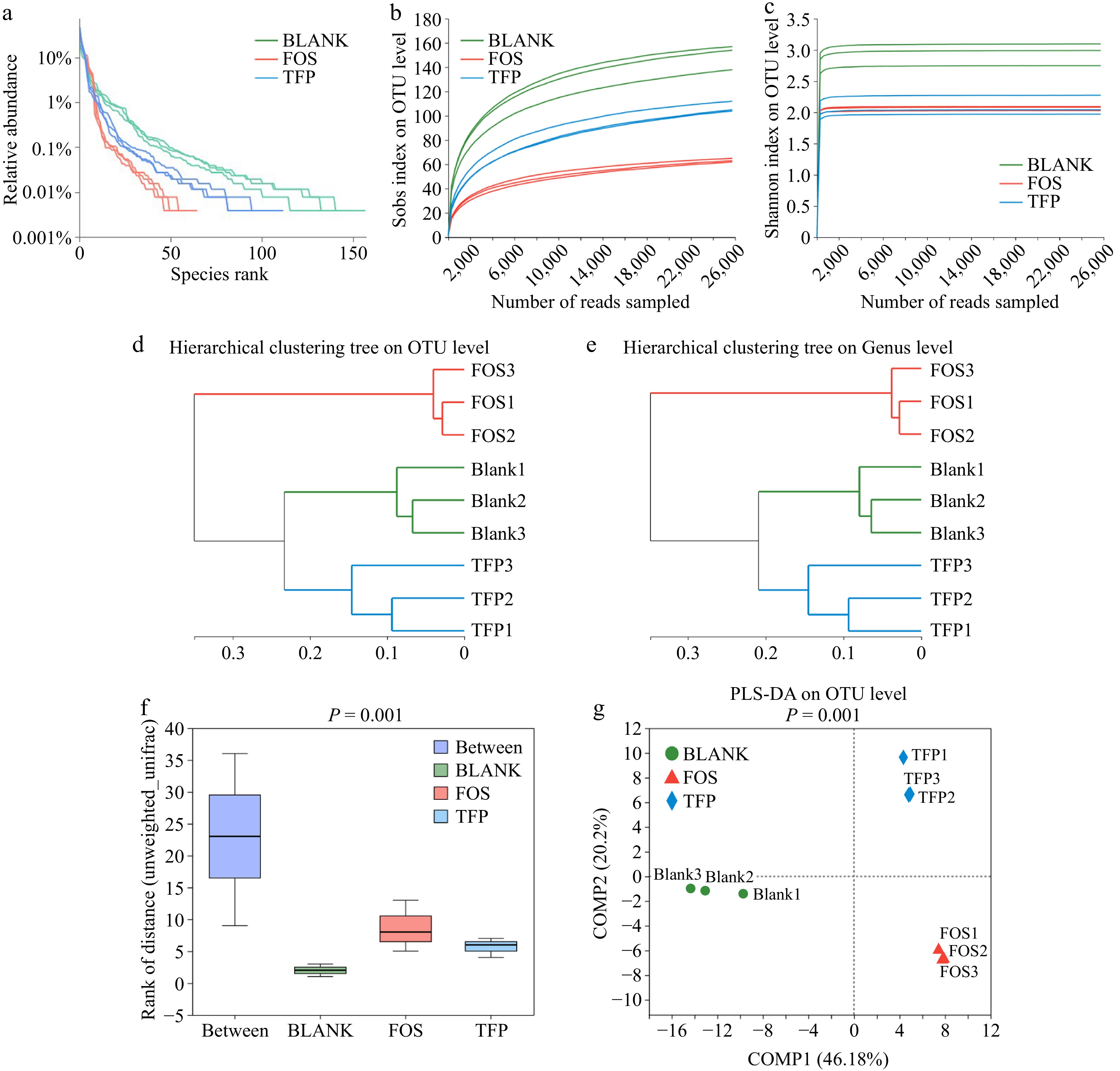
Figure 4.
Correlation curve of species diversity and between-group similarity analysis of gut microflora in vitro fermentation for 48 h. (a) Rank-Abundance curve. (b), (c) Rarefaction curve. (d), (e) Hierarchical clustering tree based on OUT and Genus levels. (f) ANOSIM/Adonis analysis. (g) PLS-DA analysis.
The samples in the TFP and FOS groups had much lower Sobs, Shannon, ACE, and Chao indices than the BLANK group, as presented in Figs. 5a−d, indicating that supplying the gut microbiota with inulin and TFP as carbon sources can result in various degrees of decline in microbial abundance and diversity[43]. The FOS group samples had the lowest microbial diversity and richness, which was similar to the findings of Yu et al.[50]. These findings displayed that the microbial community composition might be changed by both FOS and TFP interventions. In addition, PCA analysis, PCoA analysis, and NMDS analysis (Fig. 5e−g) demonstrated that the samples from the TFP, FOS, and BLANK groups exhibited a certain distance, suggesting that the gut microbiota compositions of the three groups varied. The results indicated that TFP, together with gut microbiota could change the microbial community composition, and the impact on gut microbiota varies when using carbohydrates from different sources for in vitro fermentation.
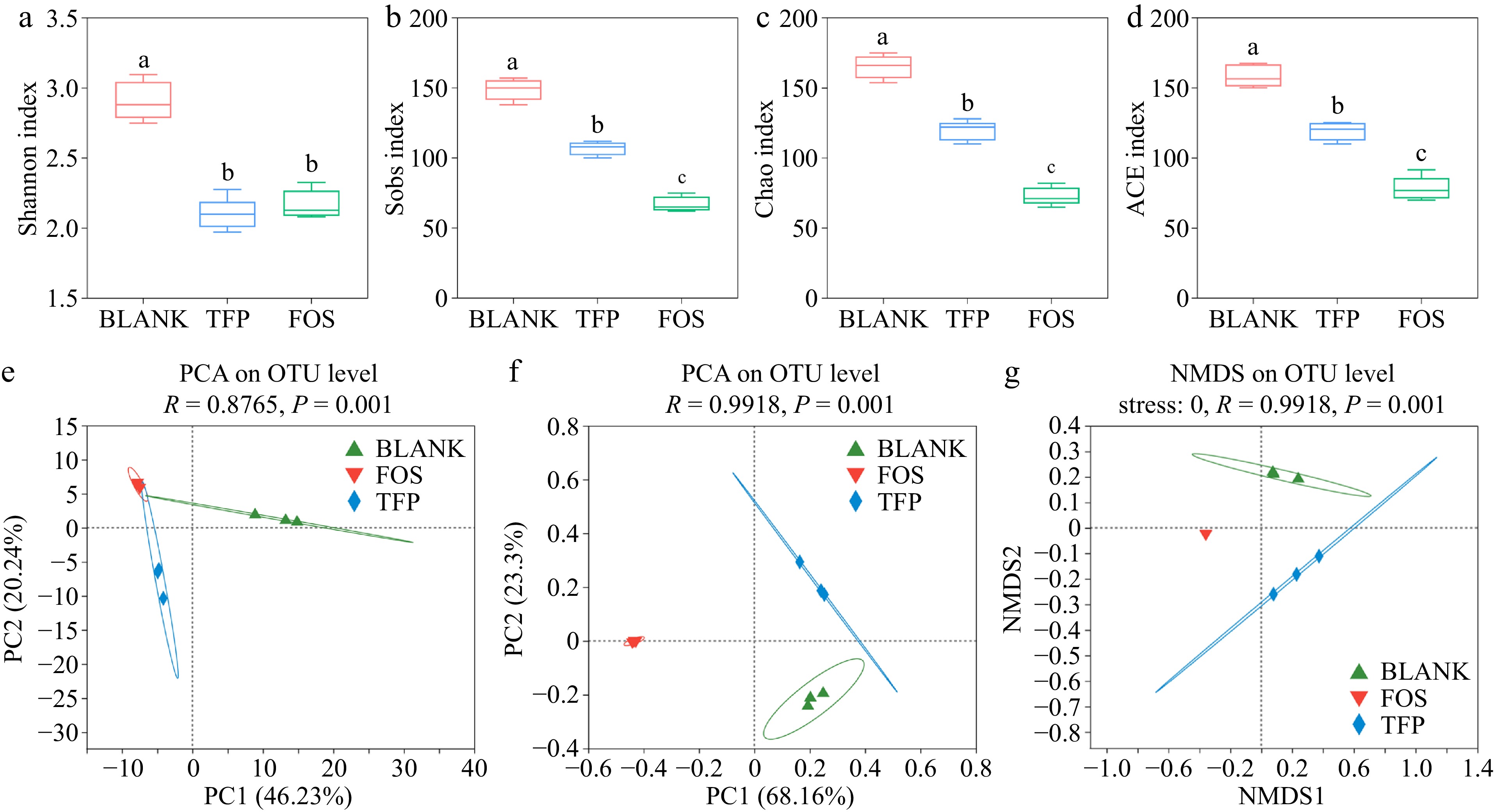
Figure 5.
Analysis of α and β diversity after 48 h of fermentation in vitro in the gut microbial community. (a)-(d) α diversity indices. (e) PCA analysis. (f) PCoA analysis. (g) NMDS analysis.
Figure 6 displayed the changes in gut microbiota after 48 h of in vitro fermentation. As illustrated in Fig. 6a, c & e, the BLANK group at the genus level consisted mainly of Phascolarctobacterium, Bacteroides, Escherichia-Shigella, Klebsiella and Fusobacterium. In comparison with the BLANK group, Bacteroide proportion significantly increased in the TFP group. Bacteroides were one of the most significant genera of intestinal microbiota and could digest dietary fiber polysaccharides and host glycans. In addition, Bacteroides acted as a key player in the fight against obesity, immune disorders, and the alleviation of intestinal inflammation[51]. The proportion of Megasphaera and Phascolarctobacterium was elevated in the TFP group versus the BLANK group, which was in line with previous findings[37]. At the same time, Escherichia-Shigella and Fusobacterium were reduced in the TFP group, which suggested that TFP could facilitate the beneficial bacteria development and suppress the harmful bacteria development. In the FOS group, the relative abundance of Escherichia-Shigella significantly increased, as Escherichia-Shigella lacked carbohydrate-active enzymes and cannot utilize polysaccharides, whereas inulin, as a low-molecular-weight carbon source, facilitated its growth[52]. Furthermore, the relative abundance of Bifidobacterium, which could degrade and apply inulin to enhance the generation of fermentation end products might be greatly raised by inulin[37].
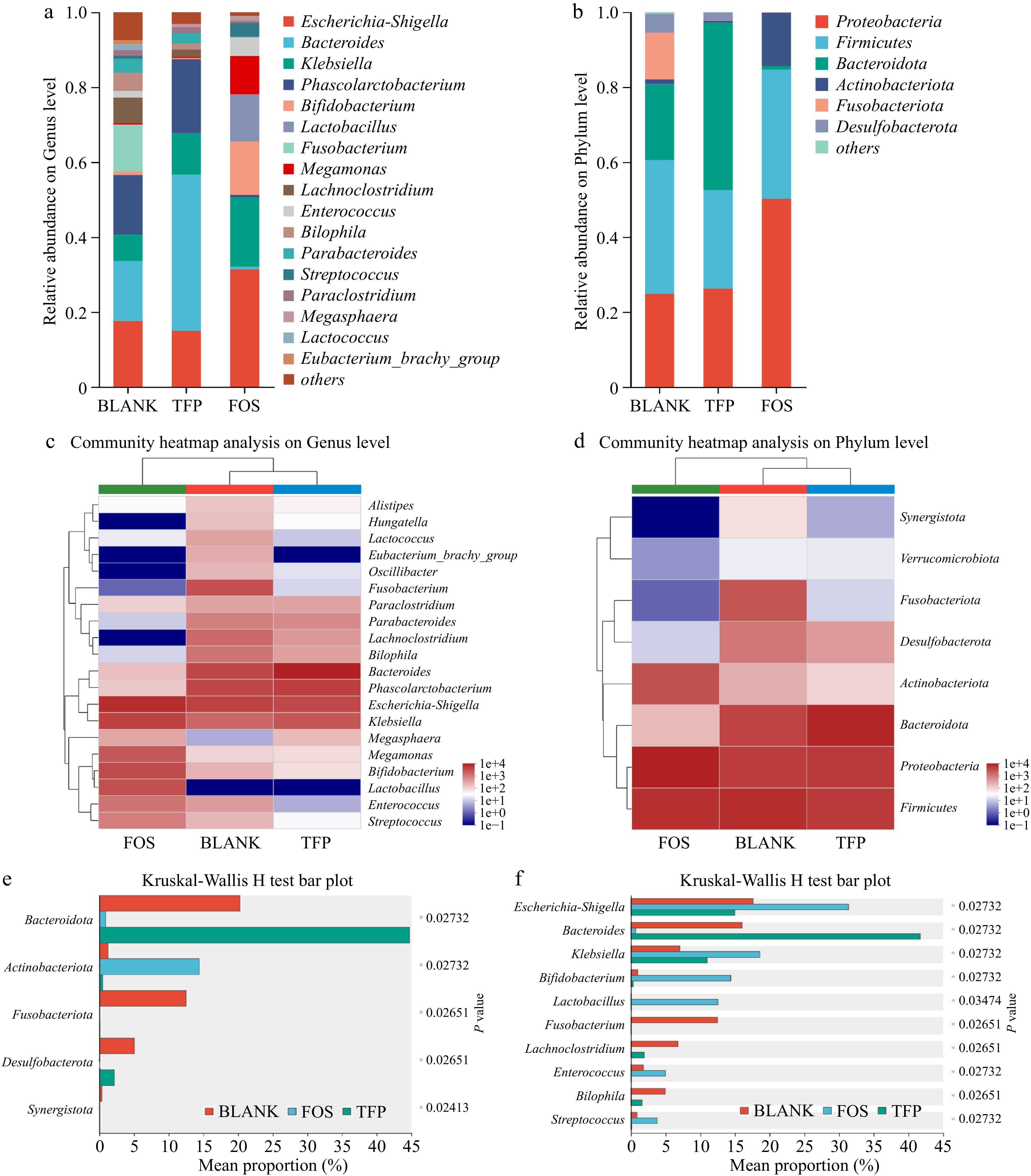
Figure 6.
Analysis of gut microbial community composition during 48 h of in vitro fermentation. (a), (b) Relative abundance. (c), (d) Community heatmap analysis. (e), (f) Kruskal-Wallis H test bar plot.
Figure 6b, d & f indicated that the major bacteria in the BLANK group at the phylum level were Proteobacteria, Firmicutes, Fusobacteriota, and Bacteroidetes. In comparison to the BLANK group, the TFP group had a much higher proportion of Bacteroidetes, but a markedly lower proportion of Firmicutes. Bacteroidetes was one of the main intestinal bacteria that were responsible for degrading polysaccharides[53]. When degrading substrates, it released polysaccharide hydrolases and glycoside hydrolases for the degradation of the Gal side chain structures along with the RG-I backbone of polysaccharides[37]. In addition, a rise in the ratio of Bacteroidetes to Firmicutes may reduce the risk of insulin resistance and obesity[54]. Therefore, TFP may play a role in anti-obesity and reducing insulin resistance by regulating the ratio of Bacteroidetes to Firmicutes. Fusobacteriota was generally considered to be associated with some opportunistic pathogens. The low proportion of Fusobacteriota in both TFP and FOS groups indicated that the addition of TFP and inulin could inhibit certain opportunistic pathogens. Proteobacteria and Actinobacteriota were the most varied bacterial phyla, which are often present in the fecal microbiota of healthy individuals[52]. The relative abundance of Actinobacteriota in the FOS group was substantially higher than the BLANK group. Actinobacteriota were Gram-positive bacteria that could convert carbohydrates into non-toxic acidic substances and were believed to promote intestinal health[55]. In summary, inulin and TFP have the potential to alter the gut microbiota composition, especially by encouraging the growth of beneficial bacteria. However, there were differences in their effects on gut microbiota. Compared to the FOS group, the addition of TFP had less interference with the normal community structure and was more conducive to maintaining gut homeostasis in the short term.
-
In conclusion, the present research demonstrated that TFP partially degraded under circumstances resembling salivary gastrointestinal digestion, leading to a notable rise in CR content and a fall in Mw. Furthermore, indigestible TFP-I may be extensively applied by the human gut microbiota during in vitro fecal fermentation. TFP demonstrated the ability to modulate both α and β diversity in the intestinal microbiota and induce changes in the community composition at the phylum and genus levels. This included a decrease in the growth of harmful bacteria for instance Escherichia-Shigella and Fusobacterium and a rise in the abundance of beneficial bacteria like Megasphaera, Phascolarctobacterium, and Bacteroides. These findings indicated that TFP had the potential to be a functional food that enhanced the intestinal microbiota environment, thereby promoting health and preventing disease, e.g., prebiotic.
-
The authors confirm contribution to the paper as follows: conceptualization, methodology, software, investigation, formal analysis, visualization: Zhu X; writing - original draft: Zhu X; writing - review & editing: Su J, Zhang L, Si F, Li D, Jiang Y, Zhang C; supervision: Jiang Y, Zhang C; resources: Zhang C. All authors reviewed the results and approved the final version of the manuscript.
-
This published article and associated supplementary information files contain all of the data generated or analyzed during this work.
This work was financially supported by the National Natural Science Foundation of China (31901644) and the University Innovation Team of Shandong Province (2022KJ243).
-
The authors declare that they have no conflict of interest.
- Supplemental File 1 Changes in molecular weight of TFP during in vitro digestion. (a) TFP. (b) TFP-S. (c) TFP-G. (d) TFP-I.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhu X, Su J, Zhang L, Si F, Li D, et al. 2024. Gastrointestinal digestion fate of Tremella fuciformis polysaccharide and its effect on intestinal flora: an in vitro digestion and fecal fermentation study. Food Innovation and Advances 3(2): 202−211 doi: 10.48130/fia-0024-0018
Gastrointestinal digestion fate of Tremella fuciformis polysaccharide and its effect on intestinal flora: an in vitro digestion and fecal fermentation study
- Received: 05 April 2024
- Revised: 05 June 2024
- Accepted: 12 June 2024
- Published online: 25 June 2024
Abstract: In this work, the gastrointestinal digestive outcome of Tremella fuciformis polysaccharide (TFP) was examined using in vitro simulated experiments, together with its effect on the intestinal microbiota. TFP did not significantly alter during the stage of oral digestion, according to an in vitro digestion investigation. Nevertheless, glycosidic connections of TFP were broken throughout the intestinal and stomach digesting phases, which resulted in the dissociation of macromolecular aggregates, a marked rise in decreasing sugar content (CR), as well as a drop in molecular weight (Mw). Additionally, microbial community analysis following fecal fermentation in vitro indicated that TFP might control the alpha and beta diversity of gut microbiota and change the genus- and phylum-level community composition. It increased the abundance of beneficial bacteria including Megasphaera, Phascolarctobacterium, and Bacteroides, and suppressed the growth of harmful bacteria like Escherichia-shigella and Fusobacterium, thus contributing to maintaining gut homeostasis. These results suggested that TFP could have a positive impact on health through enhancing the gut microbiota environment, giving a theoretical basis for its use as a prebiotic.