-
Lemon, as a typical non-climacteric fruit, is the third most significant citrus species and is popularly favored and widely grown worldwide. Lemon is rich in various vitamins and amino acids, phenolic compounds, flavonoids, etc., which have antioxidant and anticancer effects[1]. During storage, weight loss, acidity loss, and the development of brown spots on the fruit surface are the primary challenges. Moreover, lemons in the yellow stage have a shorter storage lifetime and shelf life than those in the green stage, despite being more mature. Lemons were harvested at the green stage and stored in cold storage to be sold after degreening. However, overcapacity from the conversion of large quantities of lemons to yellow can result in a waste of resources. Sun et al.[2] concluded that lemons harvested at the green stage could be stored for an extended 30 d compared with those harvested at the yellow stage when stored at 10 °C. This was mainly due to the larger maintenance of fruit antioxidant capacity and fruit quality in the green stage of lemons, which in turn delayed the onset of fruit senescence.
Fruit senescence is accelerated by not only inappropriate environmental conditions, such as low relative humidity (RH), high temperature, and oxidative stress, but also influenced by phytohormones in its tissues, such as abscisic acid (ABA), gibberellins (GAs), and brassinolides (BR)[3−5]. Oxalic acid has been shown to extend the shelf life of lemons by increasing the activities of antioxidant enzymes such as ascorbate peroxidase (APX), catalase (CAT), and peroxidase
(POD)[6]. However, it requires application at the preharvest stage. As reported, γ-aminobutyric acid (GABA) could be applied to 'Verna' lemons during both preharvest and postharvest stages, which could reduce the weight loss and extend the shelf life up to 7 d at 10 °C[7]. In addition, it has been studied that exogenous ABA treatment could regulate the sugar and organic acid metabolism processes to accelerate citrus fruit coloring and ripening[8]. GA pretreatments have been used in common horticultural management practices, while most studies focused on seed development, anthesis, and fruit set[9]. In addition, exogenous GAs treatment could delay the ripening of climacteric and non-climacteric fruits, such as persimmons, kiwifruits, and strawberries, during the fruit ripening stage[10]. Specifically, it could reduce the ripening of kiwifruits and maintain fruit quality by delaying the firmness reduction, cell-wall degradation, the rapid increase of soluble solid content (SSC), and the decline of titratable acidity (TA) content, and volatile organic compounds (VOCs)[11]. The GAs preserve tomato fruit quality by regulating the cuticular wax biosynthesis[12]. Exogenous GAs treatment delays the senescence and ripening of strawberries by reducing respiration intensity, anthocyanin content, and the activities of key enzymes involved in chlorophyll degradation[13]. GAs also regulate the metabolism of pigments, including chlorophyll a and b, in Valencia oranges[14].Water typically accounts for 80% to 95% of fruit mass and plays a vital role in maintaining their biological functions[15]. Transpiration is a key physiological process contributing to weight loss in fruits and vegetables during storage. Water vapor diffuses from the intercellular spaces, and evaporates from the fruit surface to the surrounding environment, which is greatly influenced by RH[15]. The pigmented flavedo and the spongy internal white layer of the albedo in citrus pericarp are used to transport water vapor and are anatomically separated from juice vesicles[16]. RH could regulate the physiological and biochemical reactions in fruits and vegetables, such as inhibiting fruit browning and anthocyanin degradation, maintaining cell integrity, and preventing chilling injury[17]. Moreover, high RH promoted D-Limonene production and terpene synthesis of the Torreya grandis[10]. In addition, high RH maintained the number and morphology of waxes and stomata in Kur pear and 'Navelina' oranges[18,19]. The epidermal and flavedo cells of the 'Navelate' oranges stored at high RH maintained almost intact, but moving the fruits from low RH to high RH-induced peel pitting[20].
Overall, GAs have been proven to prevent chlorophyll degradation and delay senescence in citrus, while high RH is beneficial for maintaining the appearance of the pericarp[21]. In this study, an attempt was made to extend the green stage of lemons to control the maturation and senescence process and to maintain the balance between supply and demand in the lemon market while minimizing waste. GAs were applied to maintain the greenness of lemons. Taking the quality loss resulting from weight loss, different methods of RH storage were compared, thus, RH 90% storage relieves the weight loss caused by water loss and maintains visual quality by protecting the outer colored exocarp (flavedo). Moreover, the combination of GA pretreatment and high RH storage was applied to lemons harvested at the green stage to retain storage quality and extend shelf life.
-
'Eureka' lemons (Citrus limon (L.) Burm. f.) were harvested at the green stage from Chongqing, Sichuan province, China (30°24'25'' N, 107°55'22'' E). Green lemons with uniform size (single lemon weight of 75−90 g) without defects, diseases, blemishes, or mechanical damage were selected and packed in PVC boxes (40 cm × 30 cm × 25 cm), and cold-chain transported to the laboratory at Tianjin University of Science and Technology (Tianjin, China), and were immediately pre-cooled at 14 °C for 24 h.
A total of 40 kg of lemons were equally divided into four groups, three of which were soaked in 100 mg∙L−1 of GAs (Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China) solution for 5 min and one group was soaked in distilled water for 5 min. The treated lemons were then air-dried and stored in four intelligently controlled devices (LAC-250HPY-2, Shanghai Long Yue Instrument Inc., Shanghai, China) equipped with temperature and RH control modules. The treatments can be summarized as: (1) no GAs + RH 60%, (2) GAs + RH 30%, (3) GAs + RH 60%, (4) GAs + RH 90%. All samples were stored at a temperature of 14 ± 1 °C, and were randomly sampled at 6-d (0, 6, 12, 18, 24, 30, and 36 d) intervals to assess fruit color, nutritional quality, microstructure, and aroma. The isolated pericarp was quickly frozen in liquid nitrogen and stored at −80 °C for further use. Each sample included 8−10 fruits from each group.
Measurement of pericarp color
-
A precise chromameter (HP-200, Hanpu Photoelectric Technology Co., Ltd, Shanghai, China) was utilized to measure the color of the lemons at the equatorial area, and the results were recorded as L*, a*, and b* values. Formulas for calculating hue angle (H°) and chroma (C*) were derived using the following equations[19].
$ {H}\text{°} ={\text{tan}}^{{-1}}\left(\dfrac{{{b}}^{\text{*}}}{{{a}}^{\text{*}}}\right) $ $ {C}\text{*}\text{}={\left({\left({{a}}^{\text{*}}\right)}^{\text{2}}+{\left({{b}}^{\text{*}}\right)}^{\text{2}}\right)}^{\frac{{1}}{{2}}} $ Visual appearance and determination of chlorophylls and carotenoids
-
The visual appearance of lemons was captured by a Canon EOS 6D to capture the images of the lemon pericarp. The citrus color index (CCI) was computed using the following formula[22].
$ {CCI}= 1000\left(\dfrac{{{a}}^{\text{*}}}{{{L}}^{\text{*}}\times {{b}}^{\text{*}}}\right) $ The chlorophyll and carotenoid contents of lemons were quantified by referring to the method of Yuan et al.[23] with slight modifications. In smmary, freeze-dried powder of lemon pericarp (1.0 g) was collected, added to 10 mL of 80% acetone, and then extracted at 4 °C in the dark for 1 d. The supernatant was collected, and the residues were further extracted and treated in the same manner until they became colorless. A microplate reader (Multiskan FC, USA) was used to measure the absorbance values at 470, 645, and 663 nm. Subsequently, the total chlorophyll and carotenoid contents were calculated as follows:
$ {\text{C}}_{\text{a}}={ 12.7 }\times {\text{A}}_{\text{663}}-{2.69} \times {\text{A}}_{\text{645}} $ $ {\text{C}}_{\text{b}}={22.91}\times {\text{A}}_{\text{645}}-{4.68}\times {\text{A}}_{\text{663}}\text{} $ $ \mathrm{A}=1000\times {\text{A}}_{\text{470}}+{563.05}\times {\text{A}}_{\text{663}}-{2085.05}\times {\text{A}}_{\text{645}} $ $ \begin{split} & \rm{Total\; chlorophyll\; content}\; (mg\cdot g^{-1}) \\ & =\rm{\left(C_a+C_b\right)}\times dilution\; factor\times \left(\dfrac{V_t}{M\cdot1000}\right) \end{split} $ $ \mathrm{T}\mathrm{o}\mathrm{t}\mathrm{a}\mathrm{l}\; \mathrm{c}\mathrm{a}\mathrm{r}\mathrm{o}\mathrm{t}\mathrm{e}\mathrm{n}\mathrm{o}\mathrm{i}\mathrm{d}\; \mathrm{c}\mathrm{o}\mathrm{n}\mathrm{t}\mathrm{e}\mathrm{n}\mathrm{t}\; \rm{(}mg\cdot g^{-1})=A\times\left(\dfrac{\text{V}_{\text{t}}}{229\times M\times1000}\right) $ where, Vt: total volume of extract; M: mass of sample.
Weight loss and malondialdehyde (MDA) content
-
Lemons were weighed every 3 d during storage. The method of weight loss referenced
Zhou et al.[24]. The formula for the calculation is listed as follows:$ \rm{Weight \;loss}\;(\text{%})=\dfrac{{\text{m}}_{\text{n}}-{\text{m}}_{\text{0}}}{{\text{m}}_{\text{0}}}\times{100}\text{%} $ where,
$ {\text{m}}_{\text{0}} $ $ {\text{m}}_{\text{n}} $ To evaluate the MDA content of lemon pericarp, the method of Li et al.[25] was employed with some modifications. Briefly, the lemon pericarp (5 g, fresh weight (FW)) was homogenized in trichloroacetic (5 mL of 100 g∙L−1 TCA) and centrifuged at 10,000 g for 15 min. The supernatant was mixed with thiobarbituric acid (TBA, 6.7 g∙L−1) in equal proportions and heated in a boiling water bath for 20 min. After the solution was cooled to room temperature, the supernatant was collected, and absorbances at 450 nm, 532 nm, and 600 nm were measured, respectively. The MDA content was quantified and reported in units of μmol∙kg−1. Five different fruits were used as a single replicate, each with three technical repeats.
Soluble solid content (SSC), titratable acidity (TA) content, and the SSC/TA ratio
-
To measure the SSC and TA content, three lemons were peeled, and juice was extracted using a juicer (Kaijie Instrument Manufacturing Co., Ltd., Shanghai, China), and a portable refractometer (GR15, Shanghai, China) was used to determine the SSC of lemon juice. SSC was expressed as %. The TA content in fresh lemon pulp was determined by acid-base titration and expressed as a percentage of citric acid. The SSC/TA ratio was calculated as the ratio between SSC and TA content.
Pericarp structure of lemons
-
A sample of approximately 1 cm × 1 cm was cut from lemon pericarp along the equatorial zone. A scalpel was used to make an incision in the pericarp following a line from the flavedo to the albedo. The medial and lateral structures of the flavedo were visualized and photographed using an electron microscope (WST-S200, Shenzhen Wilks Optoelectronics Co., Ltd, Shenzhen, China).
Ultrastructure of lemon pericarp by scanning electron microscope (SEM)
-
Small sections (30 mm × 30 mm) of the lemon pericarp were cut at the equatorial zone using a razor blade. Then, the lemon pericarp was lyophilized in a vacuum freeze dryer (LGJ-10, Beijing, China) for 24 h and attached to a sample stage with conductive tape. The scanning electron microscope (SEM, SU1510 Hitachi, Japan) was employed to evaluate the ultrastructure of the pericarp.
Volatiles determined by gas chromatography-mass spectrometry (GC-MS)
-
Modified headspace solid phase microextraction (SPME) was employed for the isolation and concentration of the volatiles[26]. Frozen lemon peel samples (1.0 g) were accurately weighed and then placed in a 20 mL headspace vial and infused with 2 mL H2O, and then 2 μL of cyclohexanone (99.9%) was added as an internal standard. Next, the samples were placed in a water bath at 45 °C and then stirred for 15 min, and the volatile compounds were separated on the HP-5MS capillary column (30 m × 0.25 mm × 0.25 μm). The PDMS/DVB SPME fiber (65 μm, fused silica 24 Ga) after activation and the fiber was inserted through the cap of the vial into the headspace for 30 min and exposed to the GC inlet to desorb for 15 min at 250 °C. The data system library (NIST11) was used to identify compounds for qualitative analysis. In addition, for quantitative analysis, the volatile compounds were normalized by comparing their peak areas to the internal standard (cyclohexanone).
Statistical analysis
-
Results were expressed as mean ± standard deviation (SD). All experiments were performed in triplicate and were conducted as a randomized design. Data analysis was performed using SPSS 19 (INM) and Origin 2018 (Origin Lab Corp., USA). Significant differences (p ≤ 0.05) were identified as least significant differences (LSD).
-
Generally, external color changes are associated with internal ripening in citrus[21]. The fruit color indexes (L*, a*, b*, a*/b*, H°, C*) were determined to evaluate the initial maturity and the postharvest ripening of lemons. L* indicates the lightness or darkness of the color, which increases as the fruit ripens[27]. Although there was an increase in L* values for the GAs + RH 60%, a more significant (p ≤ 0.05) increase was observed in the no GAs + RH 60% (Fig. 1a), indicating that the GAs pretreatment maintained the lightness of green lemons. In previous studies, higher water loss in fruit has been found to cause faster senescence[28]. Compared with the GAs + RH 60% and GAs + RH 90%, the L* of the GAs + RH 30% remained essentially unchanged, which may be related to the disruption of the waxy layer. At the end of the storage, the L* values for the no GAs + RH 60%, GAs + RH 30%, GAs + RH 60%, and GAs + RH 90% were 55.67 ± 1.94, 41.97 ± 0.95, 47.76 ± 1.69, and 53.65 ± 1.53, respectively.
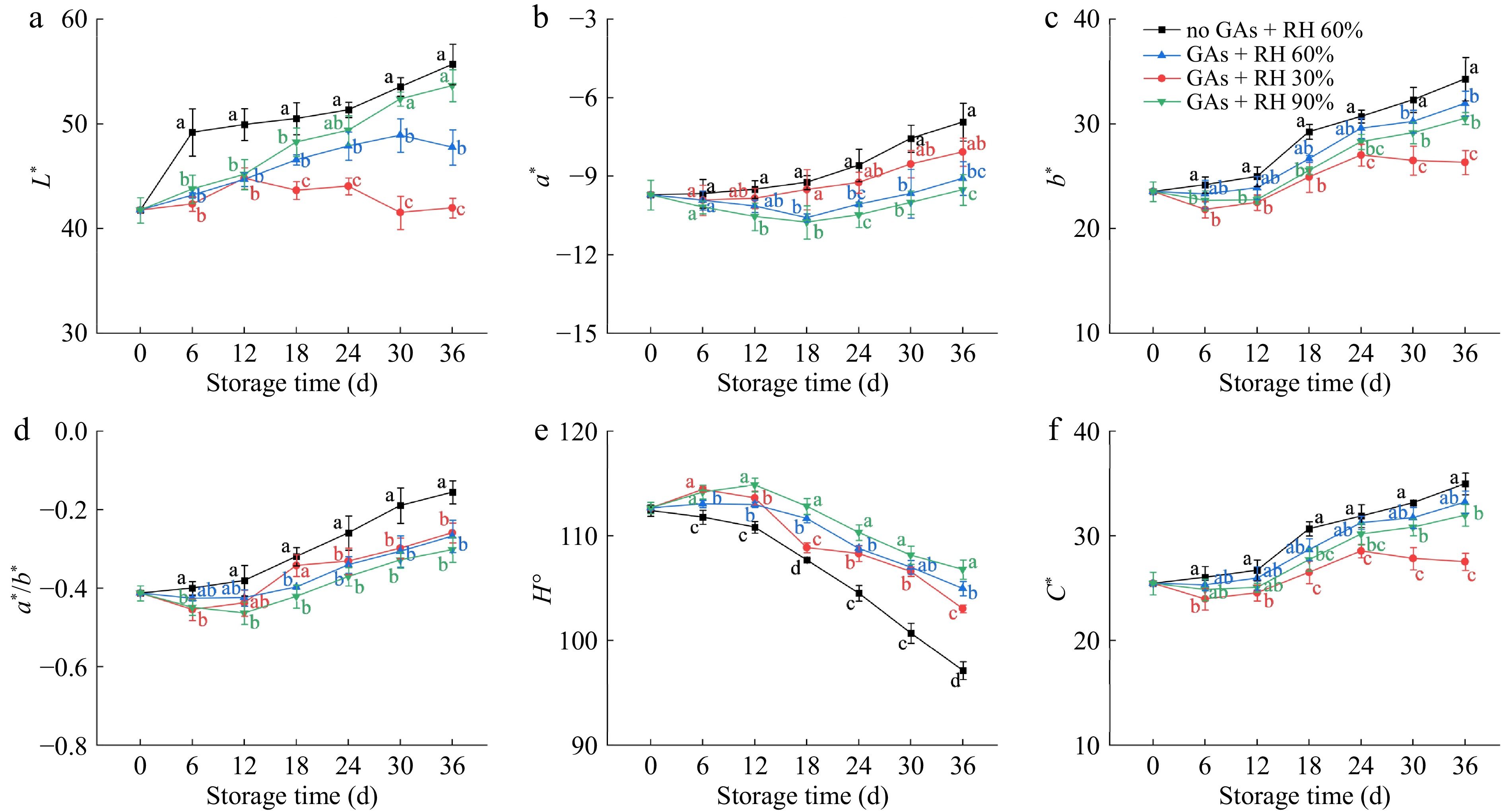
Figure 1.
Fruit color attributes of the pericarp of lemons treated with GAs + RH 30%, GAs + RH 60%, GAs + RH 90%, and the no GAs + RH 60% 3 during storage at 14 ± 1°C for 36 d. (a) L*; (b) a*; (c) b*; (d) a*/b*; (e) H°; (f) C*. Different letters denote significant differences between four treatments within each time interval (p ≤ 0.05).
With the ripening and senescence of lemons, their color changes from green to yellow, and the a* and b* values increased. The a*/b* is a composite response to skin yellow-greenness. On 0 d, the a* and b* values of lemons were −9.7 and 23.5, which represents the color of lemons was green. The a*, b*, and a*/b* values for the GAs + RH 30%, GAs + RH 60%, and GAs + RH 90% treatments declined slightly during the first 6−18 d and then showed a slow upward trend (Fig. 1b−d). Thus, GA pretreatment decreased the yellowing process and delayed color change[14]. In addition, the a*, b*, and a*/b* values of the no GAs + RH 60% on 36 d of storage increased by 28.66%, 45.61%, and 62.31%, respectively, compared with values on 0 d, indicating that the colors of lemons changed from greenish to yellow-green. In contrast, the a*, b*, and a*/b* values of the GAs + RH 60% increased by 6.48%, 35.75%, and 35.16% during the storage of 36 d. Furthermore, the a* and a*/b* values of GAs + RH 30% were consistently higher than GAs + RH 60% and GAs + RH 90%, while the L* and b* were consistently lower than others during the whole storage (Fig. 1a−d). This was consistent with the study that low humidity accelerates the senescence of zucchini fruit, leading to color change[15].
H° is a color parameter that indicates the position or angle of the color wheel. It was also used to define the difference between a specific color and a grey color with the same lightness[29]. The H° of the lemon was 112.68° on 0 d, which means that the lemon was green and shiny (Fig. 1e). Generally, the no GAs + RH 60% showed the fastest rate of reduction in H° compared with other treatments, which also showed the fastest rate of color change from green to yellow. The H° of the GAs + RH 30%, GAs + RH 60%, and GAs + RH 90% had a slight increase during the first 6−12 d and then showed a slow downward trend, which is consistent with the a*, b* and a*/b* values in Fig. 1b−d. After 36 d, the no GAs + RH 60% treatment had the yellowest color, indicating the highest degree of ripening and senescence in lemons. C* stands for saturation and purity, which represents the color sensation[29]. Figure 1f showed that the C* of GAs + RH 30% showed a continual down-regulation tendency from 24−36 d. On 36 d, C* of GAs + RH 30% was 1.20-fold and 1.16-fold lower than GAs + RH 60% and GAs + RH 90%, indicating that the main cause of color desaturation might be the low RH.
Relationship between color parameter and pigment content
-
During color development, citrus fruits typically change from green to yellow or red, which is a typical feature of fruit ripening[30]. During the first 6 d at 14 ± 1 °C, the lemons pretreated with GAs showed no significant color change, but the green color of the no GAs + RH 60% began to fade (Fig. 2a). On 18 d, the no GAs + RH 60% began to show the yellow color to the naked eye, whereas no significant color change was observed among other treatments (Fig. 2a). On 36 d, lemons in the no GAs + RH 60% were yellower than the GAs + RH 60%, GAs + RH 90%, and the GAs + RH 30% lost their shiny appearance (Fig. 2a). Slight wilting symptoms were also observed on the GAs + RH 30% compared with the GAs + RH 60% and the GAs + RH 90% on 18 d, and the GAs + RH 30% appeared severely wilting, shriveling, and showed signs of senescence on 36 d (Fig. 2a). Compared with GAs + RH 30%, GAs + RH 60%, and GAs + RH 90%, it was found that lemons stored at higher RH had a better appearance. Lemons pretreated with GAs exhibited a prolonged retention of green color at RH 60%. However, this observation did not fully elucidate the impact of GAs on lemon color transition during storage at RH 30% and RH 90%.
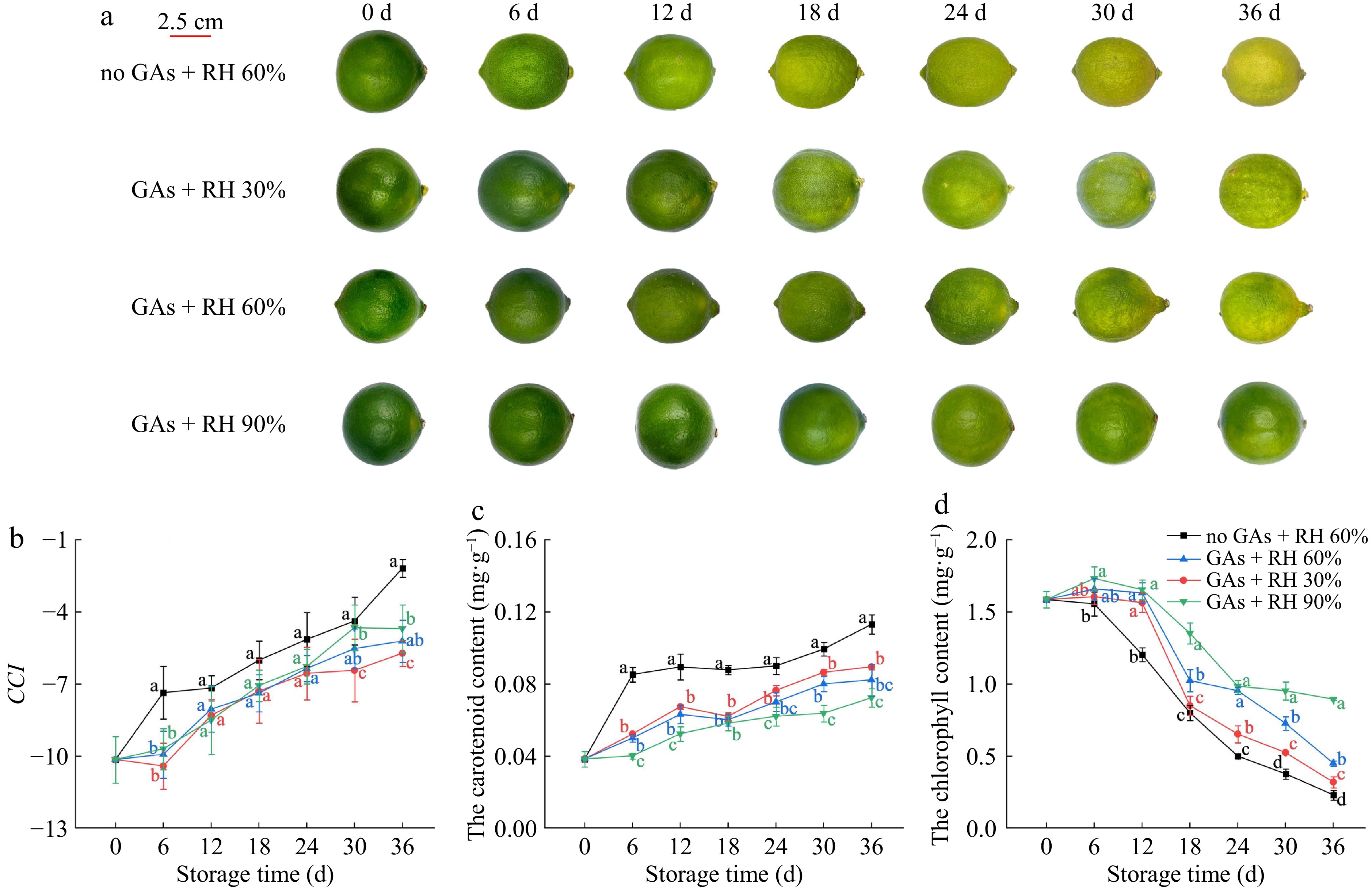
Figure 2.
The visual appearance and the relationship between color parameter and pigment content of lemons treated with GAs + RH 30%, GAs + RH 60%, GAs + RH 90%, and the no GAs + RH 60% during storage at 14 ± 1 °C for 36 d. (a) Photographs of lemons; (b) CCI; (c) the carotenoid content; (d) the chlorophyll content. Different letters indicate significant differences (p ≤ 0.05) compared with the control group under the same storage time. The scale bar of (a) is 2.5 cm.
The CCI value served as a standardized parameter for assessing the color of citrus fruits. CCI was a ratio based on HunterLab color coordinates, which determined whether fruits attained commercial maturity[31]. In Fig. 2b, the CCI of all the treatments increased continuously during storage, while the most significant change in CCI was observed in the no GAs + RH 60%. The CCI values of the no GAs + RH 60%, GAs + RH 30%, GAs + RH 60%, and GAs + RH 90% on 36 d were −2.19 ± 0.37, −5.72 ± 0.54, −5.21 ± 0.88, and −4.69 ± 0.98, respectively. The value of no GAs + RH 60% was 1.97-fold higher than the GAs + RH 60%, indicating that GA treatment played a major role in delaying the senescence of lemons[32].
Lemon pericarp color change is a vital part of fruit ripening and is mainly associated with chlorophyll degradation and carotenoid biosynthesis[14]. Overall, Fig. 2c & d showed that the carotenoid content increased during storage while the chlorophyll content decreased, which was consistent with the results presented in Fig. 2a. On 36 d, the carotenoid content was 27.22% lower in the GAs + RH 60% than in the no GAs + RH 60%. The carotenoid content of GAs + RH 60% and GAs + RH 90% were 8.1% and 19.1% lower than GAs + RH 30% (Fig. 2c), which were consistent with b* (Fig. 1c). The chlorophyll content of the no GAs + RH 60% decreased, and other treatments increased first and then decreased during storage (Fig. 2d). Therefore, the color of the no GAs + RH 60% changed from green to yellow gradually, but other treatment groups remained almost unaltered during the first 12 d of storage (Fig. 2d). In previous studies, exogenous GAs have been shown to elevate chlorophyll levels and reduce carotenoid concentrations, and subsequently delay citrus peel discoloration by inhibiting the expression of natural pheophorbide a oxygenase (PaO) and phytoene synthase (PSY)[21]. Low RH causes rapid quality deterioration of the lemons, which was similar to jujube fruits[33].
Correlation analysis of color attributes, weight loss, MDA content, SSC, TA content, and the SSC/TA ratio of lemons
-
Correlation analysis was presented in Fig. 3a, where the H° was negatively correlated with the L*, a*, b*, a*/b*, and C*, carotenoid (−0.56 ≤ r2 ≤ −0.95), and positively correlated with chlorophyll (r2 = 0.93). Besides, C* was positively correlated with L*, a*, b*, and a*/b*, and carotenoid (0.54 ≤ r2 ≤ 0.97), and was negatively correlated with the chlorophyll (r2 = −0.83). Furthermore, the carotenoid content was significantly and positively correlated with a*, b*, a*/b*, and C* (0.58 ≤ r2 ≤ 0.83), and was significantly negatively correlated with H° (r2 = −0.84). Besides, the chlorophyll content was significantly and positively correlated with H° (r2 = 0.93) and significantly negatively correlated with a*, b*, a*/b*, and C* (−0.75 ≤ r2 ≤ −0.94). In summary, fruit color variations were mainly associated with the carotenoid and chlorophyll metabolism of fruits.
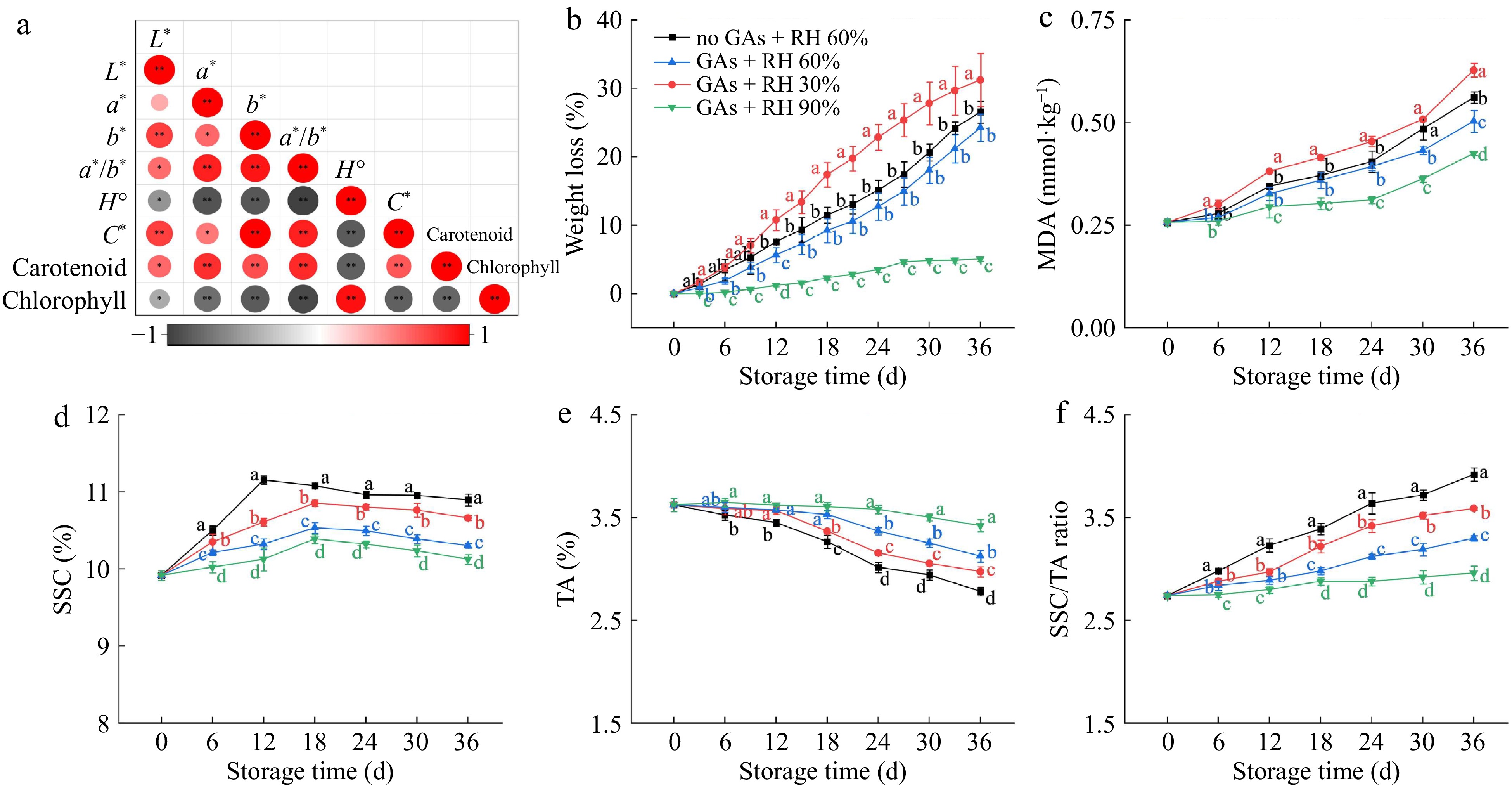
Figure 3.
Quality attributes of lemons treated with GAs + RH 30%, GAs + RH 60%, GAs + RH 90%, and no + RH 60% during storage at 14 ± 1 °C for 36 d. (a) Correlation analysis of color attributes includes L*, a*, b*, a*/b*, H°, C*, the carotenoid content, and the chlorophyll content. The red and grey colored dots respectively represent positive and negative correlations, * p ≤ 0.05, ** p ≤ 0.01. (b) Weight loss; (c) MDA content; (d) SSC; (e) TA; (f) SSC/TA ratio. Different letters indicate significant differences (p ≤ 0.05) compared with the control group under the same storage time.
The weight loss of fruit primarily results from the water loss through transpiration and the consumption of nutrients during respiration[24]. Water loss is a crucial factor affecting the quality of citrus fruits during storage, which mainly occurred in the pericarp due to the absence of functional vascular connections between the pericarp and pulp tissue[34]. Figure 3b showed that the weight loss of lemons in the four groups increased gradually with the storage periods. The weight loss of GAs + RH 90% was the lowest among all the treatments, whereas the weight losses were 21.56%, 26.14%, and 19.18% higher in the no GAs + RH 60%, GAs + RH 30%, and GAs + RH 60% on 36 d, respectively (Fig. 3b). The weight losses of the GAs + RH 60% were compared with that of the no GAs + RH 60% during storage (Fig. 3b), and GAs pretreatment showed lower weight losses than untreated with the same RH during the whole storage. Therefore, both GAs pretreatment and higher RH inhibited weight loss of green lemons, which is consistent with previously reported results that higher RH inhibited the weight loss rate of red bell peppers and GAs maintained the fruit weight of sweet cherries[35,36]. In addition, Fig. 2a showed similar results, indicating that water loss caused by low RH leads to lower turgor pressure, affecting the mechanical properties and color changes of the 'Shine Muscat' grapes[24].
The degree of membrane lipid peroxidation was indicated by measuring the MDA content. As shown in Fig. 3c, the MDA content of all groups increased along the storage duration, and the MDA content of the GAs + RH 30% and GAs + RH 60% were 0.20 and 0.08 mmol·kg-1 higher than the GAs + RH 90% on 36 d. In previous studies, high RH reduced water loss, inhibited electrolyte leakage, and alleviated damage to the cell membrane of pepper[37]. Therefore, GAs + RH 90% could inhibit the increase in MDA content, and then maintain the structure of the flavedo and prevent the efflux and degradation of chlorophyll than other treatments, which best retained the visual appearance and pericarp structure (Figs 2a & 3c).
To evaluate the influence of GAs pretreatment and RH on fruit quality during storage, the SSC, TA, and SSC/TA were determined. The SSC of the no GAs + RH 60% was significantly (p ≤ 0.5) higher than that of the other treatments during storage (Fig. 3d). Figure 3d showed that the SSC of the no GAs + RH 60% reached the maximum on 12 d (11.16% ± 0.03%), while the GAs + RH 30%, GAs + RH 60%, and GAs + RH 90% reached their maximums on 18 d (10.86% ± 0.04%, 10.54% ± 0.07%, 10.39% ± 0.06%). Therefore, GA pre-treatment delayed the appearance of the SSC maximum. The TA content in the no GAs + RH 60%, GAs + RH 30%, GAs + RH 60%, and GAs + RH 90% showed decline tendencies during storage and reached the minimum values of 2.78% ± 0.04%, 2.97% ± 0.05%, 3.12% ± 0.06%, 3.42% ± 0.06%, respectively, on 36 d (Fig. 3e). Generally, citrus fruits under low RH conditions led to higher SSC but lower TA content during storage, and then caused the consumption of nutrients and reduced metabolic activity[38]. Since 12 d, the no GAs + RH 60% had the highest SSC/TA ratio compared to the GAs + RH 60%, which could be attributed to the decreased TA content accompanied by increased SSC content, suggesting that GAs may have hindered fruit ripening (Fig. 3d,e). Furthermore, the SSC/TA ratio of the GAs + RH 90% remained unchanged compared with other treatments on 36 d, which was 24.49% higher than the no GAs + RH 60% (Fig. 3f). Therefore, GAs pretreatment delayed fruit senescence and quality deterioration of lemons[39], and low RH storage conditions showed the converse effects (Fig. 3d−f).
Flavedo structure of lemons
-
The pericarp structure of lemons is mainly composed of the outer pigmented flavedo and the white albedo[40]. Among them, the flavedo was generally constructed with parenchymatous cells, cuticles, oil glands, and vascular bundles. Excessively low humidity causes water stress on fruits, thus pericarp tissues cannot make proper adjustments for water balance, which results in cell collapse, tissue damage, and reduces the appearance values and the overall quality of fruit[16]. Figure 4 showed the lateral (Fig. 4a-1−a-9) and medial (Fig. 4b-1−b-9) of the flavedo structure during storage, a closer observation of which indicated the degree of crumpling and the profiles of color changes in green lemons. On 0 d, the lateral of flavedo was green and shiny with a uniform size and uniformly distributed oil gland pits (Fig. 4a-1). On 18 d, compared with the GAs + RH 90%, the color of the no GAs + RH 60% treatments turned yellow, and the oil gland pits of the GAs + RH 30% treatment became shallower and dull (Fig. 4a-2−a-5). Furthermore, compared with the GAs + RH 90%, the no GAs + RH 60% treatment significantly became yellow, and the GAs + RH 30% induced some degree of wrinkles at the lateral of flavedo, and the middle portion of the oil gland turned yellower (Fig. 4a-6−a-9). In Fig. 4b-1, the oil glands can be seen on the medial of the flavedo. During storage, the color of oil glands in lemons from no GAs + RH 60% changed from green to yellow (Fig. 4b-2 & b-6), which may correlate with changes in chlorophyll and carotenoid content. The GAs + RH 90% medial of flavedo maintained darker green, compared with the GAs + RH 30% and GAs + RH 60% (Fig. 4b-7 & b-9) on 36 d. Previous studies indicated that GAs pretreatment significantly delayed flavor chlorophyll degradation and promoted the late ripening of Citrus cultivars, and low RH showed the opposite pattern for zucchini fruit[17,41].
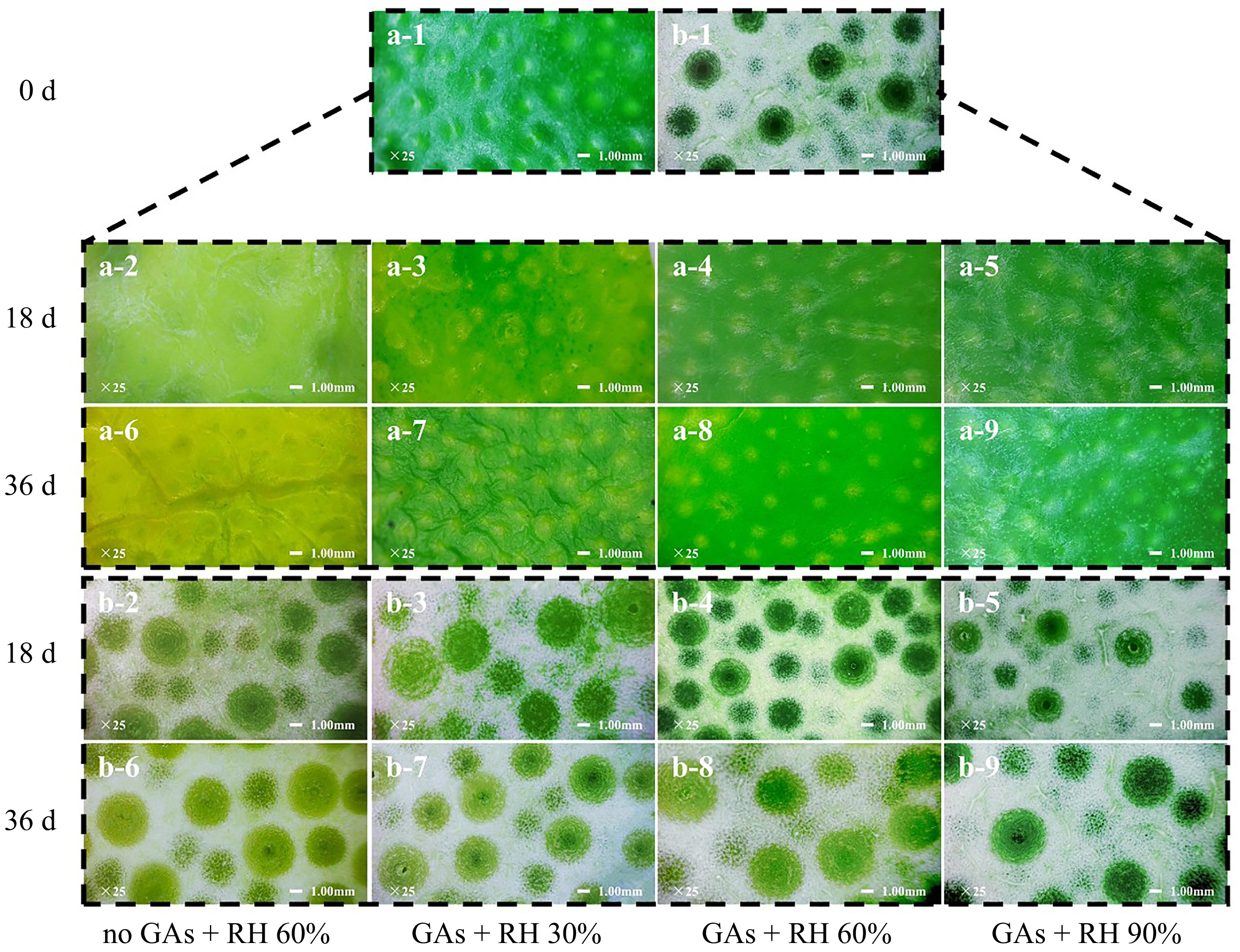
Figure 4.
Microscopic structures of flavedo treated with GAs + RH 30%, GAs + RH 60%, GAs + RH 90%, and no GAs + RH 60% during storage at 14 ± 1 °C for 36 d. (a-1) 25× (bar = 1 mm) the outer side and (b-1) 25× (bar = 1 mm) the inner side of the flavedo on 0 d; (a-2)−(a-5) 25× (bar = 1 mm) the outer side and (b-2)−(b-5) 25× (bar = 1 mm) the inner side of the flavedo on 18 d; (a-6)−(a-9) 25× (bar = 1 mm) the outer side and (b-6)−(b-9) 25× (bar = 1 mm) the inner side of the flavedo on 36 d.
Surface morphology of pericarp
-
Fruit transpiration primarily occurs through the cuticular and stomatal regions of the pericarp, with the cuticle serving as the principal barrier to water movement between the fruit and the atmosphere[42]. Stomata and waxes have the role of maintaining water balance and preventing water loss[43]. The oil gland is also enriched with plant secondary metabolites and is linked with flavor and aroma[44]. The effect of GAs pretreatment and storage at different RH on the structure of the oil gland, stomata, and cuticular waxes of lemon pericarp were analyzed. After storage for 36 d, compared with the GAs + RH 90%, the epidermal cuticle and stomata of GAs + RH 30%, GAs + RH 60%, and the no GAs + RH 60% were wrinkled and distorted in structure, and the number and morphology of wax crystals were changed (Fig. 5). From the overview of SEM images in Fig. 5a-6−a-9), the lemon pericarp of treatment of GAs + RH 90% on 36 d had a smooth and uniform surface and well-developed oil gland pits compared with others. On 36 d, the epidermal cuticle integrity of the GAs + RH 30% and no GAs + RH 60% was compromised, resulting in the oil gland being disorganized and ruptured (Fig. 5a-6 & a-7). Besides, cracks on the surface of the lemon pericarp from the GAs + RH 30% were wider and deeper than those from the GAs + RH 60% and GAs + RH 90% (Fig. 5a-7−a-9). On 36 d, the wax layers of lemons became loose and flaky under the GAs + RH 30%, and the no GAs + RH 60% (Fig. 5b-6 & b-7). However, the wax layer exhibited a smooth and nearly intact surface under the treatment of GAs + RH 90% (Fig. 5b-9). Different from GAs + RH 90%, the wax layer of other groups was severely fragmented, and the density and size of platelet-like epicuticular wax crystals increased obviously on 18 or 36 d (Fig. 5b-2−b-9). There were abundant agglomerations of small pieces and smooth ridges arrayed compactly around the fruit peel stomata of GAs + RH 30% (Fig. 5b-7) on 36 d. These findings suggested that the morphology of the wax layer and stomata of the pericarp stored under low RH changed, while the number and morphology of the wax crystals stored under high RH were preserved[3]. Moreover, the reduced glossiness of the pericarp may also be related to the destruction of the wax layer on the surface of the pericarp (Figs 1a & 5). As reported, water stress due to low environmental humidity during the postharvest storage affects the waxes of citrus fruit[45].
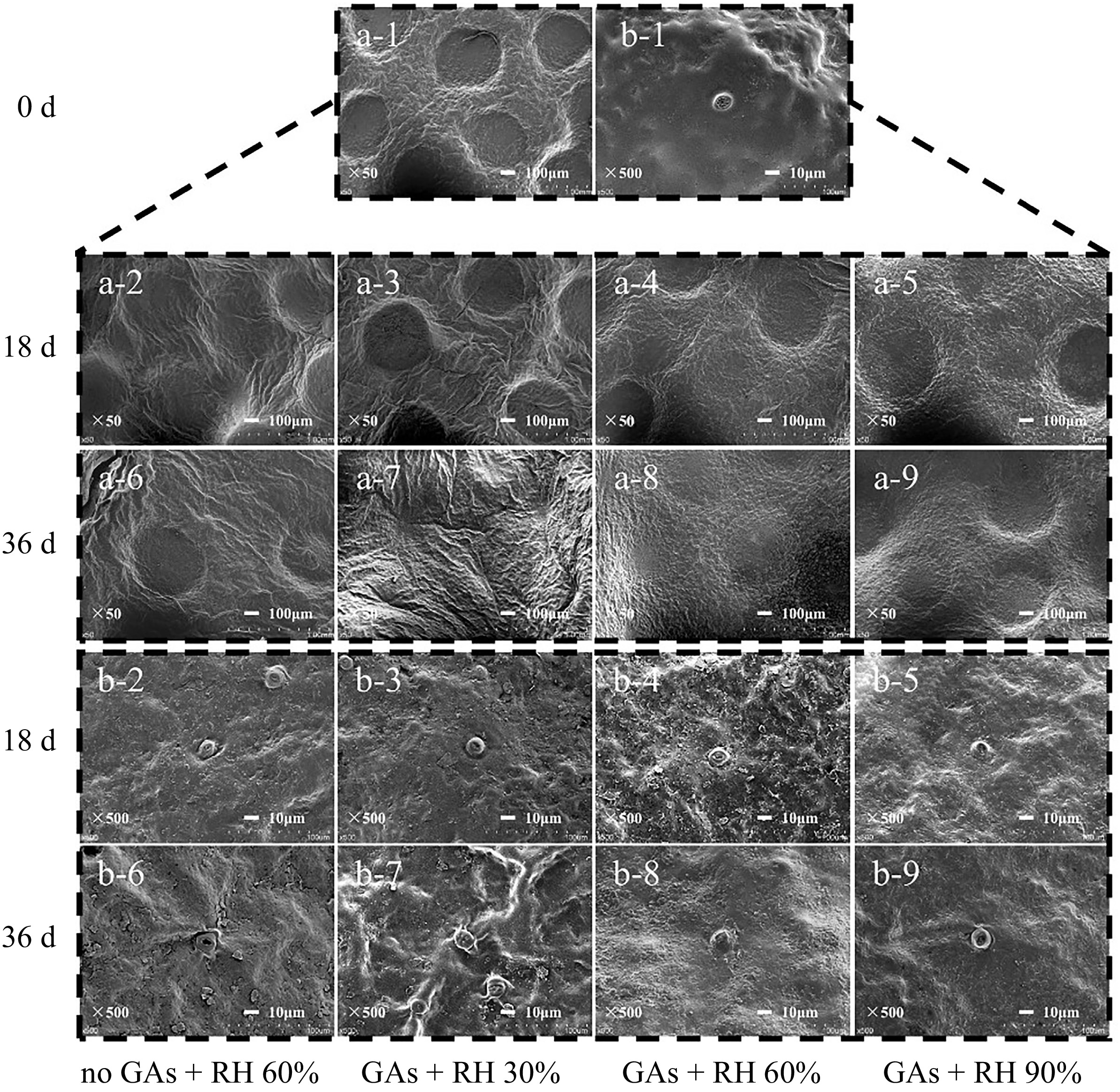
Figure 5.
The SEM analysis of the flavedo treated with GAs + 30% RH, GAs + 60% RH, GAs + 90% RH, and no GAs + 60% RH treatments during storage at 14 ± 1 °C for 36 d. (a-1) 50× and (b-1) 500× (bar = 1 mm) SEM analysis of the pericarp on 0 d. (a-2)−(a-5) 50× and (b-2)−(b-5) 500× (bar = 1 mm) SEM analysis of the pericarp on 18 d. (a-6)−(a-9) 50× and (b-6)−(b-9) 500× (bar = 1 mm) SEM analysis of the flavedo on 36 d.
Regulation of volatile aroma compounds in the pericarp
-
The volatile aroma of citrus was one of the main contributors to consumers' preferences and was mainly determined by the content and composition of the VOCs[18]. In Fig. 6, over 100 different volatile compounds of lemons were identified through GC-MS analysis, among which 25 major components were examined, including seven alkenes, two alcohols, four ketones, four acids, four aldehydes, and three esters. The variety and content of volatile components of lemon peel varied during storage at 14 ± 1 °C. Limonene is the highest content in alkenes, which is also the highest content among all VOCs during storage[46]. The total contents of α-santalene, β-myrcene, D-limonene, γ-terpinene, β-phellandrene, o-cymene, and β-bisabolene of the no GAs + RH 60%, GAs + RH 30%, GAs + RH 60%, and GAs + RH 90% on 36 d of storage were reduced by 49.74%, 60.89%, 35.98%, and 5.55%, respectively, compared with their contents on 0 d (Fig. 6a). Furthermore, along with alkenes, aldehydes have been identified as a significant factor in forming the aroma and flavor of orange juice[47], and aldehydes was a precursor for the synthesis of VOCs[48]. During storage, the aldehyde content including 5-hydroxymethylfurfural, citral, glyceraldehyde, and citronellal decreased in lemons stored at 14 ± 1 °C (Fig. 6b). On 36 d, the primary aldehydes content of the GAs + RH 90% was 1.85-fold, 4.41-fold, and 0.87-fold than the no GAs + RH 60%, GAs + RH 30%, and GAs + RH 60%, respectively (Fig. 6b). The alcohol and ketone contents remained nearly constant in lemons of GAs + RH 90%, but their contents were lower than those of alkenes or aldehydes during storage. Esters have a positive effect on fruity top notes in fresh juice and the furfural such as 5-hydroxymethylfurfural identified as the characterized odorants[49]. The acid and ketone contents decreased with the storage duration for all groups, while the GAs + RH 90% varied the least (Fig. 6c). The primary acids (acetic acid and n-hexadecanoic acid, octadecanoic acid, and tetradecanoic acid) content of the GAs + RH 90% was 1.71-fold, 0.80-fold, and 0.59-fold than the no GAs + RH 60%, GAs + RH 30%, and GAs + RH 60%, respectively (Fig. 6c). In summary, GAs + RH 90% was optimal to maintain the VOC contents and fruit quality.
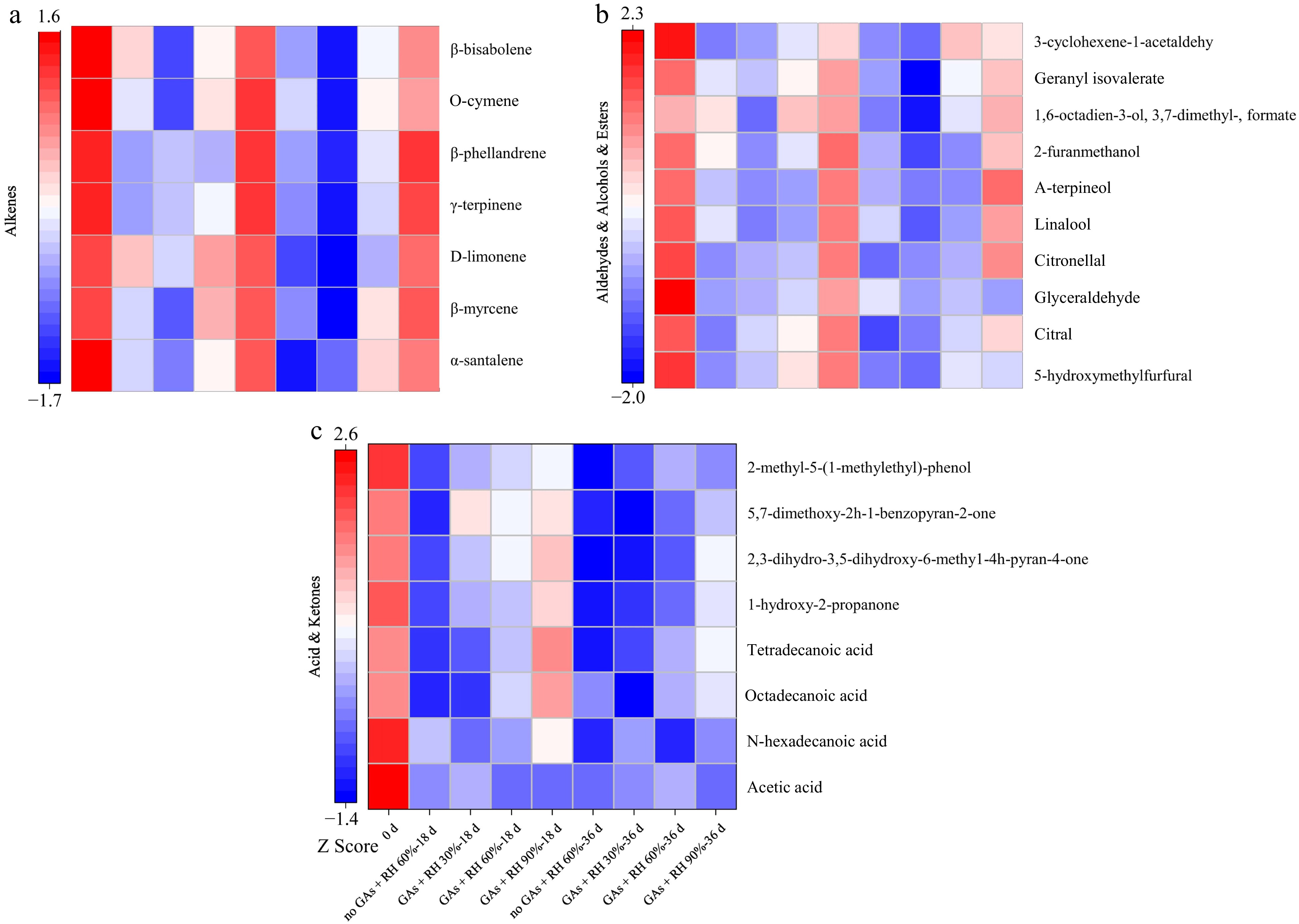
Figure 6.
Heatmaps of volatile compounds from lemon pericarp treated with GAs + RH 30%, GAs + RH 60%, GAs + RH 90%, and no GAs + 60% RH during storage at 14 ± 1 °C for 36 d. (a) Alkenes; (b) aldehydes, alcohols, and esters; (c) acid and ketones. The no GAs + RH 60% on 0 d represents untreated lemons. Z score shows the relative contents after the normalization processing. Z-score color scale represents the differential content of each compound after the implementation of normalization.
-
The integration of GAs pretreatment and RH 90% storage maintained the visual appearance, microstructure, and aroma of lemons during 36 d storage period at 14 °C. Moreover, the combined treatment maintained the green color by delaying the chlorophyll degradation and carotenoid biosynthesis. GAs + RH 90% inhibited the increase of the weight loss and MDA content, regulated the SSC/TA ratio and membrane peroxide, and hindered fruit ripening. In addition, the GAs + RH 90% treatment mitigated the wrinkling degrees of pericarp, maintained the microstructures of the oil glands, stomata, and wax, and then maintained an excellent appearance and commodity value. The contents of volatile aroma components by GAs + RH 90% were maintained, especially alkenes. The relative convenience, practicableness, and effectiveness of the GAs pretreatment combined with RH 90% storage make it a critical and valuable method in the postharvest storage of green stage lemons, which might meet different commercial needs and market demands for lemon growers, packers, and exporters.
This work was supported by the Key Science and Technology Planning Program of Tianjin (22ZYJDSS00090). Yingying Zhang appreciates funding from the Open Project of State Key Laboratory of Food Nutrition and Safety, Tianjin University of Science and Technology (Grant No. SKLFNS-KF-202204).
-
The authors confirm contribution to the paper as follows: conceptualization: Li D, Li L; data curation: Du J; methodology: Miao Z, Liu L, Farouk A, Li L; writing-original draft: Li D; writing-review & editing: Li X, Jiang Y; supervision: Li X; investigation: Cheng J, Li Y, Zhang Y; validation: Jiang Y; project administration: Hu S; funding acquisition: Zhang Y. All authors reviewed the results and approved the final version of the manuscript.
-
The data that support the findings of this study are available from the corresponding author upon reasonable request.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of China Agricultural University, Zhejiang University and Shenyang Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Li D, Li X, Miao Z, Du J, Cheng J, et al. 2024. Gibberellins pre-treatment and storage at high relative humidity improved the quality of 'Eureka' lemon (Citrus limon (L.) Burm. f.). Food Innovation and Advances 3(4): 416−425 doi: 10.48130/fia-0024-0040
Gibberellins pre-treatment and storage at high relative humidity improved the quality of 'Eureka' lemon (Citrus limon (L.) Burm. f.)
- Received: 26 June 2024
- Revised: 03 November 2024
- Accepted: 25 November 2024
- Published online: 13 December 2024
Abstract: The loss of pericarp greenness, wrinkling of the pericarp, and alteration of aroma are indicators of the ripening and senescence of lemons. In this study, lemons were soaked in 100 mg∙L-1 of gibberellin (GA) solutions for 5 min and stored at 14°C for 36 d under three relative humidity (RH) levels of 30%, 60%, and 90%, respectively. The changes in visual appearance, pigment metabolism, pericarpic microstructure, and volatile compounds of lemons during storage were evaluated. The results showed that GA pretreatment inhibited the color transformation from green to yellow of the flavedo and restrained fruit senescence. In addition, RH 90% effectively maintained the structural integrity of the oil gland, waxes, and stomata in the flavedo. GAs + RH 90% treatment maintained the fruit color index (L*, a*, b*, a*/b*, H°, C*) by inhibiting chlorophyll degradation and regulating carotenoid biosynthesis. Green lemons treated with GAs + RH 90% also showed reduced epidermal wrinkling, well-preserved cuticle, and stomatal structure, with a smooth and intact wax layer on the lemon pericarp. In addition, GAs + RH 90% treatment maintained the content of volatile aroma compounds, especially terpene. GAs + RH 90% had a great advantage in maintaining visual quality, delaying the deformation of tissue microstructure, preserving nutritional quality, and improving aroma. Thus, this treatment is potentially applicable for maintaining the storage quality of green lemons and extending their shelf life.
-
Key words:
- Relative humidity /
- Gibberellins /
- Visual appearance /
- Nutritional quality /
- Microstructure /
- Volatile compounds












