-
Brazil has the largest effective herd in the world, with about 214,69 million head of cattle, and in 2019, it was the world leader in exports and sales of beef to several countries[1]. Because of the growing demand, and because the fresh meat is highly perishable by microorganisms during the transport, handling, and storage[2], the maintenance of the quality of the beef is becoming increasingly important.
The application of ionizing irradiation in foods is widely known for its effects on microbial control, against pathogens and deteriorators, ensuring safety and prolonging the time to market. Some studies have evaluated the effects of doses considered relatively low (< 5 kGy) on the conservation and quality of raw meat[3−7], although higher doses (up to 10 kGy) applied post-rigor have also been studied as a tool in favoring tenderness[8−10]. However, the application of higher doses of irradiation in high protein content and fatty products such as meat is limited, as it can result in accelerated oxidation of lipids and proteins[6], which induces important meat quality changes as the development of off-flavors and off-odors, discoloration, and loss of nutrients[11−13]. Rodrigues et al.[9] reported that samples irradiated at 9 kGy presented higher lipid oxidation and lower oxymyoglobin proportion, color redness and chroma values. The oxidative chemical changes induced by the irradiation, can also lead to an increase in the production of volatile organic compounds (VOCs), such as hydrocarbons, alcohols, ketones, aldehydes, and carboxylic acid gases[14], thus reflecting on the meat quality. Ahn et al.[15] proposed that VOCs including dimethyl sulfide, dimethyl disulfide, and dimethyl trisulfide sulfur compounds are mainly responsible for the off-odor of irradiated pork meat described by a sensory panel as a 'barbecued corn-like' odor. These changes are related to muscle condition (pH, reducing equivalents, etc.) and to applied irradiation dose.
In addition to being dose-dependent, oxidative chemical changes induced by ionizing irradiation are favored in the presence of oxygen, and the effects are minimized when the meat is irradiated under vacuum[16]. The aqueous electron radicals generated by water molecule radiolysis can react with sulfur-containing amino acids, such as methionine and cysteine, and, or hydrogen sulfide, contributing to the off-flavor and off-odor of irradiated meat[11]. However, water radiolysis can be minimized when the frozen meat is irradiated, as the diffusion of these radicals is reduced and its access to other components of food will be limited. The process of freezing and thawing vacuum-packed meat has been suggested to increase the action of endogenous proteases during aging[8,17,18], favoring their tenderness. Thus, the proteolysis and lipolysis induced by these processes release peptides, free amino acids, and free fatty acids that represent important precursors of VOCs[19], also affecting the odor and flavor of fresh meat.
Identifying and correlating the volatile compounds of meat subjected to new technological processes is of utmost importance, since the aromatic characteristics of cooked meat play a very important role in its palatability, influencing consumer acceptance and preference[20]. Moreover, there are still no studies evaluating the combined effects of freezing and gamma irradiation on the VOC profile in beef before and after a period of commercial aging. Thus, this study aimed to evaluate the gamma irradiation of frozen and unfrozen vacuum-packed beef and subsequent aging on the volatiles profile.
-
The animals and sampling used in the present work were the same as described previously[8,9]. Briefly, both left and right striploin (Longissimus lumborum muscle, LL) from four Nellore cattle, with similar age, rearing system, and slaughter conditions, were obtained 48 h postmortem in a slaughterhouse with Federal Inspection, in the city of Contagem, Minas Gerais state (MG), Brazil. Sixteen steaks 5 cm thick per animal were obtained from both striploin, individually vacuum-packed (nylon-polyethylene 10 μm-thick package; BS420 packer, R. Baião, Ubá, MG, Brazil) and randomly distributed evenly between two groups: control (never-frozen; NF), refrigerated samples and submitted to irradiation treatments before aging; and frozen/thawed (FT), samples frozen in a conventional freezer (−18 °C/24 h) and subjected to irradiation treatments, then thawed (4 °C/24 h) before aging.
Ionizing radiation and aging
-
Within each group (NF and FT), the samples (eight steaks) were randomly distributed in four thermal boxes (two steaks of each animal per box) and subjected to ionizing radiation in the Gamma Radiator IR-214 (MDS, Nordion; cobalt-60 source and 1,925.8 Gy/h rate) at the Nuclear Technology Development Center of the National Commission of Nuclear Energy (CDTN/CNEN), in Belo Horizonte, MG, Brazil. The target doses were 3, 6, and 9 kGy, with the samples subjected to FT treatment irradiated still frozen (approximate irradiation time of 1.5, 3.0, and 4.5 h, respectively). The non-irradiated samples (0 kGy) were kept in the thermal boxes out of the irradiator chamber during the entire process. As the target dose was reached, the respective box was removed from the irradiator and kept (together with the non-irradiated sample boxes) until the last dose (9 kGy) was reached.
After the irradiation, the boxes were taken to the Laboratory of Meat Science and Technology (LabCarnes), of the Food Science Department (DCA) at Federal University of Lavras (UFLA) in Lavras, MG, Brazil. The samples were removed from boxes and stored at 1.0 °C in a climatic chamber (model EL202, EletroLab, São Paulo, SP, Brazil) for a commonly used commercial aging time of 14 d.
Volatile compounds identification
-
The VOCs were conducted as a screening analysis 1 d after irradiation and at the end of the aging process (14 d) according to procedures described by Haddad et al.[18]. The separation and identification of VOCs were conducted at the Chemical Analysis and Prospecting Center (CAPQ/UFLA), using a gas chromatograph coupled to a mass spectrometer (GC-MS, QP2010 Plusmodel; Shimadzu®, Kyoto, Japan), equipped with an automatic injector for liquids and gases (AOC-5000; Shimadzu®, Kyoto, Japan) and a SLB®-5MS capillary column (5% phenyl- 95% dimethylsiloxane; 30 m × 0.25 mm i.d., 0.25 μm film thickness; Supelco, Bellefonte, PA, USA).
To allow for exploratory analysis of the data, individual portions of the four steaks (one from each animal) were ground at once to obtain a combined sample and used as representative samples of all treatments (radiation dose and aging time). About 2.5 g of this pooled sample were weighed into a 22 mL headspace vial and sealed with a PTFE-faced silicone septum (Supelco, Bellefonte, PA, USA). The volatile organic compounds (VOCs) were extracted by headspace-solid phase micro-extraction (HS-SPME), using a DVB/CAR/PDMS (Divinylbenzene/Carboxen/Polydimethylsiloxane, 10 mm length, 50−30 μm thick layer; Supelco Bellefonte, PA, USA) fiber as stationary phase.
Before the VOCs extraction, the fiber was cleaned at 250 °C for 5 min to prevent possible contamination. The vials were preheated for 10 min at 60 °C in a heating block, and then the SPME fiber was exposed in the headspace for 45 min to extract the compounds. Thermal desorption was performed at 250 °C in a splitless mode injector for 2 min. Ramped oven temperature was used to improve volatile separation. The initial oven temperature of 35 °C was held for 2 min. After that, the oven temperature was increased to 80 °C at 2 °C per min, to 150 °C at 4 °C per min and finally to 230 °C at 8 °C per min. The carrier gas used was He 5.0 with a flow rate of 1.0 mL/min. The ionization potential of MS was 70 eV, the interface and ion source temperatures were 250 and 200 °C, respectively, and the scan range was 45 to 350 Da with a solvent cut in 0.55 min.
The identification of volatiles was achieved by comparing mass spectral data of samples with those of the MS software library (Wiley 8 and FFNSC 1.2). A homologous series of alkanes (Supelco, Bellefonte, PA, USA) were used as standards to confirm the identification by the mass-selective detector. The area of each peak was integrated using the GCMS Solutions Software (Shimadzu®, Kyoto, Japan) and the data presented as total ion counts.
Statistical analysis
-
Statistical analyses were performed using the Statistica® 8.0 software (StatSoft Inc., Tulsa, OK, USA). The results, expressed in arbitrary units (total ion counts × 104), were evaluated by analysis of variance, considering a significance level of 5%. Significant differences among means were separated using the Tukey's multiple-range test.
An exploratory examination of VOCs at different irradiation doses in the studied effects (frozen and non-frozen), within each aging time, was also conducted by multivariate technique principal component analysis (PCA). The PCA is among the statistical techniques most used for the analysis of VOCs obtained by HS-SPME associated with GC-MS, allowing the analysis of interrelationships between many variables, and explaining these variables in terms of their inherent dimensions (components).
VOCs were transformed to Log10 to normalize the data before being subjected to PCA, favoring the check of any separation between groups within the effects studied. The matrix arrangement was made of eight lines (treatments x irradiation doses) and up to 37 columns (compounds identified). The matrix of Pearson correlation was used to perform the PCA, conducted using Sensomaker® software version 1.91 (UFLA, Brazil).
-
A total of 37 volatile organic compounds (VOCs) associated with bovine striploin steaks were identified for treatments on days 1 and 14 of aging (Table 1): six alcohols, nine aldehydes, two esters, one ether, 14 hydrocarbons, four ketones, and one sulfur compound. However, 15 VOCs (3-methylbutanol, nonanol, 2-methylbutanal, 3-methylbutanal, 2-ethylhexyl salicylate, ethyl acetate, dodecane, tridecane, undecane, 2,2,5-trimethylhexane, nonane, 2,3,4-trimethylpentane, 1,3-bis(1,1-dimethylethyl) benzene, dodecanone, and heptanone) were observed only after 14 d of aging, which is due to the chemical and enzymatic reactions that continually degrade lipids and proteins during the aging process, especially influenced by the treatments (irradiation and freezing) used.
Table 1. Average values (total ion count ×104) of volatile compounds identified in irradiated (up to 9 kGy) and non-irradiated Nellore cattle muscles (L. lumborum) regard aging and freezing treatments.
Volatile compound Non-irradiated Irradiated > Aging (d) SEM NF FT NFI FIT 1 14 Alcohols 1-Butanol, 3-methyl 18 nd 2A 29B nd 28 7 1-Heptanol 22 11 5 5 4 12 4 1-Hexanol, 2-ethyl- 107 165 123 142 6A 261B 20 1-Nonanol 118 139 35 27 nd 111 19 1-Octanol 352A 196B 203 174 172A 248B 24 Ethanol 27 29 10 15 10 23 4 Aldehydes Butanal 11 nd 18A 4B 3A 17B 4 Butanal, 2-methyl nd 18 nd 6 nd 9 3 Butanal, 3-methyl nd 32 nd 12 nd 17 5 Decanal 100A 27B 45 70 14A 104A 12 Heptanal 145A 46B 135 137 125 127 15 Hexanal 264A 35B 275A 208B 245A 192B 23 Nonanal 1,821A 1236B 1,358 1,511 1,166A 1,750A 126 Octanal 435A 246B 368 372 326 399 40 Tetradecanal 183 147 250 223 98A 339A 20 Esters 2-Ethylhexyl salicylate nd 8 47 15 nd 48 9 Ethyl acetate 36 nd 25A 3B nd 30 9 Ethers Propane, 2-methoxy-2-methyl 24A 266B 102A 320B 40A 335B 46 Hydrocarbons (aliphatic) 1-Dodecene 860 nd nd 5 nd 218 107 1-Tridecene 20 nd 7 nd nd 10 3 1-Undecene 33A 112B 235A 313B nd 447 77 Heptane nd 12 8A 18B 1A 21B 1 Hexane 218A 687B 453 452 192A 712B 93 Hexane, 2,2,5-trimethyl nd 7 nd 5 nd 5 2 Nonane 9 nd 4 1 nd 6 2 Octane 135A 92B 101 89 31A 169B 15 Pentane, 2,3,4-trimethyl nd 11 nd 5 nd 7 2 Pentane, 3-methyl 18A 162B 82 112 16A 174B 26 Hydrocarbons (alicyclic) Cyclopentane, methyl 19A 264B 186 194 29A 327B 46 Hydrocarbons (aromatic) Benzene nd nd 19 4 4 13 3 Benzene, 1,3-bis(1,1-dimethylethyl) nd nd 149A 19B nd 126 22 Benzene, methyl (Toluene) 72A 752B 726A 664B 122A 1127B 137 Ketones 2-Butanone, 3-hydroxy (Acetoin) 247A 184B 23A 322B 105A 262B 69 2-Dodecanone nd nd 15 17 nd 24 7 2-Heptanone 99 nd 11 12 nd 42 13 2-Propanone 60 35 40 34 44 35 4 Sulfur compounds Sulfide, dimethyl 39 38 96 54 48 84 10 Σ Alcohols 644 540 378 393 192 682 47 Σ Aldehydes 2,959 1,786 2,450 2,543 1,977 2,954 204 Σ Esters 36 8 72 18 nd 78 14 Σ Ethers 24 266 200 204 40 335 46 Σ Hydrocarbons 1,383 2,098 1,970 1,882 396 3,363 304 Σ Ketones 407 219 89 386 150 364 73 Σ Sulfur compounds 39 38 96 54 48 84 10 Total 5,491 4,956 5,255 5,481 2,803 7,861 455 Treatments (from four animal pooled samples): NF = chilled (never-frozen) samples; FT = frozen (−18 °C/24 h) and thawed (4 °C/24 h) samples; NFI = never-frozen irradiated (3, 6 and 9 kGy) samples; FIT = frozen (−18 °C/24 h), irradiated (3, 6, and 9 kGy) and thawed (4 °C/24 h) samples; SEM = standard error of the mean (n = 12). Uppercase letters A and B represent followed by different letters in each row, corresponding to different groups (non-irradiated, irradiated, and aging), differ (p < 0.05) by F test. The action of endogenous proteases during aging results in the formation of small free peptides and amino acids which are important flavor precursors. They also accelerate chemical changes in the lipid fraction that result in the formation of multiple reactive substances (such as acids, alcohols, aldehydes, or ketones) which, directly and indirectly, contribute to the flavor development[18,21]. Moreover, the microbial action during storage also induces changes in the profile of VOCs[13,22], which can affect mainly non-irradiated samples. However, according to Rodrigues et al.[7], despite there being a large reduction in bacterial count on the first day after gamma irradiation (3 kGy), microbial cells can be detected at 14 d of aging, especially at temperatures higher than 1°C. Haddad et al.[18] reported that frozen/thawed and aged beef had a higher number of VOCs than the never-frozen aged ones.
The changes observed in VOCs associated with freezing was also clear. In the non-irradiated samples, six compounds (2-ethylhexyl salicylate, 2-methylbutanal, 3-methylbutanal, heptane, 2,2,5-trimethylhexane, and 2,3,4-trimethylpentane) were not identified in the frozen samples but were identified in the non-frozen ones; another seven compounds (3-methyl-1-butanol, 1-dodecene, 1-tridecene, 2-heptanone, butanal, ethyl acetate and nonane) identified in the frozen samples were not identified in the non-frozen ones. These differences are also due to differences caused by freezing in the degree of lipid oxidation and, or proteolysis during aging[18,23]. The loss of the myofibrillar structural integrity due to the ice crystal formation (cryo-damage) increases the contact of lipids with oxidation catalysts and of proteolytic enzymes with their substrates[23]. The activity of calpains, the main meat proteolytic enzymes (calcium-activated), could also be favored by the increase in free calcium concentration in the sarcoplasm and by the suppression of its inhibitor (calpastatin), both induced by the freezing process[24,25]. Moreover, Haddad et al.[18] reported higher microbial multiplication in frozen/thawed aged meats than in non-frozen ones, which may also have contributed to the differences in the VOCs formed.
Finally, the irradiation process also affects the meat's endogenous enzyme action and the lipid oxidation reactions during aging, creating highly oxidizing conditions along with the formation of free radicals by water radiolysis[11] and, therefore, affecting the VOCs formed during aging. Rodrigues et al.[9] and Sales et al.[8] reported that beef irradiated at 9 kGy had higher TBARS index values (higher lipid oxidation) than beef irradiated at lower doses (3 and 6 kGy), whether they are irradiated frozen or not. The effects of freezing and the different doses of irradiation applied will be discussed in the following items by maturation time, which allows evaluation of the effects directly due to irradiation (in unaged products) and those arising from the interactions of irradiation with postmortem changes (aged).
VOCs 1-day post-irradiation
-
At day 1 post-irradiation, 22 volatiles including four alcohols, seven aldehydes, one ether, seven hydrocarbons, two ketones, and one sulfur compound were identified from the meat samples (Table 2). In non-irradiated samples, except for 2-ethyl-1-hexanol (present in small quantities in non-frozen samples), all compounds identified were present in both treatments (frozen and non-frozen). Thus, the freezing process had little impact on the formation of VOCs on the first day of aging. This was expected, since, as mentioned earlier, the influence of the freezing process on the formation of VOCs is through the promotion of oxidation reactions and microbial action, which are very dependent on the storage time.
Table 2. Average values (total ion count ×104) of volatile compounds identified in Nellore cattle muscles (L. lumborum) 1-day post-irradiation.
Volatile compound Non-irradiated Irradiated SEM NF FT NFI FIT Alcohols 1-Heptanol nd nd nd 10 3 1-Hexanol, 2-ethyl- 7a nd nd 14b 5 1-Octanol 179 189 178 159 26 Ethanol 32a 18ab 6b 4b 5 Aldehydes Butanal nd nd 7 nd 2 Decanal nd nd 11 27 6 Heptanal 90b 26b 159a 136a 22 Hexanal 172b 63c 294a 281a 30 Nonanal 1,029ab 940b 1,221a 1,232a 150 Octanal 253b 218b 367a 346a 50 Tetradecanal 59b 48b 115a 111a 9 Ethers Propane, 2-methoxy-2-methyl 49b 82a 48b 15c 9 Hydrocarbons (aliphatic) Heptane nd nd 3 nd 1 Hexane 436a 362a 152b 96b 38 Octane 22 23 38 29 3 Pentane, 3-methyl 35 44 10 8 4 Hydrocarbons (alicyclic) Cyclopentane, methyl 37ab 54a 31b 15c 7 Hydrocarbons (aromatic) Benzene nd nd 8 4 2 Benzene, methyl (Toluene) 145ab 113b 168a 72b 28 Ketones 2-Butanone, 3-hydroxy (Acetoin) 468a 369b nd 2c 73 2-Propanone 44 39 43 48 3 Sulfur compounds Sulfide, dimethyl nd nd 78 49 10 Σ Alcohols 218 208 184 187 28 Σ Aldehydes 1,602 1,295 2,173 2,134 245 Σ Ethers 49 82 48 15 9 ΣHydrocarbons 674 595 409 224 69 Σ Ketones 512 408 43 50 74 Σ Sulfur compounds nd nd 78 49 10 Total 3,056 2,588 2,933 2,659 319 Treatments (from four animal pooled samples): NF = chilled (never-frozen) samples; FT = frozen (−18 °C/24 h) and thawed (4 °C/24 h) samples; NFI = never-frozen irradiated (3, 6, and 9 kGy) samples; FIT = frozen (−18 °C/24 h), irradiated (3, 6, and 9 kGy) and thawed (4 °C/24 h) samples. SEM = standard error of the mean (n = 6). Lowercase letters a−c represent followed by different letters in each row differ (p < 0.05) by Tukey test. In the irradiated samples, six compounds (1-heptanol, butanal, decanal, heptane, benzene, and dimethyl sulfide) identified were not found in the non-irradiated samples. The origin of these compounds comes from the interaction of free radicals, derived from the radiolysis of the water molecule, with proteins, amino acids, and lipids present in meat[11,20,26]. Of these compounds two are important: benzene and dimethyl sulfide.
Benzene, as well as methylbenzene (toluene), another hydrocarbon identified, are important because both are considered carcinogens. Benzene was found in low concentrations and only in irradiated samples, while toluene was identified in all samples. The formation of benzene in irradiated foods has been constantly described in the literature, being attributed to the decomposition of the amino acid phenylalanine during the ionization process. Benzene is likely formed when free radicals from the oxidative reactions (lipid and protein oxidation) react with peptide chains or migrate to the phenylalanine amino acid side chains[27]. This process can be catalyzed in muscle, due to the presence of iron and oxidative enzymes, so the formation of benzene has been reported in meats when higher irradiation doses are applied[3,6,28]. Although the irradiation could produce both volatile compounds benzene and toluene by direct cleavage of the phenylalanine[26], Ahn[29] reported that toluene could be produced from the components naturally present in meat even without irradiation.
The importance of dimethyl sulfide lies in the recognized role that sulfur compounds play as the key volatile compounds in the irradiation odor[29]. Dimethyl disulfide is formed by the radiolysis of methionine, and is considered to be the typical off-odor compound in irradiated meat[3]. The aqueous electrons (eaq−) generated by the water molecule radiolysis can promote the release of sulfur by reacting with sulfur-containing amino acids, such as methionine and cysteine, and, or hydrogen sulfide (H2S) and other VOCs that contribute to the off-flavor and off-odor of irradiated meat[11]. Depending on the amount and radiolytic degradation of sulfur-containing amino acids, odors similar to 'cabbage', 'sulfur', 'spoiled vegetable' or 'barbecue corn-like' can be produced[11]. In small amounts, sulfur compounds can decompose into compounds of lower molecular weight and then react with each other or with other substituents, which give a characteristic 'roasted' odor to the meat[30]. As observed in this experiment, dimethyl sulfide was only detected in irradiated raw beef[6,31], pork[31], and turkey[32] samples. Kwon et al.[31] also indicate the use of dimethyl sulfide as an irradiation marker for raw and frozen meats. Kim et al.[33] reported that, among animal species, irradiated pork produced more sulfur-containing volatiles than the irradiated turkey and beef under vacuum packaging conditions.
A PCA (Fig. 1) was created to illustrate the differences between each treatment (irradiation × frozen) based on individual volatile compounds. The two PCA components explained 72.09% of the variation: the first principal component (PC1) explained 42.37% and the second principal component (PC2) explained 29.72%. Three groups were formed: the non-irradiated samples were in the upper left quadrant, while the samples irradiated in lower doses (3 and 6 kGy) were grouped in the lower left quadrant and those irradiated with a high dose (9 kGy) in the upper and bottom right quadrants.
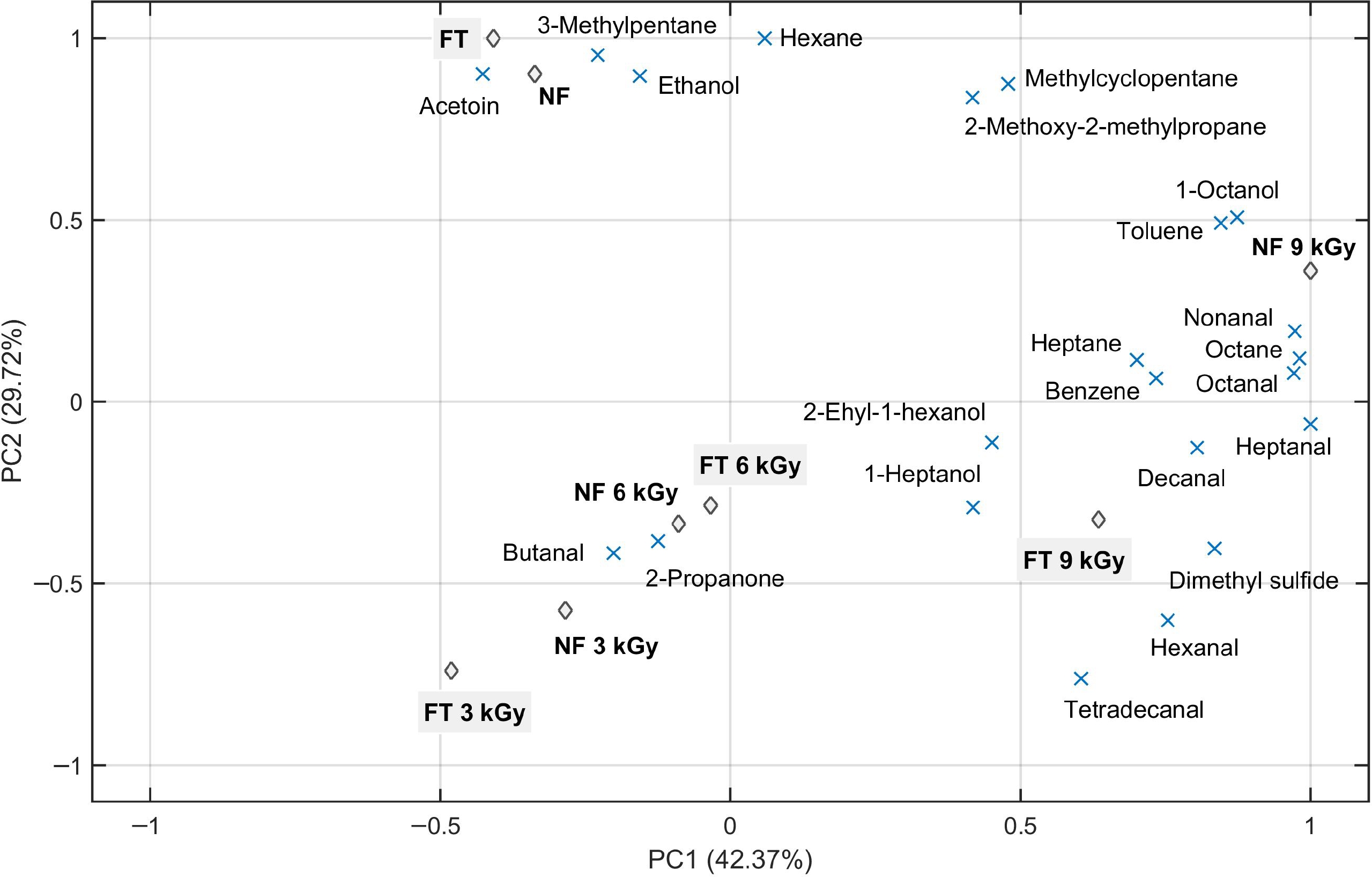
Figure 1.
Bi-plot principal component analysis (PCA) of volatile compounds in muscles (L. lumborum) of Nellore cattle 1-d post-irradiation (3, 6, and 9 kGy). NF = chilled (never-frozen) samples; and FT (filled in gray) = frozen (−18 °C/24 h), irradiated (or not) and thawed (4 °C/24 h) samples.
The irradiated unfrozen and frozen beef at 3 and 6 kGy were highly associated with the ketone 2-propanone and the aldehyde butanal. Also, besides the irradiated unfrozen and frozen beef at 9 kGy were associated with benzene and dimethyl sulfide (especially by PC1), the major volatiles associated with higher doses were aldehydes. Moreover, butanal and decanal were detected only in the irradiated samples. The association of these aldehydes in the irradiated samples is indicative of a higher lipid oxidation. According to Ross & Smith[14], from the wide range of secondary compounds formed from lipid peroxidation (including alkanes, alkenes, aldehydes, ketones, alcohols, esters, acids, and hydrocarbons), aldehydes are considered the most important because they possess low threshold values and are the major contributors to the development of rancid off-flavors and odors. In situations where oxidation occurs at an accelerated rate, like in an irradiated environment, an accumulation of secondary products occurs quickly.
The sum of aldehydes was higher in the irradiated samples, while the sum of hydrocarbons and ketones formed, both associated with the oxidation process, decreased with irradiation. The biggest change in the ketone group was basically due to the presence of the compound 3-hydroxy-2-butanone (acetoin) in much greater quantity in the non-irradiated samples. The acetoin is typically thought of as a Maillard reaction component and, although it may result from microbial catabolism of sugars, this compound may be present in raw beef[34].
Of the aldehydes detected at day 1 in this experiment, hexanal, octanal, and heptanal are products from the autoxidation of linoleic acid and are associated with the grassy, rancid, and fatty odors as well as lesser amounts of 8–10 carbon aldehydes (butanal). Nonanal and decanal (and even octanal and heptanal) are volatile products produced from the autoxidation of oleic acid being related to rancid, fatty, waxy, pungent, green, and burnt odors[11]. From these compounds, hexanal is the dominant aldehyde produced during oxidation, indicating lipid oxidation of meat more effectively than any other volatile component[14]. Feng et al.[6] identified the appearance of three aldehydes in raw beef round eye when irradiated (up to 4.5 kGy): hexanal; 2-metylbutanal; and 3-metylbutanal. The authors reported that the significant increase in lipid oxidation (measured by the TBARS index) and hexanal in the irradiated vacuum-package raw beef further confirms the use of this aldehyde as an indicator of lipid oxidation. The use of hexanal as an indicator of lipid oxidation in irradiated meat has been suggested. Some authors have reported the appearance of this aldehyde in different porcine muscles (L. thoracis et lumborum, Psoas major and Rectus femoris)[35] and in raw turkey breast meat[32] after irradiation (4.5 kGy), together with a significant increase in TBARS values.
However, lipid oxidation products (e.g., hexanal, heptanal, etc.) do not seem to have contributed to the off-odor in irradiated meat[36]. Although irradiation up to 4.5 kGy increased lipid oxidation by 44% and protein oxidation by 11% in raw beef, Feng et al.[6] ponders that, since the total amount of protein is about 10 times higher than lipids, the higher oxidative changes occur in proteins than lipids. Ahn et al.[15] reported that the amounts of lipid oxidation products, such as aldehydes, ketones, and alcohols, were either not influenced or decreased by irradiation on the first day of storage. According to these authors, their results indicate that the major contributor of off-odor in vacuum-packaged irradiated meat is not lipid oxidation, but radiolytic breakdown of sulfur-containing amino acids (such as cysteine and methionine). This is consistent with the detection of dimethyl sulfide only in the irradiated samples (Table 1) and its high relationship with the samples irradiated by the highest doses (Fig. 1).
Overall, observing the PC1 in Fig. 1, the irradiates submitted to the lowest dose (3 kGy) can be applied in the lower left quadrant and distributed towards the right quadrants as the highest doses were applied. This indicates a certain dose-dependence of the compounds 2-propanone, hexanal and dimethyl sulfide, as improved by other authors in irradiated meats up to 5 kGy[6,31,32].
VOCs after 14-d aging
-
As discussed earlier, changes in qualitative and quantitative volatile contents after aging is expected due to proteolysis and oxidation processes and microbial action over time. Fourteen days after irradiation, another 15 VOCs not previously identified on day 1 were identified: two alcohols, two aldehydes, two esters, seven hydrocarbons, and two ketones (Table 3). In addition to the presence of esters, not detected on day 1, the largest increases in the sum of VOCs after 14 d of maturation were in the groups of hydrocarbons (8.5×) and ethers (8.4×). Despite a relatively low increase after aging (1.5×), the aldehydes generated were also very important for the final meat flavor, since they made up the majority (37.4%) of the total VOCs formed along with hydrocarbons (42.7%). It is also important to highlight the increase of 3.7× and 2.4× of compounds formed after 14 d of aging in the groups of alcohols (9.0% of total VOCs) and ketones (4.6% of total VOCs), respectively.
Table 3. Average values (total ion count ×104) of volatile compounds identified in Nellore cattle muscles (L. lumborum) 14-d post-irradiation.
Volatile compound Non-irradiated Irradiated SEM NF FT NFI FIT Alcohols 1-Butanol, 3-methyl 36a nd 3b 59a 13 1-Heptanol 44a 21ab 11b nd 7 1-Hexanol, 2-ethyl- 207b 330a 246ab 270ab 24 1-Nonanol 236a 278a 70b 54b 35 1-Octanol 525a 202b 228b 190b 39 Ethanol 21 41 15 27 8 Aldehydes Butanal 22a nd 30a 8b 7 Butanal, 2-methyl nd 36a nd 12b 5 Butanal, 3-methyl nd 63a nd 24b 9 Decanal 200a 55b 79b 114ab 20 Heptanal 201a 66b 112b 138ab 22 Hexanal 356a 7d 256b 134c 34 Nonanal 2,614a 1,532b 1,496b 1,789b 189 Octanal 616a 274b 369b 398b 62 Tetradecanal 306b 246b 385a 335ab 25 Esters 2-Ethylhexyl salicylate nd 16b 94a 29b 17 Ethyl acetate 71a nd 50a 6b 17 Ethers Propane, 2-methoxy-2-methyl nd 450a 352b 392ab 85 Hydrocarbons (aliphatic) 1-Dodecene 1,719a nd nd 10b 215 1-Tridecene 40a nd 14b nd 6 1-Undecene 65d 224b 470b 626a 145 Heptane nd 24 11 36 9 Hexane nd 1,012a 754b 808b 171 Hexane, 2,2,5-trimethyl nd 14 nd 10 3 Nonane 18 nd 7 3 3 Octane 249a 160b 164b 149b 24 Pentane, 2,3,4-trimethyl nd 21 nd 11 3 Pentane, 3-methyl nd 280a 155a 217a 48 Hydrocarbons (alicyclic) Cyclopentane, methyl nd 474a 340b 373ab 84 Hydrocarbons (aromatic) Benzene nd nd 31a 4b 6 Benzene, 1,3-bis(1,1-dimethylethyl) nd nd 299a 38b 41 Benzene, methyl (Toluene) nd 1,391a 1,284a 1,256a 243 Ketones 2-Butanone, 3-hydroxy (Acetoin) 27b nd 47b 643a 117 2-Dodecanone nd nd 30 35 14 2-Heptanone 198 nd 22 25 26 2-Propanone 77a 31b 37b 21b 7 Sulfur compounds Sulfide, dimethyl 78b 77b 115a 59b 16 Σ Alcohols 1,069 872 572 599 66 Σ Aldehydes 4,315 2,278 2,727 2,953 306 Σ Esters 71 16 144 36 26 Σ Ethers nd 450 352 392 85 Σ Hydrocarbons 2,091 3,600 3,530 3,541 478 Σ Ketones 302 31 135 723 126 Σ Sulfur compounds 78 77 115 59 16 Total 7,927 7,324 7,575 8,302 572 Treatments (from four animal pooled samples): NF = chilled (never-frozen) samples; FT = frozen (−18 °C/24 h) and thawed (4 °C/24 h) samples; NFI = never-frozen irradiated (3, 6, and 9 kGy) samples; FIT = frozen (−18 °C/24 h), irradiated (3, 6, and 9 kGy) and thawed (4 °C/24 h) samples. SEM = standard error of the mean (n = 6). Lowercase letters a−d represent followed by different letters in each row differ (p < 0.05) by Tukey test. Meat develops its aroma flavor characteristics during cooking as the result of the complex interaction of precursors derived from both the lean and fat compositions of meat generating volatile flavor compounds that contribute to meat flavor[20]. While alcohols are believed to be derived from the thermal degradation of phospholipids, hydrocarbons, ketones, and esters are formed through the oxidation of lipids and the thermal degradation of fats during cooking[37]. Aldehydes are formed through the oxidation of unsaturated fatty acids such as oleic, linoleic, and linolenic acid. Additionally, aldehydes can be formed from the Strecker degradation reaction of certain amino acids such as isoleucine, leucine, methionine, phenylalanine, and valine[38]. In addition, the volatile compounds formed during aging may be the source of microbial degradation of the meat and may contribute to off-odor. In meats stored in vacuum packaging, different microorganisms (Enterobacteria, mainly Serratia; Carnobacterium spp.; Pseudomonas, mainly P. fragi; and clostridia) can lead to the formation of these compounds from the metabolism of proteins, lipids, amino acids, and even other volatile compounds (as in the case of the formation of esters from alcohols)[13]. As previously reported, all of these factors are likely to be affected by freezing treatments and, or irradiation before aging.
To illustrate the differences between each treatment (irradiation × frozen), a PCA graph (Fig. 2) was also created for VOCs on day 14. The two PCA components explained 59.89% of the variation (PC1 explained 38.67%, while PC2 explained 21.22%). In contrast to what was observed on the first post-irradiation day, the freezing process had an impact on the formation of VOCs after the aging period. Three groups were formed: (1) samples that were frozen but not irradiated (FT) or frozen and irradiated by a lower dose (FT3, and 6 kGy) were located in the upper left quadrant; (2) samples that were not subjected to freezing or irradiation (NF) were located in the upper right quadrant; and (3) non-frozen but irradiated samples (NF 3, 6, and 9 kGy) and frozen and irradiated with a high dose (9 kGy) were located in the center of the lower quadrants. The dispersed compounds close to these groups are highly related to the respective treatments.
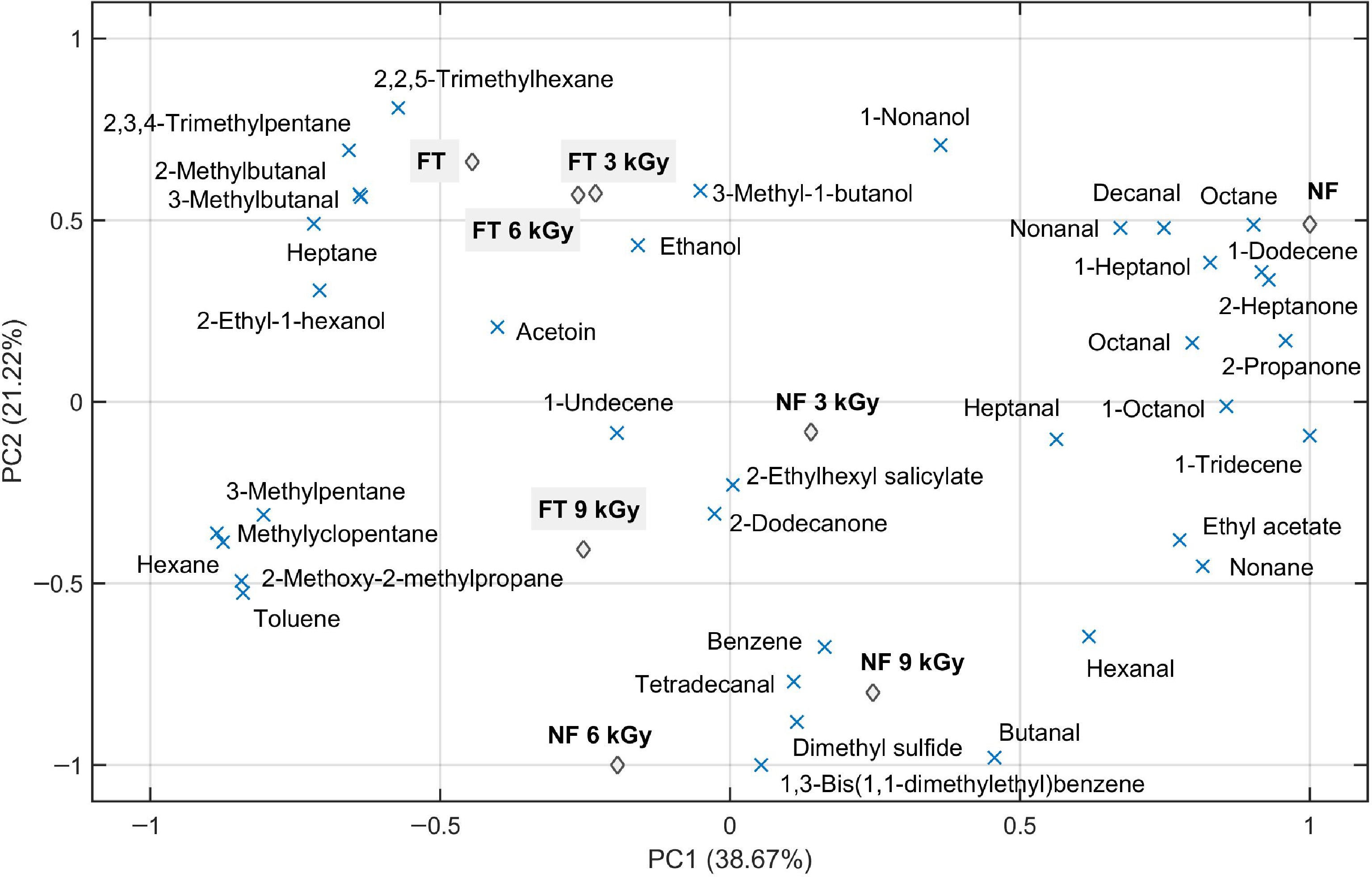
Figure 2.
Bi-plot principal component analysis (PCA) of volatile compounds in muscles (L. lumborum) of Nellore cattle 14-d post-irradiation (3, 6, and 9 kGy). NF = chilled (never-frozen) samples; and FT (filled in gray) = frozen (−18 °C/24 h), irradiated (or not) and thawed (4 °C/24 h) samples.
Two esters (2-ethylhexyl salicylate; and ethyl acetate) and two aldehydes (2-metylbutanal; and 3-metylbutanal) identified in samples aged for 14 d were not identified 1-d post-irradiation (Table 1). According to Casaburi et al.[13], ethyl acetate was one of the major esters found in naturally spoiled meat and inoculated model meat systems, contributing with their sharp/acrid notes to the spoilage meat odor. It can be synthesized from alcohol through strains of Pseudomonasspp (mainly P. fragi). Although Pseudomonas species are often isolated from spoiled meat stored aerobically, they can also occur at a lower extent in meat stored in vacuum packaging[22]. In this experiment, the ethyl acetate was not identified in the frozen and irradiated samples (Table 3), being related by PC1 (Fig. 2) to the control samples (NF; non-frozen and non-irradiated).
According to Feng et al.[6], 2-methylbutanal and 3-methylbutanal are aldehydes formed in raw beef 24 h post-irradiation (up to 4.5 kGy). The formation of both aldehydes by irradiation could occur through the Strecker degradation, a sub-reaction within the Maillard reaction, from the amino acids leucine (3-methylbutanal) and isoleucine (2-methylbutanal)[26]. The 3-methylbutanal has been related to cheese and pungent apple-like odor[39]. However, in this experiment these aldehydes were identified only in the frozen samples (Table 3), being highly related by PC1 and PC2 (Fig. 2) to the non-irradiated and, or, irradiated low-dose samples (up to 6 kGy). Thus, these compounds may also be related to greater proteolysis and, or, to the expected microbial action in frozen samples when compared to non-frozen ones[18]. The 3-Methylbutanal, as well as other aldehydes (hexanal, nonanal, heptanal, and benzaldehyde) commonly found in spoiled meat, can be produced by microorganisms such as Pseudomonas spp., Carnobacterium spp., and Enterobacteriaceae[13]. According to these authors, Carnobacterium spp. are one of the lactic acid bacteria predominantly involved in meat spoilage stored under a vacuum.
The compounds formed can also be related to the predominance and development of different microorganisms during aging in frozen and non-frozen meats, despite the short storage time. This is reinforced by looking at PC1 (Fig. 2), where control samples (NF) were more related to most aldehydes and alcohols (1-heptanoland 1-octanol) described by Casaburi et al.[13] as most commonly associated with microbial deterioration in vacuum-packed meat. A high relationship was also observed with ketones (2-heptanone and 2-propanone) frequently found in spoiled vacuum-packed meat[40]. Other alcohols and ketones identified, including that also belonging to the list of the most common alcohols (3-methyl-1-butanol, 2-ethyl-1-hexanol) and ketone (acetoin) found in spoiled meat[13,40], were more related by PC1 and PC2 to the frozen ones non-irradiated samples (FT) and irradiated in low doses (3 and 6 kGy). Pseudomonas spp. and Carnobacterium spp. appear as the bacteria most involved in the production of alcohols and ketones, but the alcohol biosynthesis could also be from the proteolytic activity as well as the reduction of aldehydes coming from lipid oxidation[13].
It is important to emphasize that the proteolysis of meat during aging is accelerated by the previous freezing/thawing process, being possible to reduce the tendering time between 7 and 14 d[18]. Postmortem aging is an important process in which myofibrillar structures are hydrolyzed by endogenous proteases, which in addition to the increase in meat tenderness and juiciness results in the formation of important flavor precursors. The formation of free amino acids and peptides during aging leads to the development of the flavor and, as the concentration increases, they react with other degradation products to form volatiles responsible for the meat's aroma[21,37]. Therefore, as beef ages, meat shows a significant alteration in the level of flavor precursors.
Thus, the differences in the profile of the compounds formed in frozen samples when compared to non-frozen ones are probably due to differences in the rates of formation of precursor compounds, either by microbial multiplication or even by proteolysis by endogenous enzymes. In addition, freezing altered the profile of volatiles formed in irradiated meats, with only samples frozen and irradiated by high doses (FT 9 kGy) correlating with irradiated non-frozen samples (Fig. 2). This probably occurred because most of the chemical changes in the meat from the irradiation are associated with reactions of free radicals formed. Ionizing radiation induces lipid peroxidation by generating hydroxyl radicals in an aqueous system and this phenomenon is known to be dose-dependent[9]. Besides the formation of these radicals being less when the free water is limited, free radicals tend to recombine when water in food is frozen also because they are less likely to diffuse and react with other food components. Therefore, because it limits free radical formation and oxidation initiation, freezing meat before irradiation retards autoxidation and extends shelf life[11,12].
It is also important to emphasize that the freezing-induced increase in postmortem proteolysis during aging could be neutralized by the radiation process, as reported by Sales et al.[8]. According to these authors, the irradiation may have created highly oxidizing conditions along with the formation of free radicals by water radiolysis, affecting meat endogenous enzymes. Thus, in addition to changing the profile of VOCs, the use of freezing seems to have favored the formation of a smaller number of compounds that can be associated with lipid and, or protein oxidation of irradiated and aged meat.
Products formed through lipid oxidation contributing to flavor development include numerous saturated and unsaturated hydrocarbons, alcohols, aldehydes, ketones, and esters[20], all identified in the aged samples. However, Rodrigues et al.[9] reported that irradiation at doses up to 6 kGy did not affect the lipid peroxidation in vacuum-packed beef after 14 d of aging but when 9 kGy was applied a significant effect was observed. The same observation was made by Sales et al.[8] in frozen/irradiated/thawed and aged vacuum-packed beef. Of the aldehydes that were associated with those irradiated by high dose (9 kGy, irradiated frozen or not) on day 1 (Fig. 1), only hexanal and tetradecanal were again related after 14 d of maturation (Fig. 2). Another aldehyde related to those irradiated by intermediate doses (3 and 6 kGy) on day 1, butanal was also related to those irradiated at 9 kGy after 14 d. However, of these three aldehydes, only the tetradecanal was associated (by PC1 and PC2) with those irradiated at 9 kGy still frozen. Although hexanal is highly associated with irradiated meats, this VOC is also influenced by other reactions, making its presence as a marker of irradiation limited in aged meats.
In addition to aldehydes, products formed in the lipid oxidation include carbonyls and hydrocarbons[14]. These compounds could be generated by the primary and secondary reactions after the chemical bonds in fatty acids are broken by irradiation in fat-containing foods like meat[31]. Of the six aliphatic hydrocarbons formed after 14 d: two (2,2,5-trimethylhexane and 2,3,4-trimethylpentane) were related to frozen non-irradiated (FT) and frozen and irradiated low-dose samples (FT 3 and 6 kGy); three (1-dodecene, 1-tridecene andnonane) were associated by PC1 with non-frozen and non-irradiated (NF) samples; and only one (1-undecene) was associated with the irradiated frozen samples (FT 3, 6, and 9 kGy) and non-frozen irradiated samples at low dose (NF 3 kGy). In the non-frozen samples irradiated at 6 and 9 kGy, the main associated hydrocarbons were aromatic 1,3-bis (1,1-dimethylethyl) benzene and benzene; the latter being still related (mostly by PC2) to samples irradiated in low dose (NF 3 kGy) and frozen and irradiated at high dose (FT 9kGy). Among these compounds, benzene emerges as a good irradiation marker, since it was detected only in irradiated samples, regardless of the aging time (Tables 2 & 3). The 1,3-bis(1,1-dimethylethyl)benzene can also be used as an irradiation marker, but it was not detected in any 1 day post-irradiation sample.
Despite being highly associated with irradiated samples, dimethyl sulfide was also detected in non-irradiated samples after 14 d of aging (Table 3). Although the mean total ion count was similar in the non-irradiated samples (frozen and non-frozen), the total ion count of this compound was about 1.47 higher in the non-frozen irradiated samples. Kim et al.[33] reported that the sulfur-containing compounds, such as dimethyl disulfide, can also be detected in non-irradiated samples, but the amount increases in these samples when irradiated. Due to the low threshold for odor detection, even small amounts of sulfur compounds are important for irradiation off-odor[37]. However, in the frozen irradiated samples of this experiment, the ion count was even lower (0.76 times) than in the non-irradiated ones. According to Kwon et al.[31], the ratios among sulfur volatiles in meat also changed during storage, which should have a significant impact on the overall odor characteristics of irradiated meat because each sulfur compound has its characteristic odor notes. Thus, regardless of the degree of lipid oxidation, it was expected that all irradiated meat produces an odor characteristic of irradiation.
-
In general, all factors evaluated (irradiation, freezing, and aging) changed the volatile compounds profile in beef. The volatile profile of meat with 1-d storage was influenced only by irradiation, with a dose-dependent effect. After 14 d of aging, a greater number of volatile compounds were identified, probably originating from the highest proteolysis and microbial action during storage, especially in the non-irradiated and frozen/thawed samples. Moreover, freezing altered the volatile profile formed in irradiated meat at relatively low doses (3 and 6 kGy).
Benzene was the only compound identified only in irradiated samples, indicating that this compound can be used as a potential irradiation marker for vacuum-packaged beef. However, although it was also identified in non-irradiated samples after 14 d of aging, dimethyl sulfide was present in greater quantity in irradiated samples, indicating that all irradiated meat produces an odor characteristic of irradiation. Despite this, the irradiation of frozen beef reduced the formation of volatile compounds related to lipid and microbial deterioration during aging. Since volatile compounds play an important role in the meat odor and flavor, influencing consumer acceptance and preference, the irradiation of vacuum-packaged frozen steaks can be an alternative to overcome the adverse effects of irradiation on meat quality.
The authors would like to thank the Nuclear Technology Development Center (CDTN/CNEN) for the sample's irradiation and to the Chemical Analysis and Prospecting Center (CAPQ/UFLA) for supplying the equipment and technical support for chromatographic analyses. We also thank the Government of Mozambique and the Brazilian agency Higher Education Personnel Improvement Coordination (CAPES) for the scholarship grant to the second author (Doctorate; PEC-PG/CAPES) and to the National Council for Scientific and Technological Development (CNPq/Brazil) for granting a postdoctoral scholarship to the first author (CNPq; 152596/2022-4) and a Research Productivity Fellow (PQ) for the last two authors.
-
The authors confirm contribution to the paper as follows: conceptualization, methodology, validation: Paula MMO, Rodrigues LM, Ramos ALS, Ramos EM; investigation, writing - original draft preparation: Paula MMO, Buchili AFM, Guimarães AS; writing - reviewing and editing: Ramos ALS, Ramos EM; supervision, project administration, funding acquisition: Ramos EM. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during the current study are available from the corresponding author on reasonable request.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Paula MMO, Buchili AFM, Rodrigues LM, Guimarães AS, Ramos ALS, et al. 2024. Effects of freezing, irradiation, and aging processes on the volatile profile of Nellore beef. Food Materials Research 4: e028 doi: 10.48130/fmr-0024-0021
Effects of freezing, irradiation, and aging processes on the volatile profile of Nellore beef
- Received: 29 June 2024
- Revised: 01 October 2024
- Accepted: 09 October 2024
- Published online: 05 November 2024
Abstract: The application of ionizing radiation is a potential tool for extending meat shelf life during transport, handling, and storage. However, its deleterious effect on meat quality is a concern, especially related to the development of off-flavors. This study aimed to evaluate the volatile profile of frozen and unfrozen vacuum-packed beef irradiated by different doses (0, 3, 6, and 9 kGy) and subsequent aging (at 1 °C) for 1 and 14 d. Volatile organic compounds (VOCs) were extracted by head-space solid-phase micro-extraction and quantified by gas-chromatography coupled to mass spectrometry. The freezing process had little impact on VOCs on the first day, with butanal, decanal, benzene, and dimethyl sulfide compounds being identified only in irradiated meats, especially at high doses (9 kGy). Benzene was the only compound identified only in irradiated samples and can be considered an irradiated meat marker. Of the 37 VOCs identified, 15 were observed after 14-d aged, especially from the groups of hydrocarbons, esters, ethers, and aldehydes. VOCs related to microbial deterioration were identified in 14-d aged beef, especially in those frozen and non-irradiated. The irradiation of frozen beef reduced the formation of VOCs related to lipid and microbial deterioration during aging, indicating an alternative to overcome the adverse effects of irradiation on meat quality.












