-
Black cumin (Nigella sativa L.), belongs to the family Ranunculaceae with various medical benefits, despite its commercial importance as a spice. The plant is cultivated and distributed throughout India. It can also be found in the Middle East, the Mediterranean basin, South Europe, Bangladesh, Turkey, Syria, Lebanon, and Israel[1−3]. The seeds are widely used as a spice in Middle Eastern and Indian cuisines, and have a strong bitter flavor and aroma; for this reason, they are used to flavor curries, vegetables, and legumes[4]. N. sativa grows up to 20−30 cm tall, with finely divided, linear leaves. The flowers are square, delicate and typically colored pale blue and white, with 5−10 petals. The fruit could be a massive and inflated capsule composed of 3−7 united follicles, each containing various seeds (Fig. 1)[5].
The top nine producers of spices worldwide are India, (1,939,000 tonnes annually), Turkey (199,018 tonnes), Bangladesh (180,993 tonnes annually), China (113,359 tonnes), Indonesia (110,387 tonnes), Pakistan (73,472 tonnes), and Ethiopia (36,754 tonnes annually)[6].
Black cumin and its primary ingredient, thymoquinone, have been shown to have anti-nociceptive, hypotensive, bronchodilator, anti-histaminic, anti-fertility, spasmolytic, immune-stimulating, choleretic, uricosuric, hypoglycemic, hepatoprotective, neuroprotective, milk production, anti-tussive, anti-inflammatory, anti-carcinogenic, anti-hyperlipidemic, anti-cancer, antioxidant, antidiabetic, anti-hypertensive, analgesic, and anticonvulsant properties, in recent pharmacological research[3,7,8]. In and outside of the Islamic world, black cumin seed oil is rising in popularity[9].
Black cumin seed oil (BCSO) is a significant source of oils with nutritional, commercial, and medicinal importance. The traditional and folklore applications of black cumin seeds (BCS) have been documented throughout human history, suggesting that this plant can be a useful source of future medicines. For centuries, people all over the world have used BCS and oil to cure a wide range of illnesses. Thymoquinone and its isoforms are the main chemical constituent of the essential oil of Nigella and are responsible for the majority of its medicinal benefits[2,3,10]. According to Fidan et al.[11], the major amino acids in Nigella sativa L. are glutamic acid, arginine, and leucine. Niacin content in BCS was found to be high. Ca, K, as well as P, are the three main minerals. For the food business, BCSs might be recommended as a possible source of new products and services with high nutritional content.
Fresh fruit and vegetable consumption has risen in recent decades as consumers’ knowledge of the need of eating properly has grown. However, such items are significantly more perishable since cutting can cause a sequence of senescence-related injury reactions and are more vulnerable to microbial deterioration. Edible coverings that aim to inhibit ripening processes while also protecting the fruit from water loss and deterioration may be an effective strategy to extend the shelf life of these items. Recently, the incorporation of additives including essential oils and their components with antibacterial and antioxidant properties into such edible coatings to enhance their efficiency has been described and patented[12]. The introduction of alginate coatings enhanced with black cumin seed extracts reduced guava rate of respiration, loss of weight, firmness loss, and skin color alterations[13].
-
The BCS of the variety Ajmer nigella-1 (AN-1) were purchased from National Research Centre on Seed Spices (NRCSS), Ajmer. The BCS were botanically identified by visual inspection at the laboratory of the Department of Food Technology in Guru Jambheshwar University of Science & Technology, Hisar, Haryana. The black cumin seeds were washed, cleaned, dried, and sealed in a zip-lock plastic bag.
Formulation of powder
-
The black cumin seeds were powdered using an electric grinder (SUJATA) and then the powder was passed through a sieve to obtain more uniform particles as a fine powder.
Proximate analysis of black cumin seeds
Moisture content
-
The moisture content of the sample was determined according to the AOAC[14]. Two grams of sample was weighed in triplicate and placed in a forced-air hot oven at 105 °C for 3 h. The samples were removed from the oven and cooled in desiccators. The change in the weight was determined and recorded as moisture content. The percentage of moisture was calculated using the following equation:
$ {\text{Moisture content}}\;({\text{%}})=\dfrac{{W}_{2}-{W}_{3}}{{W}_{1}}\times{100} $ where, W1 = weight of the fresh sample, W2 = weight of sample and crucibles, and W3 = weight of dried sample and crucible.
Ash content
-
The ash content was determined according to AOAC[14]. A sample of 2 g was weighed in crucibles of known weight and incinerated in the muffle furnace at 550 °C for 3 h. The samples were removed from the muffle furnace and were placed to cool in the desiccator and then weighed. The percentage of ash content was calculated by the following equation:
$ {\text{Ash}} \;({\text{%}})=\dfrac{{W}_{3}-{W}_{1}}{{W}_{2}} \times {100} $ where, W1 = weight of the empty crucible, W2 = weight of the fresh sample, and W3 = weight of ashed sample and crucibles.
Essential oil extraction
-
Essential oil (volatile) was extracted by hydro-distillation using a Clevenger apparatus as described in AOAC[14]. The 100 g sample of BCS powder was boiled with 1 L of distilled water in a Clevenger apparatus at a temperature of 100 °C until oil distillation ceased after 5−6 h. The volume of essential oils was determined from a calibrated trap. Finally, the oil content of BCSs was calculated by the following formula using the volume of the essential oil V and the weight of sample W:
$ Volatile\;oil,{\text{%}}\;(v/w)=\dfrac{\mathrm{V}}{W\left(sample\right)}\times 100 $ Crude fat
-
The crude fat extraction was carried out by using the soxhlet extraction method[14]. The sample of BCS powder of 2 g was weighed. The weight of the cooled flasks was determined using a weighing balance (W1). Each of the cotton-lined thimbles was weighed with about 2 g of the sample. The Soxhlet extraction apparatus was used to place the sample-filled thimbles. Petroleum ether was added to each extraction flask in a volume of 90 mL. The extraction method required around 3−4 h, following which the flasks containing the remaining contents were removed from the Soxhlet and dried for 1 h at 80 °C in a hot air oven. The flasks were then cooled for 30−45 min in desiccators. The fat content and the weight of each flask were calculated (W2). The below-given formula was used to calculate the crude fat content:
$ {\text{%}}\;Crude\;Fat=\dfrac{{W}_{2}-{W}_{1}}{\mathrm{W}\mathrm{e}\mathrm{i}\mathrm{g}\mathrm{h}\mathrm{t}\;\mathrm{o}\mathrm{f}\;\mathrm{s}\mathrm{a}\mathrm{m}\mathrm{p}\mathrm{l}\mathrm{e}}\times 100 $ where, W2 = weight of flask and fat extracted, W1 = weight of the dried flask, and W = weight of sample
Crude protein
-
The Kjeldahl method was used to determine the crude protein content. 0.5 g of sample weighed to an accuracy of ± 0.1 mg into a 250 mL digestion flask. Two Kjeldahl tabs CT 3.5 (or 7 g K2SO4 + 0.210 g CuSO4 × 5 H2O + 0.210 g TiO2) and 25 mL concentrated H2SO4 were added and shaken gently to wet the sample. The sample was digested for 60 min and left to cool for at least 15 min, then diluted with 80 mL H2O, and 25−30 mL of receiver solution were added to the receiver flask. Fifty mL of 40% NaOH was added to diluted digestion and the reaction was allowed to settle (delay) or use the SAFE feature (Steam Addition for Equilibration), to avoid a violent reaction. It was distilled for the prescribed time and titrated distillate with standardized titrant (0.2 N). A reagent blank was performed before each batch of samples. Finally, the result was calculated using the following formula:
$ {\text{%}}Nitrogen=\dfrac{(\mathrm{T}-\mathrm{B})\times \mathrm{N}\times \mathrm{14,007}\times 100}{\mathrm{W}\mathrm{e}\mathrm{i}\mathrm{g}\mathrm{h}\mathrm{t}\;\mathrm{o}\mathrm{f}\;\mathrm{s}\mathrm{a}\mathrm{m}\mathrm{p}\mathrm{l}\mathrm{e}} $ where, T = Sample titration, B = Blank titration, and N = Normality of the titrant. % Protein = % N × F, F = Protein Factor (6.25 in oilseeds).
Extraction of black cumin oil using different solvents
-
The procurement of seeds was done from the National Research Centre on Seed Spices (NRCSS), Ajmer. After procurement of BCS, cleansing of seeds was followed by washing and air drying. After the cleansing process, the seeds were crushed using an electric grinder (SUJATA), then the powder of BCS was passed through a sieve. Now by making a thimble, the sample was filled into it. Next solvent (250 mL) was added into the round bottle flask and the temperature was adjusted according to the boiling point of the solvents. The Soxhlet apparatus was operated by sample, 20 g seeds were crushed and extracted with various solvents, including ethanol (EtOH), n-hexane (n-Hex), and methanol (MeOH) at their particular boiling temperatures (Table 1) for 4 h. A rotary evaporator was used to remove solvent from the extracts. The recovered oil was placed in a hot air oven for 1 h at 80 °C to remove the moisture content and then weighed.
Table 1. Solvents used for oil extraction.
Solvent used Sample weight (g) Boiling temperature (°C) Ethanol 20 78 n-hexane 20 64 Methanol 20 69 Quality analysis of black cumin seed oil (BCSO)
Moisture content
-
The moisture content of the oil was determined according to AOAC[14] official method 926.12. The 2 g of oil was weighed in a dish that had already been dried and tared. The dish's cover was taken off and heated for 2 h at 105 °C in the oven. After removal from the hot air oven, it was cooled in a desiccator, and weighed. Moisture content was then calculated using the following equation:
$ Moisture\;content\;({\text{%}})=\dfrac{{W}_{1}}{W}\times 100 $ where, W1 = Loss in weight (g) of the material on drying, W = Weight in g of the material taken for the test.
Viscosity
-
By using a viscometer, the viscosity was read directly by Brookfield viscometer, which was maintained at 1 min and 100 rpm at 25 °C.
Refractive Index (RI)
-
The American Oil Chemists Society (AOCS) official method (Cc7-25) was used to determine the RI of each seed oil sample.
Free fatty acid content (as oleic acid) %
-
The (AOCS) scheme Ca 5a-40 was used as a determinant of free fatty acid (FFA) content. The defused isopropyl alcohol was then combined with a total of 5 g oil sample placed in a conical flask and heated to 40 °C and titrated vs a standard sodium hydroxide solution. The following formula used to calculate the FFA%[15]:
$ \mathrm{F}\mathrm{F}\mathrm{A}{\text{%}}\;\mathrm{a}\mathrm{s}\;\mathrm{O}\mathrm{l}\mathrm{i}\mathrm{e}\mathrm{c}\;\mathrm{a}\mathrm{c}\mathrm{i}\mathrm{d}=\dfrac{20\times \mathrm{N}\times \mathrm{V}}{W} $ where, V = mL of NaOH, N = NaOH normality (Eq/L), and W = sample weight (g).
Iodine value
-
The AOCS official method Cd 1–25 surplus, of about 0.5 g oil sample was put in a 500 mL flask, with the addition of 15 mL alicyclic hydrocarbon, and 25 mL of iodine monochloride solution, which was used to calculate the IV of each oil sample. Subsequently, the vacuum flask was placed in a dim area for 60 min. After an hour of incubation, 20 mL 10% KI with 150 mL of boiled steam water was added to the mixture. Later, 1 mL of 1% of starch solution was added, followed by a continuous titration until the cerulean color disappeared.
$ Iodine\; value=\dfrac{12.69\times\mathrm{N}(\mathrm{V}\mathrm{_b}-\mathrm{V}_{\mathrm{s}})}{W} $ where, N = Na2S2O3 normality (Eq/L), Vb = mL of Na2S2O3 used for blank test, Vs = mL of Na2S2O3 used for the sample, and W = sample weight (g).
Saponification value
-
By using the AOAC method[14], saponification value (SV) was found by taking 2 g of the oil weighed out in a conical flask and to 5 mL of culture, the filtrate was added and incubated for 24 h. To this solution, 5 mL of 0.2 N alcoholic KOH was added. The flask was then heated with a reflux air condenser in a water bath for 30 min. The flask was intermittently shaken throughout the boiling and after 30 min cooled and diluted to 25 mL in a measuring flask. It was then titrated against 0.1 N HCl using phenolphthalein as an indicator. A blank was run by refluxing 5 mL of the alcoholic KOH solution, 2 g of oil, and 5 mL of distilled water. Since the saponification value is the amount of KOH in milligrams to saponify 1 g of fat, it was calculated as the saponification value:
$ \text{Saponification value}=\dfrac{\mathrm{m}\mathrm{L}\; \mathrm{o}\mathrm{f}\; \mathrm{H}\mathrm{C}\mathrm{l}\times\mathrm{N}\; \mathrm{o}\mathrm{f}\; \mathrm{H}\mathrm{C}\mathrm{l}\times56.1}{\mathrm{W}\mathrm{eight}\; \mathrm{o}\mathrm{f}\; \mathrm{t}\mathrm{h}\mathrm{e}\; \mathrm{s}\mathrm{a}\mathrm{m}\mathrm{p}\mathrm{l}\mathrm{e}\left(\mathrm{g}\right)} $ Peroxide value
-
Five grams of organic coconut oil was added with 30 mL of CH3COOH and 20 mL of CHCl3 in a 250 mL conical flask. The solution was completely swirled in the measuring cylinder. 0.5 mL of saturated KI solution was added into the conical flask and the solution was shaken for 60 s then 30 mL of distilled water was added to the flask and the mixture was shaken vigorously. The flask was then stored for 2−3 min. The solution was then titrated using a solution of 0.01 mol·dm−3 of sodium thiosulfate after adding 1 mL of starch solution. Titration was stopped when the blue-grey color of the upper layer disappeared and the lower layer became milky. The above experiment was repeated five times to attain maximum accuracy. The experiment was repeated every 5 d to measure the trend of peroxide values.
$ \text{Peroxide value}=\dfrac{\mathrm{S}\times\mathrm{N}\times100}{\mathrm{Weight\; of\; the\; sample\; \left(g\right)}} $ where, S is the volume of Na2S2O3 used in the test volume of Na2S2O3 used in the blank (mL), and N is the normality of thiosulphate Na2S2O3.
Edible coating of black cumin seed oil on lemons
Raw material
-
Lemons (Citrus limon) were collected from the local market of Hisar, based on maturity, uniformity of size, and absence of physical damage. After harvest, the lemons were directly brought to the laboratory (in the same city of Hisar) and the lemons were transported to the laboratory in clean, sterile, and ventilated containers to ensure freshness and prevent contamination or spoilage during transit. The transportation time did not exceed 35 min to maintain the integrity of fruit. After that, lemons were packed in LDPE (Low Density Polyethylene) packages of 250 g each. The LDPE packages with lemons were placed in a cool, dry place to minimize exposure to direct sunlight or temperature fluctuations.
Treatments given
-
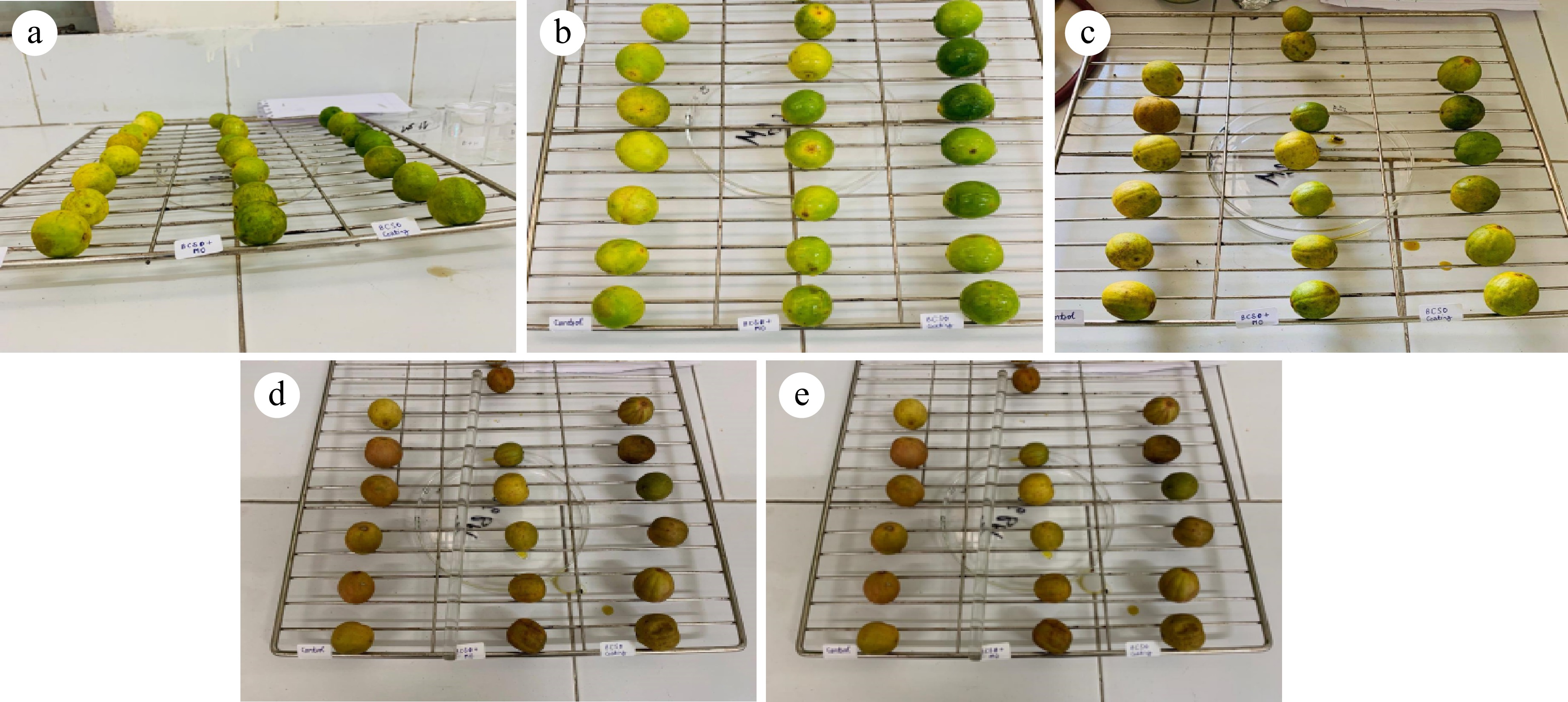
These treatments, T (control without BC), T1 (50 BC:50 MS, 1:1), and T2 (100 BC:0 MS) were used for the edible coating of lemons by using the dipping method for edible coating. Lemons were kept open in crates at room temperature after edible coating. Measurements of various physical and chemical attributes were carried out on the day of the experiment setting and the 3rd, 6th, 9th, and 12th day of storage.
Process of edible coating
-
Fresh citrus lemons were procured from the market with equal physical parameters and no physical damage. The lemons were sorted and graded according to their size. Then cleansing of lemons was followed by air drying. The edible coating was made by adding BCO in different ratios and a control sample was also taken to compare with other treated samples. By using the dipping method coating lemons were coated and then left to dry the edible coating. The lemons were stored at room temperature conditions. Then all the quality testing was performed on the day of the experiment set up. Finally, shelf life was studied at 0 day and then on the 3rd, 6th, 9th, and 12th day with a few quality tests.
Quality analysis of lemon coatings
Physiological weight loss
-
The initial weight of 10 fruits from each replication was taken immediately after treatment and then 3 days interval throughout storage time. It was calculated by the weight difference between initial and specific time intervals divided by initial weight and finally denoted by percentage.
$ \begin{split}&\mathrm{\rm{P}hysiological\; weight\; loss}=\\&\rm\dfrac{\text{Initial weight − Weight after storage}}{\text{Initial weight}}\times 100\end{split} $ pH value
-
The pH values were assessed using a digital pH meter. Ten mL of lemon juice from coated fruits and controls were added to a 50 mL beaker and pH was recorded at the interval of 3 days.
Total soluble solids
-
The total soluble solids were determined by using a Hand Refractometer to measure the total soluble solids (TSS) of lemon juice and expressed as °Brix. The prism was cleaned with distilled water after taking every reading[16].
Titratable acidity
-
Titratable acidity was measured using 1 mL of lemon juice after dilution with 4 mL of distilled water[17]. After the addition of 2−3 drops of phenolphthalein as an indicator, the conical flask was shaken, and titration was carried out with 0.1 N NaOH. All the readings were taken in triplicate and the results were expressed as g of citric acid/100 mL of lemon juice using the following equation:
$ \text{Titratable acidity}=\dfrac{\mathrm{V}\times \mathrm{ }0.067\mathrm{ }\times \mathrm{ }0.1\mathrm{ }\times \mathrm{ }100}{\mathrm{M}} $ where, V = volume of 0.1 N NaOH used, and M = mass of lemon juice.
Statistical analysis
-
All values obtained were compared using ordinary One-way ANOVA with a Newman-Keuls Multiple Comparison post-test using GraphPad Prism 6 Software, Inc. (San Diego, CA, USA). Differences at p < 0.05 were considered statistically significant.
-
The experimental study was investigated from the variety Ajmer Nigella-1 (AN-1) of black cumin. The proximate analysis of black cumin seeds were moisture, ash, essential oil, crude fat, crude protein, and crude fibre.
From the results presented in Table 2, proximate analysis of black cumin seeds showed a mean composition of 5.6% moisture, 0.3% essential oil, 4.3% ash, 21.7 % protein, 34.4% fat, and 7.8% the crude fibre. These values were much higher, smaller, or quite similar than the values reported in the literature. The result obtained for the moisture content was similar to the one obtained by Haron et al.[18] for two varieties Sanna (5.7%) and Habsyah (5.6%). The essential oil content of the present sample of black cumin was similar to the one obtained by Kinki[19] and Fidan et al.[11] (0.4%). Ash content result (4.3%) was similar to that of Haron et al.[18] for two varieties from Sudan (4.34%) and from Iran (4.36%), while slightly better than the one obtained by Sultan et al.[20] which found 4.20%. These results were very small when compared to the one obtained by Kabir et al.[21] (7.4%). Sultan et al.[20] found quite similar levels of crude protein and fat content, with 22.80% and 31.16% respectively. The fat content of the present sample is then higher than the one of Sultan et al.'s sample, while Babayan et al.[22] obtained 35.4% for the crude fat content, which is in the same range as the present study. Thilakarathne et al.[23] obtained crude fibre content of 6.03% for the sample from Ethiopia, which are almost similar to the results obtained by Sultan et al.[20] (6.04%) and smaller than the present result (7.8%). However, the result of crude fibre content is quite similar to the findings of Thilakarathne et al.[23] for the Indian sample. The difference observed between all these values can be attributed to different varieties of seeds and climatic conditions.
Table 2. Proximate analysis of black cumin seeds.
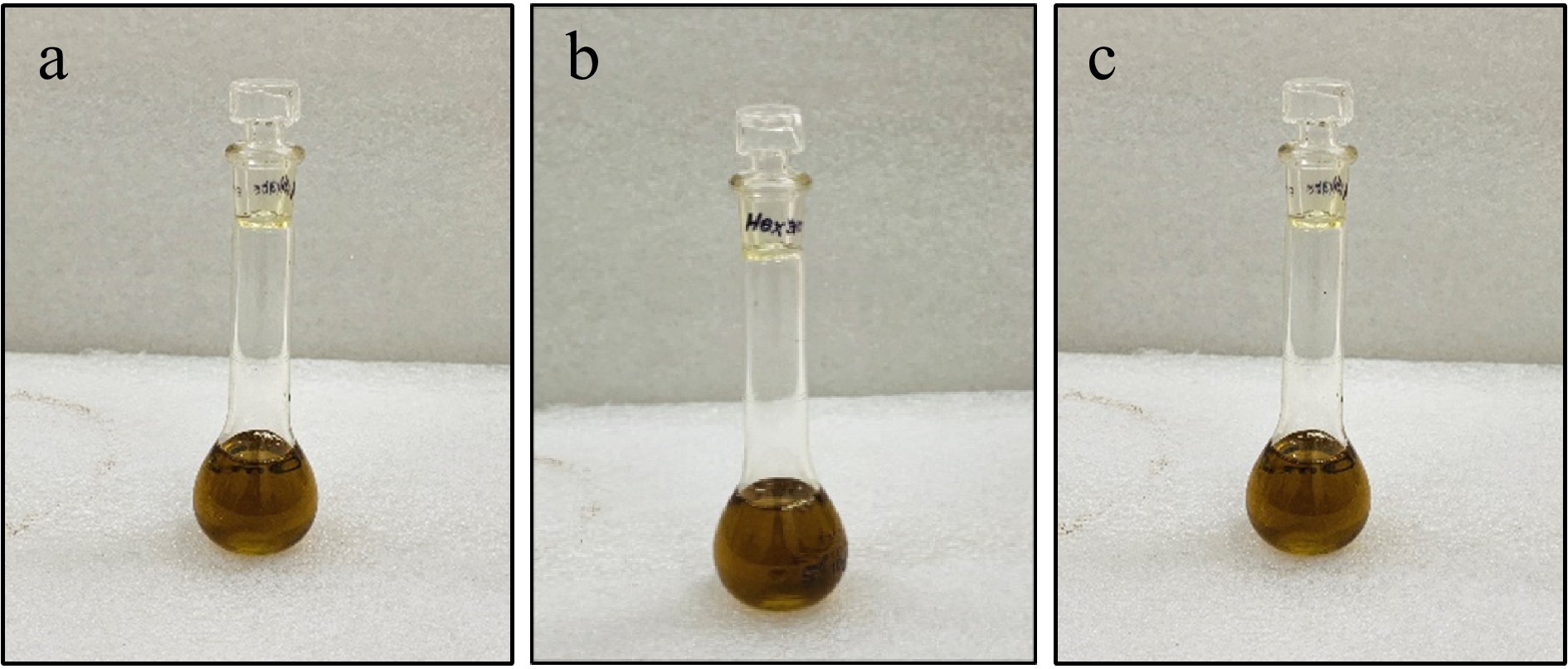
Parameters Percentage Moisture 5.60% ± 0.25% Ash 4.30% ± 0.28% Essential oil 0.30% ± 0.01% Crude fat 34.40% ± 0.51% Protein 21.70% ± 0.25% Crude fibre 7.80% ± 0.15% * Data presented as mean values for triplicates ± SD. Black cumin oil was extracted by different solvents, ethanol (EtOH) (Fig. 2a), n-hexane (n-Hex) (Fig. 2b), and methanol (MeOH) (Fig. 2c) at their specific boiling point using a soxhlet apparatus, resulting in the oil yield of 36%, 39.56%, and 32.30% respectively (Table 3). Among the three solvents used to extract the black cumin oil, n-Hex (39.56%) showed the highest oil efficiency as compared to other solvents, while MeOH showed the lowest oil yield. Therefore, the present results are in the range of 31%−45% as reported by several studies[15,20,22,24]. The difference that can be observed between the results may be due to different varieties and climatic conditions of harvest regions.
Table 3. Black cumin oil yield using different solvents.
Solvent used Oil recovery Ethanol 36.00% n-hexane 39.56% Methanol 32.30% Quality analysis of black cumin seed oil (BCSO)
-
The quality analysis of the seed oil was performed, by determining physical and chemical properties of all three extracts. As physical properties, moisture content, viscosity, and refractive index where determined, while for chemical properties, free fatty acid, iodine, saponification, and peroxide values were determined. The results are presented in Table 4.
Table 4. Quality analysis of black cumin seed oil.
Oil sample Moisture (%) Viscosity Refractive index FFA (as oleic) % Iodine Value Saponification value Peroxide value Ethanol 4.46 ± 0.05a 70.33 ± 0.57a 1.45 ± 0.004a 13.88 ± 0.03a 112.1 ± 0.1a 205.19 ± 0.03a 9.16 ± 0.01a n-hexane 4.06 ± 0.05a 65.3 ± 0.50b 1.43 ± 0.001b 13.20 ± 0.02b 123 ± 1.00b 188.25 ± 0.02b 8.14 ± 0.01b Methanol 3.97 ± 0.06a 51.0 ± 1.00c 1.42 ± 0.002c 19.27 ± 0.02c 101.6 ± 1.15c 208.17 ± 0.02c 13.7 ± 0.02c * Data presented as mean values for triplicates ± SD. Mean values followed by the same letter superscripts in a column are not significantly different (n = 3, p < 0.05). From these results, it was observed that EtOH showed the highest moisture, viscosity, and refractive index. Moisture content, viscosity, and refractive index of all these three extracts were similar to the results found by Alrashidi et al.,[15] which also found that, EtOH extract had the highest moisture content. Moisture values of these three extracts (EtOH, n-Hex, and MeOH) were not significantly different (p < 0.05), while their values of viscosity and refractive index were significantly different for each parameter.
Methanol extract was found to contain the highest values of free fatty acid, while EtOH and n-Hex extracts had similar values. Similar results were found by Alrashidi et al.[15] where the iodine value of n-Hex extract was found to be higher than others. Djilani and Dicko's[25] results showed iodine values of 111 and 117 respectively for extract obtained by Conventional Extraction (CE) using n-Hex and surfactant assisted extraction (SAE), which are quite similar to the results given in Table 4. Saponification values of EtOH and MeOH were closer to each other while, n-Hex had less saponification value. Alrashidi et al.[15] obtained saponification values of 188.27, 205.15, and 208.18, respectively for n-Hex, EtOH, and MeOH extracts, which are similar to the results given in Table 4. Peroxide value of MeOH extract was higher than others. Djilani & Dicko[25] found peroxide values of 9.6 and 8.1, respectively for extract obtained by CE using n-Hex and SAE which are similar to the results given in Table 4. Small differences observed could be due to different varieties of seeds and climatic conditions.
Quality analysis of lemon edible coatings
-
The lemons were coated with the following treatments: T (control, without BC), T1 (50 BC:50 MS, 1:1), T2 (100 BC:0 MS).
In the quality analysis, physiological loss of weight (Fig. 3a), pH value (Fig. 3b), Total soluble solids (TSS) (Fig. 3c), and titratable acidity (Fig. 3d) was observed on the day of the experiment setup then on the 3rd, 6th, 9th, and 12th day. Values on the day of the storage period are as given in Figs 3a−d respectively.
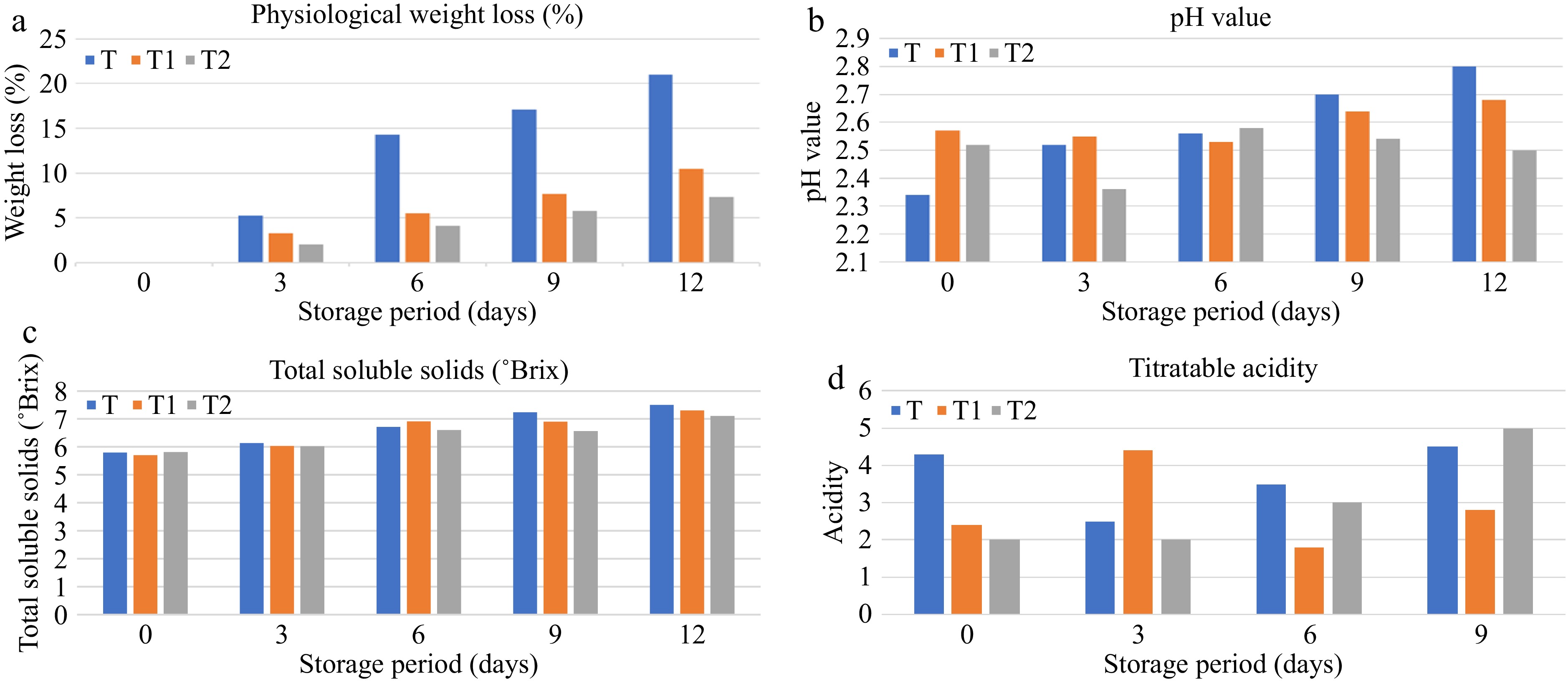
Figure 3.
Influence of edible coating stored at room temperature, on (a) physiological weight loss, (b) pH value, (c) total soluble solids, and (d) titratable acidity.
The study of the influence of edible coating on physiological weight loss at room temperature showed that the weight loss results were observed from all the treatments. Control sample (T, without BC) was found to have higher weight loss, while lemon coated with black cumin oil was having lowest weight loss followed by the T1 (50 BC:50 MS) sample (Fig. 4). Fruit dehydration causes weight loss as a result of alterations in the surface interfacial area to vapors, and respiratory rate. Fresh fruit weight loss is mostly driven by water loss from metabolic processes including respiration and osmosis[26]. On the last day of storage (i.e., 12th day), the pH value of the control sample was the highest, while, in others, no significant change in pH value was observed. During storage, the pH value first increased and then decreased. A similar trend was observed by Porras et al.[27]. TSS values on the first day were determined around 5.85 to 5.82 while on the last day, TSS increased up to 7.24 which was the highest TTS value observed in uncoated lemon. It was increasing irrespectively of the coating treatment; similar results were observed by Nasrin et al.[28]. The titratable acidity of all the treatments decreased throughout the storage period. A major decrease was found in the control sample. The black cumin oil coated was found to have the best shelf life compared to the other two treatments control and black cumin oil mixed with mustard oil. Similar results were observed by Nasrin et al.[28]. Finally, in the edible coating, the three treatments given are T (control, without BC), T1 (50 BC:50 MS), and T2 (100 BC:0 MS) and observed shelf life until the lemons were spoiled. The three treatments of black cumin oil given to edible coating from which T2 (100 BC:0 MS) showed the longest shelf life under room temperature conditions and the lowest weight loss was observed.
-
The study on the extraction and application of black cumin (Nigella sativa L.) seed oil as a surface coating for extending the shelf life of lemons has demonstrated promising results. The experimental findings indicate that lemons coated with black cumin seed oil exhibited a marked reduction in weight loss, delayed onset of spoilage, and maintained higher levels of ascorbic acid and other essential nutrients compared to the untreated control group. The evaluation further confirmed that the coated lemons retained their freshness, and firmness over a prolonged storage period. In conclusion, the utilization of black cumin seed oil as a natural and eco-friendly coating agent presents a viable alternative to synthetic preservatives for extending the shelf life of fresh produce, particularly lemons. This approach not only enhances the longevity and quality of the fruit but also aligns with the growing consumer demand for natural and health-promoting food preservation methods. Further research could explore the optimization of coating concentrations and application techniques to maximize the efficacy and commercial viability of this preservation method.
-
The authors confirm contribution to the paper as follows: conceptualization: Barmanray A, Kaushik N; supervision: Barmanray A; Writing - draft manuscript preparation: Kaushik N; writing - manuscript revision, language editing: Nyemb JN, Boum Bindebe SMLK; data collection and experimentation: Yadav A; statistical analysis, data verification and plagiarism detection: Kaushik N, Nyemb JN. All authors reviewed the results and approved the final version of the manuscript.
-
The data used to support the findings of this study are included within the article.
-
Authors want to thank Guru Jambheshwar University of Science & Technology, and The University of Maroua for technical assistance.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Barmanray A, Kaushik N, Nyemb JN, Yadav A, Boum Bindebe SMLK. 2024. Study on extraction and surface coating of black cumin (Nigella sativa L.) seed oil on the shelf life of lemons. Food Materials Research 4: e033 doi: 10.48130/fmr-0024-0025
Study on extraction and surface coating of black cumin (Nigella sativa L.) seed oil on the shelf life of lemons
- Received: 14 July 2024
- Revised: 22 October 2024
- Accepted: 11 November 2024
- Published online: 11 December 2024
Abstract: The primary goals of the research were to evaluate the quality of black cumin oil, develop an efficient way to increase its efficiency using various solvents, and then apply the oil to the lemons as an edible coating. The oil extraction was carried out with three solvents which are ethanol, n-hexane, and methanol at their different boiling points. The results indicated that among the solvents used to extract the black cumin oil, n-hexane (39.56%) showed the highest oil efficiency. Quality analysis of black cumin seed oil (BCSO) was determined. In edible coating, the three treatments given are T (control, without BC), T1 (50 BC:50 MS, 1:1), and T2 (100 BC:0 MS), and observed shelf life until the lemons became spoiled. Among the three black cumin oil treatments under room temperature, T2 (100 BC:0 MS) exhibited the longest shelf life. The findings of the study confirmed that black cumin oil applied as an edible coating could maintain the quality of lemons. The physicochemical parameters and results of quality analysis of black cumin oil edible coating assessment demonstrated the significant potential of BCSO to extend the shelf life of lemons. The three treatments of black cumin oil given to edible coating from which T2 (100 BC:0 MS) showed the longest shelf life under room temperature conditions and the lowest weight loss was observed. Therefore, treatment (T2) using pure BCSO exhibited a longer shelf life and it may be used commercially to enhance the preservation of lemons to prolong their shelf life without using harmful synthetic chemical additives.
-
Key words:
- Black cumin /
- Proximate analysis /
- Extraction /
- Quality analysis /
- Edible coating