-
Hypertension, characterized by a persistently elevated systolic/diastolic blood pressure (BP) (SBP/DBP) exceeding 130/80 mmHg, poses a significant risk for cardiovascular diseases (CVDs)[1]. Several fundamental mechanisms, such as heightened activity of the renin-angiotensin system (RAS) including angiotensin-converting enzyme (ACE), inflammation, and oxidative stress, play important roles in the onset and development of hypertension[2,3]. Although several pharmaceuticals are available for treating hypertension, they are associated with notable side effects, which is a concern in a condition necessitating lifelong therapy[4]. Therefore, an increasing focus has emerged on the exploration of novel therapies for hypertension derived from natural sources.
Egg proteins, acknowledged for their high quality, are also recognized for their biological activities such as antioxidant, anti-inflammatory, antimicrobial, and immunomodulatory activities as well as an exceptional reservoir of bioactive peptides[5−7]. One such protein is ovotransferrin, from which three potent ACE-inhibitory tripeptides, Ile-Arg-Trp (IRW), Ile-Gln-Trp (IQW), and Leu-Lys-Pro (LKP), were identified after the protein is hydrolyzed by thermolysin and pepsin[8]. All three peptides could significantly reduce blood pressure (BP) of spontaneously hypertensive rats (SHRs) but via different mechanisms[5,9]. However, ovotransferrin only accounts for about 12% of total egg white proteins; to reduce the cost of peptide preparation, the results showed the feasibility of using whole egg white, instead of ovotransferrin, as the starting material to prepare the hydrolysate[10]. Although the contents of three novel peptides are lower than that of ovotransferrin hydrolysate, this hydrolysate significantly reduced BP in SHRs[11]. Later, to further reduce the cost of peptide preparation, the whole egg white hydrolysate was prepared using thermolysin only (without pepsin) and found that this hydrolysate could also reduce BP in SHRs, suggesting that endogenous pepsin could replace its exogenous counterpart in releasing antihypertensive peptides from egg white proteins in vivo[12]. Subsequently, eight new ACE-inhibitory peptides with great gastrointestinal stability were identified from the hydrolysate; surprisingly, many were not derived from ovotransferrin but the rest of the egg white proteins (mostly ovalbumin). These findings indicated that egg white proteins, after depleting ovotransferrin, are also good sources of antihypertensive peptides. In addition, many previous studies have demonstrated that ovalbumin is a good source of antihypertensive peptides, such as YAGGRYPIL, ovokinin, and others[13−16].
The potential of ovotransferrin in treating osteoporosis was recently indicated[17]. To maximize the value-added application of egg white, we plan to develop ovotransferrin for the prevention of osteoporosis and utilize the rest of egg white (ovotransferrin-depleted fraction) for that of hypertension. Before that, we need to validate the antihypertensive effect of ovotransferrin-depleting egg white protein hydrolysate prepared by thermoase (OD-EWH). Therefore, this study was designed to investigate the antihypertensive effect and its underlying mechanisms of OD-EWH in SHRs. The whole egg white hydrolysate prepared by thermoase (EWH) was used as the reference. Changes in BP, the RAS, inflammation, and oxidative stress were analyzed.
-
Egg white (pasteurized) was purchased from Egg Solutions (Lethbridge, Alberta, Canada). All the materials and reagents used are described in their respective sections below. Chemicals are of analytical grade unless otherwise mentioned.
Preparation of EWH and OD-EWH
-
Ovotransferrin was extracted according to our recent study[18]. The ovotransferrin-depleting egg white proteins were then subjected to denaturation (95°C for 15 min) with continuous stirring. Subsequently, hydrolysis was performed using thermoase (E/S (enzyme-to-substrate ratio) 1%, pH 8.0, for 3 h), followed by termination of thermoase activity (95 °C for 15 min). The resulting mixture was then centrifuged at 10,000 g to separate the supernatant from the precipitate. The supernatant was freeze-dried for further experiments. The protein content of the OD-EWH was 88.1% ± 0.9%. EWH was prepared by denaturing the whole egg white proteins and then hydrolyzed using thermoase under similar conditions as used for preparing OD-EWH. Afterward, the mixture was centrifuged at 10,000 g to separate the supernatant, and then freeze-dried for subsequent experiments.
Ethics statement, animal model, telemetry recording, and experimental design
-
Twelve- to 14-week-old SHRs weighing 290 ± 10 g were procured from Charles River (Senneville, QC, Canada). Upon arrival, the rats were acclimatized for a week, provided with a standard rat chow and water ad libitum, and were exposed to a 12:12 h light-dark cycle in controlled humidity and temperature conditions. Then, the rats underwent surgery for the implantation of telemetry transmitters (HD-S10, Data Sciences International, St. Paul, MN, USA). The transmitter was positioned in the left groin area, with the cannula inserted into the common femoral artery and advanced into the abdominal aorta, with it further secured to the vessel using a vicryl 5-0 suture. The rats were individually caged and allowed a 7-d post-operative recovery period.
Rats were randomly divided into three groups: untreated (control), OD-EWH (1 g/kg BW/d), and EWH (1 g/kg BW/d), with each group comprising six animals. BP was monitored continuously for 24 h (10 s every min) every 3 d until day 9, with the baseline BP recorded on day 0. OD-EWH and EWH were dissolved in Ensure (Abbott Nutrition, QC, Canada) and orally administered to rats once daily from day 1; the untreated group received Ensure only. Recordings were made for SBP, DBP, mean arterial pressure (MAP), and heart rate (HR). Animals were euthanized through cardiac blood collection under anesthesia. Blood and tissues were collected and stored at −80 °C.
Immunostaining
-
Aortas were preserved in Tissue-Tek® O.C.T Compound (Sakura Finetek USA, Torrance, CA, USA) and promptly frozen at –80 °C. Tissue sections, measuring ten micrometers in thickness, were generated and affixed to glass slides using a Leica CM1860 cryostat (Leica Biosystems, Concord, ON, Canada). Subsequently, the sections underwent fixation in cold acetone (–20 °C), followed by a wash with phosphate-buffered saline (PBS, pH 7.5). They were then subjected to blocking in a solution of 1% bovine serum albumin (BSA) with 0.1% Triton-X-100 in PBS for 60 min at room temperature within a humid chamber. The sections were immunostained overnight at room temperature using a nitrotyrosine primary antibody (dilution 1:20 in 2% BSA in PBS; Novus Biologicals, Oakville, ON, Canada). Following PBS washing, the sections were incubated with a secondary antibody (dilution 1:250; Alexa Fluor 546 - red; Invitrogen, Burlington, ON, Canada) in 2% BSA in PBS for 60 min (protected from light). After another round of PBS washing, the sections were covered with glass coverslips and a VECTASHIELD mounting medium containing DAPI (Vector Laboratories, Burlingame, CA, USA). They were then promptly examined under an Olympus IX81 fluorescence microscope (Olympus, Tokyo, Japan). Quantification of immunofluorescence in the obtained images was conducted using Image Studio Lite 5.2 (Licor Biosciences, Lincoln, NE, USA).
Western blotting
-
Aorta and kidney tissue proteins were extracted using a protein extraction buffer with an EDTA-free protease inhibitor cocktail (Sigma, St. Louis, MO, USA) and RIPA buffer (Radio-Immunoprecipitation Assay). The homogenates were centrifuged at 15,000 g for 15 min at 4 °C. Protein concentrations in the supernatants were measured using a bicinchoninic acid (BCA) assay (Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts of protein were loaded onto a 9% separating gel and transferred to a nitrocellulose membrane (0.45 μm; Bio-Rad, Montreal, QC, Canada). The membrane was incubated with antibodies against ACE (Abcam, Toronto, ON, Canada), ACE2 (Abcam), AT1R (Novus Biologicals, Oakville, ON, Canada), Ang II type 2 receptor (AT2R; Abcam), MasR (Novus Biologicals), inducible nitric oxide synthase (iNOS; BD Biosciences, San Jose, CA, USA), cyclooxygenase 2 (COX2; Abcam), intracellular adhesion molecule-1 (ICAM-1; Santa Cruz, Dallas, TX, USA), vascular cell adhesion molecule-1 (VCAM-1; Abcam), and phospho-endothelial NOS (p-eNOS; Abcam). The protein bands were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Abcam). Band visualization was performed using Donkey-anti-mouse 800 CW or Goat-anti-rabbit IRDye 680 RD secondary antibodies on a Licor Odyssey BioImager, and the fluorescence signal was quantified using Image Studio Lite 5.2 (Licor Biosciences).
Statistical analysis
-
Data were presented as mean values with standard errors of the means (SEM). BP and HR were examined using a two-way ANOVA with Tukey's test, while Western blot, underwent analysis via one-way ANOVA with Dunnett's post hoc test, utilizing GraphPad Prism Version 6 (San Diego, CA, USA). A significance level of p < 0.05 was considered statistically different.
-
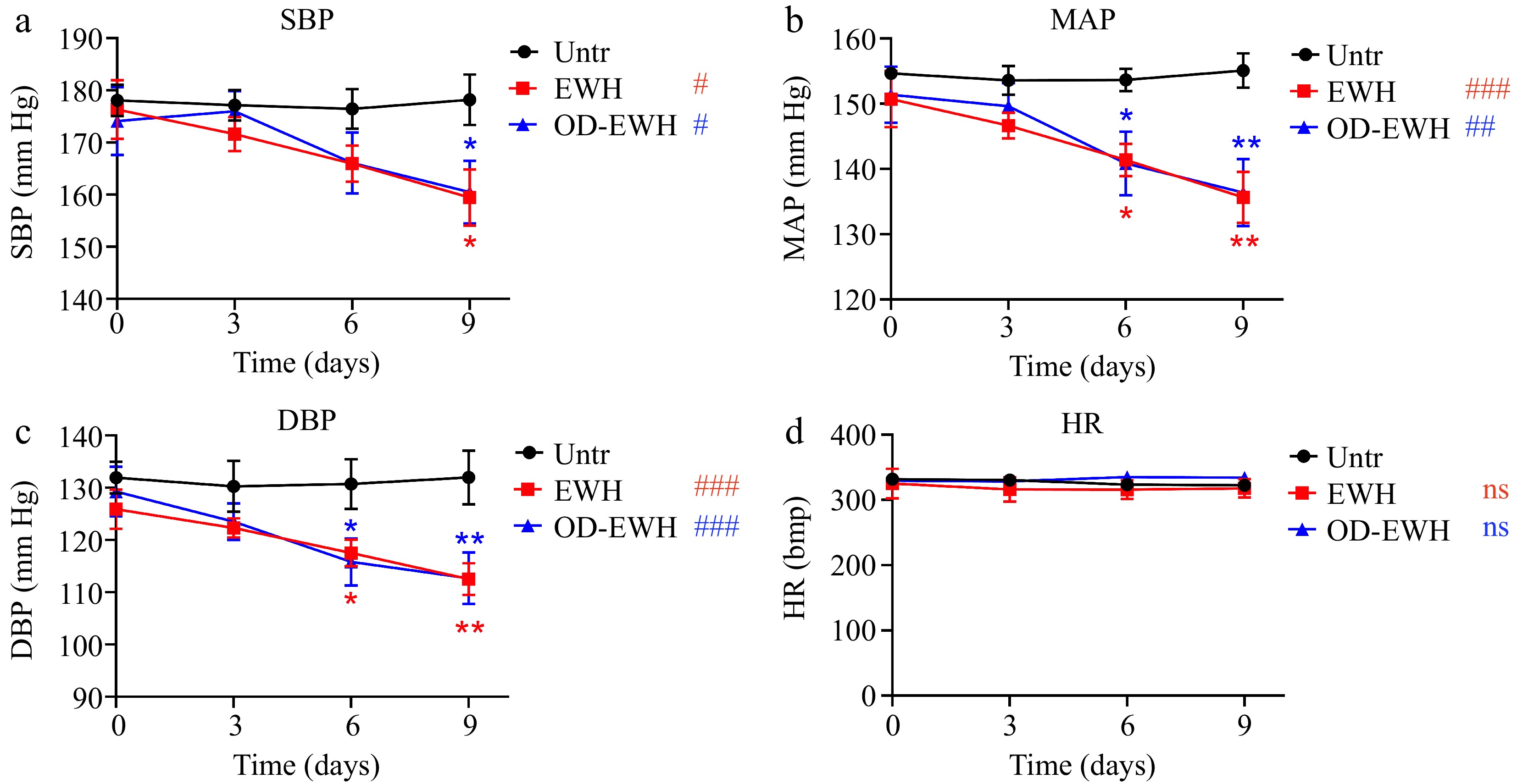
The initial SBP of SHRs on day 0 was recorded at 178.07 mmHg. The untreated group maintained this pressure over the experimental period (Fig. 1). EWH treatment (1 g/kg BW/d) significantly reduced SBP to 159.42 mmHg (p < 0.05), while the OD-EWH treatment (1 g/kg BW/d) reduced that to 160.47 mmHg (p < 0.05). Similarly, both treatments significantly reduced MAP and DBP over the treatment period (Fig. 1). Notably, neither treatment had a impact on the HR (p > 0.05) (Fig. 1). Results have shown that OD-EWH reduced BP to a similar extent to that of EWH.
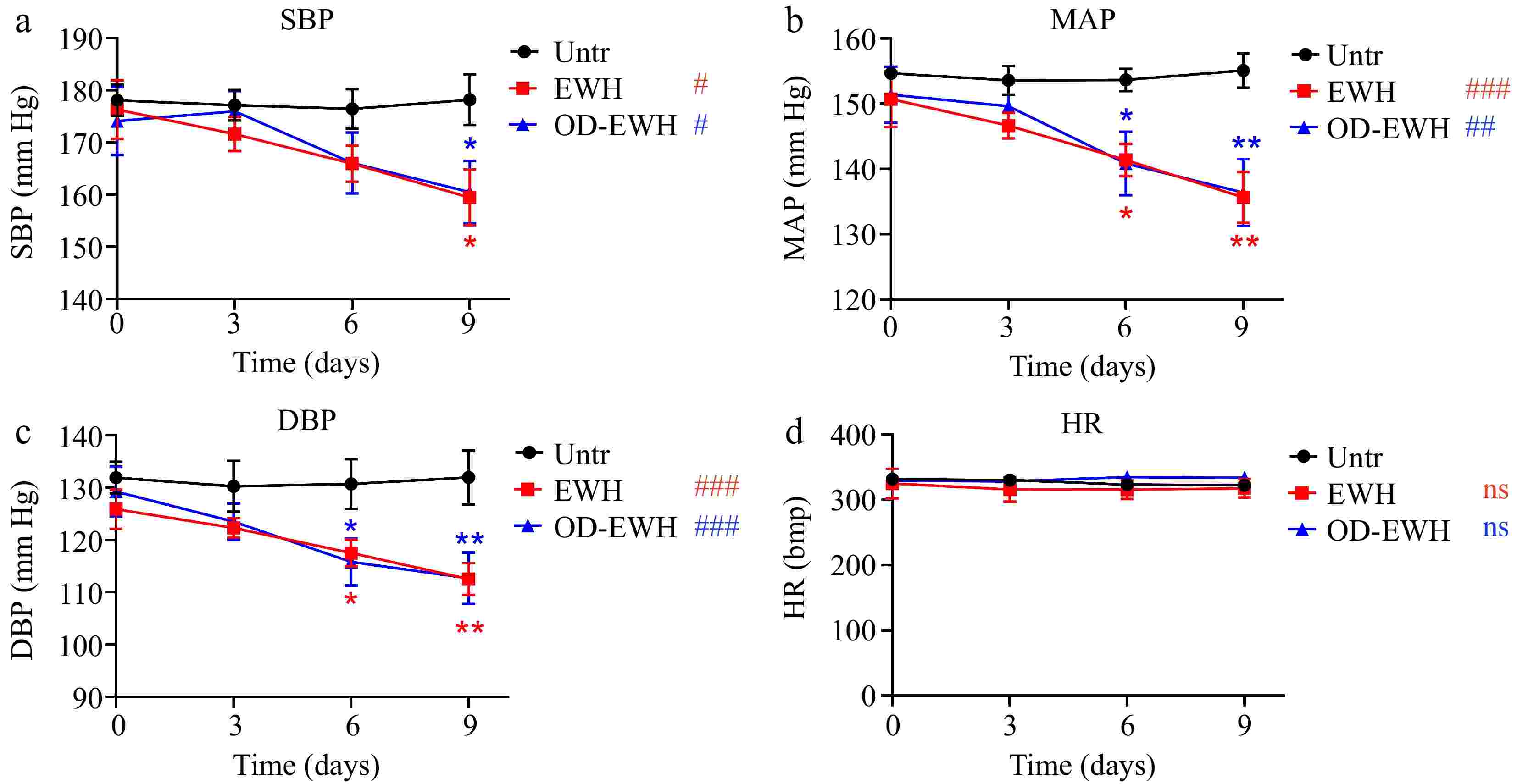
Figure 1.
Effect of oral administration of EWH or OD-EWH (at a dose of 1 g/kg BW/d) on blood pressure and heart rate in spontaneously hypertensive rats. Blood pressure and heart rate at each time point represented the mean values recorded over a 24-h period. Data were expressed as mean ± SEM from n = 6 animals per group. * p < 0.05, ** p < 0.01, compared to the untreated group at a specific time point; # p < 0.05, ## p < 0.01, ### p < 0.001, ns, not significant (p > 0.05), compared to the untreated group over 9 days. Abbreviations: DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; SBP, systolic blood pressure.
OD-EWH regulates the aortic RAS components
-
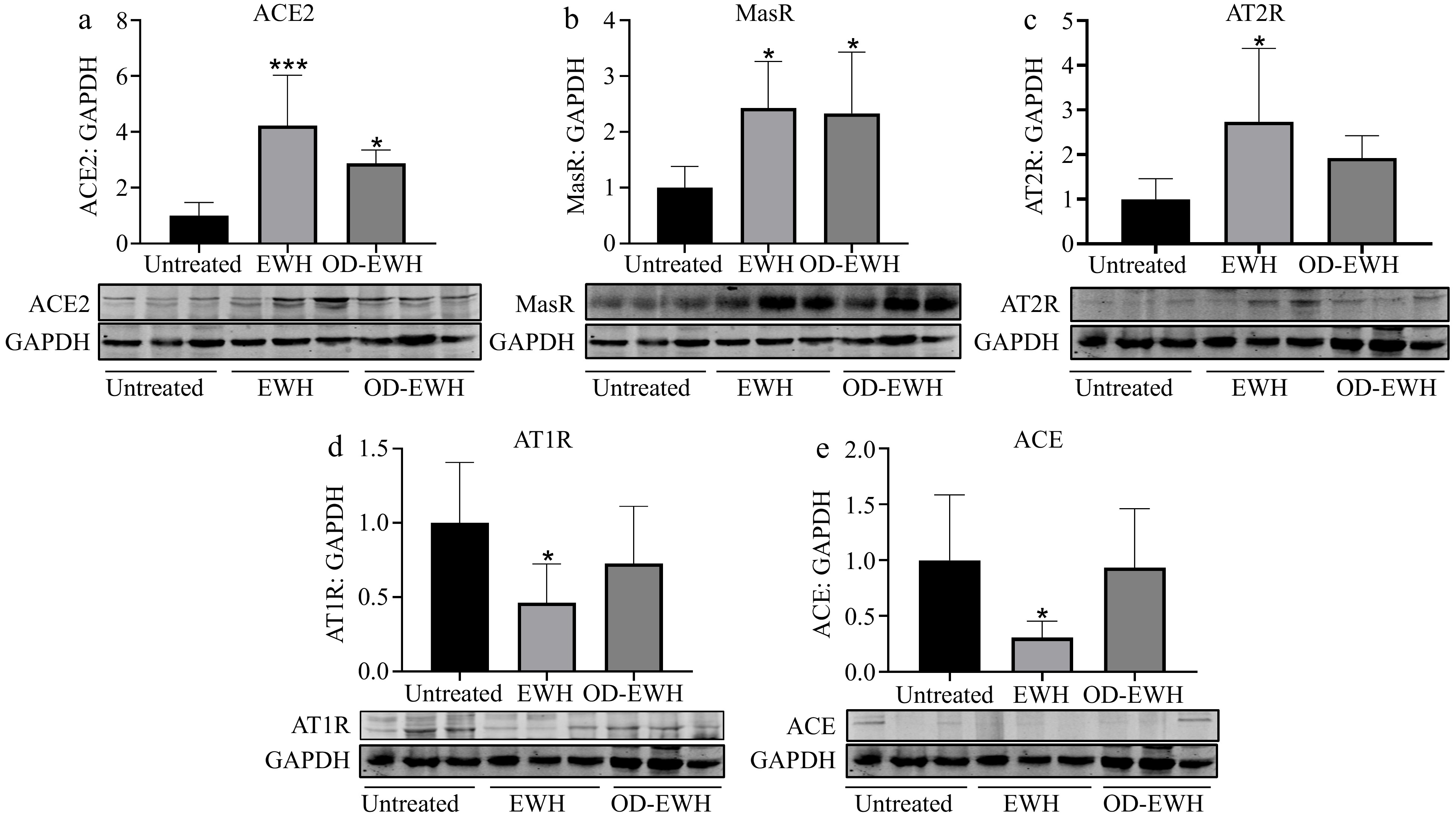
The ACE2-Ang-(1-7)-Mas receptor (MasR) plays a crucial role in suppressing oxidative stress, proliferation, and inflammation within vascular smooth muscle[19,20]. To investigate the potential of OD-EWH and EWH in enhancing ACE2 in the aorta, the protein level of ACE2 was assessed. ACE2 level was significantly increased in both OD-EWH and EWH groups compared to the untreated group (p < 0.05) (Fig. 2).
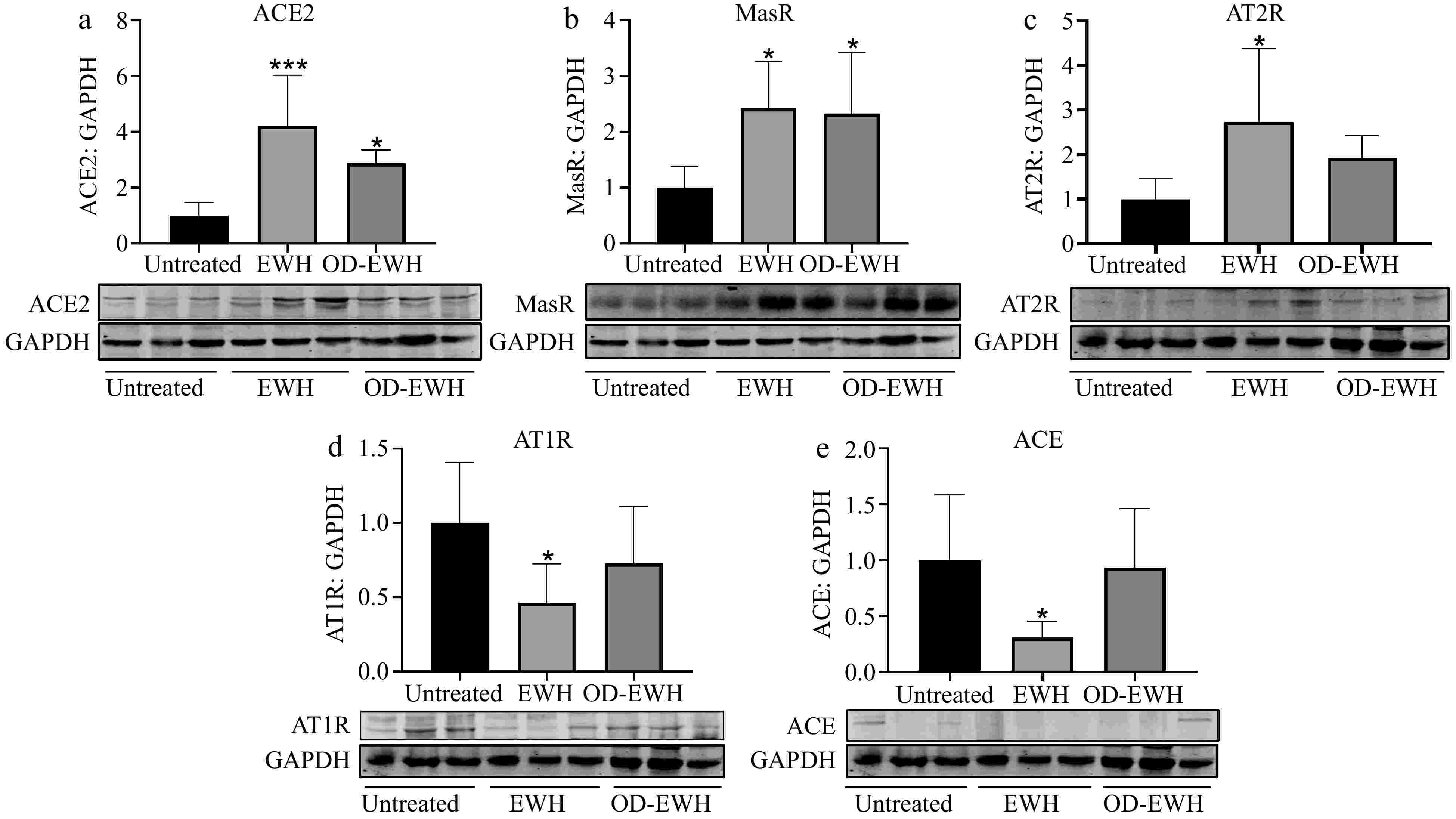
Figure 2.
Effect of oral administration of EWH or OD-EWH (at a dose of 1 g/kg BW/d) on the levels of renin–angiotensin system (RAS) components in the aorta. Vascular levels of (a) ACE2, (b) MasR, (c) AT2R, (d) AT1R, and (e) ACE were normalized to GAPDH and were then normalized to that of the untreated group. Data were expressed as mean ± SEM from four to six animals per group. * p < 0.05, *** p < 0.001, compared to the untreated group.
The harmful effect of Ang II primarily occurs via the ACE-Ang II-AT1R pathway[21], while binding of Ang II to the AT2R induces opposing effects[22]. The findings indicate that only EWH reduced the ACE and AT1R levels (p < 0.05). Additionally, EWH increased both MasR and AT2R levels while OD-EWH increased AT2R level (p < 0.05). The levels of ACE, AT1R, and AT2R were not affected by OD-EWH (Fig. 2).
OD-EWH regulates the renal RAS components
-
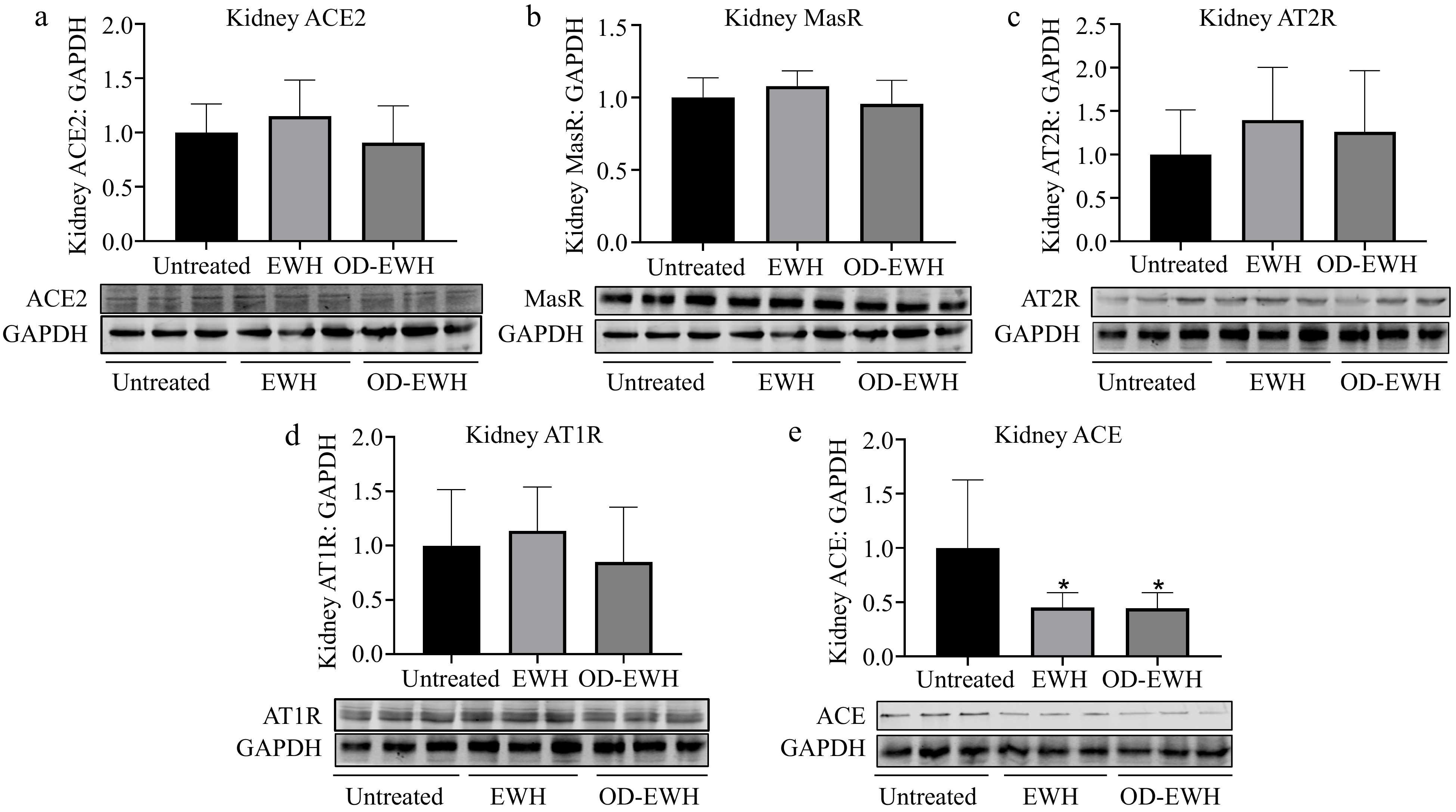
The kidney encompasses all elements of the RAS, establishing it as a vital organ for the regulation of BP[23]. Since EWH and OD-EWH affected RAS in the aorta, it was investigated whether they could also affect RAS in the kidney. No significant differences were observed in the protein levels of renal ACE2, MasR, AT1R, and AT2R in rats treated with either OD-EWH or EWH (p > 0.05). However, a significant decrease in renal ACE level was detected in both OD-EWH- and EWH-treated groups compared to untreated groups (p < 0.05) (Fig. 3).
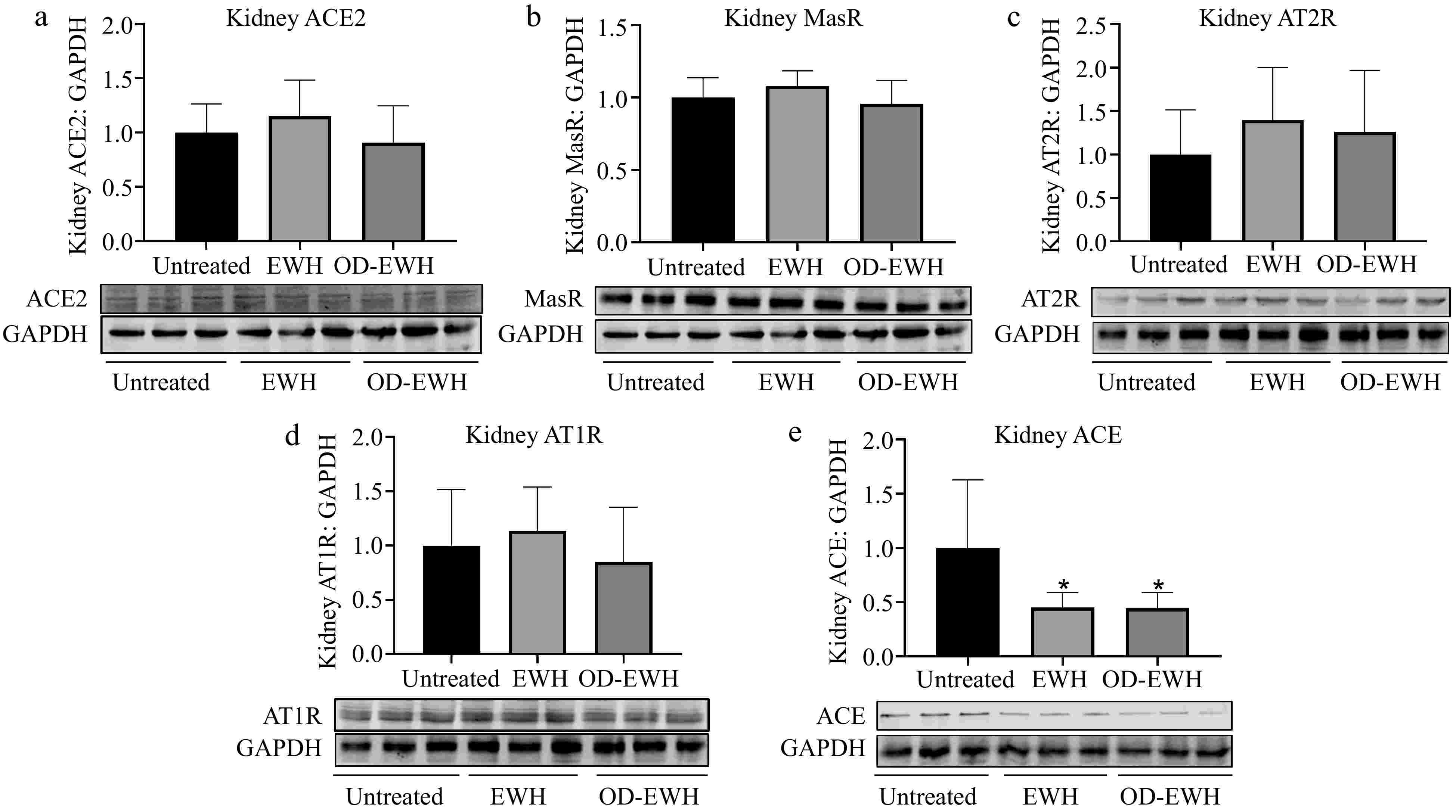
Figure 3.
Effect of oral administration of EWH or OD-EWH (at a dose of 1 g/kg BW/d) on the levels of renin–angiotensin system (RAS) components in the kidney. The protein levels of (a) ACE2, (b) MasR, (c) AT2R, (d) AT1R, and (e) ACE were normalized to GAPDH and were then normalized to that of the untreated group. Data represented as mean ± SEM from four to six animals per group. * p < 0.05, compared to the untreated group.
OD-EWH enhances eNOS expression
-
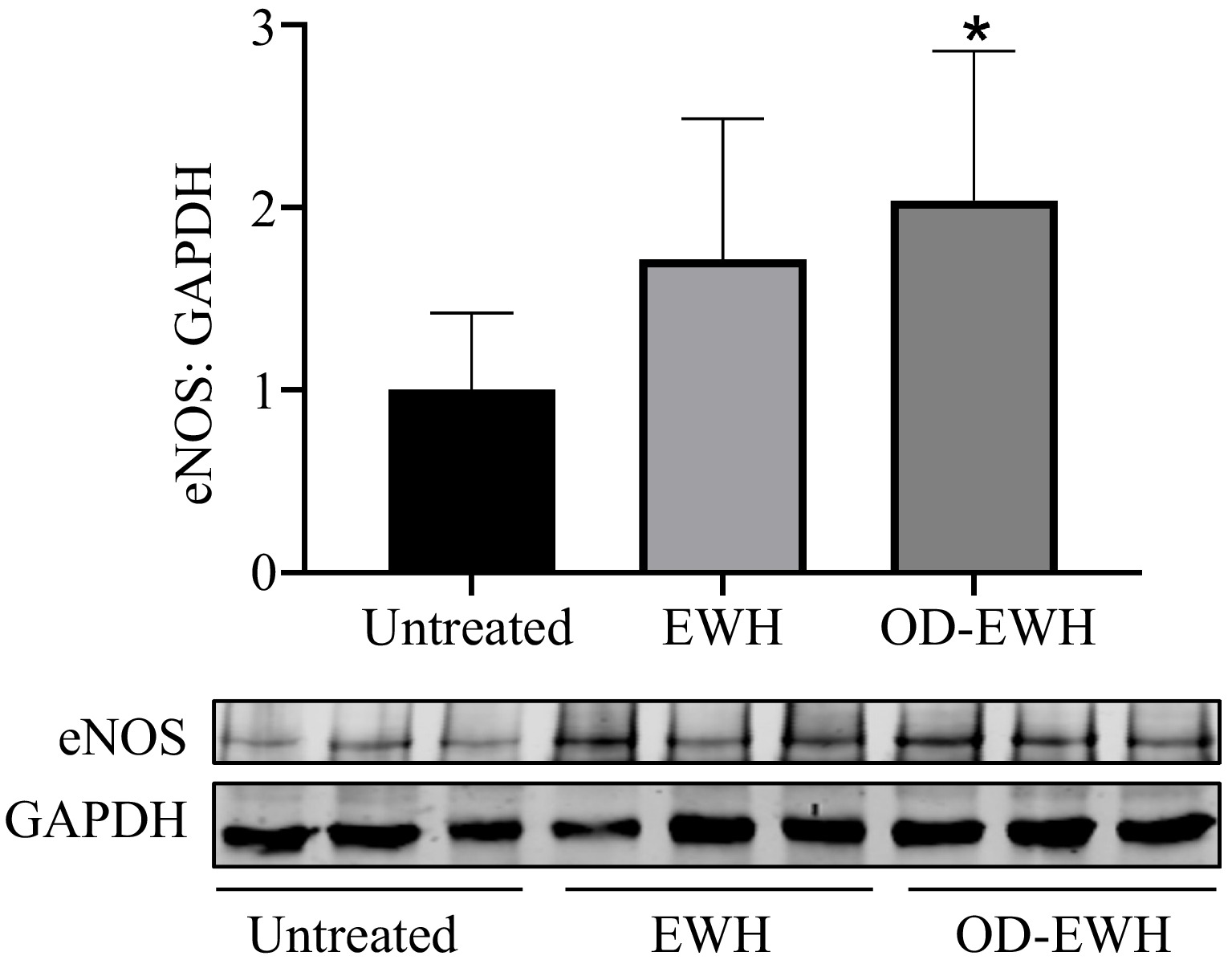
eNOS is the crucial enzyme for generating NO that triggers vasorelaxation and thus BP reduction. In this study, OD-EWH treatment increased the eNOS level in the aorta of SHRs (p < 0.05). EWH appeared to increase the aortic level of eNOS despite insignificantly in comparison with the untreated group, indicating the role of OD-EWH in decreasing BP through eNOS (Fig. 4).
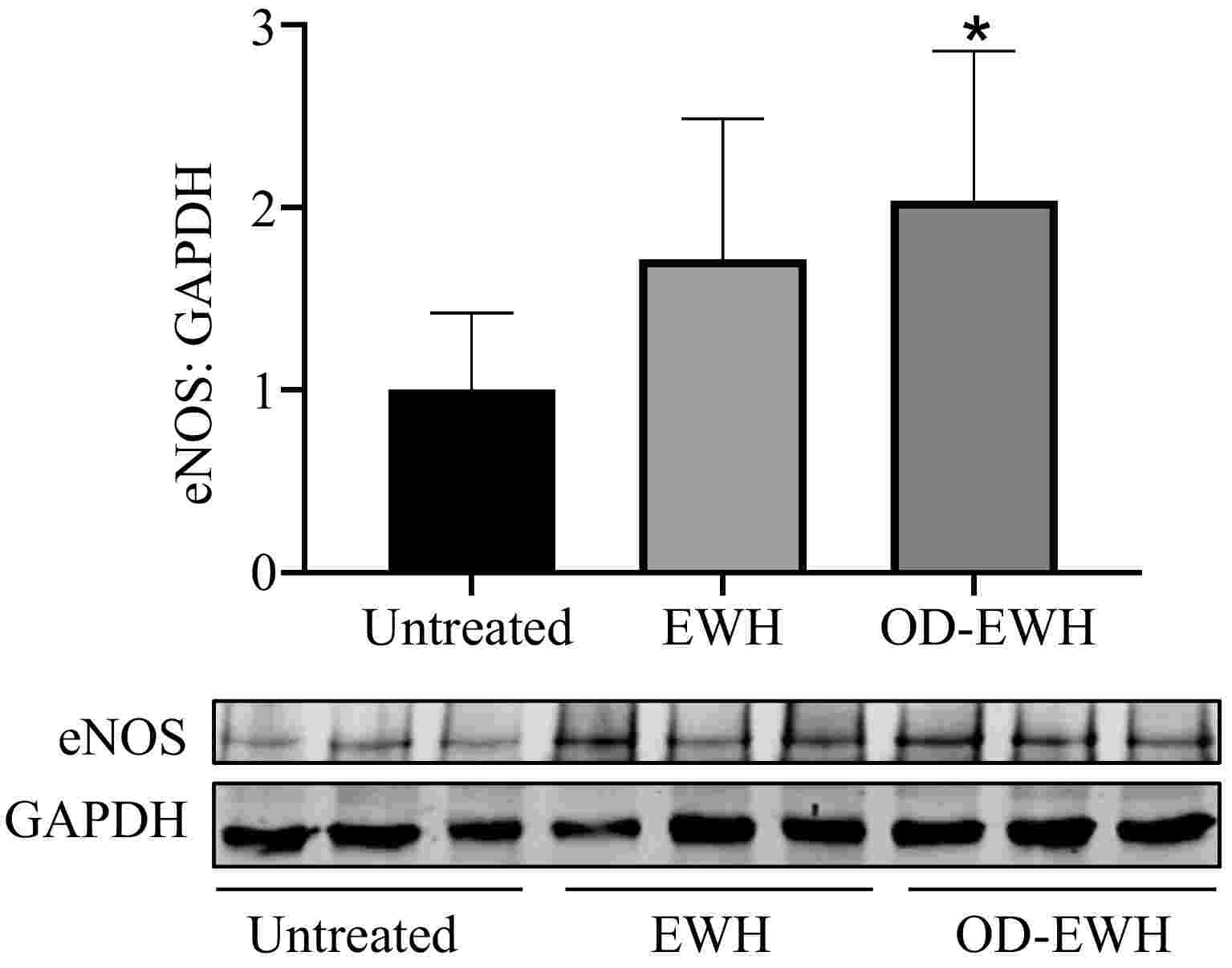
Figure 4.
Effect of oral administration of EWH or OD-EWH (at a dose of 1 g/kg BW/d) on the endothelial nitric oxide synthase (eNOS) level in the aorta. Vascular protein level of eNOS expression was normalized to GAPDH and was then normalized to that of the untreated group. Data were expressed as mean ± SEM from four to six animals per group. * p < 0.05, compared to the untreated group.
OD-EWH attenuates vascular oxidative/nitrosative stress and inflammation
-
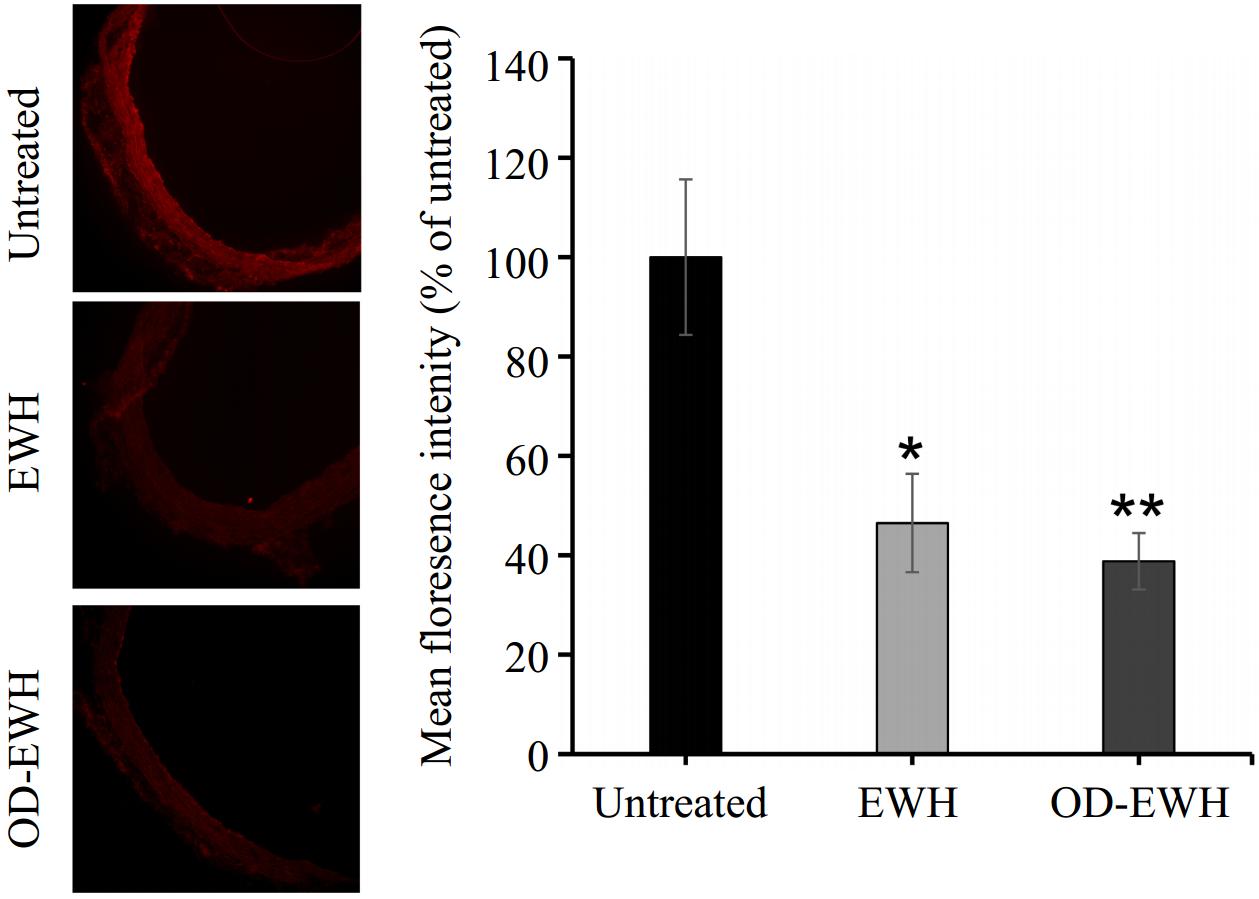
A marked reduction in nitrotyrosine staining (a marker of increased peroxynitrite generation and oxidative/nitrosative stress) was observed in the aortas of OD-EWH and EWH-treated SHRs (p < 0.05) (Fig. 5).
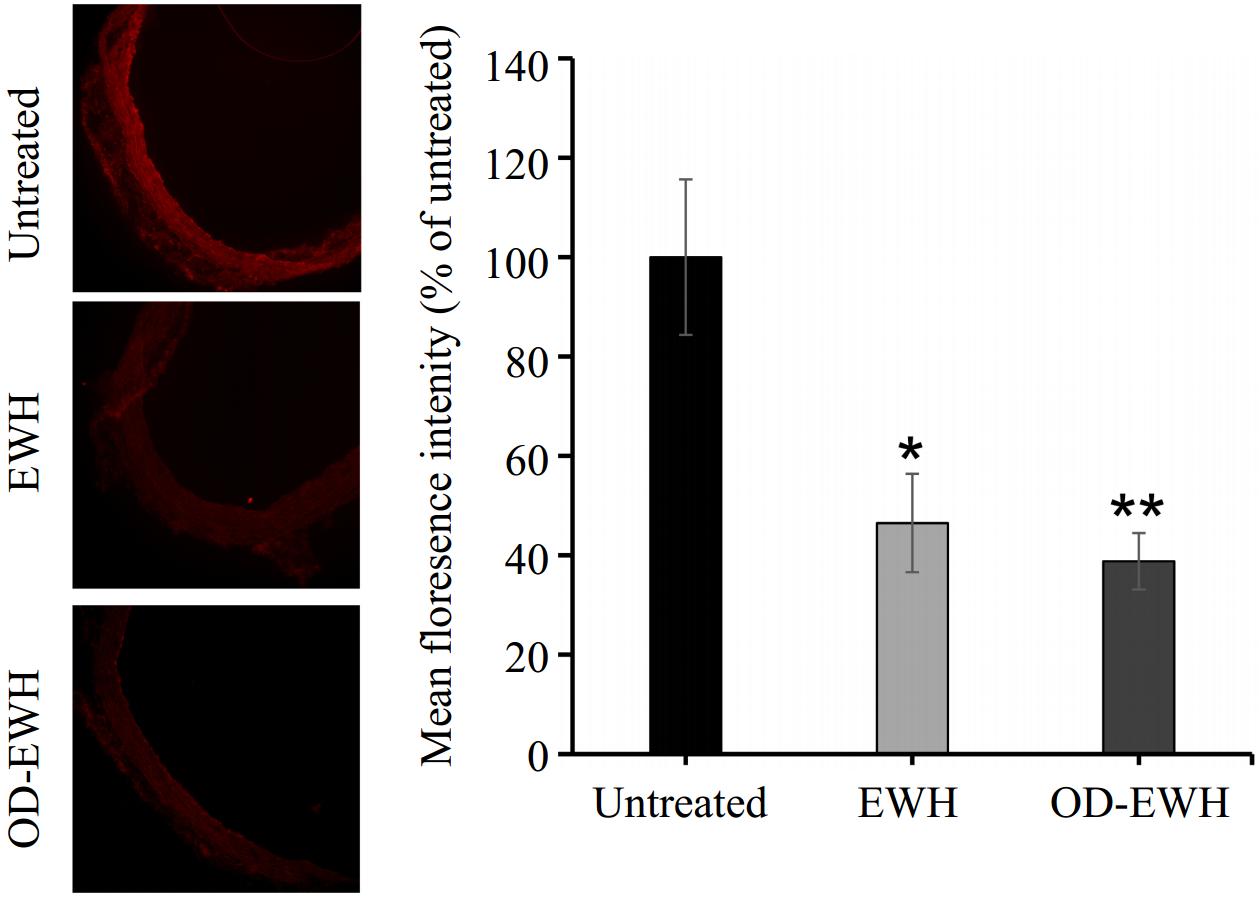
Figure 5.
Effect of oral administration of EWH or OD-EWH (at a dose of 1 g/kg BW/d) on nitrotyrosine level in the aorta. Representative fluorescence images of aortic sections indicate nitrotyrosine level. The level of nitrotyrosine was normalized to tissue without primary antibody treatment (background fluorescence) and the fluorescence signal strength was expressed as the percentage relative to the untreated group. Data represented as mean ± SEM from four to six animals per group. * p < 0.05 and ** p < 0.01, compared to the untreated group.
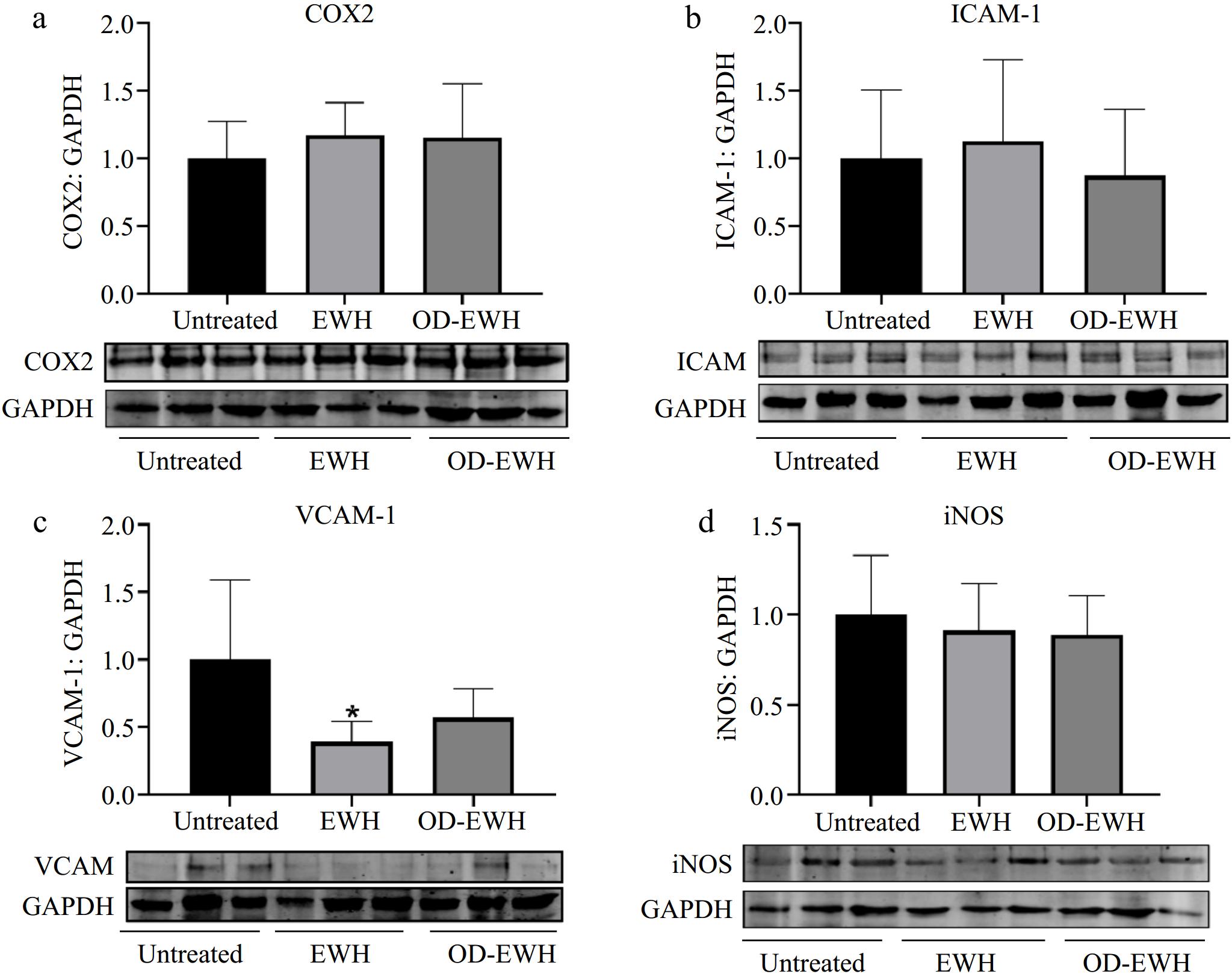
The level of adhesion molecules, ICAM-1 and VCAM-1, plays a crucial role in the initiation and progression of vascular inflammation[24]. EWH significantly reduced the level of VCAM-1 (p < 0.05) but did not affect those of COX2, ICAM-1, and iNOS (p > 0.05) (Fig. 6). OD-EWH did not affect the levels of VCAM-1, ICAM-1, COX2, and iNOS. These findings indicate that EWH could function as both an anti-inflammatory and antioxidant agent, potentially providing further benefits in improving cardiovascular disease conditions.
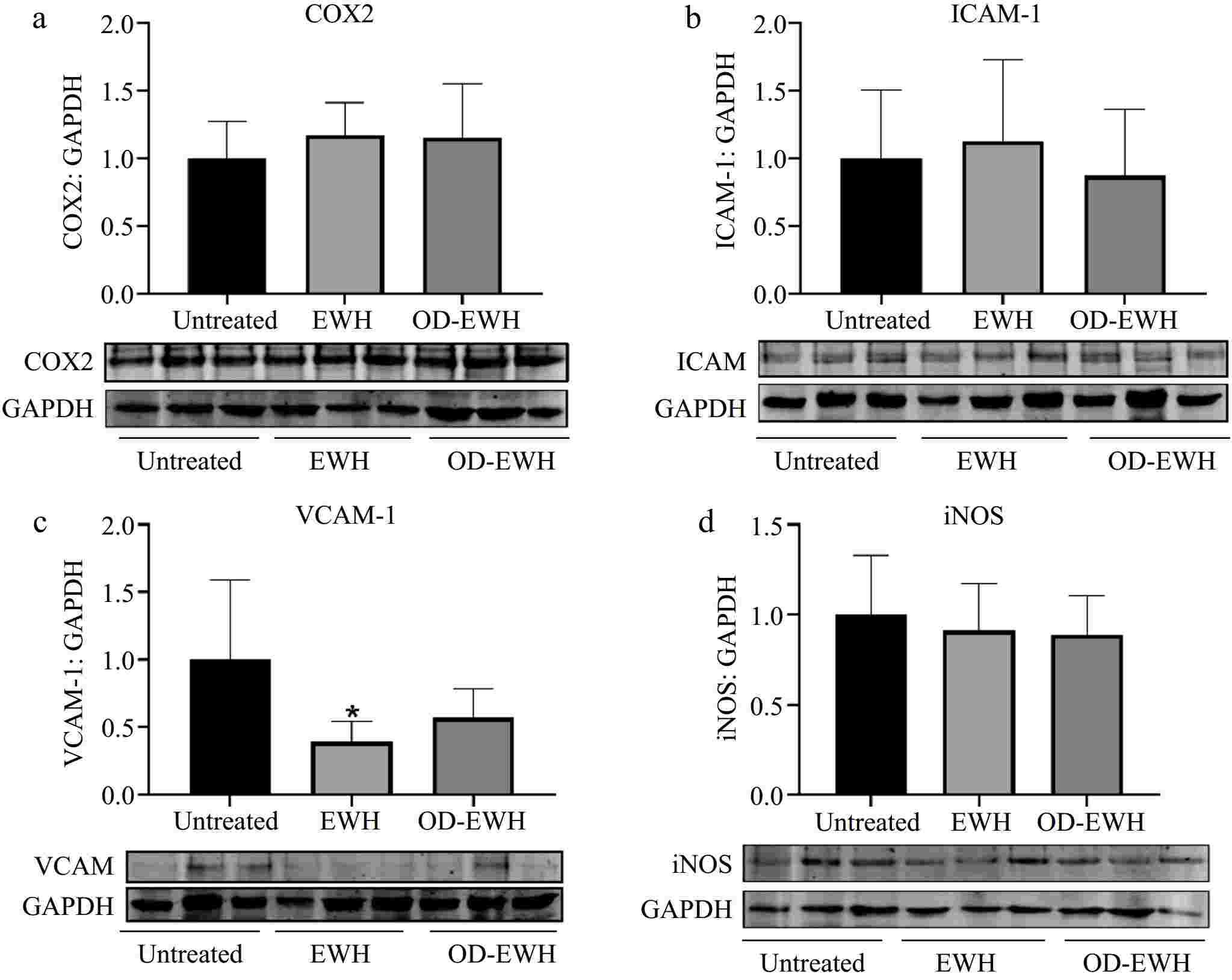
Figure 6.
Effect of oral administration of EWH or OD-EWH (at a dose of 1 g/kg BW/d) on the levels of inflammatory proteins in the aorta. (a) Vascular levels of cyclooxygenase 2 (COX2), (b) intracellular adhesion molecule-1 (ICAM-1), (c) vascular cell adhesion molecule-1 (VCAM-1), and (d) inducible nitric oxide synthase (iNOS) were normalized to GAPDH and were then normalized to that of the untreated group. Data were expressed as mean ± SEM from four to six animals per group. *p < 0.05, compared to the untreated group.
-
Since our previous study reported that many potent ACE-inhibitory peptides are present in non-ovotransferrin egg white proteins[12], in the present study the antihypertensive effect of OD-EWH in SHRs was explored. EWH was used as the reference since it reduced BP substantially in SHRs[11]. Both hydrolysates could significantly reduce the BP of SHRs at a dosage of 1 g/kg BW/d; importantly, neither treatment impacted the HR of SHRs, suggesting the preservation of cardiac function. These results demonstrated the comparable efficacy of OD-EWH to that of EWH in hypertension treatment.
Protein hydrolysates obtained from various types of foods have been shown to decrease BP[11,25]. OD-EWH reduced SBP by approximately 18 mmHg in SHRs, showing a similar effect to that of an egg white hydrolysate (25–30 mmHg) administered at a dosage of 1,000 mg/kg BW/d for 18 d and a milk protein hydrolysate (30 mmHg) given at 1,200 mg/kg BW/d for 15 d[11,26]. The present results also aligned with a previous investigation that oral administration of a chicken muscle protein hydrolysate prepared by thermoase at the same dosage decreased ~25 mmHg in SBP over 20 d[27]. Besides, peptides with antihypertensive properties sourced from chicken after oral administration (10 mg/kg BW), Ala-Val-Phe-Gln-His-Asn-Cys-Gln-Glu (AVFQHNCQE) and Gln-Val-Gly-Pro-Leu-Ile-Gly-Arg-Tyr-Cys-Gly (QVGPLIGRYCG) reduced SBP by 25 and 11 mmHg, respectively[28]. Certainly, both protein hydrolysates and isolated peptides have demonstrated great efficacies in regulating BP. Nevertheless, protein hydrolysates surpass pure peptides considering various factors for commercial production including cost-effectiveness, energy efficiency, and scalability[10,27].
Increased activity of RAS results in elevated BP and cardiovascular diseases[29]. Both EWH and OD-EWH treatments modulate the RAS. A notable decrease in aortic ACE was observed in the SHRs treated with EWH, while that of ACE2 and MasR was significantly increased by either OD-EWH or EWH treatment (p < 0.05). Moreover, EWH, but not OD-EWH, decreased the aortic AT1R and increased that of AT2R. Therefore, OD-EWH may reduce BP in SHRs by modulating the vascular RAS, involving the upregulation of vascular ACE2 and MasR, independent of vascular expressions of ACE, AT1R, and AT2R. Neither EWH nor OD-EWH regulated any RAS components in kidneys except for ACE. These results suggested that OD-EWH and EWH could modulate both vascular and renal RAS despite being slightly different due to different peptide compositions. It reduced ACE level in the kidneys without affecting its level in the aortas; instead, it upregulated ACE2 and MasR levels in the aortas. Such variations suggest the gap between in vitro and in vivo activity and the complexity of the mechanisms of antihypertensive peptides in vivo due to many factors such as biotransformation[30]. Compared to OD-EWH, EWH also modulated vascular ACE and AT2R levels, which aligned with our previous results[11], likely due to the effect of peptides released from ovotransferrin[31].
OD-EWH elevated the aortic eNOS level, indicating the restoration of NO-mediated vasorelaxation, one of the mechanisms reducing BP by antihypertensive peptides. For example, a chicken foot protein-derived nonapeptide, AVFQHNCQE, could reduce BP in SHRs by enhancing NO generation[32]. A rice-derived tripeptide, Leu-Arg-Ala (LRA), a NO-dependent vasorelaxant peptide lowered BP significantly in SHRs[33]. Besides, some peptides also appear to enhance eNOS including thermoase-prepared chicken muscle protein hydrolysate[27] and EWH in the present study (Fig. 4). Lack of enhancing NO generation by EWH contradicted our previous findings that the hydrolysate could enhance NO-mediated vasorelaxation[11]. This ambiguity was likely due to either the EWH was orally administered to SHRs for only 9 d, much shorter than 18 d as reported by Jahandideh et al.[11], or because there might be a concurrent increased NO bioavailability due to mitigated oxidative stress by EWH in the vasculature[27]. These questions await further clarification. Moreover, OD-EWH increased eNOS significantly (p < 0.05) but EWH did not, indicating that egg white proteins, excluding ovotransferrin, might be great protein sources for preparing eNOS-regulating peptides.
Elevated levels of ROS and chronic inflammation are implicated in the development of hypertension. ROS, such as superoxide anion, can impede the bioavailability of NO by producing peroxynitrite (–ONOO), which induces a pro-inflammatory phenotype through tyrosine nitration of diverse proteins. Previous research has demonstrated that lowering –ONOO level and tyrosine nitration in SHRs through the inhibition of ROS formation contribute to BP reduction[34]. In the present study, treatment with EWH or OD-EWH significantly reduced nitrotyrosine levels in the aorta of SHRs (Fig. 5). EWH treatment reducing oxidative stress was consistent with our previous findings[11]. OD-EWH appeared to have a higher antioxidant effect than that of EWH, suggesting that egg white proteins excluding ovotransferrin might be a greater protein source of antioxidant peptides. Therefore, characterizing the antioxidant effect of OD-EWH followed by the identification of the responsible antioxidant peptides is also one of our future works. EWH alleviated vascular inflammation especially the expression of VCAM-1 while OD-EWH did not affect vascular inflammation in SHRs. Peptides have been reported to reduce BP by mitigating vascular inflammation. Val-Pro-Pro (VPP), Ile-Pro-Pro (IPP), and IRW suppressed the levels of ICAM-1 and VCAM-1 in human umbilical vein endothelial cells[35,36]. In SHRs, a chicken muscle protein hydrolysate and its derived peptide mitigated vascular inflammation by lowering iNOS and VCAM-1[27]; however, a peptide derived from the hydrolysate, Val-Val-His-Pro-Lys-Glu-Ser-Phe (VVHPKESF), did not reduce iNOS and VCAM-1, but only that of COX2[30]. Therefore, a peptide might exert its biological functions via different mechanisms when being administered alone or together with other peptides to animals. This might explain the varying functions between OD-EWH and EWH in SHRs since EWH also contains peptides from ovotransferrin. In addition, OD-EWH did not reduce vascular inflammation in SHRs, which might suggest that ovotransferrin may be the protein containing more anti-inflammatory peptides in egg whites. Therefore, EWH may function as both an anti-inflammatory and antioxidant agent, while OD-EWH may be more feasible to develop as an antioxidant agent.
-
Using EWH as the reference, OD-EWH demonstrated similar activity in reducing BP in SHRs as that of EWH, without impacting heart rate. Compared with EWH, OD-EWH also increased the levels of ACE2 and MasR but did not affect ACE, AR1R, and AT2R; both hydrolysates lowered ACE levels in the kidneys. Moreover, only OD-EWH increased eNOS and only EWH ameliorated vascular inflammation, while both hydrolysates mitigated the vascular oxidative stress. Our results suggested the complexity of the actions of various peptides in modulating biological functions in vivo. Nevertheless, these findings emphasize the diverse potential of EWH and OD-EWH in hypertension management.
-
The AUP#00001571 animal protocol was approved by the University of Alberta Animal Welfare Committee, adhering to the guidelines provided by the Canadian Council on Animal Care and the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health.
This work was supported by the Natural Sciences and Engineering Research Council of Canada, Grant No. CRDPJ 532150-18, Egg Farmers of Canada, and the Global Egg Corporation.
-
The authors confirm contribution to the paper as follows: conceptualization, writing—editing: Ashkar F, Fan H, Wu J; formal analysis, investigation: Ashkar F, Fan H, Fernando I, Wang Z; data curation, writing—original draft preparation: Ashkar F, Fan H; Supervision, project administration, funding acquisition, resources: Wu J. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated or analyzed during the current study are available from the corresponding author on request.
-
The authors declare that they have no conflict of interest.
-
# Authors contributed equally: Fatemeh Ashkar, Hongbing Fan
- Copyright: © 2024 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Ashkar F, Fan H, Wang Z, Fernando IPS, Wu J. 2024. Ovotransferrin-depleted egg white hydrolysate reduces blood pressure in spontaneously hypertensive rats. Food Materials Research 4: e035 doi: 10.48130/fmr-0024-0026











 DownLoad:
DownLoad: