-
The king coconut (Cocos nucifera var. aurantiaca) locally known as 'Thambili' is one of the native and rare types of coconuts grown abundantly only in Sri Lanka[1]. Specifically, the king coconut which has a golden orange-colored husk is widely acclaimed culturally and historically in Sri Lankan history tracing back to pre-historic periods. One of the most cherished qualities of the king coconut is its extra-sweet, fragrant nut water, renowned for its high concentration of total sugars (5−6 g/100 mL), predominantly inverted sugars (glucose and fructose)[2]. This natural beverage is not only refreshing but also highly hydrating, containing natural electrolytes (Na+, K+, Ca+2, and Mg+2), vitamins, and minerals with a neutral pH[3]. The unique flavor, sweetness, and health benefits of king coconut water have contributed to its immense popularity in Sri Lanka, from roadside stalls to luxurious hotel offerings. It has become a symbol of Sri Lankan culture and a sought-after export commodity, with a substantial commercial market potential globally (Table 1).
Table 1. Export performance of king coconut nuts in Sri Lanka[4].
Period Volume of king coconut nut export (MT) Value generated from king coconut nut exportation
(LKR. millions)January to September 2022 8,236,427 1,516.58 January to September 2023 9,991,401 2,556.24 Change (%) 21 69 However, the increasing demand for bottled king coconut water has led to a significant accumulation of immature king coconut husk waste (KCHW) from export factories[5]. This waste cannot be used for value addition due to the immaturity of the husk. While KCH has been traditionally used as a mulching material around crops, offering benefits such as moisture retention, weed control, and soil erosion prevention, it has limitations compared to other organic mulching materials[6]. KCH exhibits lower moisture absorbance ability and slower decomposition rates, which can hinder its effectiveness as a mulch. The unsustainable management of this agricultural waste poses a threat to the ecosystem, serving as a breeding ground for insects and mosquitoes, and potentially causing health issues such as dengue and elephantiasis[1]. Moreover, mulching and burying in agricultural lands may not be economical or sustainable solutions for managing this generated agro-industrial bio-waste. Nonetheless, major king coconut water exporters generally utilize this resource as a biofuel feedstock in boilers to generate power for their operations. However, this practice may not be environmentally friendly or sustainable in the long run.
It is vital to investigate long-term solutions for the valuation of immature KCHW in light of these difficulties. One possible strategy that has to be considered is the utilization of this waste to produce goods that may be beneficial to the coconut plantations and other coconut-based agroforestry systems for instance through the production of biochar and ash, which are widely used as soil conditioners for increase in overall productivity[7,8]. This can be a good source of potassium (K) nutrients[1]. And it increases the nutrient retention ability of soil[9]. Ultimately, it contributes to achieving Sustainable Development Goals (SDGs) by enhancing soil fertility and crop yields, thereby ensuring food security in the global scenario[10−16].
This study aims to investigate the nutritional properties of KCHW biochar and ash as potential soil conditioners with different pyrolysis temperatures and times including 300, 400, and 500 °C for 1 h for biochar and 400, 500, and 600 °C for 4 h for ash production. Therefore, this will extend understanding and recommend their application in coconut production to reduce the detrimental effects of king coconut cultivation.
-
The investigation was carried out in the Agronomy Division of the Coconut Research Institute situated in Lunuwila, Sri Lanka. The steps were to place the KCHW in small chips and expose the chips to oven drying at a temperature of 60 °C for 72 h. Following this, the dried chips underwent pyrolysis utilizing a Muffle Furnace (Model P330, Nabertherm, Germany) at three different heating levels: Those were 300, 400, and 500 °C for making biochar production. In the production of ash, 400, 500, and 600 °C temperatures were used. Over these hours, the pyrolysis process was conducted in a regulated setting with a restricted air supply, maintaining a heating rate of 7 °C per minute. This biochar was permitted to cool to room temperature and more analysis was done at a later time after cooling.
Characterization of KCH biochar and ash
-
The properties of KCHW ash and biochar were examined through various analyses. The samples' electrical conductivity (EC) and pH were measured using standard protocols with an Edge meter (Hanna, Romania)[17]. The Kjeldahl method determined the total nitrogen (N) content[18]. In contrast, the modified dry ash method was used to analyze the levels of phosphorus (P), potassium (K), magnesium (Mg), calcium (Ca), and micro-nutrient components [iron (Fe), sodium (Na), lead (Pb), zinc (Zn), nickel (Ni), and copper (Cu)] in the samples[19]. Proximate analysis was conducted using the muffle furnace pyrolysis method.
Statistical analysis
-
R software (4.1.3) was utilized for all statistical studies. A One-way Analysis of Variance (ANOVA) was performed on the data mean values at a 5% significance level. Tukey's honestly significant difference (HSD) comparison test was then used to conduct statistical comparisons.
-
The biochar's nutrient composition is controlled by the temperature at which it is produced, for the amount of N, P, K, Ca, and Mg that is both total and available (Table 2). The total N content of produced KCHW-biochar decreased significantly with rising pyrolysis temperature, from 1.99% at 300 °C to 0.58% at 500 °C. However, the available N content remained constant (0.08%) across all temperatures. Higher pyrolysis temperatures lead to a significant reduction in the total N level of the KCHW-biochar. As the pyrolysis temperature increases, these nitrogen-containing compounds undergo further decomposition and transformation, leading to a decline in the total nitrogen content of the biochar[20]. The N amount in biochar was found to be positively correlated with the N content in the biomass and negatively correlated with the pyrolysis temperature[21]. However, the available N content remains unaffected by the temperature, suggesting that the remaining N is present in a stable form. This stabilization could occur through: (1) the formation of recalcitrant nitrogen-containing aromatic structures; (2) the creation of nitrogen-rich heterocyclic compounds; and (3) the conversion of labile nitrogen compounds into more resistant forms.
Table 2. Macronutrient composition of KCHW-biochar at different pyrolysis temperatures.
Pyrolysis temperature (°C) Total nutrient content (%) Available nutrient content (%) N P K Ca Mg N P K Ca Mg 300 1.99a 0.42 2.41 0.29b 0.37 0.08 0.05 2.37b 0.21 0.03b 400 1.37b 0.46 3.02 0.35b 0.41 0.08 0.16 2.62ab 0.21 0.08b 500 0.58c 0.46 3.33 1.16a 0.41 0.08 0.16 2.93a 0.38 0.24a p-value 0.000 0.560 0.116 0.000 0.688 0.980 0.478 0.047 0.055 0.017 CV 47.55 13.92 19.27 71.42 12.55 25.72 132.38 11.58 40.68 96.38 * In each column, means that do not share a letter differ significantly at p < 0.05. The total and available P content did not show significant variations across the different pyrolysis temperatures in the process of KCHW-biochar production. This indicates that P is relatively stable and retained in the biochar across the temperature range studied[22]. Wang et al. mentioned that volatilization of P-containing compounds happens at nearly around 760 °C of the pyrolysis temperature[23]. The same results were observed in the coconut husk-biochar production process[1].
The total K content enhanced slightly with rising temperature, from 2.41% at 300 °C to 3.33% at 500 °C in produced biochar. The available K content followed a similar pattern, increasing from 2.37% at 300 °C to 2.93% at 500 °C. Some studies show that processes associated with temperature sensitivity are involved in the release of readily available K. The rate of thermal decomposition of K correlates with temperature, and the amount of K made more available at 500 °C is greater than that of the less available K implants. Nevertheless, the release of available K is less efficient at lower temperatures, which causes the output of less[24,25]. This paper further highlights that pyrolysis temperature influences the chemical contents and processes of K compounds within the biochar and its release to the soil solution[26]. Hence, the established differences in available K availability at various temperatures reflect on the temperature deposition on the mechanisms from which available K can be released in the compounds in the soil[27].
The total Ca level increased significantly with rising temperature, from 0.29% at 300 °C to 1.16% at 500 °C in here. As organic components volatilize at higher temperatures, the relative concentration of mineral elements increases. This is not necessarily due to more Ca being added, but because other volatile components are being removed[28]. The process creates a 'concentration effect' where the same amount of Ca appears to increase as a percentage due to the reduction of other components. The available Ca content also showed an increasing trend, but the differences were not statistically significant. Comparable characteristics were noted in various forms of biochar generated from seaweed, orange pomace, chicken litter, and vine pruning[29].
The total Mg amount of KCHW-biochar remained relatively constant across the temperatures. However, the available Mg content increased significantly with rising temperature, from 0.03% at 300 °C to 0.24% at 500 °C. At higher temperatures, the biochar matrix undergoes significant structural changes that fundamentally alter its composition and characteristics. These thermal transformations break down complex organic structures that previously encapsulated Mg, creating a more porous and open matrix. As the structure becomes increasingly fragmented and permeable, Mg becomes more readily accessible and extractable. Consequently, the enhanced porosity facilitates improved availability and extraction of Mg, revealing more of the mineral that was previously locked within the intricate organic complexes. The same results have been obtained for Gliricidia sepium wood-biochar also[30].
These findings can help optimize the pyrolysis conditions to produce biochar with desirable nutrient profiles for specific applications, such as soil conditioning or fertilizer production.
Micronutrient composition of KCHW-biochar
-
The content of the total of different micronutrients and trace elements in the biochar obtained from KCHW at various pyrolytic temperatures are summarized in Table 3. It shows that the process temperature does not affect the total content of the micronutrient (Na, Fe, Cu, and Zn) and potentially toxic heavy metal ions (Pb, Ni) in the biochar prepared at the studied temperature range of pyrolysis (300−500 °C). On this basis, it can be concluded that the pyrolysis temperature within this range, may not influence the concentrations of the micronutrients and the trace elements in the biochar. Nonetheless, it must be pointed out that the solubility or the extent of leaching of these elements, elements which are more related with the bio-availability and possible effects on the soil and plant systems in the present investigation were not determined.
Table 3. Micronutrient composition of KCHW-biochar at different pyrolysis temperatures.
Pyrolysis
temperature
(°C)Total nutrient content Na (%) Fe (%) Cu (ppm) Zn (ppm) Pb (ppm) Ni (ppm) 300 0.69 0.59 20.56 82.47 1.85 14.45 400 0.98 0.21 17.37 92.33 0.49 14.16 500 1.04 0.40 19.66 75.24 0.56 16.51 p-value 0.142 0.078 0.724 0.377 0.154 0.442 CV 25.83 54.96 23.26 16.92 100.63 15.13 Nutrient composition of KCHW-ash
Macronutrient composition of KCHW-ash
-
When the pyrolysis temperature was raised, the total N content of KCHW-ash dropped dramatically, going from 2.27% at 400 °C to 0.77% at 600 °C (Table 4)[31]. As with the KCHW-biochar, an increase in the pyrolysis temperature might desirably result in a massive cut in the total N content of the KCHW-ash. This could be because of losses of nitrogen compounds at higher temperatures and relatively higher stabilities of N compounds in KCHW[21]. However, the available N content did not show a statistically significant difference across temperatures. Though N-containing compounds undergo structural changes at higher temperatures, these transformations convert labile (easily decomposable) nitrogen into more stable forms. Also, some N compounds become embedded within complex C structures. These structures act as a protective matrix, preventing nitrogen from being completely lost.
Table 4. Macro-nutrient composition of KCHW-ash at different pyrolysis temperatures.
Pyrolysis temperature (°C) Total nutrient content (%) Available nutrient content (%) N P K Ca Mg N P K Ca Mg 400 2.27a 1.58b 11.79b 2.29 1.30b 0.07 0.82b 8.01 1.13b 0.45 500 1.15b 2.36a 16.16a 3.11 1.78a 0.05 1.35ab 12.40 1.87a 0.41 600 0.77b 2.47a 15.49ab 2.19 1.71a 0.04 2.27a 10.60 1.46ab 0.15 p-value 0.000 0.003 0.027 0.229 0.016 0.381 0.042 0.154 0.010 0.150 CV 49.68 21.20 16.84 27.68 16.30 42.58 53.19 27.15 24.45 62.62 * In each column, means that do not share a letter differ significantly at p < 0.05. The total P content of KCHW-ash, got higher by elevating the degree of heating, from 1.58% at 400 °C to 2.47% at 600 °C (Table 4). The available P level followed a similar trend, increasing from 0.82% at 400 °C to 2.27% at 600 °C. The increased P content with rising temperature may be attributed to the higher reactivity and availability of phosphorus at higher temperatures. At higher temperatures, the reaction kinetics are more favorable for forming phosphorus compounds, leading to a higher total P content in the samples[32].
The total K content raised with elevated temperatures, from 11.79% at 400 °C to 16.16% at 500 °C, but the difference between 500 and 600 °C was not statistically significant in KCHW-ash (Table 4). Due to the volatilization of organic components at higher temperatures, a relative K concentration happens. Moreover, structural transformations in the ash matrix caused by higher temperatures, can release K from complex organic compounds and create more compact mineral structures potentially altering the chemical form of K. The available K content did not show a significant variation across temperatures in the present study. It suggests that thermal processing does not significantly affect K extractability. K may be bound in forms that are relatively resistant to temperature-induction. At 300 °C during manufacture, KCHW ashing revealed the highest K level (6.50%) at 300 °C[33].
Even though the total Ca content did not show a statistically significant difference across temperatures, the available Ca content increased considerably from 1.13% at 400 °C to 1.87% at 500 °C, but the difference between 500 and 600 °C was not statistically significant (Table 4). This suggests that higher temperatures enhance the availability or extractability of Ca in the ash. Both the total and available Mg content in KCHW-ash, increased with higher pyrolysis temperatures, from 1.30% (total) and 0.45% (available) at 400 °C to 1.78% (total) and 0.41% (available) at 500 °C, indicating that higher temperatures promote the concentration and availability of Mg in the ash. The differences were not statistically significant between 500 and 600 °C.
Table 5 presents data on the total content of various micronutrients and trace elements present in ash derived from KCHW at different treatments. The total Na, Fe, and Pb contents did not exhibit a statistically significant variation across the pyrolysis temperatures studied. However, the total Cu content, expressed in ppm (mg/kg), increased significantly with higher pyrolysis temperatures, from 63.34 ppm at 400 °C to 88.79 ppm at 600 °C. Other than that, the total Zn content, also expressed in ppm, showed a significant increase with higher temperatures, ranging from 197.91 ppm at 400 °C to 333.09 ppm at 500 °C. The increases in Cu and Zn are not about adding more metals, but about making existing trace metals more concentrated and accessible through thermal processing.
Table 5. Micronutrient composition of KCHW-ash at different pyrolysis temperatures.
Pyrolysis
temperature
(°C)Total nutrient content Na (%) Fe (%) Cu (ppm) Zn (ppm) Pb (ppm) Ni (ppm) 400 4.13 0.68 63.34b 197.91b 0.75 10.65b 500 5.79 0.88 88.31a 333.09a 2.29 17.47ab 600 5.39 1.57 88.79a 320.32a 1.36 21.74a p-value 0.082 0.132 0.024 0.008 0.157 0.048 CV 19.53 55.58 18.66 25.52 67.27 36.52 * In each column, means that do not share a letter differ significantly at p < 0.05. Proximate analysis of KCHW-biochar and KCHW-ash
-
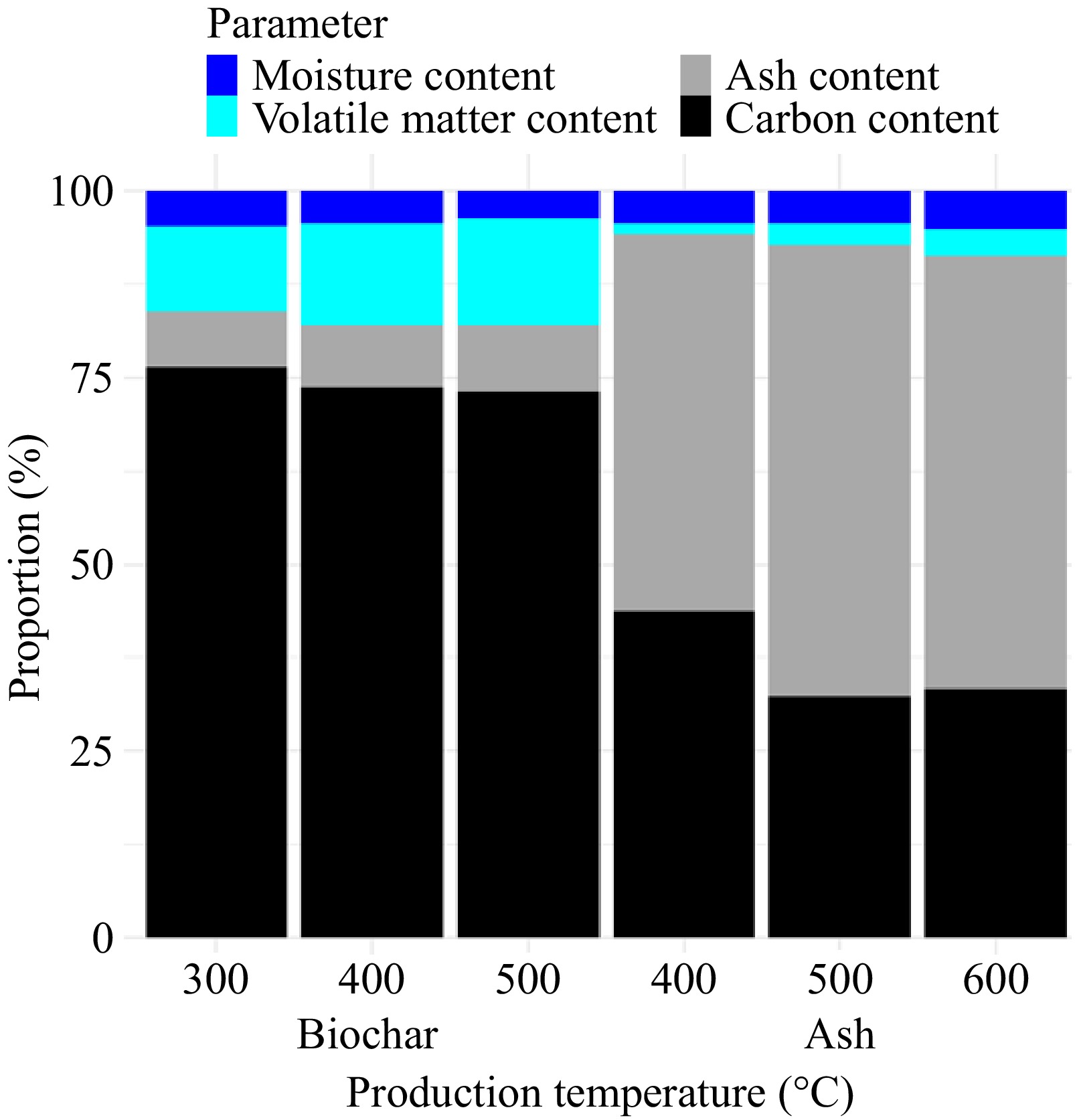
Proximate analysis for both KCHW-biochar and ash was carried out (Fig. 1). Pyrolysis temperature significantly affects the moisture content of the biochar, with higher temperatures resulting in lower moisture content. This is likely due to increased water evaporation at higher temperatures[34]. Volatile matter, ash content and fixed carbon percentages do not show statistically significant changes across the temperature range studied. This suggests that these properties are relatively stable within this temperature range. The high percentage of fixed carbon (> 70% across all temperatures) indicates that biochar has good potential for carbon sequestration and stability[35]. As confirmed by previous literature, cellulose primarily decomposes into volatile compounds, whereas lignin contributes more significantly to the formation of fixed carbon in the resulting product[36]. The volatiles mainly contain CO, CO2, H2, CH4, N2, and other gaseous carbohydrates. The low ash content (< 9% across all temperatures) suggests that the biochar may have minimal impact on soil mineral content when used as an amendment[37]. The ash content indirectly gives an idea about its alkaline properties[36].
When considering the KCHW-ash, pyrolysis temperature does not significantly affect the moisture content and volatile matter of the ash within the studied range. Ash content shows a trend of increasing with temperature, but the differences are not statistically significant. This suggests that the inorganic matter concentration tends to increase at higher temperatures, possibly due to the loss of organic compounds. Production temperature has a major impact on fixed carbon content, with a maximum of 400 °C and decreased contents at higher degrees. This suggests that higher temperatures cause a greater amount of carbon to be lost, most likely as a result of enhanced oxidation and volatilization. The material appears to be rich in inorganic compounds, which could be advantageous if applied as a soil amendment to supply minerals, based on the high ash content (> 50% at all temperatures)[38].
-
Producing biochar and ash from immature KCHW is a safe approach for the management of agricultural waste. It has the potential of an effective soil conditioner that contributes to improving coconut yield. This study demonstrates that the pyrolysis temperature affects the qualitative and quantitative characteristics of the obtained products as well as their nutritional value. For biochar, higher pyrolysis temperatures led to decreased total N content but increased available K, Ca, and Mg. The high fixed carbon content of biochar (> 70%) suggests excellent potential for carbon sequestration and soil amendment applications. Similar patterns in the nutrient composition variations with increasing pyrolysis temperature were seen in KCHW ash. While higher temperatures (500 °C for biochar and 600 °C for ash) generally show improved availability of certain nutrients, the optimal temperature is not universally applicable. The selection of the most suitable pyrolysis temperature should be guided by the nutrient needs of the target crop, existing soil nutrient profile, and specific deficiencies in the agricultural system.
Results of this study revealed that producing soil conditioners from immature KCHW resolves concerns about the safe disposal of agricultural wastes while promoting ecological coconut farming. Through pyrolysis, the production of biochar and ash and its application contributed to the recycling of soil nutrients as well as the improvement of the soil fertility of the soils of the coconut-based farming system. Since these soil conditioners are time and cost-effective, future research should focus on field experiments to determine the long-term effects of these conditioners on the health and productivity of the coconut plantations. In addition, economic and environmental effects assessment could be useful in determining the feasibility on an increased scale of applying this kind of waste valuation approach. This strategy is in harmony with the ideas of the circular economy and can contribute greatly to the further development of even more eco-friendly ways of coconut cultivation.
-
The authors confirm contribution to the paper as follows: study conceptualization: Dissanayaka NS, Rajaratnam K, Atapattu AJ; methodology: Udumann SS, Jayamali A, Gayadari MP, Gunarathna NK, Shamila SK; software: Atapattu AJ; validation: Dissanayaka NS, Udumann SS, Atapattu AJ; formal analysis: Udumann SS; investigation: Shamila SK; resources: Shamila SK; data curation: Nuwarapaksha TD, Dissanayaka NS, Udumann SS; writing—original draft preparation: Dissanayaka NS, Rajaratnam K; writing—review and editing: Udumann SS, Shamila SK; visualization: Nuwarapaksha TD, Udumann SS; supervision: Atapattu AJ; project administration: Atapattu AJ. All authors reviewed the results and approved the final version of the manuscript.
-
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
This research received no external funding. We would like to express our appreciation to the technical personnel from the Agronomy Division at the Coconut Research Institute.
-
The authors declare that they have no conflict of interest.
- Copyright: © 2025 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Dissanayaka NS, Rajaratnam K, Udumann SS, Nuwarapaksha TD, Shamila SK, et al. 2025. Evaluation of the nutritional composition of king coconut husk waste biochar and ash soil conditioners: a comprehensive analysis. Technology in Agronomy 5: e003 doi: 10.48130/tia-0024-0034
Evaluation of the nutritional composition of king coconut husk waste biochar and ash soil conditioners: a comprehensive analysis
- Received: 27 August 2024
- Revised: 05 December 2024
- Accepted: 10 December 2024
- Published online: 13 February 2025
Abstract: An enormous amount of immature king coconut husk waste (KCHW) has accumulated as a result of the rising demand for king coconut water worldwide, creating environmental problems. This study investigates the potential of KCHW as a sustainable resource for producing soil conditioners through pyrolysis. Biochar, produced at 300, 400, and 500 °C for 1 h, and ash, produced at 400, 500, and 600 °C for 4 h, analyzed for their nutritional composition and physical properties. The results revealed that increasing pyrolysis temperature significantly influenced nutrient profiles, leading to higher available potassium, calcium, and magnesium concentrations. Biochar exhibited high fixed carbon content (> 70%), indicating potential for carbon sequestration, while ash samples showed high inorganic matter content (> 50%), suggesting value as a mineral-rich amendment. In conclusion, this study confirms biochar's potential to enhance available potassium, calcium, and magnesium levels in soil. The study underscores the critical importance of systematically refining pyrolysis parameters to develop biochar with optimal nutritional characteristics that precisely match specific soil nutrient deficiencies. It provided insights for devising future sustainable waste management approaches for coconut industries and seemingly made suggestions for the enhancement of the state of the plantation soil for coconut production.