-
Plant growth is seriously affected by abiotic stresses such as drought, low temperature and soil salinity. Drought is of particular concern in view of the predicted consequences of global climate change[1]. Severe episodes of drought stress lead to a shut down of photosynthesis, disturb the plant's core metabolism and can lead to plant death[2]. Plants have evolved a range of strategies, such as physical (leaf surface morphology), biochemical adaption and transcriptional reprograming, to combat drought stress[3,4].
In the process of long-term evolution, plants have formed a series of physical defenses with their own organizational structures to resist the damage of the external environment, such as trichome and waxy cuticles[5]. Trichomes are hairy appendages on the surface of plants, which protect plant tissues from insects and ultraviolet (UV), and increase the tolerance of plants to drought stress[6]. The development of the cuticle, comprising a lipid layer (cutin) intermeshed and coated with wax, is one of the major adaptations for withstanding short term drought stress[7]. The cutin molecule is composed of cross-linked C16 and C18 ω-hydroxyl fatty acids, while the wax is a complex mixture of long-chain fatty acids and their derived alcohols, aldehydes, alkanes, ketones, and wax esters[8,9]. An increased deposition of cuticular wax has been associated with higher levels of drought tolerance in both rice and Arabidopsis thaliana[10,11]. Under drought conditions, the phytohormone abscisic acid (ABA), a key regulator of leaf stomatal conductance, is triggered[12,13]. Due to increase of ABA level under drought, the guard cell forcibly closes the stomata to reduce transpirational water loss, and inhibits photosynthesis by preventing the entry of CO2[2,14,15]. At the same time, the shrinkage in cell volume caused by water shortage increases the viscosity of the cellular content, hindering normal enzymatic function as a consequence[16]. A drought stress-induced loss in photosynthetic activity can also generate oxidative stress on account of the build-up of reactive oxygen species (ROS)[17,18]. Under normal conditions, plants scavenge ROS by a range of enzymatic and non-enzymatic means[19]. The capacity to neutralize ROS has been associated with the level of drought tolerance in a number of plant species[20−22]. Some plant species also show a pronounced capacity to adjust the cellular osmotic environment in response to drought stress by accumulating highly soluble non-toxic compounds such as sugars (sucrose, trehalose and sorbitol), free amino acids (proline) and amines (glycine betaine and polyamines)[23,24].
The ornamental species chrysanthemum (Chrysanthemum morifolium) is widely appreciated as a source of cut flowers and pot plants. Most chrysanthemum cultivars are very vulnerable to drought stress, but some of the wild relatives of C. morifolium have been identified as important reservoirs of genetic variation relevant for drought tolerance improvement[25,26]. Since the physiological response of Chrysanthemum spp. to drought stress is poorly understood, we set out to study leaf surface morphology and the response of key antioxidant enzymes, photosynthesis and endogenous levels of ABA to artificially induced drought stress in C. nankingense and C. japonense, two species characterized by a differential level of drought tolerance.
-
The accessions of C. nankingense (drought-sensitive) and C. japonense (drought-tolerant) were obtained from the Chrysanthemum Germplasm Resource Preserving Centre, Nanjing Agricultural University, China. Rooted cuttings (six leaf stage) were grown hydroponically in Hoagland solution (pH 5.8) under a 12 h photoperiod (300 µmol·m−2·s−1 photosynthetically active radiation), a relative humidity of 70% and a day/night temperature of 25/20 °C. The material was acclimated to these conditions for six days before the imposition of polyethylene glycol (PEG)-induced drought stress. Following the method of Zhang et al.[27], the plants were transferred for two, four, six, eight or 10 h into a solution of 20% w/v PEG 6000 dissolved in Hoagland, generating a potential of ~ −0.52 MPa. Control plants were retained in half strength Hoagland's solution (−0.01 MPa). The experiment was set out as a completely randomized split-plot with three replications (six plants per species per replication). The physiological and biochemical assays were conducted on the third or fourth leaves below the apex of the shoot.
Water status
-
Leaf wilting was rated visually on a scale of zero (no observable wilting) to five (severely wilted)[27]. The relative water content (RWC) of leaves was estimated following the methods of Galmés et al.[28]. Each data point represented the mean of three independent leaves.
Characterization of the leaf surface and its cuticular wax
-
The morphology of the leaf surface was observed by scanning electron microscopy, according to the methods of He
et al.[29]. To calculate the density of trichome and stomata, each sample was observed under six different scope visual fields. Cuticular waxes were extracted from 0.2 g fully expanded leaves by incubating in 10 ml chloroform for 30 s at room temperature. An internal standard was provided by adding 5 µg n-tetracosane (C24) to each sample. The solvent was evaporated under a mild nitrogen stream, then redissolved in a mixture of 100 μl pyridine, 100 μl bis-N,N-(trimethylsilyl)-trifluoroacetamide (Macherey-Nagal, Düren, Germany). After heating at 70 °C for 1 h, the solvent was evaporated again under nitrogen and the samples redissolved in 200 μl chloroform. Qualitative and quantitative composition analyses followed the methods of Lee et al.[30]. A 1 μl aliquot was separated by GC–MS (Agilent 7890A-5975C, USA) and quantification was based on the internal standard. Enzyme assays
-
Leaf samples were stored at −80 °C after quick freezing in liquid nitrogen. The frozen leaf segments (0.25 g) were ground to a powder in liquid nitrogen, and soluble protein was extracted by homogenization in 1 ml 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA and 1% w/v polyvinyl pyrrolidone 40. The supernatant of centrifuged homogenate (12,000 g, 15 min, 4 °C) is directly used for subsequent enzyme analysis. Total protein content was determined according to the Bradford dye-binding method[31]. Superoxide dismutase (SOD) activity assay was performed following the method of Giannopolitis & Ries[32] with minor modifications. Each 3 ml reaction mixture (50 mM potassium phosphate buffer (pH 7.8), 13 mM L-methionine, 75 μM nitroblue tetrazolium (NBT), 2 μM riboflavin, 1 mM EDTA and 100 μl supernatant) was illuminated for 10 min in white fluorescent light (100 µmol·m−2·s−1). Then the SOD activity was measured at 560 nm. Peroxidase (POD) activity was measured by monitoring the increase in absorbance at 470 nm caused by the oxidation of guaiacol, which was slightly modified according to the method of Li[33]. Each 3 ml reaction was initiated by adding 20 μl 40 mM H2O2 into 2.9 ml 50 mM phosphate buffered saline (PBS) (pH 7.0), 50 μl 20 mM guaiacol and 30 μl supernatant. PBS was used as blank control instead of supernatant. The catalase (CAT) assay was based on method of Beers & Sizer[34] with minor modifications. Each 3 ml reaction was initiated by adding 50 mM potassium phosphate buffer (pH 7.0), 15 mM H2O2 and 100 μl supernatant. Ascorbate peroxidase (APX) activity was assayed following the method of Nakano & Asada[35] with minor modifications. Each 3 ml reaction was initiated by adding 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbate, 0.1 mM H2O2 and 100 μl supernatant.
Cell membrane stability, malondialdehyde (MDA) and free profine content
-
Cell membrane stability was determined by measuring electrolyte leakage (EL). Following the method of Hu et al.[36], whole fully expanded leaves were sliced and incubated in 10 ml distilled deionized water on a shaker for 24 h. The conductance of the solution at 24 h was taken as the initial level (Ci). Thereafter, the material heated to 100 °C for 10 min, and the conductance of the solution (Cmax) was determined again. The EL was calculated by the expression (Ci/Cmax) × 100%. For lipid peroxidation analysis, the MDA content was measured using the thiobarbituric acid (TBA) method described by Heath & Packer[37] with minor modifications. Fresh leaf tissue (0.5 g) was ground and extracted in 5 ml 5% w/v trichloroacetic acid (TCA). The homogenate was centrifuged (12,000 g, 5 min), and 2 ml of the supernatant was added to 2 ml 0.67% w/v TBA (prepared in 10% v/v TCA). The mixture was rapidly cooled after heating to 100°C for 30 min, and centrifuged (12,000 g, 10 min). The absorbance of the supernatant was monitored at 532 nm. Correction of non-specific turbidity was obtained by subtracting the absorbance value taken at 600 nm. The level of lipid peroxidation was expressed as nmol per g fresh weight. Free proline was extracted and determined as described by Bates et al.[38] with minor modifications.
Chlorophyll content and photosynthesis related parameters
-
Chlorophyll (0.1 g) was extracted in 95% ethanol for 48 h and the absorbance of the supernatant detected at 470, 649 and 665 nm. The quantity of total chlorophyll (a + b) was determined as described by Li[33]. The net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) of fully expanded leaves were monitored using a LI-COR 6400 portable photosynthesis system (LI-COR, Lincoln, NE, USA). The CO2 concentration in the chamber was 380 ± 10 µmol/mol and a photosynthetic photon flux density of 1000 µmol·m−2·s−1 at the leaf surface was provided by an LED red-blue light source (LI-COR 6400-02). The maximum quantum efficiency of PSII photochemistry (Fv/Fm) was determined in the same intact leaves according to the method of Liu et al.[39]. For each treatment, Pn, Gs, Tr, Ci and Fv/Fm values were obtained from five leaves at each time point.
Endogenous ABA level
-
Frozen leaf (~1 g fresh weight) was ground in liquid nitrogen and homogenized for 12 h in 10 ml pre-cooled 80% v/v aqueous methanol under low light. The mixture was centrifuged (12,000 g, 4 °C, 10 min) and the pellet extracted twice in 10 ml 80% methanol at 4 °C under low light. The supernatant was filtered through a Sep-Pak C18 gel cartridge and freeze dried. The lyophilisate was redissolved in 1 ml methanol and passed through a 0.45 μm filter. Quantification of ABA was conducted by high performance liquid chromatography (HPLC) (Agilent Technologies 1100) as described by Ciha et al.[40] with minor modifications. The separation column was supplied by Agilent (HC-C18, 5 μm, 250 mm × 4.6 mm). The solvents were 0.6% v/v glacial acetic acid (A) and 100% methanol (B); the initial solvent was 100% A, moving to 50% A, 50% B over the subsequent 10 min, where it was held for 20 min. The solvent flow rate was 1 ml/min, the detection wavelength 254 nm and the column temperature 30 ± 0.2 °C. Quantification was based on calibration with known ABA standards (Sigma-Aldrich Chemie, Munich, Germany).
Statistical analysis
-
All data are mean ± standard deviation (SD). IBM SPSS Statistics 17.0 software and Microsoft Excel 2007 was used for statistical analysis. A one-way analysis of variance, followed by Duncan’s multiple range test (with P set at 0.05/0.01), was employed to assess whether treatment means differed significantly from one another.
-
The wilting index of unstressed plants was zero, and the stress induced wilting in both species (Fig. 1a). After 2 h of stress, the wilting index of C. japonense rose to one, and the lower leaves had wilted and had begun to droop (Y1 in Fig. 1a). Wilting set in earlier and was more severe in C. nankingense. By 2 h, its wilting index had already reached two, and its lower leaves were wilted and drooping (N1 in Fig. 1a); after 10 h, the wilting index was five and all the leaves appeared dehydrated and withered (N5 in Fig. 1a). At this stage, the wilting index of C. japonense was still only three and its uppermost leaves remained turgid (Y5 in Fig. 1a).
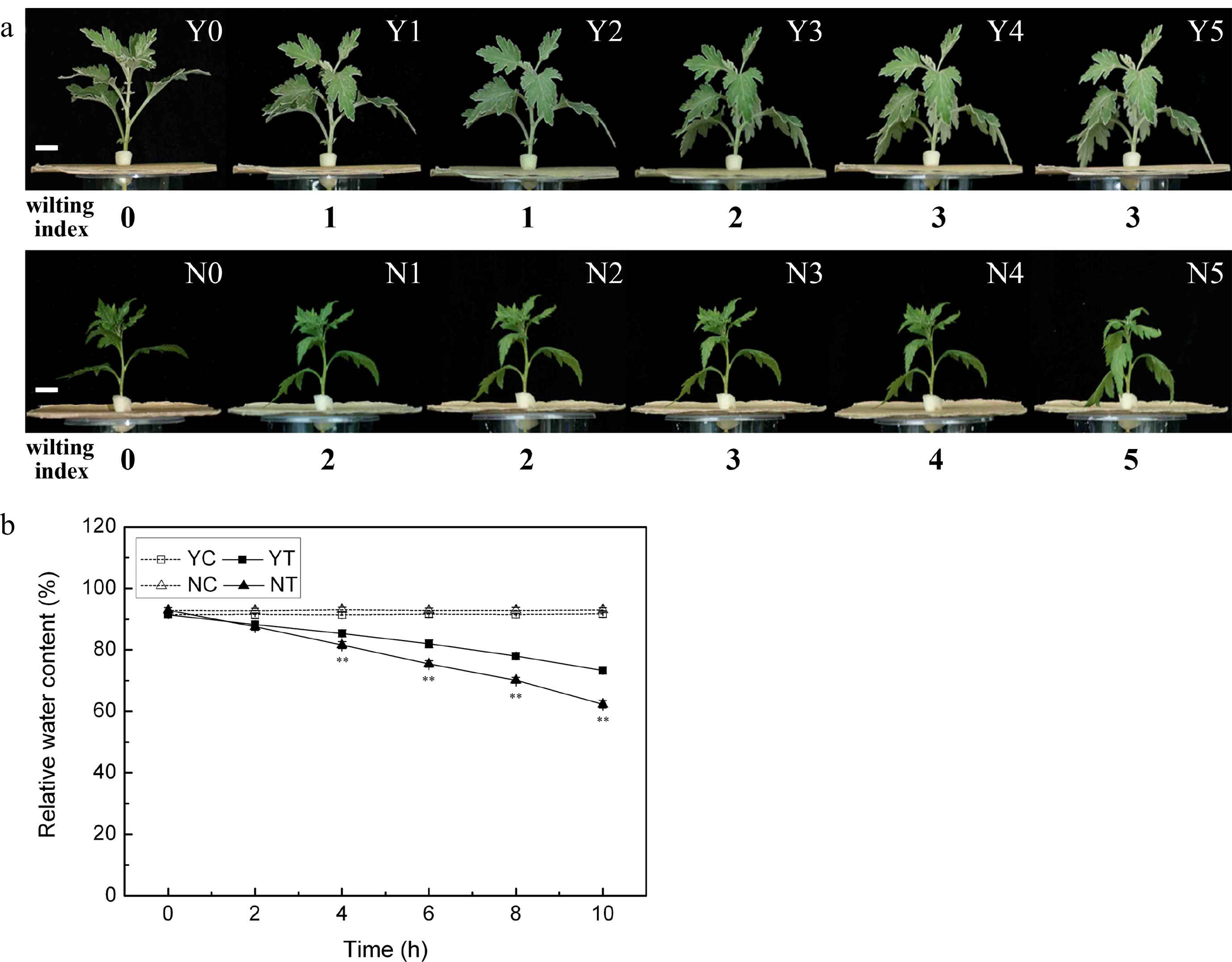
Figure 1.
The response of C. japonense and C. nankingense to PEG-induced drought stress. (a) The morphological response of C. japonense and C. nankingense to PEG-induced drought stress. Y0-Y5, N0-N5: C. japonense (Y) and C. nankingense (N) plants subjected to, respectively, 0, 2, 4, 6, 8 and 10 h of stress. The wilting index ranges from 0 (no observable wilting) to 5 (severely wilted). Scale bars = 1 cm. (b) The response of leaf RWC to PEG-induced drought stress. Y: C. japonense, N: C. nankingense, C: Control (no PEG), T: PEG treatment. ** Value significant at P ≤ 0.01. Values given as mean ± SD (n = 3).
The RWC of both species was maintained at the same level under non-stressed conditions (Fig. 1b), but declined markedly as a result of the stress treatment. The decline was more acute in C. nankingense than in C. japonense. The RWC in the leaf of the latter was significantly higher than in the former after only 4 h of PEG treatment, while after a 10 h exposure, the RWCs had fallen to, respectively, 62.3% and 73.3%.
Leaf surface morphology
-
A marked difference in the appearance of the leaf surface was observed between the two species. The trichome density on the upper and lower leaf surface of the C. nankingense leaf was low (0.10 and 1.79 per mm2 respectively) (Table 1), while in contrast, C. japonense developed many trichomes especially on the lower leaf surface - the density on the upper leaf surface was 33.45 per mm2, while that on the lower surface was too high to count. The abundance of trichomes prevented the measurement of stomatal density, but on the upper leaf surface, stomatal density in the C. japonense was significantly greater than on the equivalent C. nankingense leaf surface (76.57 vs 11.96 per mm2, respectively) (Table 1), and the C. japonense gland cells were larger than those on the C. nankingense leaf (Fig. 2d, h).
Table 1. Variation in leaf surface morphology in C. japonense and C. nankingense.
Species Upper epidermis of leaf Lower epidermis of leaf Trichome density (mm−2) Stoma density (mm−2) Trichome density (mm−2) Stoma density (mm−2) C. japonense 33.45 ± 1.46A 76.57 ± 11.72 A ∞ N C. nankingense 0.11 ± 0.12B 11.96 ± 10.81B 1.79 ± 0.47 346.94 ± 24.73 Values (given as mean ± SD) labeled with a different letters differed significantly (P ≤ 0.01) (n = 6). ∞ means too much to calculate. N means unable to observe because of the well-developed trichome layer covering lower epidermis of leaf. 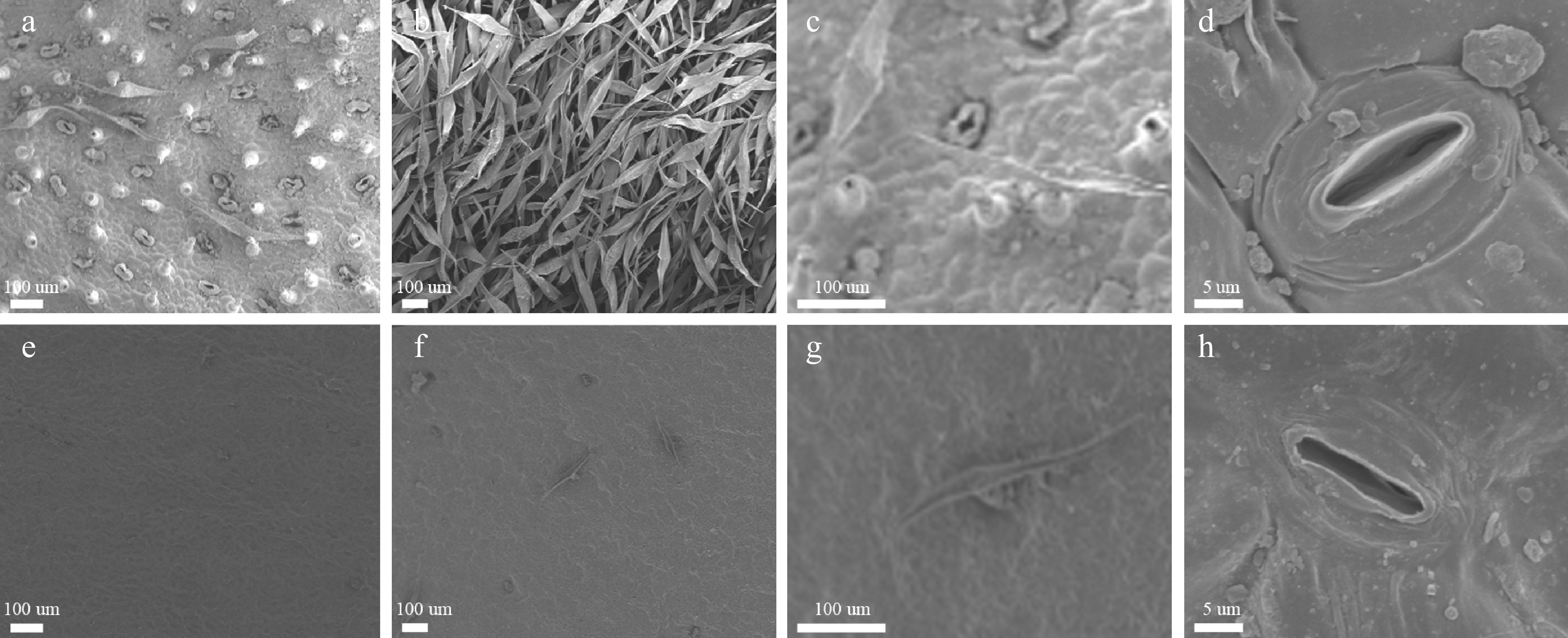
Figure 2.
Scanning electron microscopic images of the leaf surface of C. japonense (a-d) and C. nankingense (e-h). (a) and (e): upper leaf surface, (b) and (f): lower leaf surface, (c) and (g): a single trichome, (d) and (h): a single stomate.
Cuticular wax amount and composition
-
The total wax load on the C. japonense leaf was ~6.6 fold greater than on the C. nankingense leaf (Fig. 3a). There was also a significant difference between the species for cuticular wax composition. Fatty alcohols (include primary alcohols and secondary alcohols) were the predominant component (39.9%) of the C. japonense leaf wax, followed by esters (33.1%), alkanes (21.3%) and fatty acids (5.7%). In C. nankingense, fatty alcohols were even more predominant (49.8%), while the remainder was composed of alkanes (35.4%) and esters (14.8%). The level of fatty acids in C. nankingense cuticular wax was below the level of detection. Nine components were specific to the cuticular wax of C. japonense, namely C20 and C24 fatty acids, C14, C22 and C24 primary alcohols, and C16, C17, C31 and C32 esters. A C20 ester was the only component specific for C. nankingense. Eight components were shared: C26 and C28 primary alcohols, C30 secondary alcohol, C17, C24 and C32 alkanes, C30 ester (although its content was greater in C. japonense) and C30 primary alcohol (the content of this component was greater in C. nankingense) (Fig. 3b).
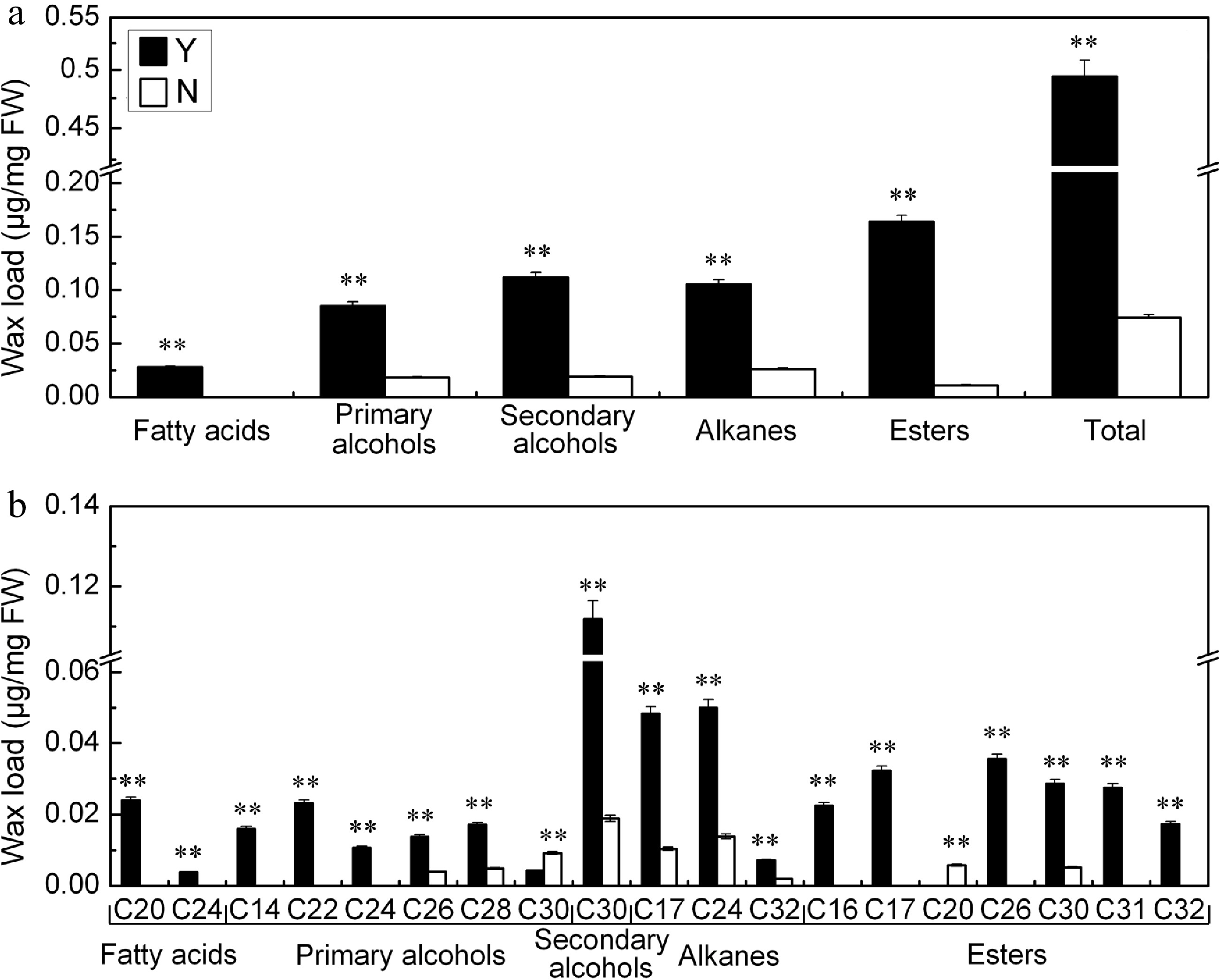
Figure 3.
(a) Quantity and (b) composition of cuticular wax on the C. japonense (Y) and C. nankingense (N) leaf. ** Value significant at P ≤ 0.01. Bars indicate the SD of the mean (n = 3).
Antioxidant enzyme activity
-
The PEG treatment enhanced the activity of SOD, POD, CAT, and APX in both species. SOD activity was greater in C. japonense than in C. nankingense throughout the stress treatment (Fig. 4a). In C. japonense, it rose to 2.0 fold its background level after 8 h exposure and to 1.6 fold after 10 h, while in C. nankingense, the equivalent levels were 1.1 and 1.2 fold. POD activity tended to be greater in C. nankingense, although after 4 h of treatment it reached 1.9 fold of the background level in C. japonense, representing 1.3 fold the C. nankingense level (Fig. 4b). The background level of CAT activity was higher in C. nankingense than in C. japonense. In response to PEG treatment, it increased markedly in both species (Fig. 4c), reaching 1.3 and 1.6 fold of the background level in C. nankingense after, respectively, 2 h and 4 h of treatment. In C. japonense, CAT activity rose to 1.2 and 1.8 fold of the background after 2 h and 4 h of treatment, respectively. After 6 h of exposure, activity had risen to 1.4 (C. nankingense) and 2.0 (C. japonense) fold of the background level, although these levels were not statistically different from one another. As the stress was prolonged, CAT activity in C. japonense rose to nearly two fold the background level, but in C. nankingense, the increase was much more modest. APX activity was also greater in C. nankingense than in C. japonense under non-stressed conditions (Fig. 4d). The PEG treatment rapidly induced APX activity in C. nankingense,while that in C. japonense increased slowly. APX activity in C. nankingense reached 1.7 fold of background by 8 h, and 1.4 fold by 10 h, while in C. japonense, the equivalent levels were 3.0 fold and 3.3 fold.
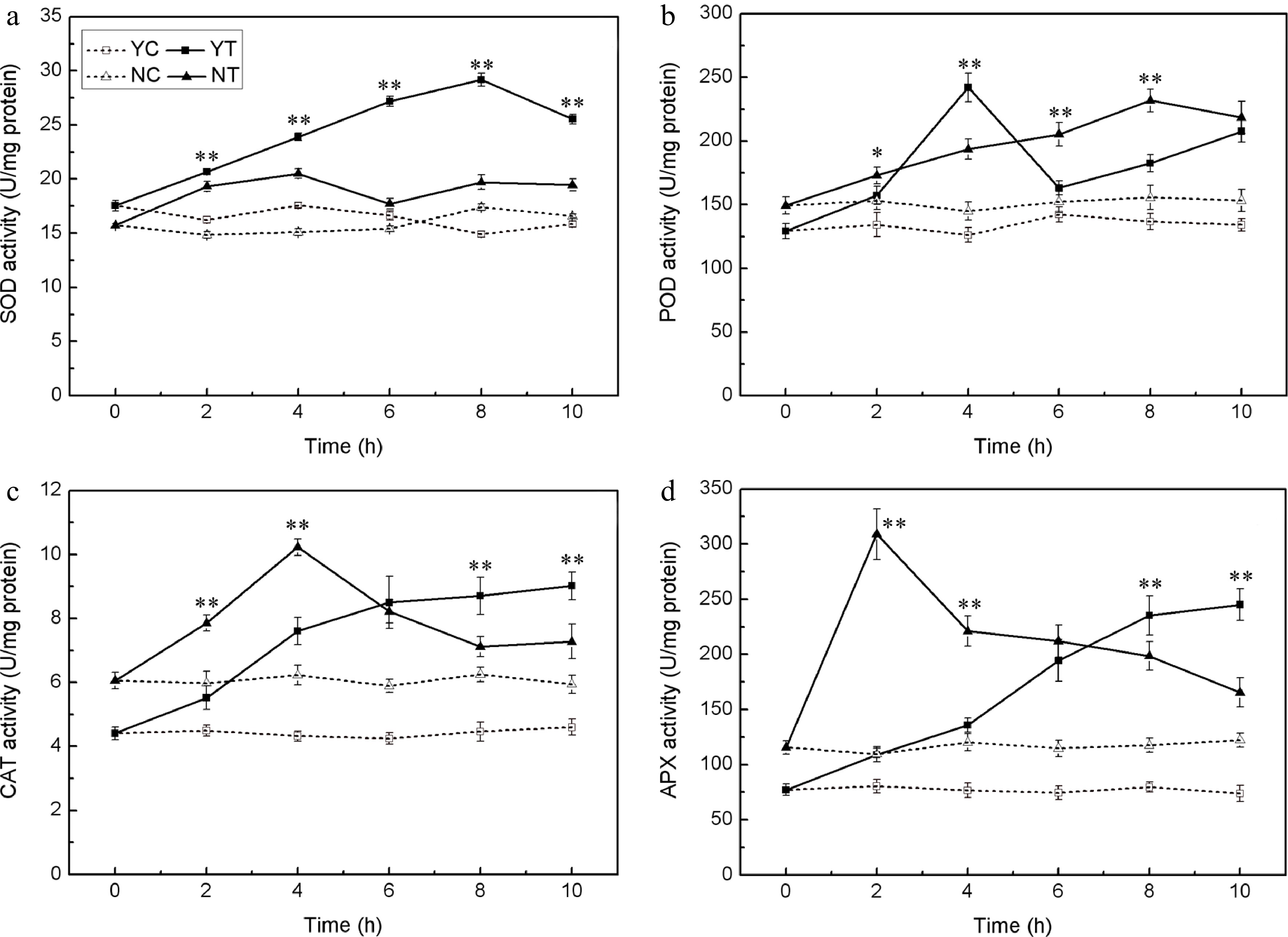
Figure 4.
Enzymatic activity (SOD (a), POD (b), CAT (c), and APX (d)) in the leaf of droughted C. japonense (Y) and C. nankingense (N) plants. C: Control (no PEG), T: PEG treatment. *, ** Value significant at P ≤ 0.05 or 0.01. Values given as mean ± SD (n = 3). SD’s indicated by a bar.
EL and MDA content
-
Under control conditions, EL was maintained at a constant low level in both species (Fig. 5a). However, when subjected to PEG treatment, it increased as the time of exposure was lengthened. The C. nankingense EL was significantly higher than that of C. japonense throughout the whole period. By the end of the stress period treatment, it had reached 3.4 fold the control level in C. japonense and 3.8 fold in C. nankingense. The leaf MDA content behaved in a similar fashion (Fig. 5b), increasing in both species as the plants were exposed to stress. The increase set in earlier and was more pronounced in C. nankingense. After 2 h, the MDA content in the C. japonense leaf was no different from the background level, while in C. nankingense it had risen by 1.3 fold. By the end of the stress treatment, the MDA content of the C. japonense and C. nankingense leaves were, respectively 1.7 and 2.7 fold that of the non-stressed controls, indicating that the membrane lipid of C. nankingense was highly peroxidized and the cell membranes system seriously damaged.
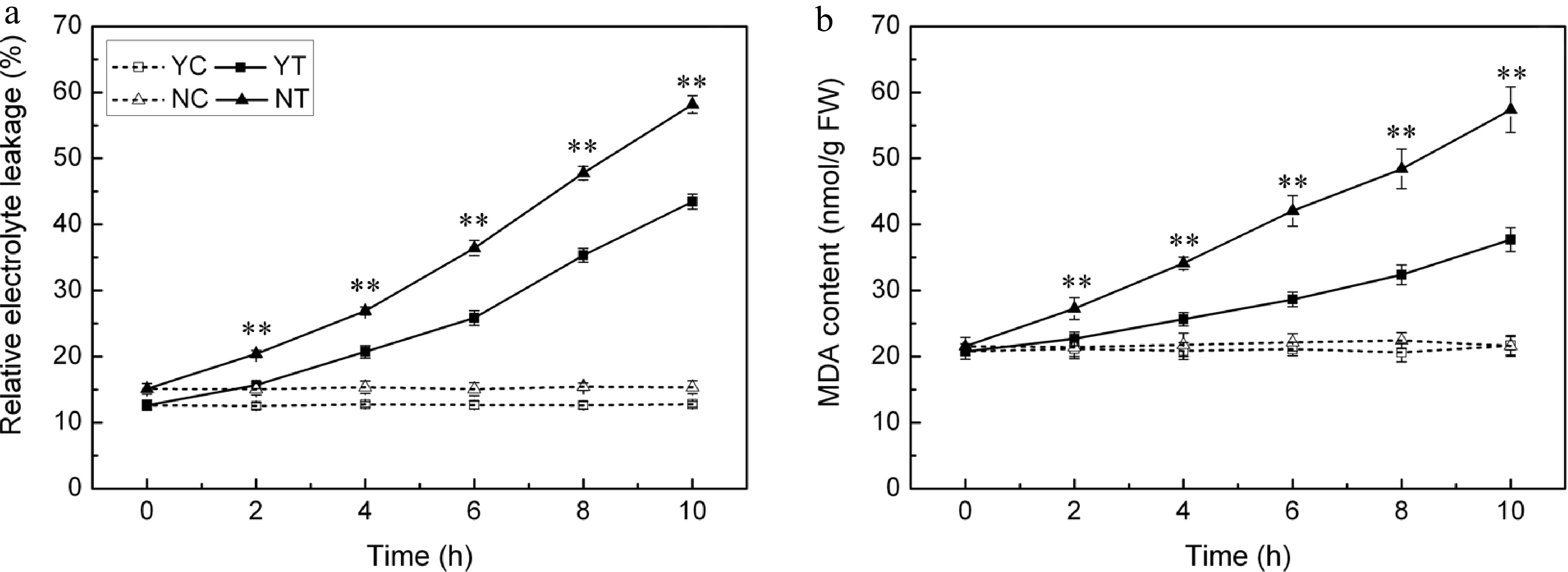
Figure 5.
(a) Electrolyte leakage and (b) MDA content in droughted leaves of C. japonense (Y) and C. nankingense (N). C: Control (no PEG), T: PEG treatment. ** Value significant at P ≤ 0.01. Values given as mean ± SD (n = 3).
Free proline content
-
The accumulation of proline was negligible under control conditions, but the PEG treatment induced a significant accumulation in proline. C. japonense responded to water deficient stress more quickly, and accumulated more proline than C. nankingense (Fig. 6). The proline content in the C. japonense leaf was 1.6 and 2.4 fold of background at 2 h and 4 h respectively, and the corresponding levels were 1.2 and 1.6 fold in C. nankingense. The proline level in the C. japonense leaf was higher than that in the C. nankingense leaf throughout the stress treatment.
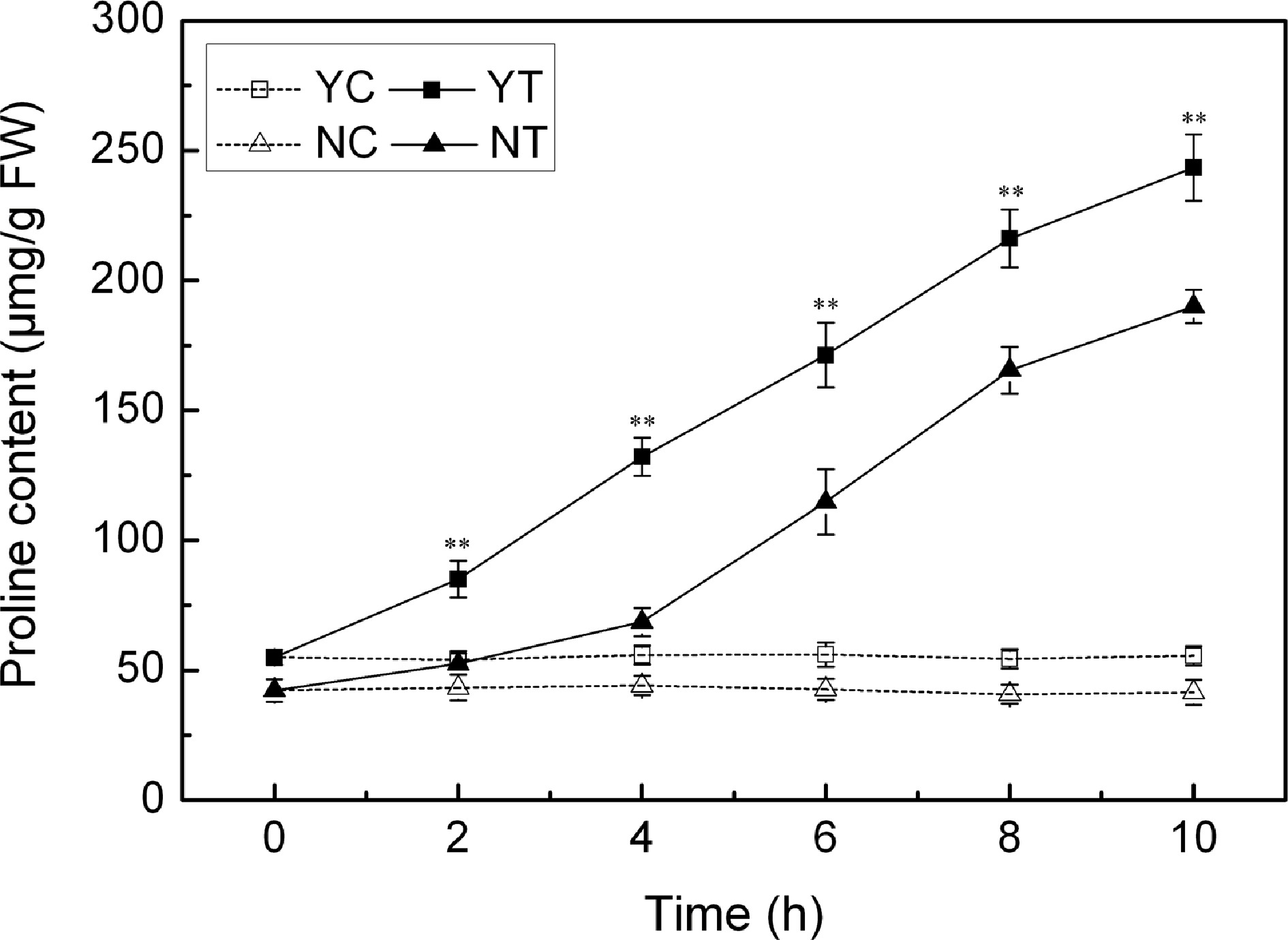
Figure 6.
Free proline content in droughted leaves of C. japonense (Y) and C. nankingense (N). C: Control (no PEG), T: PEG treatment. ** Value significant at P ≤ 0.01. Values given as mean ± SD (n = 3). SD's indicated by a bar.
The effect of drought stress on photosynthetic parameters
-
Pn, Gs, Tr, Fv/Fm and chlorophyll content were negatively affected by drought stress in both species, while the Ci parameter increased. The background level of Pn in C. nankingense was ~8.7 μmol CO2 m−2·s−1, somewhat higher than in C. japonense. In plants subjected to stress, this parameter decreased more sharply in C. nankingense than in C. japonense (Fig. 7a). By 2 h, it had fallen to 0.7 (C. nankingense) and 0.9 (C. japonense) fold of the control, and remained higher in C. japonense than in C. nankingense during the rest of the treatment. By 10 h, it had fallen to 0.1 fold in C. japonense and close to zero in C. nankingense. Gs behaved in a similar way. It decreased more rapidly in C. nankingense than in C. japonense (Fig. 7b), and over the period 6−10 h, remained higher in C. japonense than in C. nankingense. Tr followed the same pattern. Under control conditions, it was higher in C. nankingense than in C. japonense (Fig. 7c), after 2 h of stress it had fallen to 0.8 fold the background in both species. As the stress was prolonged, Tr fell in C. nankingense to 0.6 (4 h), 0.3 (6 h) and 0.1 (8 h) fold of the background level, and in C. japonense to, respectively, 0.6, 0.4 and 0.3 fold at these time points. Under control conditions, the Ci of C. japonense was higher than that of C. nankingense. It increased significantly in C. nankingense in response to PEG treatment (Fig. 7d). In contrast, in C. japonense, it fell very slightly over the first four hours of stress, only rising above the background level thereafter. Its level was higher in C. nankingense than in C. japonense throughout the stress treatment. Under control conditions, the Fv/Fm ratio remained stable at > 0.8 (Fig. 7e); exposure to PEG stress had a negative effect on both species, particularly on C. nankingense. By the end of the treatment, the Fv/Fm of C. nankingense and C. japonense were, respectively 0.5 and 0.7 fold that of the background. Under control conditions, the chlorophyll content of the leaves of C. nankingense was significantly higher than in those of C. japonense, but it decreased more quickly in C. nankingense than in C. japonense when the plants were exposed to PEG treatment (Fig. 7f). By the end of the stress treatment, the chlorophyll content of C. nankingense was 0.6 fold and that of C. japonense was 0.8 fold the initial levels, and the chlorophyll content of C. japonense was significantly higher than that of C. nankingense.
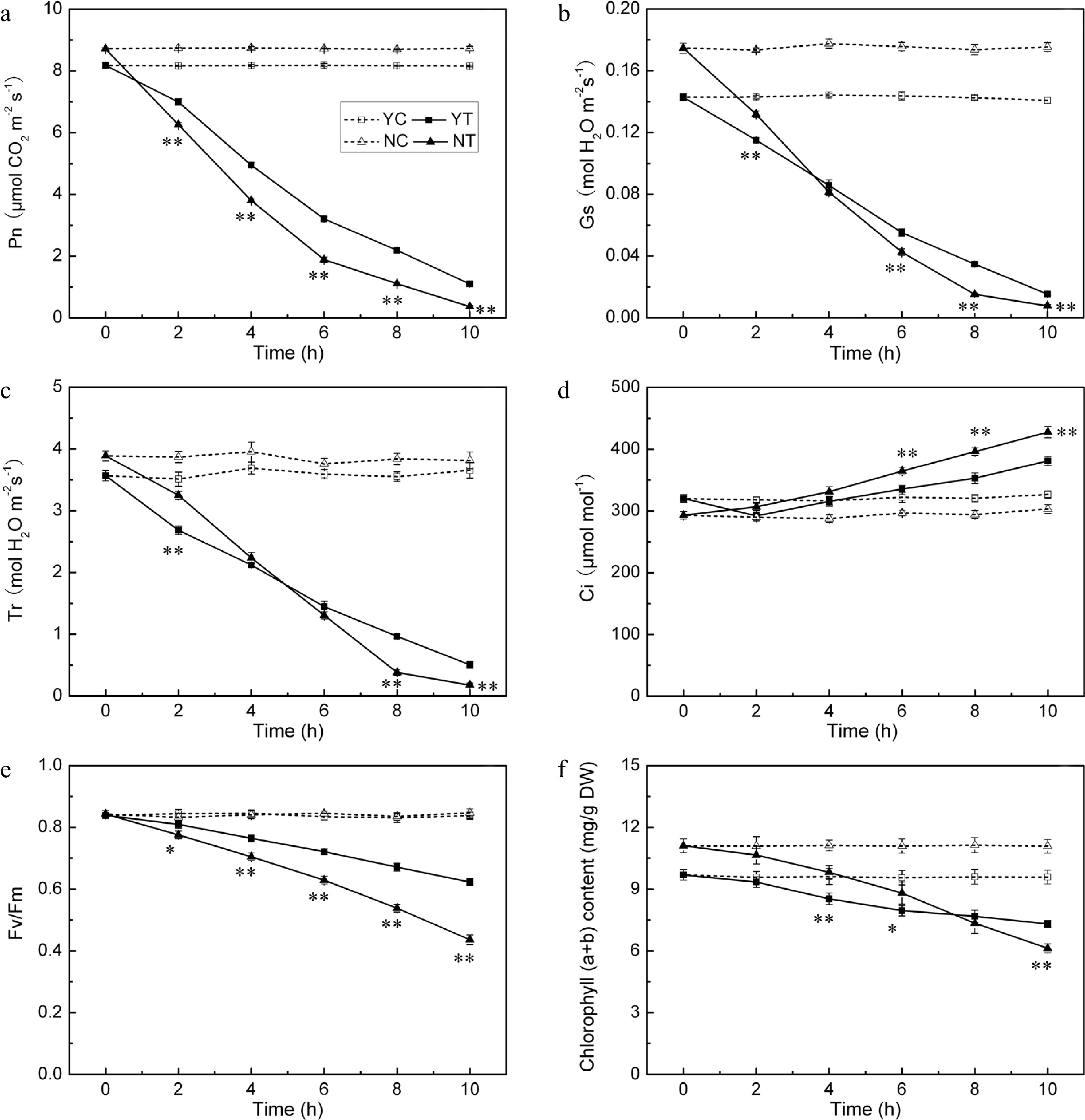
Figure 7.
Photosynthetic parameters (Pn (a), Gs (b), Tr (c), Ci (d), Fv/Fm (e) and chlorophyll (a + b) content (f)) in the droughted leaves of C. japonense (Y) and C. nankingense (N). C: Control (no PEG), T: PEG treatment. *, ** Value significant at P ≤ 0.05 or 0.01. Values given as mean ± SD (n = 5). SD’s indicated by a bar.
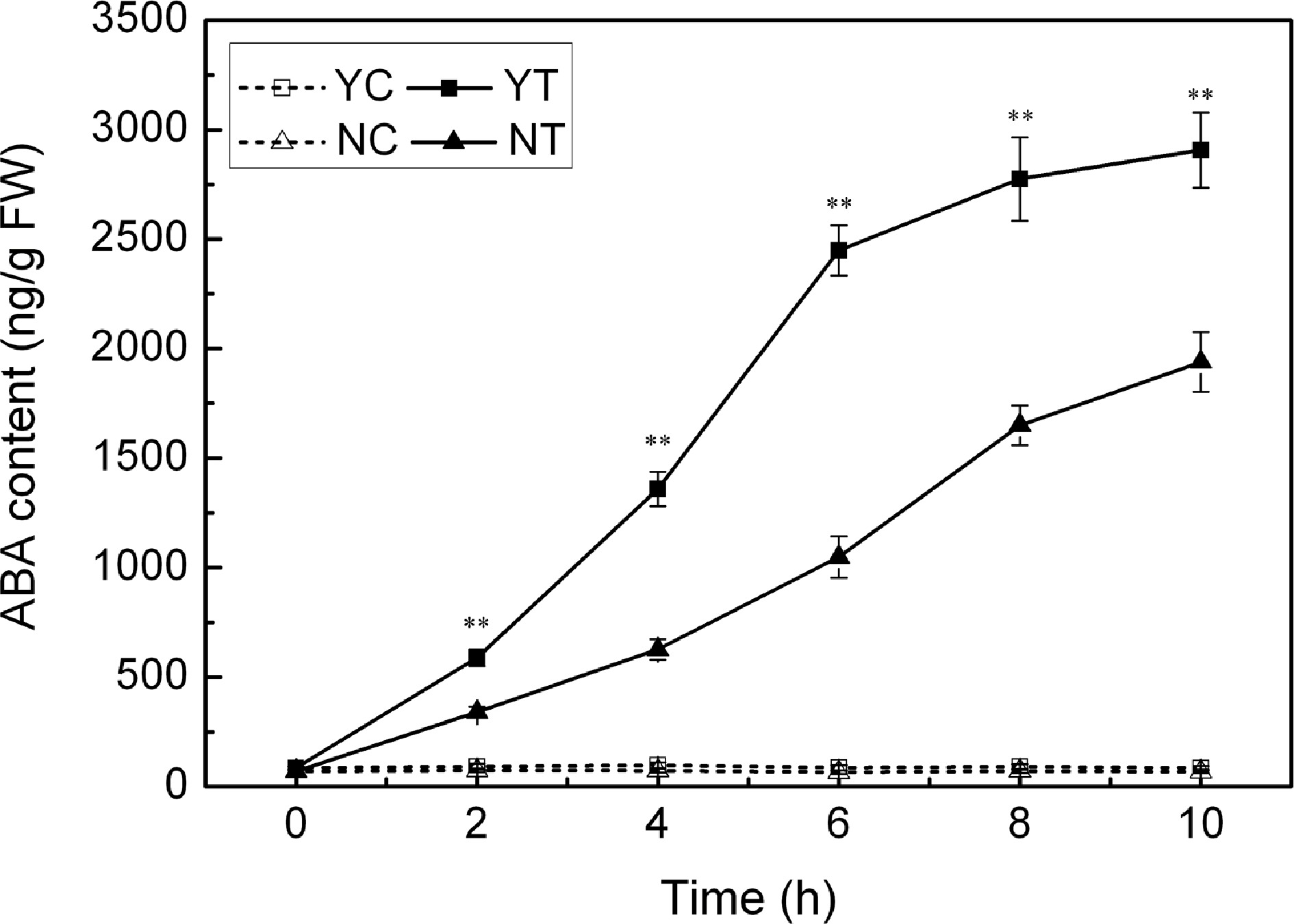
Leaf ABA content
-
The ABA content of the leaves of both species was consistently low under control conditions (Fig. 8), but increased markedly in response to PEG treatment. The response of C. japonense plants was much larger than that of C. nankingense plants. The ABA content in the C. japonense leaves increased rapidly over the first six hours of stress, and thereafter more slowly. The ABA content in the leaves of C. japonense was 1.7, 2.3 and 1.5 fold higher than in the leaves of C. nankingense at 2 h, 6 h and 10 h respectively.
-
C. nankingense, the more drought sensitive of the two Chrysanthemum spp., developed signs of drought-induced damage earlier than C. japonense and the wilting index of the former was consistently higher at each time point (Fig. 1a). In agreement with this differential response, the RWC of C. japonense was greater than that of C. nankingense (Fig. 1b), supporting the use of RWC as an indirect means of classifying crop varieties for their drought sensitivity[41,42].
The association between leaf surface features and drought tolerance
-
The leaf surfaces of the two species differs greatly. Leaf trichomes have been considered as a physical barrier against drought and high temperature stress[43]. They could increase water-use efficiency by increasing leaf boundary-layer resistance, thereby reducing transpirational water loss[44]. The more tolerant species developed a much higher density of trichomes on its leaves (Table 1). As this trait is readily visible, it would be attractive as an indirect selection criterion for improving drought tolerance[45]. Cuticular wax deposition represents an important mechanism for limiting non-stomatal water loss[46]. The quantity of cuticular wax on the surface of the leaves of C. japonense was markedly greater than on those of C. nankingense (Fig. 3a), consistent with their ranking with respect to drought tolerance. There were also significant differences between the two species with respect to the composition of cuticular wax (Fig. 3b), with the wax in the more tolerant species being richer in fatty alcohols and esters. Wax has been reported to affect the drought tolerance of plants in many species, among which, it has been reported the wax content of sunflower increased under drought condition[47]. To our knowledge, this is the first documented description of the composition of chrysanthemum cuticular wax.
The association between antioxidant enzyme activity and drought tolerance
-
Drought stress is often accompanied by the accumulation of ROS, which induce oxidative stress[48]. Plants have evolved a number of means to scavenge ROS molecules, and the enzyme SOD is considered to be part of the first line of this defence[49]. SOD, CAT, APX, POD all reduces superoxide. The activity of all four of these enzymes was increased by drought stress in both species (Fig. 4), although SOD activity was enhanced more in C. japonense than in C. nankingense. Significant increases in the activity of both APX and CAT were observed in the early phase of the stress exposure, particularly in C. nankingense, while more modest increases were observed for C. japonense; enzyme activity remained higher in C. japonense than in C. nankingense after 6 h of stress. Experiments conducted in rice have similarly shown that the more tolerant cultivars tend to express higher levels of CAT and APX activity[50]. It has been suggested that in soybean[51] , sorghum[52] and sunflower[47], drought tolerance is associated with enhanced POD activity. However, this does not appear to apply to Chrysanthemum spp., since the level of POD activity was similar in both species after 10 h of stress (Fig. 4b). Drought stress induces extensive lipid peroxidation, allowing MDA (a by-product of lipid peroxidation) content to be exploited as an indicator of stress-induced oxidative damage to membranes[53,54]. Finally, EL provides a measure of cell integrity, and so has been frequently used as a surrogate for stress tolerance[55,56]. The levels of both EL and MDA in C. japonense were uniformly lower than in C. nankingense, at least over the first 10 h of stress treatment (Fig. 5), supporting the conclusion that C. japonense is a more drought tolerant species than C. nankingense.
The association between osmotic regulation and drought tolerance
-
Plants take advantage of various molecules as osmoregulants, in particular the amino acid proline[57]. C. japonense with strong drought tolerance clearly accumulated more proline than C. nankingense when the plants were exposed to drought stress (Fig. 6), similar results were observed in other species including sunflower[47], rice[58] and Arabidopsis[24]. Proline contributes to the stabilization of sub-cellular structures, the scavenging of ROS and to buffering of cellular redox-potential under stress conditions[59]. The enhanced ability of the C. japonense leaf to accumulate proline thus may well provide a more favorable osmotic environment and a more stable cell membrane during episodes of drought stress.
The association between photosynthesis and drought tolerance
-
Photosynthesis is very sensitive to drought stress. The photosynthetic parameters Pn, Gs and Tr were all significantly compromised in both Chrysanthemum spp. by drought stress (Fig. 7a−c). Zhang et al.[60] has similarly reported that moisture stressed Atractylodes lancea suffers a reduction in photosynthesis as measured by Gs and Pn. An early response to drought stress is stomatal closure, which serves to limit transpirational loss[61]. After 2 h of stress, C. nankingense had significant higher Tr than C. japonense (Fig. 7c), thus resulting in more water loss in leaves, which might explain faster loss in RWC of C. nankingense than that in C. japonense. Changes in Gs depend on leaf RWC[62], and Gs and Tr were both correlated with leaf RWC in both species. It is generally considered that drought-induced stomatal closure would certainly have suppressed photosynthesis[63,64]. Gs and Pn decreased rapidly in both species under PEG treatment (Fig. 7a, b). Under a more prolonged period of moisture deficiency, the leaf tissue becomes increasingly dehydrated, inducing metabolic impairment and a restriction in photophosphorylation capacity[62,65]. When stomatal conductance falls below a threshold of 50 mmol H2O m−2·s−1, limitations of non-stomatal processes become more important[66]. Here, Gs remained above this threshold in the first four hours of stress, but dropped below it by 6 h in C. nankingense but not in C. japonense (Fig. 7b), suggesting that the photosynthetic apparatus of C. nankingense suffered earlier and more severe damage. Ci increased slightly in both species under PEG stress (Fig. 7d), as also observed in cotton, vetiver grass and wheat[67−69]. An overestimate in Ci could arise from heterogeneous (or 'patch') stomatal closure and cuticular conductance, which have been identified as potential sources of error in the calculation of Ci in drought affected plants[70]. This may explain why Ci rose at a time when Gs and the RWC were low. Dark-adapted Fv/Fm values and estimates of chlorophyll content decreased in both species under PEG stress (Fig. 7e, f). A decline in PSII quantum efficiency during periods of stress has been noted in a number of plant species[71−73]. Low Fv/Fm ratios have been related to photoinhibition[74], since plants frequently absorb more light energy than they require for photosynthesis, particularly under drought conditions. Due to the limited reaction capacity of converting solar energy into chemical energy, excessive light absorption exacerbates the inactivation of PSII under drought, freeing electrons for the formation of ROS[75]. Both the Fv/Fm ratio and the chlorophyll content decreased more sharply for C. nankingense than for C. japonense. After 10 h of PEG stress, C. japonense leaves retained a higher chlorophyll content and a larger Fv/Fm ratio than those of C. nankingense (Fig. 7e, f), symptomatic of C. japonense being able to maintain a higher photosynthetic capacity under drought stress. Similarly, drought tolerant bean and edamame cultivars have been reported to retain a higher chlorophyll content and a superior Fv/Fm ratio than do more susceptible ones[76,77].
The association between ABA content and drought tolerance
-
ABA, one of the most important metabolites produced under drought stress, is known to regulate plant water balance and drought stress tolerance[78]. Analysis of ABA-deficient mutants and -related genes have shown that this hormone is essential for triggering many of the important responses to drought stress[79]. Here, it was obvious that the ABA level in the leaf of both species was greatly enhanced by the imposition of drought stress (Fig. 8). The ABA content was significantly higher in C. japonense than in C. nankingense. In droughted-stressed durum wheat, Mahdid et al. have shown that a more tolerant cultivar accumulated more ABA than did a less tolerant one[80]. ABA is thought to increase hydraulic conductivity from the roots to the transpiring tissues[81], acting in conjunction with ABA-induced stomatal closure to restore a favorable water status to the leaf tissue. Gs and ABA appeared to be negatively correlated in both species. ABA may also influence osmotic regulation, ion and solute transport loading in growing cells, and so play a vital role in both water retention and protein and membrane protection[82]. Low water potential-induced proline accumulation in A. thaliana requires wild-type levels of ABA[83], while drought-induced changes in the synthesis of proline have been shown to be ABA dependent[84]. ABA plays a role in the upstream of proline accumulation by regulating the expression of key enzyme genes of proline biosynthesis, which also improves the adaptation of rice to hypoxia stress to a certain extent[85]. The present data indicate that the improved capacity to accumulate proline shown by C. japonense may be associated with its enhanced ability to accumulate ABA.
-
Overall, it is clear that these two Chrysanthemum species show contrasting responses to drought stress at the morphological, physiological and biochemical levels. The superior tolerance of C. japonense likely flows from a combination of its better developed trichome layer, its higher cuticular wax content, its more rapid and abundant accumulation of ABA, its more flexible photosynthesis capacity, and its more effective osmoprotective and antioxidative system. The evaluation of the drought tolerance of the two chrysanthemum species further enriched the drought tolerance germplasm resource bank of chrysanthemum, clarified the different physiological and biochemical responses of two chrysanthemum species with great differences in drought tolerance, which has certain guiding significance for further development and application of drought tolerance resources of chrysanthemum.
This study is supported by the National Natural Science Foundation of China (31870306), the National Key Research and Development Program of China (2020YFE0202900), the Fundamental Research Funds for Central Universities (KYZZ2022004).
-
The authors declare that they have no conflict of interest.
-
# These authors contributed equally: Yi Zhang, Jing Gu
- Copyright: © 2022 by the author(s). Published by Maximum Academic Press, Fayetteville, GA. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Zhang Y, Gu J, Xia X, Zeng J, Sun H, et al. 2022. Contrasting responses to drought stress between Chrysanthemum japonense and C. nankingense. Ornamental Plant Research 2:16 doi: 10.48130/OPR-2022-0016
Contrasting responses to drought stress between Chrysanthemum japonense and C. nankingense
- Received: 15 August 2022
- Accepted: 21 September 2022
- Published online: 25 October 2022
Abstract: The response of Chrysanthemum japonense and C. nankingense to drought stress induced by polyethylene glycol was characterized at the level of leaf water status, leaf surface morphology and cuticular wax (quantity and composition), the activity of antioxidant enzymes, the extent of membrane lipid peroxidation, the accumulation of proline, photosynthesis performance and abscisic acid (ABA) accumulation. The more tolerant species C. japonense maintained its water status more effectively than C. nankingense, probably because its leaves form more cuticular wax and are able to accumulate higher levels of ABA. Superoxide dismutase activity was higher in C. japonense than in C. nankingense, as was that of catalase and ascorbate peroxidase during the later part of the stress episode, but levels of peroxidase were not differentiated at the end of the stress period. Membrane damage, as measured by electrolyte leakage and malondialdehyde accumulation, was less severe in C. japonense, which was also able to generate higher levels of free proline after a 10 h exposure to stress. Thus the superior response of C. japonense also reflects a more adapted system of osmoprotection and antioxidation. As a result, photosynthesis was compromised less by drought stress in C. japonense than in C. nankingense. That provides a scientific basis for the development and application of drought tolerance resources of chrysanthemum.
-
Key words:
- Drought stress /
- Chrysanthemum /
- Cuticular wax /
- ABA /
- Photosynthesis /
- Polyethylene glycol