-
Cancer remains the leading cause of human death worldwide. The International Agency for Research on Cancer (IARC) 2020 report revealed approximately more than 19.3 million new cases of cancer patients, with breast cancer having the highest incidence (11.7%) followed by lung cancer (11.4%), colorectal cancer (10.0%), prostate cancer (7.3%), and gastric cancer (5.6%). Cancer is a multifactorial disease with many events involved in its initiation and progression, with inflammation being one of the major carcinogens. Notably, chronic inflammation can trigger DNA mutation and then carcinogenesis via reactive oxygen species (ROS) generation[1,2]. Thus, anti-oxidative, anti-inflammatory agents are considered useful for cancer prevention[2].
Many natural products have various biological activities, including anti-inflammation, innate immunity activation, and anticancer effects[3,4]. Natural products have been used as medicines for many diseases in which inflammation and cancer are the major targets[5]. For example, many conventional anticancer drugs, such as paclitaxel, camptothecin, and vincristine, originate from natural products[3,4]. The development and utilization of natural products for controlling inflammatory diseases and cancer has become a research hotspot. In this context, we have discovered canolol, a potent antioxidative, anti-inflammatory active component of rapeseed, which showed strong activity for removing ROS[6] and a remarkable suppressive effect against mouse ulcerative colitis induced by dextran sulfate sodium (DSS), and thus significantly inhibited colorectal cancer carcinogenesis triggered by azoxymethane (AOM)/DSS[7]. We have also investigated the activity of hot water extract of Phellinus linteus, fresh leaves of Kumaizasa bamboo, and Chaga mushroom (i.e., MeshimaMax) and found a remarkable cancer-preventive effect in both transplanted tumor and carcinogen (AOM/DSS, 7,12-dimethylbenz[a]anthracene)-induced colon cancer, which is strongly associated with the activation of macrophage and innate immunity[8]. These studies suggest potential applications of natural products for cancer prevention.
Along this line, the current study focuses on a hot water extract of seven traditional Chinese medicines (Lycii fructus, Crataegi fructus, Phyllanthi fructus, Chrysanhemi flos, Coicts semen, Ganoderma lucidum, and Zizyphi fructus), which is referred to as LLA. As a nutritional supplement drink, LLA has been used in China and Japan for more than 30 years, and its main active components are flavonoids, organic acids, and polysaccharides. Notably, phyllanthi has been shown to exhibit anti-inflammatory and antipyretic effects, and its air-dried fruit has also been used to treat cancer in Tibetan and Egyptian medicines[9]. An in vitro study using mouse melanoma B16F10 cells showed that the extracts from the roots of phyllanti strongly inhibited cell growth and differentiation[5]. Moreover, Kanglaite injection, which originates from the oil extract of Coicts semen, is widely used for treating many cancers, including pancreatic, lung, gastric, and breast[10−13]. In addition, improved superoxide dismutase (SOD) activity has been reported for polysaccharides derived from Lycium Chinese, a component of LLA, with vitamin C-like antioxidant activity[14,15].
Though many studies suggested the anti-inflammatory and anti-cancer potential of the active components in LLA as described above, very few studies have been carried to explore the beneficial effects of the whole LLA as a nutritional drink. In a previous study using LLA, we found a remarkable ergogenic capacity in aged mice, and the effect was considered mostly due to the increased anti-oxidative activity, i.e., increased superoxide dismutase (SOD) activity[16]. It is well-recognized that the anti-oxidative activity is positively related to anti-inflammation and cancer-prevention potential[1,2,17,18]. Our previous studies using the extracts of natural products[6−8], as well as ample studies from other research groups[19−21] strongly indicated that natural products with high anti-oxidative activity exert the beneficial potential for suppressing inflammation and cancer. Along this line, we considered that LLA may also exhibit anti-inflammation and cancer prevention potential, and this hypothesis was verified by using various solid tumor models, including chemical carcinogen-induced cancer that mimics the process of human cancer development. We also examined the possible mechanisms of action involved in the activity of LLA, focusing on its anti-inflammatory properties.
-
DSS was obtained from MP Biomedicals, LLC (Irvine, CA, USA). Fujifilm Wako Pure Chemical Corporation (Osaka, Japan) supplied AOM, cell culture medium (RPMI-1640, DMEM), Quantikine ELISA kit of mouse monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6). LLA was obtained from the Institute of International Kampo Co. Ltd. While Sigma-Aldrich Chemical (St. Louis, MO, USA) supplied thiazolyl blue tetrazolium bromide (MTT) and 2′,7′-dichlorofluorescein diacetate (DCDHF-DA), Dojindo Molecular Technologies, Inc. (Kumamoto, Japan) supplied GSSG/GSH quantification kit.
Animals
-
Six-week-old male ddY mice and five-week-old male ICR mice were obtained from SLC (Shizuoka, Japan). The mice were housed at 22 ± 10 °C and 55% ± 5% relative humidity with automatic lighting at a 12-h light/dark cycle. Mice were fed adaptively for 1 week before experiments. During experiments, the mice were randomly divided into different groups as indicated.
Antitumor effects of LLA
Mouse sarcoma S180 tumor model
-
A mouse sarcoma S180 tumor model was prepared by injecting S180 tumor cells (2 × 106 cells/100 uL) into the dorsal skin of a ddY mouse. LLA (0.07%, 0.2%, 0.6%) was given through drinking water; in one group, LLA was administered daily from the day of S180 cell inoculation, and in another group, daily LLA administration was carried out one week after tumor inoculation when the tumor grew to 6–8 mm in diameter. Each group included four mice. During the experiment, the width (W) and length (L) of the tumors, as well as the body weight of mice, were measured every 2–3 d during the study period, and tumor volume (mm3) was calculated as (W2 × L)/2. When the tumor grew to 4,000–5,000 mm3, the mice were euthanized by inhaling isoflurane at saturated vapor pressure.
Mouse colon cancer model
-
A carcinogen-induced mouse colon cancer model was prepared by injecting AOM (10 mg/kg) i.p. into ICR mice, followed by daily administration of 2% DSS through drinking water from one week after AOM injection for 7 d. Each group included 6−8 mice. During the experiments, LLA in commonly used doses as a healthy supplement (i.e., 0.07%, 0.2%, 0.6%) was given through drinking water; however, during DSS treatment, LLA was given via food to avoid potential interference. At 12–13 weeks after AOM administration, the mice were sacrificed by isoflurane euthanasia as described above, colon and blood were collected, and the number and size of colon cancer nodules were measured.
Anti-inflammatory effects of LLA
-
The mouse colitis model was established by feeding 2% DSS to ICR mice in drinking water. LLA (0.07%, 0.2%, 0.6%) treatment was carried out throughout the experiment by adding LLA to the mouse food. Each group included 7−8 mice. During the experiments, we observed the symptoms and severity of colitis, which were evaluated using a semi-quantitative disease activity index (DAI). The DAI was determined by score changes in the body weight of mice, the presence of occult blood, gross bleeding, and stool consistency[22]. The weight loss of mice was graded into five levels (0: either a weight gain or no weight loss; 1: 1%–5% loss; 2: 5%–10% loss; 3: 10%–20% loss; and 4: more than 20% loss). Three levels of grade were used for stool consistency (0: normal; 2: loose; and 4: diarrhea) and occult blood (0: negative; 2: occult blood-positive; and 4: gross bleeding).
On the 12th day after feeding DSS, when severe symptoms were observed, the mice were sacrificed by isoflurane euthanasia as described above, and the blood and colon specimens were collected for biochemical and pathological examinations. Serum samples were used to measure the inflammatory cytokines: MCP-1, TNF-α, and IL-6.
Cytotoxicity assay
-
The cytotoxicity of LLA was examined in both cancer cells (human cervical cancer cells A2780) and normal cells (monkey kidney epithelial cells, CCL-81). The cells were cultured in RPMI-1640 containing 10% fetal bovine serum. The cells were seeded in 96-well plates (3,000 cells/well); after overnight incubation, different doses of LLA were added, and the cells were further cultured for 48 h. Cell viability was measured using an MTT assay[23].
Effect of LLA on the phagocytotic activity of macrophages
-
The effect of LLA on the phagocytotic activity of macrophages was investigated using mouse macrophage RAW264.7 cells. RAW264.7 cells (1 × 105 cells/1.5 mL) were cultured on a 6-well plate; after overnight incubation, LLA of different concentrations were added and the cells were further cultured for 24 h. Then, yeast cells (Saccharomyces cerevisiae) (3 × 105 cells) were added to the culture media. At the scheduled time, the macrophages incorporating yeast were detected and counted using a microscope (BZ-X700, Keyence Co. Ltd., Osaka, Japan). Two hundred RAW264.7 cells were counted, and the phagocytic ability of macrophages was calculated by the following equation, phagocytosis = A / 200 × 100%, in which A was the number of macrophages that took up yeast cells.
Quantification of glutathione and ROS in cancer cells after LLA treatment
-
Colon cancers were seeded in a 12-well plate (3.5 × 105 cells/well). After overnight pre-incubation, the indicated concentrations of LLA were added and the cells were treated for 24 h. The cells were then trypsinized and collected, and the glutathione (GSH) concentrations in the cells were quantified using a GSSG/GSH quantification kit (Dojindo Molecular Technologies, Inc.) following the manufacturer's instructions.
For intracellular ROS measurement, C26 cancer cells were seeded in 12-well plates at a density of 2 × 105 cells/well and incubated for 12 h. Different concentrations of LLA were then added to the cells, and the cells were treated for 24 h. To detect intracellular ROS, a 10 μM solution of the fluorescent probe DCDHF-DA was added to the cells 30 min before harvesting. Upon internalization, DCDHF-diacetate is converted to DCDHF, which reacts with ROS to form the fluorescent compound dichlorofluorescein. Subsequently, the cells were exposed to 100 μM hydrogen peroxide (H2O2) for 30 min. The level of intracellular ROS was quantified by measuring the fluorescence intensity using flow cytometry (BD AccuriTM C6 Plus; Becton Dickinson, San Jose, CA, USA). In some experiments, LLA was administered to the cells simultaneously with H2O2 exposure. In a separate study, the same protocol was carried out without H2O2 exposure.
Statistical analyses
-
All data were expressed as mean ± SD. Data were analyzed using ANOVA, followed by the Bonferroni multiple comparison test. A difference was considered statistically significant when p < 0.05.
-
We first investigated the anti-inflammatory activity of LLA using a DSS-induced mouse inflammatory colitis model. As shown in Fig. 1a, after feeding DSS for 5 d, colitis was triggered and rapidly progressed, as evidenced by the increased DAI. Histological examination confirmed colitis formation, as evidenced by colon shortening (Fig. 1b), and destruction of colon mucosa (Fig. 1c). In parallel with these findings, a remarkable increase in inflammatory cytokines (i.e., TNF-α, MCP-1, and IL-6) was observed (Fig. 2). LLA administration significantly suppressed the above-described symptoms and histopathological changes in colitis (Fig. 1). Notably, after LLA treatment, inflammatory cytokine generation was largely inhibited, and the levels of all tested inflammatory cytokines decreased to almost normal levels at all tested doses (Fig. 2).
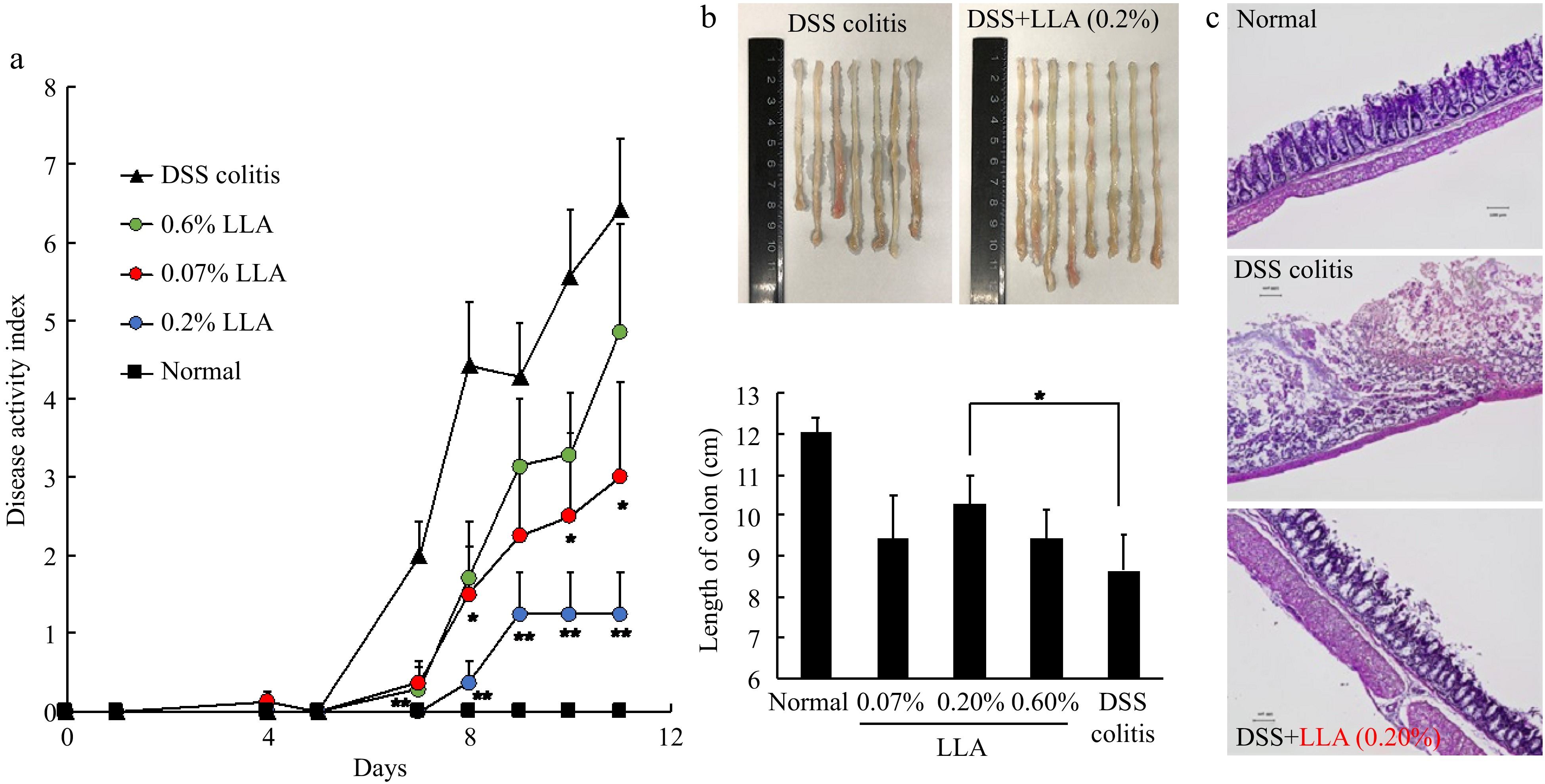
Figure 1.
Anti-inflammatory effect of LLA on DSS-induced mouse colitis. The DSS-induced colitis model was triggered by oral administration of 2% DSS. LLA treatment was carried out throughout the experiment. During the experiments, symptoms of colitis were recorded daily, and (a) the disease activity index (DAI) values were calculated. On day 11, when severe colitis appeared, mice were killed, (b) the length of the colon was measured, and (c) histological examination of the colon was carried out. Values are mean ± SD; n = 7–8. *, p < 0.05; **, p < 0.01 vs DSS colitis group. See text for details.
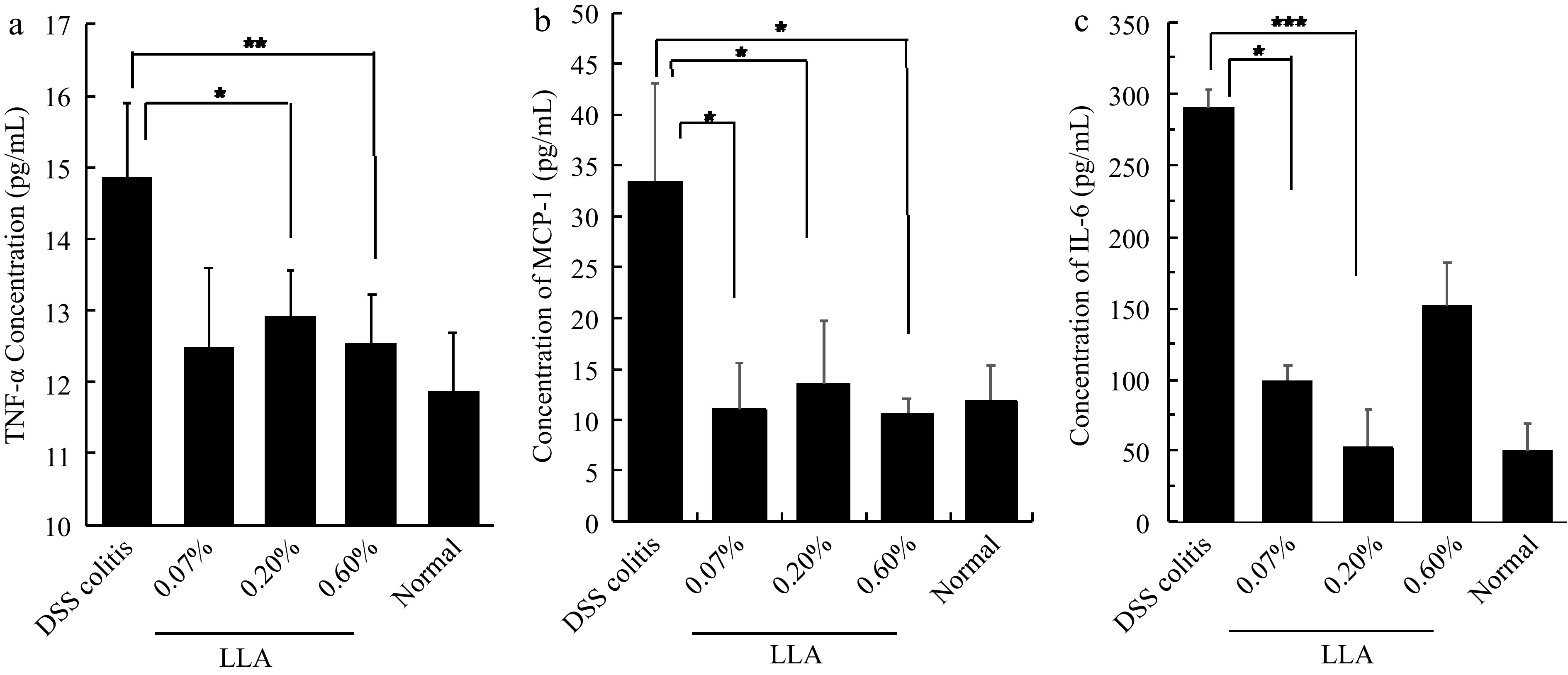
Figure 2.
(a) Inhibition of inflammatory cytokines TNF-α, (b) MCP-1, and (c) IL-6 by LLA in DSS-induced murine colitis. The experimental protocol is the same as that described in Fig. 3. On day 11 of the experiment, mice were killed, and serum samples were collected for measurement of the cytokines by using ELISA. Values are mean ± SD; n = 7–8. *, p < 0.05; **, p < 0.01; *, ***, p < 0.001. See text for details.
Cancer prevention and antitumor effects of LLA
-
In the DSS induced colitis model, as expected we clearly found the suppression of inflammation by LLA. It is thus reasonable to anticipate the potential effects of LLA for cancer, we thus performed the studies using two solid tumor models as below.
LLA suppresses the growth of mouse sarcoma S180 transplanted into the dorsal skin of mice
-
In the mouse sarcoma S180 tumor model, we first examined the cancer-preventive effect of LLA by starting the LLA treatment on the same day that the S180 cells were injected into the mice. As shown in Fig. 3a, tumor growth was significantly inhibited dose-dependently.
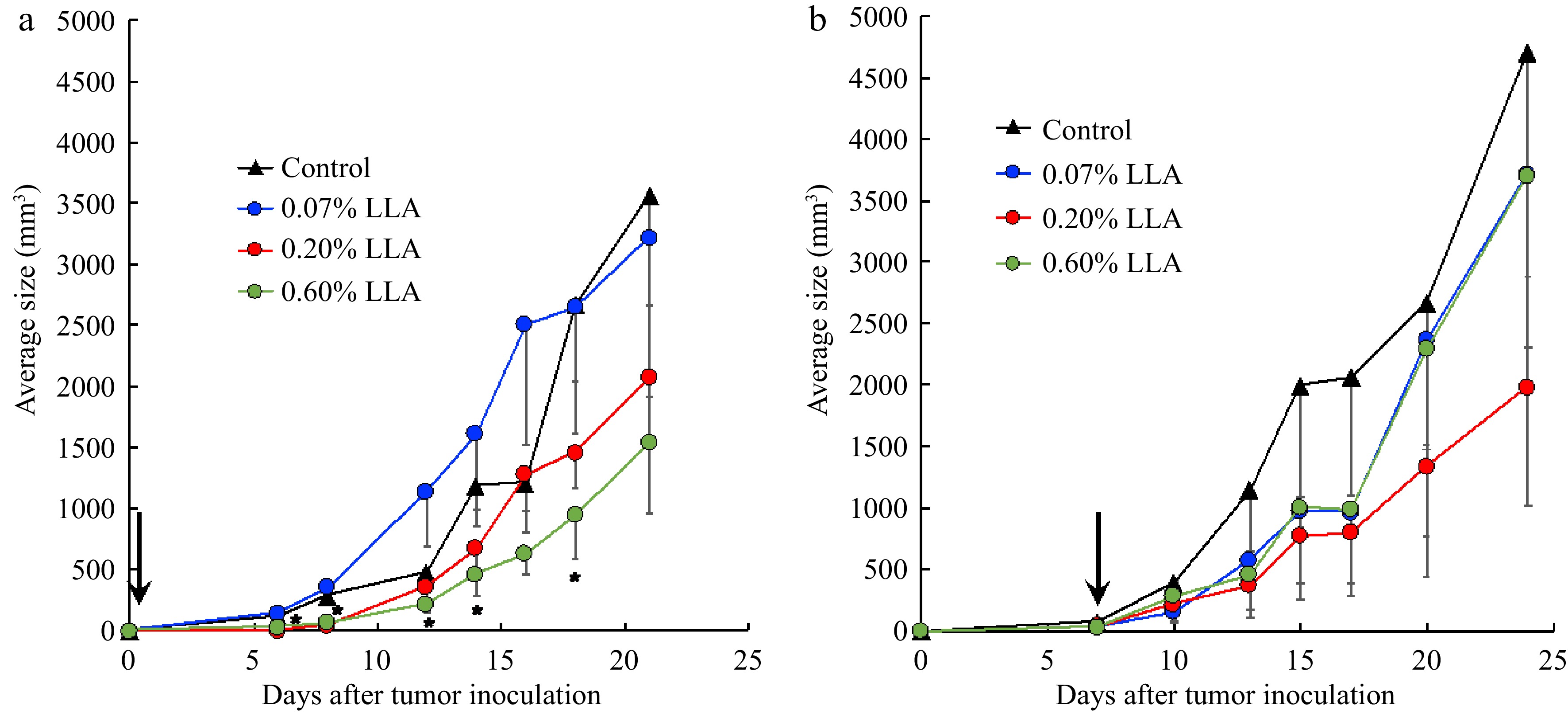
Figure 3.
Antitumor effect of Ligustrum lucidum ait (LLA) against mouse sarcoma S180 solid tumor. (a) LLA was fed to the mice from the day of mouse sarcoma S180 tumor cell inoculation. (b) LLA was fed to the mice from day 7 after S180 tumor cell inoculation when the tumor had grown to 7–8 mm in diameter. Arrows indicate the date to start the LLA treatment. Data are mean ± SD, n = 4. *, p < 0.05, 0.6% LLA group vs Control. See text for details.
However, when LLA treatment was initiated one week after tumor inoculation when the tumor grew to 7–8 mm in diameter, we did not find significant inhibition of tumor growth, although 0.2% of LLA showed an apparent trend of tumor growth inhibition (Fig. 3b). These findings suggest that LLA does not exhibit direct tumoricidal activity; that is, LLA as a healthy supplement drink did not show a significant anticancer effect.
LLA inhibits colon cancer carcinogenesis induced by ADM/DSS
-
We then focused on the cancer-preventive effect of LLA and confirmed it using a carcinogen (AOM/DSS)-induced mouse colon cancer model. In line with the results shown in Fig. 3a, we found remarkably decreased tumor nodules in the colon (Fig. 4). However, the LLA effect was not dose-dependent: 0.2% of LLA exhibited a better effect than 0.6% (Fig. 4).
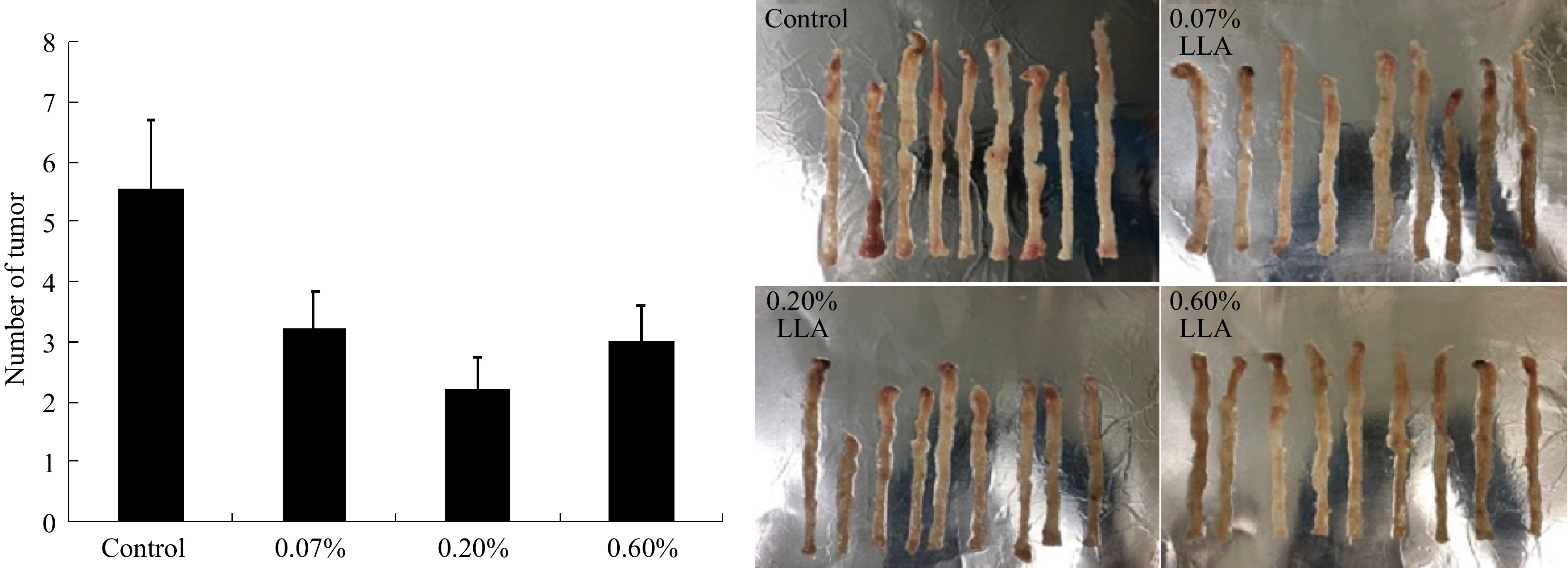
Figure 4.
Suppression of azoxymethane/dextran sulfate sodium (AOM/DSS) induced mouse colon carcinogenesis by LLA. At 12-13 weeks after AOM administration with/without LLA treatment, mice were killed and the numbers of tumor nodules in the colon were counted (left), and the photos of the colon were shown on the right Data are mean ± SD, n = 9. *, p < 0.05 vs control. See text for details.
Cytotoxicity of LLA
-
To further tackle the actions and mechanisms of LLA against cancer, we then measured the in vitro cytotoxicity of LLA using both tumor and normal cells. In both normal (CCL-81) and tumor cells (C26 cells), almost no or very little cytotoxicity of LLA was found, up to 1 mg/ml (Fig. 5), however, remarkable cytotoxicity was observed at concentration higher than 1 mg/ml). These findings, along with its anti-inflammatory, cancer preventive effect as shown in Figs 1 to 4, indicate the inverted U-shape dose effect of LLA.
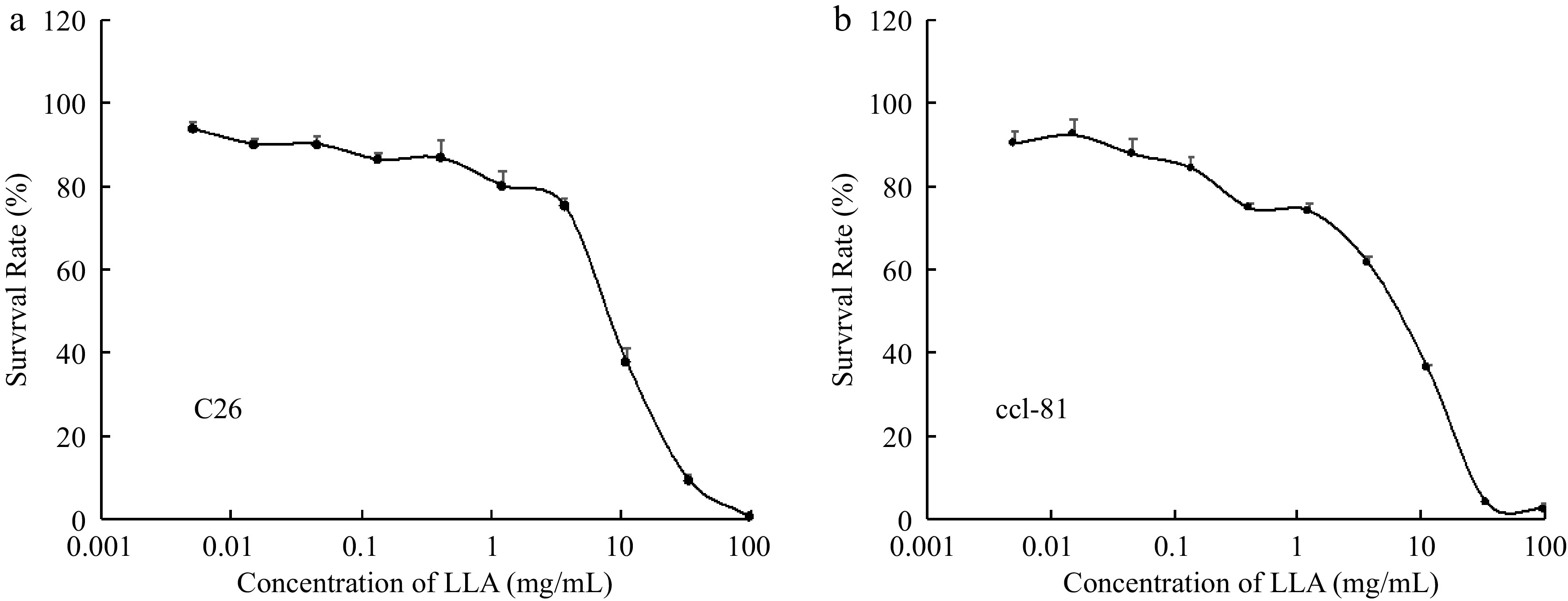
Figure 5.
(a) In vitro cytotoxicity of LLA to normal cells (monkey kidney epithelial cells CCL-81, as well as (b) tumor cells (human ovarian cancer A2780). Cell viability after LLA treatment was examined by MTT assay. Values are mean ± SD; n = 8. See text for details.
Effect of LLA on the phagocytosis of macrophages
-
Our previous study showed that extracts of natural products exhibited an antitumor effect by activating innate immunity[8]. Therefore, we measured the effect of LLA on the phagocytotic activity of macrophages. As shown in Fig. 6, macrophages gradually absorbed yeast added to the culture medium time-dependently; however, LLA treatment did not increase the phagocytosis rate of macrophages, suggesting that macrophage activation does not serve as a mechanism in LLA-induced cancer prevention.
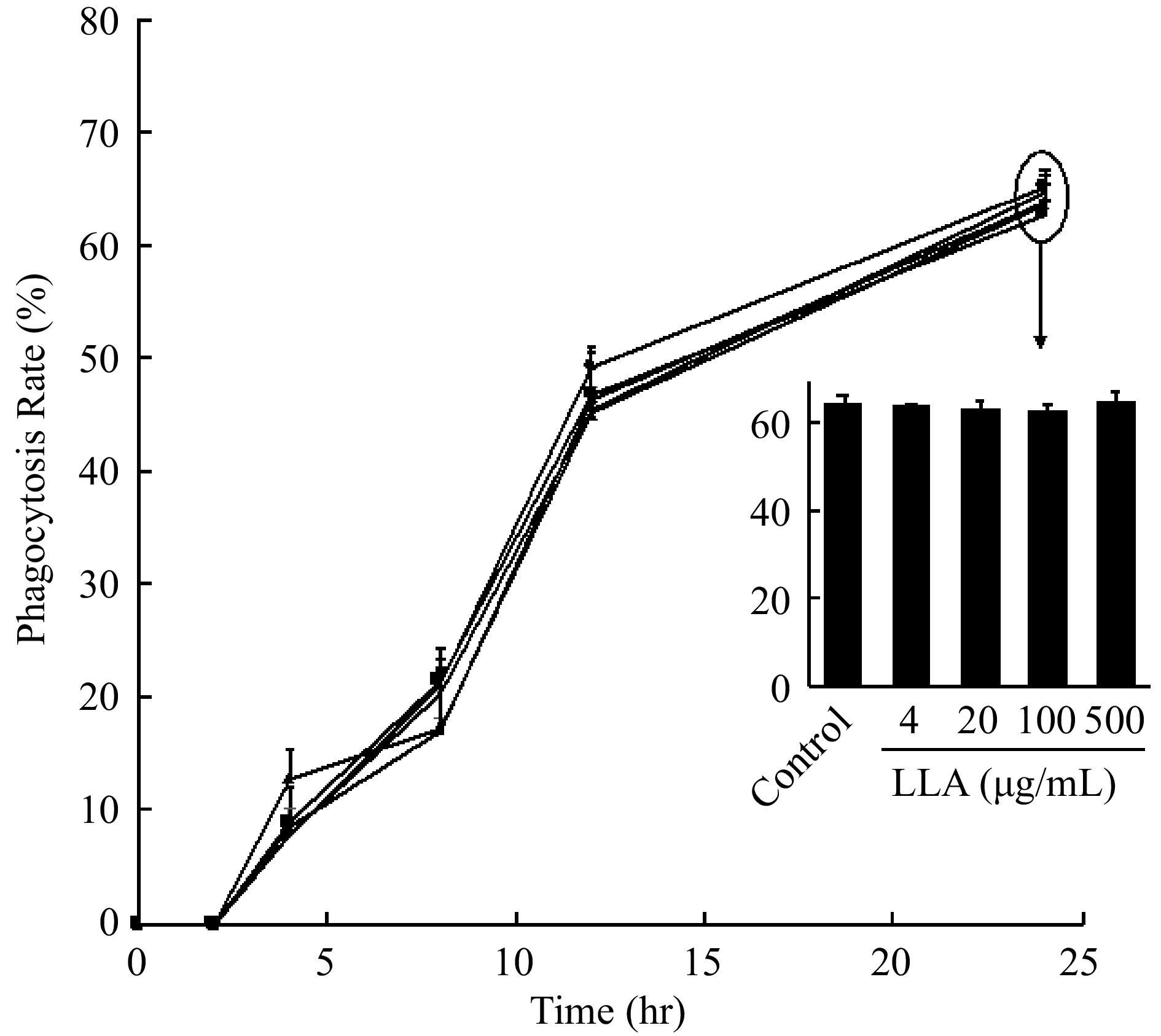
Figure 6.
Effect of LLA on the phagocytotic activity of macrophage. Engulfment of yeast by macrophages in the presence /absence (control) of LLA was observed and counted with a microscope for 12 h after adding yeast to macrophages, the phagocytosis rate of macrophages was then calculated. Values are mean ± SD; n = 3. See text for details.
The impact of LLA on cell redox status under varied conditions
-
A previous study using LLA suggested the antioxidative potential of LLA, that is, increased SOD activity and GSH levels in the mice[16]. To determine whether LLA could affect the redox status of cancer cells, we measured the antioxidant GSH and ROS levels in colon cancer C26 cells after LLA treatment. No apparent changes in GSHand ROS (Supplemental Fig. S1) were observed after LLA treatment up to 1 mg/ml. However, upon exposure of cells to hydrogen peroxide (H2O2) to induce ROS generation, we observed that LLA treatment effectively suppressed intracellular ROS levels in a dose-dependent manner (Fig. 7). This effect was observed both when LLA was administered as a pre-treatment (Fig. 7a) and when it was co-exposed with H2O2 (Fig. 7b). These results strongly suggest that LLA does not disrupt the redox homeostasis of the cells but instead exerts robust anti-oxidative effects in the face of oxidative stress.
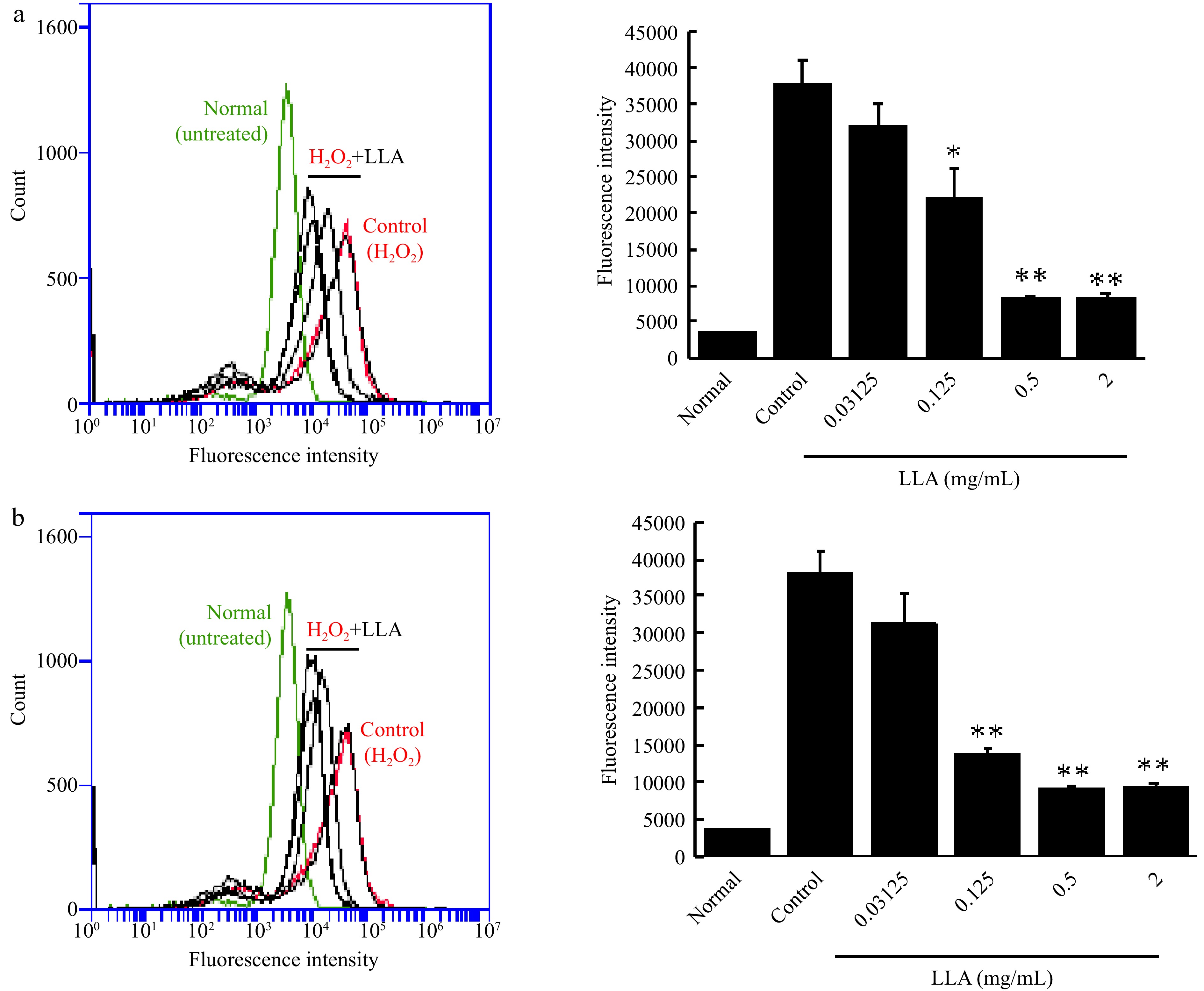
Figure 7.
Suppression of intracellular ROS induced by hydrogen peroxide (H2O2) through LLA treatment in C26 colon cancer cells. (a) Pre-treatment: C26 cells were treated with increasing concentrations of LLA for 24 h prior to H2O2 addition. (b) Co-exposure: LLA was administered simultaneously with H2O2 exposure. The intracellular ROS levels were quantified using the fluorescence ROS probe DCDHF-DA and analyzed by flow cytometry. Mean values ± standard deviation (SD) are shown; n = 4–8. *, p < 0.05, **, p < 0.01 compared to the H2O2 alone group. See text for details.
-
LLA is a well-recognized supplemental drink that has been used in Japan and China for decades. We previously found that LLA significantly increased SOD and GSH levels in the spleen and liver[16], suggesting the antioxidative activity of LLA. Notably, ROS plays an important role in the progression of inflammation, which leads to the cell's malignant transformation. Moreover, polyphenols, which are the major effector molecules in fruits, exhibit cancer-preventive effects, mostly through their anti-inflammatory properties[24]. As a result, it is reasonable to speculate that LLA may have an anti-inflammatory effect and thus aid in cancer prevention. As expected, we found that LLA exhibited a potent anti-inflammatory effect in the DSS-induced mouse inflammatory colitis model (Fig. 1), in which the symptoms and pathological changes were remarkably improved by LLA (Fig. 1), and inflammatory cytokine production was significantly suppressed (Fig. 2). In line with the anti-inflammatory activity of LLA, carcinogenesis induced by AOM/DSS, which is a typical model of inflammation-related carcinogenesis, was markedly inhibited by LLA administration (Fig. 4), indicating the strong potential of LLA for cancer prevention.
In this study, we did not find a direct anticancer effect of LLA: feeding LLA to mice bearing solid tumors did not suppress tumor growth significantly (Fig. 3b). However, previous studies have indicated the antitumor activities of traditional Chinese medicine in LLA. For example, the fruit extract of Phyllanthus emblica has long been used in traditional medicine to treat many diseases, such as constipation and cancer[25]. From the leaves, roots, and fruit juice of Phyllanthus emblica, 18 main components have been extracted and identified, such as phyllaemblicin B, phyllaemblicin C, and phyllemtannin, most of which showed potent tumoricidal effects against many tumor cells[5]. In addition, the oil extract of Coicts semen, Kanglaite injection, not only shows an anticancer effect but can also largely enhance the host's immune function, which was approved in 1997 by the Ministry of Health of China for treating many cancers: over millions of cancer patients in more than 2,000 hospitals in China have been treated with Kanglaite injection[10,26]. It is not clear why LLA had no significant anticancer effect, but we believe that dosing is the main reason. Namely, the above-mentioned studies used a condensed extract of traditional medicines at relatively high concentrations; however, the LLA used in our present study was a supplement drink containing the hot water extract of seven Chinese traditional medicines, which was used and evaluated as a whole as a healthy supplement but not as a pharmaceutical agent. This means that the active components in LLA that may have exhibited tumoricidal effects were very limited. In addition, the dosing in this study was comparable to the commonly suggested amount of this supplement drink (e.g., the dose of 0.6% is comparable to 135 ml per day in humans). Thus, by the protocol used in this study, the effector components with anticancer effects could not reach or were much lower than the cytotoxic doses. Future studies are warranted to identify the anticancer components of LLA and to elucidate the potential of LLA for cancer treatment.
Although we did not find an apparent anticancer effect of LLA, its potential for cancer prevention was found. LLA administration before tumor formation significantly inhibited tumor occurrence and growth (Fig. 3a), and feeding LLA to the mice remarkably suppressed the colon cancer carcinogenesis triggered by carcinogen AOM/DSS (Fig. 4) that mimics the process of human cancer development. Regarding the possible mechanisms of action for the cancer-preventive effect of LLA, we did not find an apparent cytotoxic activity of LLA (Fig. 5) or observe its effect on macrophage activation (Fig. 6), suggesting that cytotoxicity and activation of innate immunity are not involved in the cancer-preventive effect of LLA. In a previous study using LLA, our group found that LLA exhibited a potent antioxidative effect by increasing the activity of the antioxidative enzyme SOD and the amount of antioxidant GSH[16], indicating the anti-inflammatory potential of LLA. As expected, we found a remarkable anti-inflammatory effect of LLA in the DSS-induced mouse colitis model (Figs 3, 4). Unexpectedly, we did not observe significant changes in the cellular redox status, as measured by the levels of GSH and ROS, in cancer cells after LLA treatment under normal conditions (Supplemental Fig. S1). However, we made an important discovery when examining the response of cells to oxidative stress. LLA treatment significantly suppressed the generation of intracellular ROS when the cells were exposed to H2O2 (Fig. 7), indicating the potent anti-oxidative effect of LLA under conditions of pathological oxidative stress. Given that inflammation plays a major role in cancer development, and ROS is a critical factor in triggering inflammation and promoting cancer initiation and progression, we propose that LLA's cancer-preventive effect is largely attributed to its anti-oxidative and anti-inflammatory activities. Nonetheless, further investigations are warranted to elucidate the precise mechanisms underlying the action of LLA, and we plan to conduct these investigations in future studies.
Furthermore, while we observed LLA's anti-inflammatory and cancer-preventive effects, we discovered that the effect was not linearly dose-dependent. In both studies using the DSS-induced colitis model and AOM/DSS-induced mouse colon carcinogenesis model, 0.2% LLA showed the best effect, overwhelming both 0.07% and 0.6% (Figs 1−3). These findings provide evidence for an inverted 'U-shaped' dose response effect of LLA, where low to moderate concentrations exhibit anti-inflammatory properties, while higher doses may induce proinflammatory and cytotoxic effects. This observation may be attributed to the tumoricidal effects of the individual components within LLA, as discussed earlier. Interestingly, our in vitro cytotoxicity study revealed a striking discovery: LLA demonstrated a remarkable cytotoxic effect at concentrations exceeding 1 mg/ml (Fig. 5). This finding sheds light on the inverted U-shaped dose-response relationship of LLA and its potential implications. Furthermore, we found a similar phenomenon in our previous study using an extract mixture of Phellinus linteus, bamboo leaf, and chaga mushroom[8], and other researchers also reported similar findings using natural herbs[27]. However, the detailed mechanisms underlying this dose effect remain unclear, necessitating further investigations.
Although the cytotoxicity of LLA components has been reported in many cancer cells[5], we found no evidence of LLA cytotoxicity up to 1 mg/kg in either cancer or normal cells (Fig. 5). While these findings support our previously stated hypothesis that LLA's cancer-preventive effect is not due to its tumoricidal effect, they also support LLA's safety/nontoxicity as a supplemental drink.
Nowadays, various healthy supplements, including supplemental drinks originating from natural products and traditional Chinese medicines, have been used habitually for health-oriented purposes. While most studies regarding healthy supplements focus on the effect of a single component and active molecule in a supplement, it is also crucial to evaluate the compositive effect of the supplement as a whole under routine doses and usage. We thus believe that the current study provides insights into the effect of LLA and its future applications.
-
We have revealed that the extract of seven traditional Chinese medicines, LLA, had potent cancer-preventive activity not only in the transplanted solid tumor model but also in the carcinogen-induced colon carcinogenesis model, which mimics the actual initiation and progression of human cancer. The cancer-preventive effect of LLA is mostly the consequence of suppressing inflammation. We believe that this is the first study regarding the potential of LLA for cancer prevention, and we anticipate that this supplement drink will benefit patients suffering from inflammatory diseases as well as be useful for cancer prevention.
-
All experiments were conducted following the Laboratory Protocol of Animal Handling, Sojo University, and were approved by the Animal Ethical Committee, Sojo University (No. P/009/2019).
This research was partly funded by the research funding from Faculty of Pharmaceutical Sciences, Sojo University to Jun Fang and Kazumi Yokomizo.
-
The authors declare that they have no conflict of interest.
-
These authors contributed equally: Shanghui Gao
- Supplemental Fig. S1 Effect of LLA on the redox status of cancer cells. Mouse colon cancer C26 cells were treated by indicated concentrations of LLA for 24 h, after which the intracellular levels of GSH (A) and ROS (B) were quantified. Values are mean ± SD; n = 3. See text for details.
- Copyright: © 2023 by the author(s). Published by Maximum Academic Press on behalf of Nanjing Agricultural University. This article is an open access article distributed under Creative Commons Attribution License (CC BY 4.0), visit https://creativecommons.org/licenses/by/4.0/.
-
About this article
Cite this article
Gao S, Fang J, Zhou J, Yokomizo K. 2023. Extract essence of seven Chinese herbs potentially exhibit anti-inflammatory and cancer-preventive effects. Food Materials Research 3:19 doi: 10.48130/FMR-2023-0019
Extract essence of seven Chinese herbs potentially exhibit anti-inflammatory and cancer-preventive effects
- Received: 05 April 2023
- Accepted: 19 June 2023
- Published online: 04 September 2023
Abstract: In recent decades, natural products have gained increasing attention as potential therapies for chronic diseases, including inflammation and cancer. Among them, a hot water extract derived from seven traditional Chinese herbs (Lycii fructus, Crataegi fructus, Phyllanthi fructus, Chrysanthemi flos, Coicis semen, Ganoderma lucidum, and Zizyphi fructus), referred to as LLA, has demonstrated potent antioxidative effects. In line with this, our study aimed to investigate the anti-inflammatory properties of LLA and explore its potential for cancer prevention. To evaluate the anti-inflammatory effects of LLA, we employed a dextran sulfate sodium (DSS)-induced mouse colitis model. Daily oral administration of LLA significantly suppressed colitis symptoms, improving the disease activity index and mitigating colon tissue damage. Moreover, LLA treatment effectively inhibited the production of proinflammatory cytokines, including IL-6, TNF-α, and MCP-1. In addition to its anti-inflammatory effects, LLA exhibited remarkable efficacy in suppressing mouse colon carcinogenesis induced by azoxymethane (AOM) and DSS. Administration of LLA during the AOM/DSS treatment resulted in a significant reduction in tumor occurrence and growth, highlighting its potential as a preventive agent against colon cancer. Furthermore, we investigated the antitumor effect of LLA using a mouse sarcoma S180 solid tumor model. Although LLA did not demonstrate significant antitumor effects on established tumors, its application prior to tumor formation significantly inhibited tumor occurrence and growth. Collectively, these findings underscore the beneficial effects of LLA administration in the context of inflammatory diseases and highlight its potential as a preventive measure against cancer.
-
Key words:
- Natural products /
- Extract essence /
- Chinese herbs /
- Reactive oxygen species /
- Cancer prevention /
- Anti-inflammation













